Abstract
PAKs 4, 5 and 6 are members of the group B family of p21-activated kinases. Among this group, PAK4 has been most extensively studied. While it has essential roles in embryonic development, in adults high levels of PAK4 are frequently associated with cancer. PAK4 is overexpressed in a variety of cancers, and the Pak4 gene is amplified in some cancers. PAK4 overexpression is sufficient to cause oncogenic transformation in cells and in mouse models. The tight connection between PAK4 and cancer make it a promising diagnostic tool as well as a potential drug target. The group B PAKs also have important developmental functions. PAK4 is important for many early developmental processes, while PAK5 and PAK6 play roles in learning and memory in mice. This chapter provides an overview of the roles of the group B PAKs in cancer as well as development, and includes a discussion of PAK mediated signaling pathways and cellular functions.
Introduction
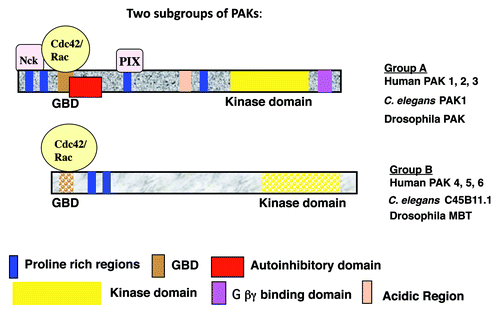
The p21 activated kinase (PAK) family of Ser/Thr kinases were first identified as effector proteins for Cdc42 and Rac, members of the Rho GTPase family, but they can also respond to Rho GTPase independent signals. The PAKs fall into two groups (A and B), based on their sequences and functions (see ).
Group A includes mammalian PAK1, PAK2 and PAK3.Citation1-Citation3 Each of these kinases has an N-terminal regulatory domain and a C-terminal kinase domain. Within the regulatory domain is a GTPase binding domain (GBD), which binds to activated Rac or Cdc42. PAK1-3 also have several other conserved motifs, as illustrated in . Cdc42 or Rac stimulates their kinase activities, by relieving an intramolecular interaction between the kinase domain and an autoinhibitory domain (AID). In addition to Rho GTPases, PAK1-3 need several other signal transducers for their full activation in cells, such as the SH3 adaptor proteins (PIX and NCK), Tyr-kinases (Fyn and ETK) and sphingolipids.Citation2,Citation4,Citation5 Furrthermore, PAK1-3 are negatively regulated by a few proteins such as merlin, nischarin and the kinase LKB1.
PAK4-6, like PAK1-3, also have N-terminal GBD and a C-terminal kinase domains, but they lack other conserved domains found in PAK1-3 [although PAK5 does contain an AID (auto-inhibitory domain)] (see ). Furthermore, the GBD and kinase domains of PAK4-6 have only approximately 50% identity with those of PAK1-3, and the regulatory domains outside of the GBD are completely different from PAK1-3. PAK4 is the first among the group B PAKs to be cloned.Citation6 PAK4 binds preferentially Cdc42, but it also binds Rac. It differs from PAK1-3 in its substrate specificity, although there is also some overlap.Citation7,Citation8 PAK4 expression is high throughout development, but in many adult tissues PAK4 protein levels are low. In contrast, PAK5 and PAK6 are expressed in a limited number of tissues, and are expressed at an especially high level in the adult brain.Citation9-Citation12 Interestingly, PAK6, which is also expressed in testes and prostate, was shown to have an important role in androgen receptor signaling, in a pathway that is not known to be linked to Rho GTPases.Citation11,Citation13,Citation14 Thus, the group B PAKs may have both Rho GTPase dependent and Rho GTPase independent functions. This chapter will focus on the group B PAKs, their cellular functions, and their roles in oncogenesis and development.
