Abstract
Endophilin B1 is a member of the endophilin family that is localized predominantly to intracellular membranes. Also known as Bax-interacting factor-1 (Bif-1), this protein has been observed to regulate the membrane dynamics of various intracellular compartments, such as the control of mitochondrial morphology and autophagosome formation in fibroblast. Endophilin B1 is expressed in the brain, but its functions in neurons had remained unknown. Recently, we have observed a novel role of endophilin B1 in neurons where it controls the trafficking of TrkA, cognate receptor for the prototypic neurotrophin nerve growth factor (NGF). Knock-down of endophilin B1 expression induces precocious targeting of NGF/TrkA to late endosomes and lysosomes, thereby leading to reduced TrkA levels. This is accompanied by marked attenuation of NGF-induced gene transcription and neurite outgrowth. Our observations suggest that endophilin B1 regulates TrkA level and downstream functions by controlling the movement of TrkA to late endosomes/lysosomes, possibly acting at the level of early endosomes.
It has been demonstrated for a myriad of surface receptors that ligand binding triggers internalization of the ligand/receptor complex. Nonetheless, various internalized receptors are trafficked in distinct manners. While some internalized receptors are rapidly recycled to cell surface, other are transported to the lysosomes for degradation.Citation1,Citation2 Endocytosis of surface receptors through a clathrin-dependent manner often leads to trafficking of the internalized receptors to the endocytic pathway. Clathrin-coated vesicles containing the endocytosed receptors enter the endocytic pathway by fusing with early endosomes, single membrane vesicular structures characterized by the recruitment of early endosome antigen 1 (EEA1) and a small GTPase Rab5. Early endosomes then serve as a triage for receptors that will be recycled or degraded. Recycling of receptors either occurs directly through vesicles that contain the small GTPase Rab4, or via trafficking to Rab11-containing recycling endosomes. On the other hand, receptors directed to the degradative pathway are transported to Rab7-containing late endosomes (also known as multi-vesicular bodies), and subsequently to the lysosomes where the receptors are degraded by lysosomal proteases.Citation1,Citation2 In a recent study, we identified endophilin B1 as a molecule that controls the movement of the neurotrophin nerve growth factor (NGF) and its cognate receptor TrkA along the endocytic pathway.Citation3 Neurotrophins are a family of trophic factors pivotal for the survival, differentiation and synaptic functions of neurons.Citation4 By staining against various markers of the endocytic pathway, we demonstrated that a portion of the internalized NGF/TrkA is directed to the degradative pathway. Interestingly, knock-down of endophilin B1 expression accelerates movement of NGF/TrkA to the late endosomes and lysosomes, in addition to enhancing the percentage of NGF/TrkA that are eventually targeted to these two compartments. The consequence of precocious targeting of NGF/TrkA to the degradative compartments is a reduction in total TrkA level in endophilin B1 knocked-down cells, and not surprisingly, attenuation of NGF-induced gene transcription and neurite outgrowth.Citation3 Since endophilin B1 forms a complex with EEA1 and also co-localizes with EEA1 immunoreactivity, it seems that endophilin B1 possibly controls the movement of NGF/TrkA through modulating their trafficking at the early endosomes/late endosomes junction. On the other hand, we found that knock-down of endophilin B1 expression also reduces targeting of NGF/TrkA to Rab4- and Rab11-positive vesicles, suggesting that endophilin B1 also affects recycling of NGF/TrkA.Citation3 Knock-down of endophilin B1 thus decreases the pool of available TrkA by reducing recycling while concurrently facilitating degradation of the receptor. Collectively, these observations indicate an essential role of endophilin B1 in coordinating the trafficking of NGF/TrkA from the early endosomes to different pathways, thereby controlling TrkA level ().
