Abstract
Membrane fusion with vacuoles, the lysosome equivalent of the yeast Saccharomyces cerevisiae, is among the best undestood membrane fusion events. Our precise understanding of this fusion machinery stems from powerful genetics and elegant in vitro reconstitution assays. Central to vacuolar membrane fusion is the multi-subunit tether the HOmotypic fusion and Protein Sorting (HOPS) complex, a complex of proteins that organizes other necessary components of the fusion machinery. We lack a similarly detailed molecular understanding of membrane fusion with lysosomes or lysosome-related organelles in metazoans. However, it is likely that fundamental principles of how rabs, SNAREs and HOPS tethers work to fuse membranes with lysosomes and related organelles are conserved between Saccharomyces cerevisiae and metazoans. Here, we discuss emerging differences in the coat-dependent mechanisms that govern HOPS complex subcellular distribution between Saccharomyces cerevisiae and metazoans. These differences reside upstream of the membrane fusion event. We propose that the differences in how coats segregate class C Vps/HOPS tethers to organelles and domains of metazoan cells are adaptations to complex architectures that characterize metazoan cells such as those of neuronal and epithelial tissues.
Fundamental Insights from the Yeast Saccharomyces cerevisiae
The HOPS complex was originally discovered in the yeast Saccharomyces cerevisiae. Genetic screens in S. cerevisiae for mutant strains deficient in the localization of lysosomal enzymes revealed phenotypes in vacuole morphology and vacuolar protein secretion.Citation1–Citation4 The gene products of these strains came to be referred to as the vacuole protein sorting (Vps) proteins.Citation3 Vps mutant strains were classified as class A–F based on vacuolar characteristics and morphology.Citation1,Citation3 Among those are the class B and C Vps genes, which encode subunits of the HOPS complex. Class B Vps strains were characterized by fragmentation of vacuolesCitation3 and class C Vps strains were characterized by the absence of any visible vacuolar compartment.Citation3 The class B proteins Vps39 and Vps41 and the class C proteins Vps11, Vps16, Vps18, and Vps33 assemble in a hexameric complex referred as the HOmotypic fusion and Protein Sorting (HOPS) complex ().Citation7–Citation9 The class C proteins Vps11, Vps16, Vps18, and Vps33 assemble in an additional hexameric complex with the Vps3 and Vps8 subunits referred as the class C core vacuole/endosome tethering complex (CORVET, ).Citation10,Citation11 In the HOPS complex the class B and C subunits play specific roles in the tethering, docking, and fusion stages of membrane fusion.Citation3,Citation5,Citation8,Citation9,Citation11–Citation15 A similar function is performed by the class C Vps proteins present in the CORVET complex.Citation10,Citation11 For outstanding reviews please refer to Nickerson et al.,Citation12 Wickner,Citation16 Brocker et al.,Citation15 and Epp et al.Citation17
Elegant studies by Ostrowicz and Plemel lead to a model for HOPS complex organization.Citation5,Citation6 In this model, the class C subunits reside as an extended core of the complex with Vps33 interacting with membrane SNAREs while the class B subunits Vps39 and Vps41 interact with the Rab7-GTPase at the apposed membrane.Citation5,Citation6 This organization of HOPS complex subunits fits with the known functions of individual subunits. The core subunit Vps33 is a member of the Sec1/Munc18 protein family.Citation18–Citation20 Interactions of Vps33 with t-SNAREs through the Sec1/MUNC18 domain may have a role in the recognition of target-specific SNAREs, thereby aiding in trans-SNARE pairing or, may inhibit non-specific trans-SNARE pairs.Citation21 The class B HOPS subunits Vps39 and Vps41 have specificity for interaction with the Rab7 S. cerevisiae homolog Ypt7. In S. cerevisiae, the HOPS subunit Vps39 has been shown to interact with Ypt7. Though Vps39 was originally proposed to serve as the GEF for this vacuolar Rab Ypt7, recent studies in vivo suggest Vps39 is not the Rab7 GEF.Citation9,Citation22,Citation23 Instead, the dimeric Mon1-Ccz1 complex is the Rab7/Ypt7 GEF.Citation24 The subunit Vps41 has also been shown to directly bind Ypt7 and act as the effector for Ypt7 in vacuolar tethering events.Citation12,Citation25 In addition to interactions with the Rab7 homolog, Vps41 has also been shown to interact with the vesicle adaptor AP-3 in S. cerevisiae.Citation23,Citation26–Citation28
Metazoan Mutations in HOPS Subunits Reveal Diverse Functions of the HOPS Complex
S. cerevisiae studies have been instrumental in understanding the role HOPS subunits have in regulating tethering, SNARE pair formation, and fusion of organelles with the vacuolar compartment.Citation1,Citation3–Citation5,Citation7–Citation9,Citation12–Citation15,Citation26–Citation42 Metazoan genetic deficiencies are consistent with the role of the HOPS complex in the delivery of vesicle contents to lysosomes and lysosome-related organelles. However, the existence of HOPS subunit isoforms, metazoan-specific HOPS subunits, such as SPE-39, as well as partially overlapping distribution or function of HOPS subunits along the endocytic route suggest a more complex picture in metazoans than in yeast.Citation43–Citation47
Vps33 exists as two isoforms (a and b) encoded by different genes in C. elegans, Drosophila melanogaster and Homo sapiens. Vps33A and Vps33B are not redundant in Drosophila suggesting distinct tissue and possibly organellar functions.Citation48 Moreover, the Hermansky-Pudlak syndrome (HPS) and Arthrogryposis, Renal Dysfunction and Cholestasis (ARC), affecting Vps33a and Vps33b respectively, further support this idea in mammals. HPS is characterized by occulocutanoeous pigment dilution, prolonged bleeding, and pulmonary fibrosis.Citation49–Citation51 Symptoms of HPS arise from defects in sorting to lysosomes and lysosome-related organelles such as the melanosome and platelet dense granules.Citation49,Citation50,Citation52 In humans, a subset of HPS patients displays mutations to the gene encoding the β subunit of AP-3.Citation53,Citation54 Models for HPS have been discovered in mouse and Drosophila.Citation53,Citation54 In mice, mutations to the genes that encode the clathrin adaptor AP-3 subunits AP-3 β1 and AP-3 δ1 as well as the class C HOPS subunit Vps33a result in decreased coat color, prolonged bleeding, and defects in lysosomal protein targeting.Citation49,Citation52,Citation55,Citation56 Drosophila homologs of