Cellular Activities and Signal Transduction Pathways Mediated by PAK4-6
Cytoskeletal organization
PAK4 promotes filopodia formation in response to activated Cdc42,Citation6 and it appears to be an important link between Cdc42 and filopodia formation. PAK4 also leads to the dissolution of stress fibers and subsequent loss of focal adhesions, partly by inactivating a Rho activator called GEFH1.Citation8 Unlike PAK1 (T423E), the “constitutively activated” PAK5 (S573N) leads to cytoskeletal changes, which include filopodia formation and the formation of neurite-like extensions in neuroblastoma cells.Citation9
Substrates
Like PAK1,Citation15 PAK4 phosphorylates LIM kinase 1 (LIMK1),Citation7 which in turn phosphorylates the actin depolymerization protein cofilin, thereby inhibiting actin depolymerization.Citation16,Citation17 The phosphorylation of LIMK1 by PAK4 may help explain how PAK4 leads to polymerized actin structures such as filopodia. In addition to direct phosphorylation of LIMK1, PAK4 also activates LIMK1 indirectly, via inhibition of SlingShot phosphatase (SSH1), a LIM kinase phosphatase.Citation18 LIMK1 phosphorylation by PAK4 may also be regulated by another protein, DGCRL (DiGeorge critical region 6). DGCRL was identified as a PAK4 binding protein, which has links to cancer and metastasis.Citation19 Binding of DGCR6L to PAK4 enhances LIMK phosphorylation in response to PAK4, and this may promote the migration of gastric cells. In addition to the formation of filopodia, PAK4 and other PAK proteins lead to the dissolution of stress fibers. In the case of PAK1, this has been shown to be mediated by myosin light chain kinase (MLCK). PAK1 directly phosphorylates MLCK, and this in turn leads to stress fiber dissolution.Citation20 In contrast, PAK4 does not phosphorylate MLCK, but it still causes stress fiber dissolution, suggesting that it operates by a different mechanism.Citation8 One possible mediator in PAK4-induced stress fiber dissolution is GefH1, a guanine nucleotide exchange factor (Gef) for Rho. PAK4 specifically phosphorylates GefH1, and this is thought to inhibit its ability to activate Rho, consequently inhibiting stress fiber formation.Citation21
Other group B PAK substrates are linked to cell adhesion. PAK5 interacts with p120- catenin in vitro and leads to its phosphorylation. A constitutively active PAK4 also leads to phosphorylation of p120.Citation22 p120 catenin has important roles in controlling cell shape and adhesion and also has a role in anchorage-independent cell growth.Citation23 The exact role for p120 downstream to group B PAKs, however, is still under investigation. Another substrate that may be important for cell adhesion is a member of the integrin family. Specifically, PAK4 has been shown to phosphorylate the cytoplasmic tail of β-5 integrin. Cytoplasmic tails of integrins have many functions, including key roles in adhesion and migration. Phosphorylation of β-5 integrin may have important implications in cell adhesion and migration in response to PAK4.Citation24
PAK4 phosphorylates a number of substrates that are involved in the regulation of cell growth and survival. For example, PAK4, as well as PAK5 and PAK1-3,Citation25-Citation28 has been shown to phosphorylate cRaf-1. In the case of PAK4, this leads to ERK MAP Kinase activation in primary cells.Citation29 PAK4 and PAK1-3Citation5,Citation30 also phosphorylate the pro-apoptotic protein Bad, which contributes to PAK mediated cell survival.Citation5,Citation30-Citation32 Xenopus and mammalian PAK4 were shown to phosphorylate the GTPase Ran, and Ran phosphorylation increases during mitosis. PAK4-mediated phosphorylation of Ran may regulate the assembly of Ran-dependent complexes on the mitotic spindle, suggesting a role for this complex in mitosis.Citation33
Mechanisms of activation of the group B PAKs
The PAKs bind to Cdc42 and Rac via their GBDs, but the contribution of these Rho GTPases to activation of the PAKs can vary. In the case of the group A PAKs, PAK kinase activity is activated in response to binding to Cdc42 and Rac. This appears to be regulated by an autoinhibitory domain (AID) located within the N-terminal regulatory domain, and overlapping the GBD. The AID may operate by interacting with the kinase domain of a dimerizing PAK. Activated Rho GTPases can bind the AID and relieve the autoinhibition, leading to PAK activation.Citation34 In contrast to the group A PAKs, the group B PAKs are not necessarily activated by Rho GTPases, and it is not clear whether the group A PAKs dimerize or operate via an autoinhibitory mechanism. Instead, Cdc42 or Rac may lead to changes in cellular localization of the group B PAKs, possibly bringing them into proximity to their substrates. An autoinhibitory site has been identified in PAK5, however, and in one study PAK5 has been shown to be activated by activated Cdc42.Citation35 Activation of the PAKs is therefore complex, and may vary among the different PAK family members.