Endophilin B1 was initially implicated in the regulation of mitochondrial morphology and apoptosis in fibroblasts.Citation5,Citation6 Recently, endophilin B1 was also found to be required for autophagy induction in fibroblasts.Citation7 Nonetheless, despite the abundant expression of endophilin B1 in the brain,Citation3,Citation8 its functions in neurons had remained unknown. Our observations revealed a novel role of endophilin B1 in neurons where it regulates the trafficking of NGF/TrkA.Citation3 Given the strategic location of endophilin B1 on early endosomes, it is plausible that endophilin B1 may regulate the trafficking of all surface receptors. Nonetheless, we found that endophilin B1 fails to affect trafficking of transferrin receptor.Citation3 Consistent with this observation, endophilin B1 does not associate with transferrin receptor, in contrast to its interaction with TrkA.Citation3 This suggests that endophilin B1 does not merely function as a master switch that sits on early endosomes to direct movement of cargos, but it only regulates the movement of certain receptors. The mechanisms underlying this selectivity will clearly be a subject of intense future investigation, but existing evidence provided some interesting hints. Since the association between TrkA and endophilin B1 occurs even in the absence of ligand stimulation, it appears that the receptor selectivity displayed by endophilin B1 occurs partly through selective binding of endophilin B1 with various receptors. In support of this possibility, preliminary observations revealed that endophilin B1 also associates with TrkB, which is structurally similar to TrkA, but not epidermal growth factor receptor. It will thus be important to further delineate the mechanisms by which selective binding of endophilin B1 to different receptors are achieved. Endophilin B1 is characterized by an N-BAR domain for lipid binding, and a SH3 domain for interaction with other SH3 domain-containing interacting proteins. It will be interesting to determine the region on endophilin B1 that is required for its association with Trk receptors. Furthermore, it will be pivotal to elucidate the specific domain that mediates the regulation of NGF/TrkA trafficking by endophilin B1, which will provide some much needed insights on the mechanisms by which endophilin B1 modulates NGF/TrkA trafficking at the early endosomes.
It should be noted that in addition to serving as an entry point to the endocytic pathway, the early endosomes have also been suggested to serve as carriers for internalized Trk receptors destined for intracellular transport, for example, along neuritis.Citation9–Citation11 Importantly, the mediation of retrograde survival signals by NGF was found to require retrograde transport of NGF/TrkA complex. Recent studies revealed that internalized NGF/TrkA complexes are transported in the form of “signaling endosomes”, where the receptors remain activated, thus enabling continued activation of signaling pathways within the cells. Inhibition of TrkA internalization and dynein-based transport of the receptor abolish NGF-mediated retrograde survival signals, thus revealing the importance of transported NGF/TrkA complexes in the role of NGF as target-derived neurotrophic factor.Citation12–Citation14 Interestingly, the signaling molecules recruited onto “signaling endosomes” are different from those activated by surface TrkA receptors.Citation15 In agreement with this observation, it was demonstrated using compartmentalized cultures that NGF applied to distal axons triggers different signaling events at the cell body compared to that induced by direct NGF treatment at the cell body.Citation16 These observations collectively suggest that signaling pathways activated by transported NGF/TrkA complexes are distinct from those initiated by cell surface TrkA, and may hence serve different functions.
Given the observed localization of endophilin B1 on early endosomes that also contain TrkA, and early endosomes being the potential carrier of transported NGF/TrkA, it will be of interest to examine if endophilin B1 also regulates retrograde transport of NGF/TrkA, thereby affecting the mediation of retrograde survival signals. Indeed, since the role of endophilin B1 is to apparently prevent premature targeting of NGF/TrkA to the degradative pathway, it is plausible that endophilin B1 may function to maintain NGF/TrkA on early endosomes to enable long distance transport. Furthermore, recruitment of Erk1/2 onto endosomes is nearly abolished when the expression of endophilin B1 was knocked-down, while recruitment and activation of Akt is essentially unaffected.Citation3 This indicates that the reduced Erk1/2 activation on endosomes is not purely a consequence of reduced TrkA level and activation on endosomes, but that endophilin B1 may also play a role in modulating the recruitment of signaling molecules onto endosomes. In light of the initiation of distinct signaling cascades by “signaling endosomes” and surface receptors, our observations hint at the interesting possibility that endophilin B1 may regulate retrograde survival signals mediated by neurotrophins through modulating signaling from endosomes. We are in the process of elucidating this possibility, which we believe will provide novel insights on the function of neurotrophins as target-derived neurotrophic factors.