class B and C HOPS subunits Vps33a, Vps41, Vps18, and adaptor protein AP-3 subunits also result in pigmentation defects.Citation48,Citation52,Citation57–Citation61 Importantly, mammalian phenotypes in AP-3 and Vps33a deficiencies are distinct from those of patients carrying mutations in Vps33b, a defect that leads to the ARC syndrome. ARC results from mutations to the gene responsible for encoding the class C HOPS subunit Vps33b or the Vps33b-interacting protein SPE-39, which is absent in yeast.Citation46,Citation62,Citation63 Spe39 is also referred as VIPAR in Homo sapiens, a nomenclature that we have disputed.Citation62,Citation64 In Drosophila SPE-39 is known as Vps16bCitation65 despite evidence indicating that SPE-39 possesses unique domains not present in Vps16.Citation45,Citation46 SPE-39 robustly immunoprecipitates Vps16,Citation46 thus suggesting that rather than a Vps16 isoform, SPE-39 is an additional component of class C Vps complexes such as HOPS and a putative mammalian CORVET complex (). Patients with ARC syndrome display a variety of phenotypes including severe contracture of joints referred to as arthrogryposis, defects in renal function, cholestasis, bleeding disorders, dry, thickened, scaly or flaky skin referred to as ichthyosis, defects in metabolic absorption, absence or severe size decrease of the corpus callosum and defective organization of the anterior horn of the spinal cord.Citation62,Citation63,Citation66–Citation84 Importantly, severe joint contracture in patients with ARC syndrome results from a neurological defect as opposed to muscular defects.Citation63,Citation75,Citation80 Analysis of ichthyosis in ARC patients suggests defects in lamellar body secretion as shown by increased amounts of lamellar granules in patients with ARC syndrome by electron microscopy.Citation68,Citation69,Citation72 Kidney epithelial cells and hepatocytes show a loss of apically targeted proteins.Citation71 All of these phenotypes are consistent with a role for Vps33b and SPE-39 in regulation of lysosome-related organelles and secretion in polarized mammalian cell types. Thus, differences between HPS and ARC phenotypes affecting isoforms of class C Vps proteins may reflect differential tissue distribution of Vps33 isoforms. Alternatively, Vps33 isoforms expressed in the same cell may mediate either distinct tethering events between organelles and target membranes whose identities are specified by Vps33a, Vps33b, and SPE-39 and/or differences in cargoes trafficked by SPE-39-, Vps33a-, or Vps33b-dependent mechanisms.
Signaling receptors are attractive cargo candidates for explanation of phenotypic differences in HPS and ARC patients. For example, epidermal growth factor and Notch receptor degradation are impaired in HOPS complex deficiencies.Citation46,Citation85 Notch signaling is critical for embryonic development of neural and epidermal tissues and in the adult Notch modulates synaptic plasticity.Citation86,Citation87 Notch regulation could contribute to the severe neuronal and neuromuscular symptoms in patients with ARC syndrome. In the canonical Notch signaling pathway, Notch binds extracellular ligands and undergoes cleavage at the extra- and intracellular domains.Citation85,Citation86 Following cleavage, the intracellular domain is transported to the nucleus where it regulates gene expression.Citation85,Citation86 In addition, there is an endosomal pathway for Notch activation where Notch receptor is internalized in the absence of extracellular ligand.Citation85 Internalized Notch is targeted into intraluminal vesicles in multi-vesicular bodies where receptor degradation terminates signaling.Citation85 However in the endosomal pathway, which is AP-3- and HOPS-dependent, Notch is sorted away from intralumenal vesicles and remains at the multi-vesicular body limiting membrane for delivery to lysosomes where the intracellular domain undergoes cleavage and translocates to the nucleus.Citation85,Citation86 The proposed role of HOPS subunits in this study was in regulating the fusion of late endosomes with the lysosome.Citation85 Such a model is consistent with the presence of HOPS in late endosomes, as demonstrated in yeastCitation88 and mammalian cells (see below). In such a model an AP-3 pool localized to incoming vesicles would encounter HOPS complexes present in late endosomes resulting in fusion of AP-3 vesicles with late endosomes (see ).Citation28,Citation88,Citation89 An alternative yet not exclusive view, considers that since HOPS and AP-3 interactCitation28,Citation47,Citation88–Citation90 and AP-3 and HOPS are present in vesicle carriersCitation47 (), perhaps the HOPS complex subunits may regulate Notch receptor sorting away from multivesicular bodies, to AP-3-HOPS clathrin-coated vesicles for delivery to lysosomes.
Comparative Cell Biology of S. cerevisiae and Metazoan HOPS-Coat Interactions
The role of HOPS in the membrane fusion event itself is well understood in S. cerevisiae thanks to in vitro vacuole fusion assays.Citation16 However, a comparable assay in a metazoan system does not yet exist, limiting our ability to draw parallels with S. cerevisiae. Thus, we limit our discussion to HOPS subcellular localization and HOPS-coat interactions where information allows a preliminary comparison between unicellular and multicellular eukaryotes. The S. cerevisiae's HOPS subunits are localized to the vacuolar compartment.Citation28,Citation31,Citation36,Citation40,Citation41,Citation91 HOPS complex subunits have not been identified at the Golgi or endosomal intermediates in S. cerevisiae.Citation28 Thus, it is postulated that in S. cerevisiae, cytosolic HOPS complex cycles on and off the target membrane.Citation23 In contrast with this unique localization of HOPS in yeast, metazoan cells yield a different localization of HOPS subunits.Citation46,Citation47,Citation92,Citation93 Vps class C proteins Vps11, 16 and 18 colocalize with early and late endosome compartment markers in mammalian cells.Citation92,Citation94 This observation is consistent with the HOPS complex interaction with mammalian late endosomal SNARE syntaxin-7 and Rab7.Citation92,Citation95 However, some studies indicate that the class C Vps proteins Vps11, 16, 18, and 33b as well as the HOPS subunits Vps39 and Vps41 mostly localize to early endosomes but minimally with late endosomal and lysosomal markers.Citation47,Citation93 These data suggest that additional mechanisms beyond what is present in yeast have evolved to localize HOPS complexes to diverse endosomal compartments in metazoans.