CDC42/RAC-independent activation of PAK4-6
Although PAKs are bind to Cdc42 and Rac, a number of stimuli which could operate independently of these GTPases may also lie upstream of the PAKs. In MDCK epithelial cells, for example, PAK4 is activated by hepatocyte growth factor (HGF) via a signaling pathway that requires PI3 kinase. HGF also causes PAK4 to localize to the cell periphery, and PAK4 is in turn thought to contribute to HGF induced changes in cytoskeletal organization and cell adhesion.Citation36 PAK4 was also shown to interact with the cytoplasmic domain of the keratinocyte growth factor (KGF) receptor in a transformed kidney cell line, and may have a role in KGFR mediated cell survival pathways.Citation37 PAK6 has been linked to androgen receptor signaling,Citation11,Citation13,Citation14 by a mechanism not known to be linked to CDC42/RAC. PAK6 was also shown to be activated by MAP kinase kinase 6 (MKK6) and by the p38 MAP Kinase.Citation38 These data suggest that multiple types of signaling pathways may converge upon PAK4-6 to modulate their activity or cellular localization.
PAK4 and Oncogenesis
Numerous studies point to a role for the PAK kinases in oncogenic transformation and in cellular processes associated with transformation. These include regulation of cell survival, proliferation, cytoskeletal organization and migration.Citation30,Citation39-Citation45 Among PAK4-6, PAK4 is most closely linked to cancer, and it is overexpressed in many cancer cells.Citation39,Citation46-Citation49 Some of the key studies linking PAK4-6 with oncogenesis are described below.
PAK4 promotes anchorage independent growth in cultured cells
Like activated Cdc42,Citation50-Citation52 activated PAK4 promotes anchorage-independent growth in immortalized fibroblasts.Citation8,Citation21 Anchorage independent growth is an important in vitro hallmark of oncogenic transformation, which can be assessed by focus formation in soft agar. Interestingly, constitutively activated PAK4 (S445N) is as efficient as oncogenic RAS mutants in this assay system.Citation8 Dominant negative PAK4 mutants can partially inhibit focus formation of fibroblasts induced by oncogenic Dbl which activates both Rho and CDC42.Citation8
PAK4 and the cell cycle
Precise control of the cell cycle is important for normal cell function. Improper regulation of the cell cycle in contrast, can be associated with uncontrolled growth and cancer. PAK4 protein levels fluctuate throughout the cell cycle. In immortalized fibroblasts they increase early in G1 phase and then decline within several hours, remaining low during the rest of the cycle. Deletion of PAK4 significantly extends the life time of p21, a CDK (cyclin-dependent kinase) inhibitor,Citation22 suggesting that PAK4 is important for p21 degradation. This could have important implications in cell cycle control and cancer. In contrast, in primary cells PAK4 was shown to promote cell cycle arrest and increased levels of cell cycle inhibitory proteins.Citation29 The role for PAK4 in cell cycle regulation is therefore complex, and may be different in primary cells as opposed to established cell lines.