Abbreviations
| Bif-1 | = | Bax-interacting factor-1 |
| EEA1 | = | early endosome antigen 1 |
| NGF | = | nerve growth factor |
Figures and Tables
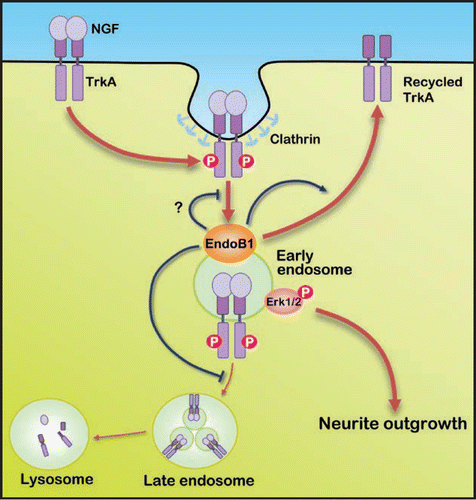
Figure 1 A schematic depicting the potential role of endophilin B1 in NGF/TrkA trafficking and downstream function. Binding of NGF to TrkA triggers internalization of the NGF/TrkA complex through clathrin-mediated endocytosis. Subsequent to clathrin uncoating, the vesicles containing the internalized NGF/TrkA complex fuse with early endosomes, where endophilin B1 interacts and co-localizes extensively with early endosome marker EEA1. Endophilin B1 possibly controls the trafficking of internalized NGF/TrkA from early endosomes to late endosomes, as knock-down of endophilin B1 expression results in premature targeting of TrkA to late endosomes and lysosomes, leading to reduced total TrkA level. Endophilin B1 also appears to facilitate recycling of NGF/TrkA to the cell surface. Nonetheless, whether endophilin B1 regulates endocytosis and the trafficking of NGF/TrkA from plasma membrane to early endosomes remain to be elucidated. Our data suggest that by preventing precocious targeting of NGF/TrkA to the degradation pathway, endophilin B1 preserves TrkA level and contributes to Erk1/2 activation on endosomes, thereby enabling NGF-induced neurite outgrowth.
Acknowledgements
This work was supported in part by the Research Grants Council of Hong Kong (HKUST 6119/04M, 6431/06M, 661007 and HKUST 1/06C) and the Area of Excellence Scheme of the University Grants Committee (AoE/B-15/01). We thank Ka Chun Lok for his excellent help in preparing the figure.
Addendum to:
References
- Gaborik Z, Hunyady L. Intracellular trafficking of hormone receptors. Trends Endocrinol Metab 2004; 15:286 - 293
- Miaczynska M, Zerial M. Mosaic organization of the endocytic pathway. Exp Cell Res 2002; 272:8 - 14
- Wan J, Cheung AY, Fu WY, Wu C, Zhang M, Mobley WC, et al. Endophilin B1 as a novel regulator of nerve growth factor/TrkA trafficking and neurite outgrowth. J Neurosci 2008; 28:9002 - 9012
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem 2003; 72:609 - 642
- Karbowski M, Jeong SY, Youle RJ. Endophilin B1 is required for the maintenance of mitochondrial morphology. J Cell Biol 2004; 166:1027 - 1039
- Takahashi Y, Karbowski M, Yamaguchi H, Kazi A, Wu J, Sebti SM, et al. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol Cell Biol 2005; 25:9369 - 9382
- Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y, et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat Cell Biol 2007; 9:1142 - 1151
- Modregger J, Schmidt AA, Ritter B, Huttner WB, Plomann M. Characterization of Endophilin B1b, a brain-specific membrane-associated lysophosphatidic acid acyl transferase with properties distinct from endophilin A1. J Biol Chem 2003; 278:4160 - 4167
- Neet KE, Campenot RB. Receptor binding, internalization and retrograde transport of neurotrophic factors. Cell Mol Life Sci 2001; 58:1021 - 1035
- Ginty DD, Segal RA. Retrograde neurotrophin signaling: Trk-ing along the axon. Curr Opin Neurobiol 2002; 12:268 - 274
- Howe CL, Mobley WC. Long-distance retrograde neurotrophic signaling. Curr Opin Neurobiol 2005; 15:40 - 48
- Delcroix JD, Valletta JS, Wu C, Hunt SJ, Kowal AS, Mobley WC. NGF signaling in sensory neurons: evidence that early endosomes carry NGF retrograde signals. Neuron 2003; 39:69 - 84
- Ye H, Kuruvilla R, Zweifel LS, Ginty DD. Evidence in support of signaling endosome-based retrograde survival of sympathetic neurons. Neuron 2003; 39:57 - 68
- Heerssen HM, Pazyra MF, Segal RA. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nat Neurosci 2004; 7:596 - 604
- Wu C, Lai CF, Mobley WC. Nerve growth factor activates persistent Rap1 signaling in endosomes. J Neurosci 2001; 21:5406 - 5416
- Watson FL, Heerssen HM, Bhattacharyya A, Klesse L, Lin MZ, Segal RA. Neurotrophins use the Erk5 pathway to mediate a retrograde survival response. Nat Neurosci 2001; 4:981 - 988