The different HOPS localization patterns observed in S. cerevisiae and mammals could be due to changes in the organization of AP-3 trafficking mechanisms that evolved concurrent with multicellularity. In S. cerevisiae, the adaptor AP-3 recruits cargoes at the Golgi compartment for inclusion into vesicles that will be targeted to the vacuole, whereas in mammals, AP-3 functions at the early endosome for vesicle formation.Citation96–Citation100 Unlike mammals, S. cerevisiae AP-3 does not bind clathrin and clathrin is not required for the proper localization of AP-3 cargoes.Citation97,Citation100,Citation101 In contrast, clathrin does bind AP-3 and clathrincoated vesicles contain AP-3 subunits in mammals.Citation90,Citation102–Citation105
Early studies of HOPS subunits suggested interaction of AP-3 with the HOPS subunit Vps41 in S. cerevisiae.Citation26,Citation27 Direct interaction of the ear domain of AP-3 with Vps41 was presumed to occur with the isolated Vps41 subunit and not with the entire HOPS complex.Citation26,Citation27 These findings suggested that Golgi-derived AP-3 vesicles would require Vps41 as a coat complex in lieu of clathrin in S. cerevisiae.Citation26 However, elegant experimentation by Angers et al.Citation28 indicate AP-3 interacts with the whole hexameric HOPS complex rather than with the isolated Vps41 subunit. Furthermore, they showed that HOPS subunits are not associated with the Golgi, where S. cerevisiae AP-3 vesicle formation occurs, or on AP-3 vesicular intermediates.Citation28 Instead the results by Angers et al. as well as Cabrera et al. suggested a model where coated or partially coated AP-3 vesicles reached a vacuole decorated with HOPS complexes. Interestingly, the AP-3 cargo kinase Yck3 phosphorylates the HOPS subunit Vps41, present at the vacuole, making HOPS permissive to bind AP-3 in the vesicle. It is at the vacuole that an AP-3-HOPS interaction materializes as a step of the membrane tethering-fusion process ().Citation28,Citation88
Like in S. cerevisiae, mammalian AP-3 and HOPS complexes interact.Citation47,Citation90 However, the temporal and spatial organization of mammalian AP-3 and HOPS complex interactions differ from yeast in the following ways:
HOPS subunits interact and colocalize on early endosomes with the coats, AP-3 and clathrin;Citation47
HOPS subunits co-fractionate with clathrin-coated vesicles;Citation47
HOPS subunits localize to endosomal compartments in a clathrindependent manner as revealed by chemical-genetic perturbation of clathrin;Citation47
HOPS subunits are distributed in a polarized manner by clathrin-dependant mechanism.Citation47
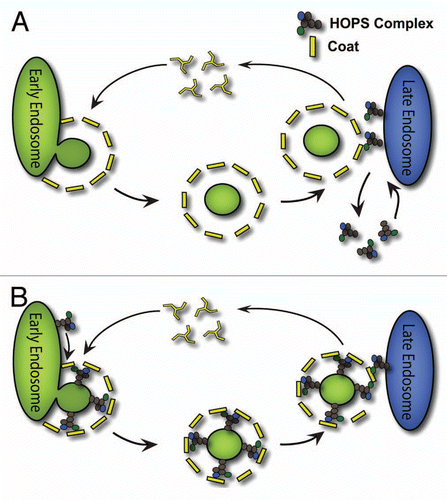
The basic tenet of HOPS complex localization to the yeast vacuole is that it occurs through recruitment from the cytoplasm directly to the vacuolar membrane (). This model supports class B and C Vps HOPS subunits localization exclusively to the vacuole in S. cerevisiae. However, this model is not sufficient to explain the clathrin-dependent subcellular localization of HOPS complexes in mammalian cells.Citation47 Thus, we propose a novel and complementary model of class B and C Vps HOPS subunit localization in mammalian cells (). In this model, HOPS localizes to early endosomes at sites of vesicle formation. HOPS complex subunits are included in clathrin-coated membranes as cargoes for traffic to the late endosome/lysosome or polarized domain of cells, such as those found in epithelial and neuronal cells ().
The prevailing notion is that coats recruit target-specific cargoes for inclusion into vesicles destined for its target.Citation106 However, how does a vesicle “know” its target location?Citation107 Coats could confer information to vesicles for specific fusion with target organelles by inclusion of tethers and SNAREs at the vesicle formation stage (). This view is not represented in canonical models of vesicle-mediated membrane traffic that have conceptually segregated vesicle formation from vesicle fusion machineries for analytical purposes.Citation106 Our work demonstrated that tethers, such as the HOPS complex, are located on coated vesicles. These findings provide a mechanism for target recognition by coupling the vesicle formation and fusion machineries (). In addition, coat-tether associations would provide a coat-dependent vesicular mechanism governing organelle-specific tether localization and delivery. These principles may be universal as suggested by the interaction between the coat COPII and the tether TRAPPI in coated vesicles targeted from the ER to the Golgi complex.Citation108
Figures and Tables
Figure 1 Organization of the class C Vps proteins into the multisubunit tethers HOPS and CORVET. Diagrams depict the proposed architecture of the HOPS and CORVET complexes based on studies in S. cerevisiaeCitation5,Citation6 and a putative organization of HOPS complexes in metazoans. Class C Vps subunits are represented by gray spheres and class B Vps subunits are diagrammed as green and blue spheres, CORVET-specific subunits are depicted by purple spheres. At least four HOPS complexes could exist defined by Vps33a and Vps33b isoforms and the binding of SPE-39 to Vps33 isoforms. The existence of the mammalian CORVET complex has not been documented yet. It may be possible that Vps33a and Vps33b isoforms and the binding of SPE-39 to Vps33 isoforms may define similarly diverse CORVET complexes.
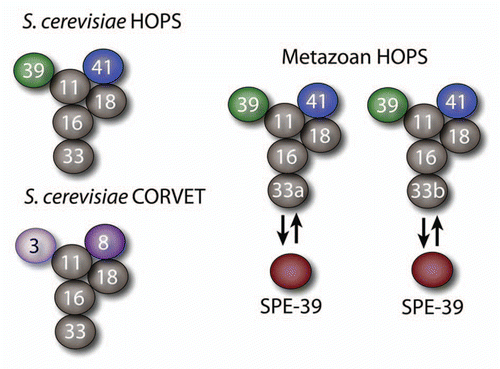
Figure 2 Models of HOPS complex localization to early and late endosome/lysosome compartments. (A) HOPS complex localization to late endosome/lysosomes is specified by the properties of the late endosome/lysosomal membrane. HOPS complexes cycle on and off of the late endosome/lysosomal membrane. Interactions between coat and HOPS occur at the fusion stage where an incoming coated vesicle encounters the acceptor membrane that already contains the HOPS tether. (B) In a second model of HOPS complex localization, the HOPS complex is incorporated into newly forming vesicles at the donor endosome by interactions with, but not restricted to, the coat. A coated vesicle delivers the HOPS complex to a late endosome/lysosomal compartment. Coats confer information to vesicles for specific fusion with target organelles by inclusion of tethers and at the vesicle formation stage in this second model of HOPS localization.