PAK4 promotes cell survival
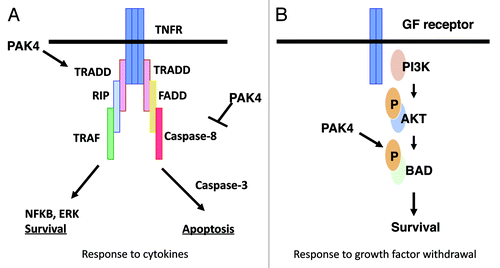
Increased survival in the face of apoptotic stimuli is an important aspect of tumorigenesis. When overexpressed, PAK4 is associated with survival and protection from apoptosis.Citation30,Citation40 Conversely, cells lacking PAK4 have an increased susceptibility toward apoptosis.Citation41 PAK4 promotes cell survival by different mechanisms, depending on the stimulus (see ). In response to serum withdrawal, PAK4 protects cells via a kinase dependent mechanism that is associated with its ability to phosphorylate the pro-apoptotic protein Bad.Citation30 In response to stimuli that activate death domain containing receptors, however, such as TNF or Fas ligand, PAK4 functions via a kinase independent mechanism.Citation40 In this case PAK4 inhibits recruitment of caspase-8 to the DISC complex that forms on the cytoplasmic side of the receptor. This leads to inhibition of caspase-8 activity.Citation40 PAK4 also has a role in activating cell survival pathways, which lead to NFκB and ERK activation.Citation41 Like PAK4, PAK5 also protects cells from apoptosis, and this may be due to direct phosphorylation of BAD.Citation53,Citation54 PAK5 localizes to the mitochondria, and this is required for its protective function.Citation53 PAK5 overexpression also inhibits camptothecin-induced apoptosis in colorectal cancer cells, and this is mediated by inhibition of caspase-8.Citation54
Figure 2. Different mechanisms by which PAK4 inhibits apoptosis. In response to cytokines PAK4 blocks caspase-8 binding to the DISC, thereby inhibiting apoptosis. It also stimulates NFκB and ERK pathways leading to survival. These pathways occur by kinase independent mechanisms. In response to serum withdrawal PAK4 acts by phosphorylating Bad, thereby promoting cell survival. This occurs by a kinase dependent mechanism.
PAK4 plays roles in invasion and migration
Invasion and migration are important parts of the oncogenic process, and they are tightly linked with metastasis. The role for PAK4 in actin cytoskeletal organization suggests an important role for in migration. In fact, when a constitutively active PAK4 mutant was overexpressed in pancreatic ductal cells the result was an increase in migratory capacity and increased invasion in in vitro assays. In contrast, PAK4 siRNA reduces invasion in an in vitro assay in a pancreatic tumor cell line.Citation55 Overexpression of PAK4 also promotes invasion, migration and proliferation of choriocarcinoma cells, and its inhibition has the opposite effect.Citation56 PAK4 was also shown to have a role in migration and adhesion of prostate cancer cells. As described above, PAK4 is regulated by HGF.Citation36 PAK4, along with LIMK1, functions downstream to HGF in prostate cancer cells and in turn mediates cell migration.Citation43 The level of auto-phosphorylated PAK4 is elevated in prostate cancer cells,Citation57 but when PAK4 levels are reduced using siRNA, the cells become deficient in the ability to migrate in response to HGF. The PAK4 deficient cells also have alterations in cytoskeletal organization and adhesion, and they display reduced cell-adhesion turnover rates. These data point to an important link between PAK4 and invasion and migration in several different types of cancer cells. This role may be closely linked to its role in regulating cytoskeletal organization, cell adhesion, and integrin phosphorylation.
Overexpression of PAK4 leads to tumor formation in mice
NIH3T3 cells that stably overexpress PAK4 cause tumors to form when injected into athymic mice.Citation58 Interestingly, while a constitutively active mutant of PAK4 causes tumors to form rapidly, even wild-type PAK4 leads to a high level of tumorigenesis. In the case of wild-type PAK4 tumors takes longer to appear, but ultimately grow to a large size.Citation58 Thus there appears to be a lag time before tumors begin to form in response to wild-type PAK4.Citation58 These results are important because there is little evidence for activating mutants of PAK4 in cancer. Rather, it may be overexpression of wild-type PAK4 that is associated with many types of cancer. It is therefore crucial to understand how wild-type PAK4 can lead to tumor formation and growth. Interestingly, PAK4 causes an increase in cell proliferation, and a corresponding decrease in apoptosis in the mouse tissue well before tumors can be detected.Citation58 These changes in growth characteristics could be part of what primes the cells to become tumor cells after the lag period.