Acknowledgments
This work was supported by grants from the National Institutes of Health to V.F. (NS42599 and GM077569) and S.W.L. (GM082932). S.A.Z. was supported by T32 GM008367, National Institutes of Health, Training Program in Biochemistry, Cell, and Molecular Biology. We are indebted to the Faundez lab members for their comments.
Addendum to:
References
- Banta LM, Robinson JS, Klionsky DJ, Emr SD. Organelle assembly in yeast: characterization of yeast mutants defective in vacuolar biogenesis and protein sorting. J Cell Biol 1988; 107:1369 - 1383; PMID: 3049619; http://dx.doi.org/10.1083/jcb.107.4.1369
- Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein sorting in Saccharomyces cerevisiae: isolation of mutants defective in the delivery and processing of multiple vacuolar hydrolases. Mol Cell Biol 1988; 8:4936 - 4948; PMID: 3062374
- Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell 1992; 3:1389 - 1402; PMID: 1493335
- Wada Y, Anraku Y. Genes for directing vacuolar morphogenesis in Saccharomyces cerevisiae. II. VAM7, a gene for regulating morphogenic assembly of the vacuoles. J Biol Chem 1992; 267:18671 - 18675; PMID: 1526999
- Ostrowicz CW, Brocker C, Ahnert F, Nordmann M, Lachmann J, Peplowska K, et al. Defined subunit arrangement and rab interactions are required for functionality of the HOPS tethering complex. Traffic 2010; 11:1334 - 1346; PMID: 20604902; http://dx.doi.org/10.1111/j.1600-0854.2010.01097.x
- Plemel RL, Lobingier BT, Brett CL, Angers CG, Nickerson DP, Paulsel A, et al. Subunit organization and Rab interactions of Vps-C protein complexes that control endolysosomal membrane traffic. Mol Biol Cell 2011; 22:1353 - 1363; http://dx.doi.org/10.1091/mbc.E10-03-0260
- Nakamura N, Hirata A, Ohsumi Y, Wada Y. Vam2/Vps41p and Vam6/Vps39p are components of a protein complex on the vacuolar membranes and involved in the vacuolar assembly in the yeast Saccharomyces cerevisiae. J Biol Chem 1997; 272:11344 - 11349; PMID: 9111041; http://dx.doi.org/10.1074/jbc.272.17.11344
- Seals DF, Eitzen G, Margolis N, Wickner WT, Price AA. Ypt/Rab effector complex containing the Sec1 homolog Vps33p is required for homotypic vacuole fusion. Proc Natl Acad Sci USA 2000; 97:9402 - 9407; PMID: 10944212; http://dx.doi.org/10.1073/pnas.97.17.9402
- Wurmser AE, Sato TK, Emr SD. New component of the vacuolar class C-Vps complex couples nucleotide exchange on the Ypt7 GTPase to SNARE-dependent docking and fusion. J Cell Biol 2000; 151:551 - 562; PMID: 11062257; http://dx.doi.org/10.1083/jcb.151.3.551
- Markgraf DF, Ahnert F, Arlt H, Mari M, Peplowska K, Epp N, et al. The CORVET subunit Vps8 cooperates with the Rab5 homolog Vps21 to induce clustering of late endosomal compartments. Mol Biol Cell 2009; 20:5276 - 5289; PMID: 19828734; http://dx.doi.org/10.1091/mbc.E09-06-0521
- Peplowska K, Markgraf DF, Ostrowicz CW, Bange G, Ungermann C. The CORVET tethering complex interacts with the yeast Rab5 homolog Vps21 and is involved in endo-lysosomal biogenesis. Dev Cell 2007; 12:739 - 750; PMID: 17488625; http://dx.doi.org/10.1016/j.devcel.2007.03.006
- Nickerson DP, Brett CL, Merz AJ. Vps-C complexes: gatekeepers of endolysosomal traffic. Curr Opin Cell Biol 2009; 21:543 - 551; PMID: 19577915; http://dx.doi.org/10.1016/j.ceb.2009.05.007
- Sato TK, Rehling P, Peterson MR, Emr SD, Class C. Vps protein complex regulates vacuolar SNARE pairing and is required for vesicle docking/fusion. Mol Cell 2000; 6:661 - 671; PMID: 11030345; http://dx.doi.org/10.1016/S1097-2765(00)00064-2
- Wang T, Ming Z, Xiaochun W, Hong W. Rab7: role of its protein interaction cascades in endo-lysosomal traffic. Cell Signal 2011; 23:516 - 521; PMID: 20851765; http://dx.doi.org/10.1016/j.cellsig.2010.09.012
- Bröcker C, Engelbrecht-Vandre S, Ungermann C. Multisubunit tethering complexes and their role in membrane fusion. Curr Biol 2010; 20:R943 - R952; PMID: 21056839; http://dx.doi.org/10.1016/j.cub.2010.09.015
- Wickner W. Membrane fusion: five lipids, four SNAREs, three chaperones, two nucleotides, and a Rab, all dancing in a ring on yeast vacuoles. Annu Rev Cell Dev Biol 2010; 26:115 - 136; PMID: 20521906; http://dx.doi.org/10.1146/annurev-cellbio-100109-104131
- Epp N, Rethmeier R, Kramer L, Ungermann C. Membrane dynamics and fusion at late endosomes and vacuoles - Rab regulation, multisubunit tethering complexes and SNAREs. Eur J Cell Biol 2011; 90:779 - 785; PMID: 21683469; http://dx.doi.org/10.1016/j.ejcb.2011.04.007
- Cai H, Reinisch K, Ferro-Novick S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev Cell 2007; 12:671 - 682; PMID: 17488620; http://dx.doi.org/10.1016/j.devcel.2007.04.005
- Jahn R, Sudhof TC. Membrane fusion and exocytosis. Annu Rev Biochem 1999; 68:863 - 911; PMID: 10872468; http://dx.doi.org/10.1146/annurev.biochem.68.1.863
- Waters MG, Hughson FM. Membrane tethering and fusion in the secretory and endocytic pathways. Traffic 2000; 1:588 - 597; PMID: 11208146; http://dx.doi.org/10.1034/j.1600-0854.2000.010802.x
- Carr CM, Rizo J. At the junction of SNARE and SM protein function. Curr Opin Cell Biol 2010; 22:488 - 495; PMID: 20471239; http://dx.doi.org/10.1016/j.ceb.2010.04.006
- Peralta ER, Martin BC, Edinger AL. Differential effects of TBC1D15 and mammalian Vps39 on Rab7 activation state, lysosomal morphology, and growth factor dependence. J Biol Chem 2010; 285:16814 - 16821; PMID: 20363736; http://dx.doi.org/10.1074/jbc.M110.111633
- Angers CG, Merz AJ. New links between vesicle coats and Rab-mediated vesicle targeting. Semin Cell Dev Biol 2011; 22:18 - 26; PMID: 20643221; http://dx.doi.org/10.1016/j.semcdb.2010.07.003
- Nordmann M, Cabrera M, Perz A, Brocker C, Ostrowicz C, Engelbrecht-Vandre S, et al. The Mon1-Ccz1 complex is the GEF of the late endosomal Rab7 homolog Ypt7. Curr Biol 2010; 20:1654 - 1659; PMID: 20797862; http://dx.doi.org/10.1016/j.cub.2010.08.002
- Brett CL, Plemel RL, Lobinger BT, Vignali M, Fields S, Merz AJ. Efficient termination of vacuolar Rab GTPase signaling requires coordinated action by a GAP and a protein kinase. J Cell Biol 2008; 182:1141 - 1151; PMID: 18809726; http://dx.doi.org/10.1083/jcb.200801001
- Darsow T, Katzmann DJ, Cowles CR, Emr SD. Vps41p function in the alkaline phosphatase pathway requires homo-oligomerization and interaction with AP-3 through two distinct domains. Mol Biol Cell 2001; 12:37 - 51; PMID: 11160821
- Rehling P, Darsow T, Katzmann DJ, Emr SD. Formation of AP-3 transport intermediates requires Vps41 function. Nat Cell Biol 1999; 1:346 - 353; PMID: 10559961; http://dx.doi.org/10.1038/14037
- Angers CG, Merz AJ. HOPS interacts with Apl5 at the vacuole membrane and is required for consumption of AP-3 transport vesicles. Mol Biol Cell 2009; 20:4563 - 4574; PMID: 19741093; http://dx.doi.org/10.1091/mbc.E09-04-0272
- Banta LM, Vida TA, Herman PK, Emr SD. Characterization of yeast Vps33p, a protein required for vacuolar protein sorting and vacuole biogenesis. Mol Cell Biol 1990; 10:4638 - 4649; PMID: 2201898
- Bugnicourt A, Froissard M, Sereti K, Ulrich HD, Haguenauer-Tsapis R, Galan J-M. Antagonistic roles of ESCRT and Vps class C/HOPS complexes in the recycling of yeast membrane proteins. Mol Biol Cell 2004; 15:4203 - 4214; PMID: 15215319; http://dx.doi.org/10.1091/mbc.E04-05-0420
- Cabrera M, Ostrowicz CW, Mari M, LaGrassa TJ, Reggiori F, Ungermann C. Vps41 phosphorylation and the Rab Ypt7 control the targeting of the HOPS complex to endosome-vacuole fusion sites. Mol Biol Cell 2009; 20:1937 - 1948; PMID: 19193765; http://dx.doi.org/10.1091/mbc.E08-09-0943
- Gerhardt B, Kordas TJ, Thompson CM, Patel P, Vida T. The vesicle transport protein Vps33p is an ATP-binding protein that localizes to the cytosol in an energy-dependent manner. J Biol Chem 1998; 273:15818 - 15829; PMID: 9624182; http://dx.doi.org/10.1074/jbc.273.25.15818
- Hickey CM, Stroupe C, Wickner W. The major role of the Rab Ypt7p in vacuole fusion is supporting HOPS membrane association. J Biol Chem 2009; 284:16118 - 16125; PMID: 19386605; http://dx.doi.org/10.1074/jbc.M109.000737
- Iwaki T, Osawa F, Onishi M, Koga T, Fujita Y, Hosomi A, et al. Characterization of vps33+, a gene required for vacuolar biogenesis and protein sorting in Schizosaccharomyces pombe. Yeast 2003; 20:845 - 855; PMID: 12868054; http://dx.doi.org/10.1002/yea.1011
- Laage R, Ungermann C. The N-terminal domain of the t-SNARE Vam3p coordinates priming and docking in yeast vacuole fusion. Mol Biol Cell 2001; 12:3375 - 3385; PMID: 11694574
- LaGrassa TJ, Ungermann C. The vacuolar kinase Yck3 maintains organelle fragmentation by regulating the HOPS tethering complex. J Cell Biol 2005; 168:401 - 414; PMID: 15684030; http://dx.doi.org/10.1083/jcb.200407141
- McVey Ward D. hVPS41 is expressed in multiple isoforms and can associate with vesicles through a RING-H2 finger motif. Exp Cell Res 2001; 267:126 - 134; PMID: 11412045; http://dx.doi.org/10.1006/excr.2001.5244
- Poupon V, Stewart A, Gray SR, Piper RC, Luzio JP. The role of mVps18p in clustering, fusion, and intracellular localization of late endocytic organelles. Mol Biol Cell 2003; 14:4015 - 4027; PMID: 14517315; http://dx.doi.org/10.1091/mbc.E03-01-0040
- Starai VJ, Hickey CM, Wickner W. HOPS proof-reads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell 2008; 19:2500 - 2508; PMID: 18385512; http://dx.doi.org/10.1091/mbc.E08-01-0077
- Wang L. Hierarchy of protein assembly at the vertex ring domain for yeast vacuole docking and fusion. J Cell Biol 2003; 160:365 - 374; PMID: 12566429; http://dx.doi.org/10.1083/jcb.200209095
- Wang L, Seeley ES, Wickner W, Merz AJ. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell 2002; 108:357 - 369; PMID: 11853670; http://dx.doi.org/10.1016/S0092-8674(02)00632-3
- Cowles CR, Snyder WB, Burd CG, Emr SD. Novel Golgi to vacuole delivery pathway in yeast: identification of a sorting determinant and required transport component. EMBO J 1997; 16:2769 - 2782; PMID: 9184222; http://dx.doi.org/10.1093/emboj/16.10.2769
- Sriram V, Krishnan KS, Mayor S. deep-orange and carnation define distinct stages in late endosomal biogenesis in Drosophila melanogaster. J Cell Biol 2003; 161:593 - 607; PMID: 12743107; http://dx.doi.org/10.1083/jcb.200210166