The studies described above indicate that PAK4 is sufficient to form tumors in athymic mice. Another important question is whether PAK4 is also necessary for tumor formation. This seems to be the case at least in part, as the oncogenic Ha-RasV12 expressed in PAK4 knockout (PAK4-/-) fibroblasts forms tumors significantly less efficiently than that in the wild-type control cells.Citation58 Tumors from PAK4-/- cells form more slowly and grow to a smaller size. Furthermore, PAK4-/- tumors that do form are especially bloody in appearance (angiogenic). One possible explanation for this is that the PAK4 null cells begin to undergo apoptosis after a certain point, due to the lack of PAK4 and its corresponding cell survival functions. This in turn would prevent the tumors from growing beyond a certain size. An even more dramatic result was obtained when cells were transfected with Cdc42V12. Although wild-type cells transfected with Cdc42V12 are highly transforming in athymic mice, virtually no tumors form when PAK4-/- cells transfected with Cdc42V12 are used. In the latter study the cells were conditionally PAK4 knockout by the Cre/LoxP system,Citation58 which may eliminate some of the compensatory effects that could be relevant in conventional knockout cells.
PAK4 is overexpressed in cancer
When PAK4 levels were tested in a panel of 60 tumor cell lines, it was found, remarkably, to be overexpressed in almost all of them.Citation12 By comparison, PAK4 is expressed at low levels in most normal tissues. This is in contrast to PAK6, which is highly expressed in a few adult tissues but is not overexpressed in most tumor cell lines.Citation12 In addition to cell lines, PAK4 has been reported to be elevated in many primary tumors. PAK4 was shown to be overexpressed in a subset of gastric tumors, and overexpression of PAK4 tends toward more poor survival rates of gastric cancer patients.Citation59 Liver cancer is frequently linked to PAK4 overexpression. PAK4 is overexpressed in human hepatocellular cancer carcinoma (HCC) samples.Citation59 In these HCC samples, PAK4 overexpression and activation correlates with overexpression of the kinase CDK5-Associated Protein CDK5RAP3. CDK5RAP3 has a role in controlling apoptosis and genotoxic stress.Citation60 It has been implicated both in enhancing and inhibiting cell growthCitation61,Citation62 and is amplified In HCC.Citation63 In HCC CDK5RAP3 is associated with more aggressive tumor behavior and increased invasiveness.Citation64 CDK5RAP3 activates PAK4, and PAK4 is in turn hypothesized to trigger cell invasiveness in HCC.Citation64 Further evidence for the role for PAK4 in liver cancer comes from studying microRNAs.Citation65 The microRNA miR-199a/b-3p is highly expressed in liver, but consistently decreased in HCC. Interestingly, this microRNA, which has an antitumor effect in cells, downregulates PAK4 and blocks the downstream ERK activation,Citation65 strongly linking PAK4 to the etiology of the disease.
Ovarian cancer cell lines have also been reported to have high levels of PAK4. High PAK4 and phospho-PAK4 levels are strongly associated with metastasis of ovarian cancers, and with poor survival and reduced chemosensitivity.Citation66 Knockdown of PAK4 in ovarian cancer cell lines abrogates cell migration, invasion, and proliferation, and it reduces a number of signaling pathways associated with cell growth. It also blocks tumor growth in mice. Overexpression of PAK4 in ovarian cancer cell lines has the opposite effect, and leads to increased cell migration and invasion.Citation66 Breast tumors also frequently express PAK4. PAK4 mRNA levels are elevated in breast cancer cell lines,Citation12 and PAK4 protein level is elevated in human breast tumors and rat mammary tumor samples.Citation58
PAK4 is clearly overexpressed in a variety of cancers and tumor cell lines as described above.Citation12,Citation58,Citation59,Citation66,Citation67 In some cases point mutations were also found in PAK4, such as in some colorectal cancers,Citation68 but in other cases overexpression of wild-type PAK4 may be sufficient for oncogenesis. The mechanism of PAK4 overexpression in cancer is thus of considerable interest. In at least some cases, PAK4 overexpression may be due to gene amplification. The Pak4 gene is located on a chromosomal region that is frequently amplified in cancer. The wild-type Pak4 gene was shown to be amplified in a panel of pancreatic cancer samples, including pancreas ductal adenocarcinomas (PDACs),Citation55,Citation69,Citation70 and squamous cell carcinomas.Citation71 The chromosomal region containing Pak4, 19q13.2, is also frequently amplified at a high rate in aggressive breast cancers with basal-like features.Citation72 The frequent amplification of the Pak4 gene in cancer could make PAK4 an excellent diagnostic tool for early detection of cancer.
PAK4 and breast cancer
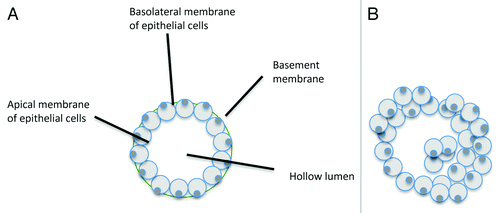
In addition to serving as a marker for cancer, PAK4 may also be a driving force in cancer and thus holds potential as a drug target. In breast cancer, for example, PAK4 is highly overexpressed, and mouse and cell culture models show that it is sufficient to lead to disease. The role for PAK4 in disrupting the normal structure of the mammary gland has been examined in vitro by studying the mouse mammary epithelial cell line iMMEC.Citation73 When grown in 3D culture, iMMECs form spherical acini, mimicking those seen in normal non-cancerous breast epitheliaCitation74 (see ). Normal iMMECs have nearly undetectable levels of PAK4, similar to the normal mammary epithelium. When they are stably transfected with an expression vector containing wild-type PAK4, the resulting acinar structures exhibit several changes that are typically associated with cancer. These include increased cell proliferation and survival, filling of the luminal space with cells,Citation73 increased acinar size, loss of cell polarity, and a thicker layer of epithelial cells surrounding the lumen (see ). Importantly, these changes are reminiscent of changes seen in the glandular epithelium during pre-cancerous conditions and early tumorigenesis. Some of the changes, such as filling of the luminal space, are particularly reminiscent of atypical hyperplasia and DCIS.Citation75 Importantly, the PAK4 expressing cells form tumors at a high frequency when implanted into the mammary fat pads of mice,Citation73 indicating that PAK4 can be a driving force in oncogenic transformation of these cells. Oncogenes such as Her2Neu (ErbB2) and oncogenic Ras also cause iMMECs to become tumorigenic.Citation74,Citation76 Interestingly, the levels of PAK4 are also strongly increased in response to ErbB2 and Ras in iMMECs,Citation73 suggesting a role for PAK4 downstream to these oncogenes in breast cancer.
Figure 3. Diagram of typical acini formed by iMMECs. (A) When grown in 3D culture conditions, wild-type iMMECs form spherical acini with a completely hollow lumen, surrounded by a single layer of polarized epithelial cells. (B) Overexpression of PAK4 leads to formation of acini, which lack a completely hollow, with a thicker outer layer of epithelial cells, and leads to disruption in cell polarity.
One interesting result from the above study was the finding that PAK4 overexpression disrupts cell polarity.Citation73 Alterations in cell polarity can be important in cancer, and the role for PAK4 in polarity is intriguing. It is not yet clear how PAK4 can alter cell polarity when it is overexpressed. It is interesting that PAK4 along with Par6b, have recently been shown to be required for regulation of apical junction formation by Cdc42 in human bronchial epithelial cells.Citation77 Par6 is a cdc42 binding protein, known to play a role in cell polarity.Citation78,Citation79 Its binding to Cdc42 is important for establishing cell polarity, and it will be interesting to determine whether PAK4 may interfere with this interaction when it is overexpressed, thereby disrupting cell polarity in cancer. The exact role for pak4 in cell polarity, and possible downstream mediators, is an area that needs to be explored further.