- Swetha MG, Sriram V, Krishnan KS, Oorschot VM, Ten Brink C, Klumperman J, et al. Lysosomal membrane protein composition, acidic pH and sterol content are regulated via a light-dependent pathway in metazoan cells. Traffic 2011; 12:1037 - 1055; PMID: 21535339; http://dx.doi.org/10.1111/j.1600-0854.2011.01214.x
- Zhu GD, L'Hernault SW. The Caenorhabditis elegans spe-39 gene is required for intracellular membrane reorganization during spermatogenesis. Genetics 2003; 165:145 - 157; PMID: 14504223
- Zhu GD, Salazar G, Zlatic SA, Fiza B, Doucette MM, Heilman CJ, et al. SPE-39 family proteins interact with the HOPS complex and function in lysosomal delivery. Mol Biol Cell 2009; 20:1223 - 1240; PMID: 19109425; http://dx.doi.org/10.1091/mbc.E08-07-0728
- Zlatic SA, Tornieri K, L'Hernault SW, Faundez V. Clathrin-dependent mechanisms modulate the subcellular distribution of class C Vps/HOPS tether subunits in polarized and nonpolarized cells. Mol Biol Cell 2011; 22:1699 - 1715; PMID: 21411634; http://dx.doi.org/10.1091/mbc.E10-10-0799
- Akbar MA, Ray S, Krämer H. The SM protein Car/Vps33A regulates SNARE-mediated trafficking to lysosomes and lysosome-related organelles. Mol Biol Cell 2009; 20:1705 - 1714; PMID: 19158398; http://dx.doi.org/10.1091/mbc.E08-03-0282
- Bonifacino JS. Insights into the biogenesis of lysosome-related organelles from the study of the Hermansky-Pudlak syndrome. Ann N Y Acad Sci 2004; 1038:103 - 114; PMID: 15838104; http://dx.doi.org/10.1196/annals.1315.018
- Pierson DM, Ionescu D, Qing G, Yonan AM, Parkinson K, Colby TC, et al. Pulmonary fibrosis in hermansky-pudlak syndrome. a case report and review. Respiration 2006; 73:382 - 395; PMID: 16490934; http://dx.doi.org/10.1159/000091609
- Hermansky F, Pudlak P. Albinism associated with hemorrhagic diathesis and unusual pigmented reticular cells in the bone marrow: report of two cases with histochemical studies. Blood 1959; 14:162 - 169; PMID: 13618373
- Huizing M, Boissy RE, Gahl WA. Hermansky-Pudlak syndrome: vesicle formation from yeast to man. Pigment Cell Res 2002; 15:405 - 419; PMID: 12453182; http://dx.doi.org/10.1034/j.1600-0749.2002.02074.x
- Di Pietro SM, Dell'Angelica EC. The cell biology of Hermansky-Pudlak syndrome: recent advances. Traffic 2005; 6:525 - 533; PMID: 15941404; http://dx.doi.org/10.1111/j.1600-0854.2005.00299.x
- Wei ML. Hermansky-Pudlak syndrome: a disease of protein trafficking and organelle function. Pigment Cell Res 2006; 19:19 - 42; PMID: 16420244; http://dx.doi.org/10.1111/j.1600-0749.2005.00289.x
- Swank RT, Novak EK, McGarry MP, Rusiniak ME, Feng L. Mouse models of Hermansky Pudlak syndrome: a review. Pigment Cell Res 1998; 11:60 - 80; PMID: 9585243; http://dx.doi.org/10.1111/j.1600-0749.1998.tb00713.x
- Suzuki T, Oiso N, Gautam R, Novak EK, Panthier J-J, Suprabha PG, et al. The mouse organellar biogenesis mutant buff results from a mutation in Vps33a, a homologue of yeast vps33 and Drosophila carnation. Proc Natl Acad Sci USA 2003; 100:1146 - 1150; PMID: 12538872; http://dx.doi.org/10.1073/pnas.0237292100
- Mullins C, Hartnell LM, Bonifacino JS. Distinct requirements for the AP-3 adaptor complex in pigment granule and synaptic vesicle biogenesis in Drosophila melanogaster. Mol Gen Genet 2000; 263:1003 - 1014; PMID: 10954086; http://dx.doi.org/10.1007/PL00008688
- Mullins C, Hartnell LM, Wassarman DA, Bonifacino JS. Defective expression of the mu3 subunit of the AP-3 adaptor complex in the Drosophila pigmentation mutant carmine. Mol Gen Genet 1999; 262:401 - 412; PMID: 10589826; http://dx.doi.org/10.1007/s004380051099
- Sevrioukov EA, He JP, Moghrabi N, Sunio A, Kramer H. A role for the deep orange and carnation eye color genes in lysosomal delivery in Drosophila. Mol Cell 1999; 4:479 - 486; PMID: 10549280; http://dx.doi.org/10.1016/S1097-2765(00)80199-9
- Shestopal SA, Makunin IV, Belyaeva ES, Ashburner M, Zhimulev IF. Molecular characterization of the deep orange (dor) gene of Drosophila melanogaster. Mol Gen Genet 1997; 253:642 - 648; PMID: 9065698; http://dx.doi.org/10.1007/s004380050367
- Warner TS, Sinclair DA, Fitzpatrick KA, Singh M, Devlin RH, Honda BM. The light gene of Drosophila melanogaster encodes a homologue of VPS41, a yeast gene involved in cellular-protein trafficking. Genome 1998; 41:236 - 243; PMID: 9644832
- Cullinane AR, Straatman-Iwanowska A, Zaucker A, Wakabayashi Y, Bruce CK, Luo G, et al. Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat Genet 2010; 42:303 - 312; PMID: 20190753; http://dx.doi.org/10.1038/ng.538
- Gissen P, Tee L, Johnson CA, Genin E, Caliebe A, Chitayat D, et al. Clinical and molecular genetic features of ARC syndrome. Hum Genet 2006; 120:396 - 409; PMID: 16896922; http://dx.doi.org/10.1007/s00439-006-0232-z
- L'Hernault SW, Faundez V. On the endosomal function and gene nomenclature of human SPE-39. Nat Genet 2011; 43:176; PMID: 21350494; http://dx.doi.org/10.1038/ng0311-176
- Pulipparacharuvil S, Akbar MA, Ray S, Sevrioukov EA, Haberman AS, Rohrer J, et al. Drosophila Vps16A is required for trafficking to lysosomes and biogenesis of pigment granules. J Cell Sci 2005; 118:3663 - 3673; PMID: 16046475; http://dx.doi.org/10.1242/jcs.02502
- Kim SM, Chang HK, Song JW, Koh H, Han SJ. Agranular platelets as a cardinal feature of ARC syndrome. J Pediatr Hematol Oncol 2010; 32:253 - 258; PMID: 20224444; http://dx.doi.org/10.1097/MPH.0b013e3181c3a8d0
- Arhan E, Yusufoğlu AM, Sayli TR. Arc syndrome without arthrogryposis, with hip dislocation and renal glomerulocystic appearance: a case report. Eur J Pediatr 2009; 168:995 - 998; PMID: 18972129; http://dx.doi.org/10.1007/s00431-008-0860-5
- Bull LN, Mahmoodi V, Baker AJ, Jones R, Strautnieks SS, Thompson RJ, et al. VPS33B mutation with ichthyosis, cholestasis, and renal dysfunction but without arthrogryposis: incomplete ARC syndrome phenotype. J Pediatr 2006; 148:269 - 271; PMID: 16492441; http://dx.doi.org/10.1016/j.jpeds.2005.10.005
- Choi H-J, Lee M-W, Choi J-H, Moon K-C, Koh J-K. Ichthyosis associated with ARC syndrome: ARC syndrome is one of the differential diagnoses of ichthyosis. Pediatr Dermatol 2005; 22:539 - 542; PMID: 16354257; http://dx.doi.org/10.1111/j.1525-1470.2005.00135.x
- Cullinane AR, Straatman-Iwanowska A, Seo JK, Ko JS, Song KS, Gizewska M, et al. Molecular investigations to improve diagnostic accuracy in patients with ARC syndrome. Hum Mutat 2009; 30:E330 - E337; PMID: 18853461; http://dx.doi.org/10.1002/humu.20900
- Gissen P, Johnson CA, Morgan NV, Stapelbroek JM, Forshew T, Cooper WN, et al. Mutations in VPS33B, encoding a regulator of SNARE-dependent membrane fusion, cause arthrogryposis-renal dysfunction-cholestasis (ARC) syndrome. Nat Genet 2004; 36:400 - 404; PMID: 15052268; http://dx.doi.org/10.1038/ng1325
- Hershkovitz D, Mandel H, Ishida-Yamamoto A, Chefetz I, Hino B, Luder A, et al. Defective lamellar granule secretion in arthrogryposis, renal dysfunction, and cholestasis syndrome caused by a mutation in VPS33B. Arch Dermatol 2008; 144:334 - 340; PMID: 18347289; http://dx.doi.org/10.1001/archderm.144.3.334
- Jang JY, Kim KM, Kim G-H, Yu E, Lee J-J, Park YS, et al. Clinical characteristics and VPS33B mutations in patients with ARC syndrome. J Pediatr Gastroenterol Nutr 2009; 48:348 - 354; PMID: 19274792; http://dx.doi.org/10.1097/MPG.0b013e31817fcb3f
- Taha D, Khider A, Cullinane AR, Gissen P. A novel VPS33B mutation in an ARC syndrome patient presenting with osteopenia and fractures at birth. Am J Med Genet A 2007; 143A:2835 - 2837; PMID: 17994566; http://dx.doi.org/10.1002/ajmg.a.32051
- Eastham KM, McKiernan PJ, Milford DV, Ramani P, Wyllie J, van't Hoff W, et al. ARC syndrome: an expanding range of phenotypes. Arch Dis Child 2001; 85:415 - 420; PMID: 11668108; http://dx.doi.org/10.1136/adc.85.5.415
- Jang WY, Cho TJ, Bae JY, Jung HW, Ko JS, Park MS, et al. Orthopaedic manifestations of arthrogryposisrenal dysfunction-cholestasis syndrome. J Pediatr Orthop 2011; 31:107 - 112; PMID: 21150740; http://dx.doi.org/10.1097/BPO.0b013e3182032c83
- Parsch K, Pietrzak S. Arthrogryposis multiplex congenita. Orthopade 2007; 36:281 - 292; PMID: 17323063; http://dx.doi.org/10.1007/s00132-007-1044-0
- Abu-Sa'da O, Barbar M, Al-Harbi N, Taha D. Arthrogryposis, renal tubular acidosis and cholestasis (ARC) syndrome: two new cases and review. Clin Dysmorphol 2005; 14:191 - 196; PMID: 16155421; http://dx.doi.org/10.1097/00019605-200510000-00005
- Tekin N, Durmus-Aydogdu S, Dinleyici EC, Bor O, Bildirici K, Aksit A. Clinical and pathological aspects of ARC (arthrogryposis, renal dysfunction and cholestasis) syndrome in two siblings. Turk J Pediatr 2005; 47:67 - 70; PMID: 15884633
- Hayes JA, Kahr WH, Lo B, Macpherson BA. Liver biopsy complicated by hemorrhage in a patient with ARC syndrome. Paediatr Anaesth 2004; 14:960 - 963; PMID: 15500499; http://dx.doi.org/10.1111/j.1460-9592.2004.01301.x
- Howells R, Ramaswami U. ARC syndrome: an expanding range of phenotypes. Arch Dis Child 2002; 87:170 - 171; PMID: 12138079; http://dx.doi.org/10.1136/adc.87.2.170-c
- Horslen SP, Quarrell OW, Tanner MS. Liver histology in the arthrogryposis multiplex congenita, renal dysfunction, and cholestasis (ARC) syndrome: report of three new cases and review. J Med Genet 1994; 31:62 - 64; PMID: 8151641; http://dx.doi.org/10.1136/jmg.31.1.62
- Denecke J, Zimmer KP, Kleta R, Koch HG, Rabe H, August C, et al. [Arthrogryposis, renal tubular dysfunction, cholestasis (ARC) syndrome: case report and review of the literature]. Klin Padiatr 2000; 212:77 - 80; PMID: 10812557; http://dx.doi.org/10.1055/s-2000-9656
- Abdullah MA, Al-Hasnan Z, Okamoto E, Abomelha AM. Arthrogryposis, renal dysfunction and cholestasis syndrome. Saudi Med J 2000; 21:297 - 299; PMID: 11533803
- Wilkin M, Tongngok P, Gensch N, Clemence S, Motoki M, Yamada K, et al. Drosophila HOPS and AP-3 complex genes are required for a Deltex-regulated activation of notch in the endosomal trafficking pathway. Dev Cell 2008; 15:762 - 772; PMID: 19000840; http://dx.doi.org/10.1016/j.devcel.2008.09.002
- Lai EC. Notch signaling: control of cell communication and cell fate. Development 2004; 131:965 - 973; PMID: 14973298; http://dx.doi.org/10.1242/dev.01074
- Pierfelice T, Alberi L, Gaiano N. Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 2011; 69:840 - 855; PMID: 21382546; http://dx.doi.org/10.1016/j.neuron.2011.02.031
- Cabrera M, Langemeyer L, Mari M, Rethmeier R, Orban I, Perz A, et al. Phosphorylation of a membrane curvature-sensing motif switches function of the HOPS subunit Vps41 in membrane tethering. J Cell Biol 2010; 191:845 - 859; PMID: 21079247; http://dx.doi.org/10.1083/jcb.201004092
- Anand VC, Daboussi L, Lorenz TC, Payne GS. Genome-wide analysis of AP-3-dependent protein transport in yeast. Mol Biol Cell 2009; 20:1592 - 1604; PMID: 19116312; http://dx.doi.org/10.1091/mbc.E08-08-0819
- Salazar G, Zlatic S, Craige B, Peden AA, Pohl J, Faundez V. Hermansky-Pudlak syndrome protein complexes associate with phosphatidylinositol 4-kinase type II alpha in neuronal and non-neuronal cells. J Biol Chem 2009; 284:1790 - 1802; PMID: 19010779; http://dx.doi.org/10.1074/jbc.M805991200
- Nakamura N, Hirata A, Ohsumi Y, Wada Y. Vam2/Vps41p and Vam6/Vps39p are components of a protein complex on the vacuolar membranes and involved in the vacuolar assembly in the yeast Saccharomyces cerevisiae. J Biol Chem 1997; 272:11344 - 11349; PMID: 9111041; http://dx.doi.org/10.1074/jbc.272.17.11344
- Kim BY, Kramer H, Yamamoto A, Kominami E, Kohsaka S, Akazawa C. Molecular characterization of mammalian homologues of class C Vps proteins that interact with syntaxin-7. J Biol Chem 2001; 276:29393 - 29402; PMID: 11382755; http://dx.doi.org/10.1074/jbc.M101778200
- Richardson SC, Winistorfer SC, Poupon V, Luzio JP, Piper RC. Mammalian late vacuole protein sorting orthologues participate in early endosomal fusion and interact with the cytoskeleton. Mol Biol Cell 2004; 15:1197 - 1210; PMID: 14668490; http://dx.doi.org/10.1091/mbc.E03-06-0358
- Chirivino D, Del Maestro L, Formstecher E, Hupe P, Raposo G, Louvard D, et al. The ERM proteins interact with the HOPS complex to regulate the maturation of endosomes. Mol Biol Cell 2011; 22:375 - 385; PMID: 21148287; http://dx.doi.org/10.1091/mbc.E10-09-0796
- Caplan S, Hartnell LM, Aguilar RC, Naslavsky N, Bonifacino JS. Human Vam6p promotes lysosome clustering and fusion in vivo. J Cell Biol 2001; 154:109 - 122; PMID: 11448994; http://dx.doi.org/10.1083/jcb.200102142
- Piper RC, Bryant NJ, Stevens TH. The membrane protein alkaline phosphatase is delivered to the vacuole by a route that is distinct from the VPS-dependent pathway. J Cell Biol 1997; 138:531 - 545; PMID: 9245784; http://dx.doi.org/10.1083/jcb.138.3.531
- Stepp JD, Huang K, Lemmon SK. The yeast adaptor protein complex, AP-3, is essential for the efficient delivery of alkaline phosphatase by the alternate pathway to the vacuole. J Cell Biol 1997; 139:1761 - 1774; PMID: 9412470; http://dx.doi.org/10.1083/jcb.139.7.1761
- Peden AA, Rudge RE, Lui WWY, Robinson MS. Assembly and function of AP-3 complexes in cells expressing mutant subunits. J Cell Biol 2002; 156:327 - 336; PMID: 11807095; http://dx.doi.org/10.1083/jcb.200107140
- Robinson MS. Adaptable adaptors for coated vesicles. Trends Cell Biol 2004; 14:167 - 174; PMID: 15066634; http://dx.doi.org/10.1016/j.tcb.2004.02.002
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell 1997; 91:109 - 118; PMID: 9335339; http://dx.doi.org/10.1016/S0092-8674(01)80013-1
- Vowels JJ, Payne GS. A dileucine-like sorting signal directs transport into an AP-3-dependent, clathrin-independent pathway to the yeast vacuole. EMBO J 1998; 17:2482 - 2493; PMID: 9564031; http://dx.doi.org/10.1093/emboj/17.9.2482
- Borner GH, Harbour M, Hester S, Lilley KS, Robinson MS. Comparative proteomics of clathrin-coated vesicles. J Cell Biol 2006; 175:571 - 578; PMID: 17116749; http://dx.doi.org/10.1083/jcb.200607164
- Peden AA, Oorschot V, Hesser BA, Austin CD, Scheller RH, Klumperman J. Localization of the AP-3 adaptor complex defines a novel endosomal exit site for lysosomal membrane proteins. J Cell Biol 2004; 164:1065 - 1076; PMID: 15051738; http://dx.doi.org/10.1083/jcb.200311064
- Theos AC, Tenza D, Martina JA, Hurbain I, Peden AA, Sviderskaya EV, et al. Functions of adaptor protein (AP)-3 and AP-1 in tyrosinase sorting from endosomes to melanosomes. Mol Biol Cell 2005; 16:5356 - 5372; PMID: 16162817; http://dx.doi.org/10.1091/mbc.E05-07-0626
- Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science 1998; 280:431 - 434; PMID: 9545220; http://dx.doi.org/10.1126/science.280.5362.431
- Bonifacino JS, Glick BS. The mechanisms of vesicle budding and fusion. Cell 2004; 116:153 - 166; PMID: 14744428; http://dx.doi.org/10.1016/S0092-8674(03)01079-1
- Behnia R, Munro S. Organelle identity and the sign-posts for membrane traffic. Nature 2005; 438:597 - 604; PMID: 16319879; http://dx.doi.org/10.1038/nature04397
- Lord C, Bhandari D, Menon S, Ghassemian M, Nycz D, Hay J, et al. Sequential interactions with Sec23 control the direction of vesicle traffic. Nature 2011; 473:181 - 186; PMID: 21532587; http://dx.doi.org/10.1038/nature09969