In addition to PAK4, PAK1 is also associated with breast cancer, and there are both similarities and differences between PAK1 and PAK4 in cell culture and animal models of the disease. Transgenic mice that express the constitutively active PAK1 mutant (T423E) in the mammary gland develop mammary tumors, but at low penetrance and with a long latency period, suggesting that other genetic events are required in the transformation process.Citation80 PAK1 was also shown to be activated in breast cancer cells, specifically in estrogen receptor (ER) negative tumors that overexpress the oncogene Erb-B2.Citation81 Blocking PAK1 activity, in contrast, inhibits transformation of MCF10A cells by ErbB2, and blocks tumor formation in mice in response to ErbB2 positive breast cancer cell lines. The T423E mutant of PAK1 can bypass the requirement for ErbB2 activity in transformation. These results suggest an important role for PAK1 in ErbB2 positive ER negative breast cancer.Citation81 One important difference between the PAK1 and PAK4 studies is that in contrast to PAK1, even overexpression of wild-type PAK4 in mammary epithelial cells and fibroblasts causes them to be tumorigenic in mice, and it does so at a high frequency.Citation58,Citation73 It can be difficult to compare these results because different types of conditions were used for the different studies, but it is intriguing that wild-type PAK4 is sufficient to cause tumorigenesis. This is important because wild-type PAKs are often overexpressed in tumors, and both in vitro studies and mouse models are likely to be increasingly valuable for studying the mechanisms behind PAK4 protein-mediated tumorigenesis.
An interesting cell culture model for breast cancer is the MCF10A progression series. MCF10A, neoT, ATI and DCIS cells are all derived from MCF10A cells. MCF10A represent normal human breast epithelium,Citation82 similar in many respects to the mouse iMMECs. The other cells in the progression series are models for increasing levels of oncogenic transformation.Citation83-Citation85 When grown in 3D culture, there is increasing derangement of normal acinar structure in the more malignant cells. PAK4 levels increase in the more malignant versions of the cells.Citation86 Likewise, PAK1 expression and auto-phosphorylation levels were also shown to increase in the more malignant versions of the cells,Citation87 and the abnormal morphologies can be partially reversed by the expression of dominant negative PAK1. Overexpression of exogenous wild-type or activated PAK1, however, has no effect on cell proliferation, invasion or acinar growth.Citation87 In contrast, as described above, overexpression of wild-type PAK4 has striking effects on mouse iMMECs and causes them to become tumorigenic.Citation73 In other words, PAK1 is essential, but not sufficient for malignant growth, whereas PAK4 is both essential and sufficient for malignant growth of human cells.
PAK4 as a drug target
There is currently a significant amount of interest by pharmaceutical companies in developing drugs that target the different PAK family members in cancer. An inhibitor that blocks PAK4 kinase activity has been generated by Pfizer Oncology. This potent pan-PAK inhibitor, PF-3758309 has growth inhibitory activity toward a large number of tumor cell lines with the IC50 raging 5-15 nM.Citation88,Citation89 The Pfizer inhibitor is currently being tested clinically for effectiveness against various solid tumorsCitation88 (clinicaltrials.gov). A newer PAK4 inhibitor, LCH-7749944 has also been reported recently.Citation90 This PAK4 inhibitor affects several cell signaling pathways. Specifically, it downregulates a pathway mediated by PAK4/c-Src/EGFR and cyclin D1, and it inhibits EGFR activity. LCH-7749944 also affects cell morphology, by blocking filopodia formation and leading to cell elongation. This may be due to the fact that it blocks the cofilin pathway, which is tightly linked to cytoskeletal organization, as well as the ERK/MMP2 (matrix metalloproteinase) pathways. Importantly, this inhibitor suppresses proliferation of human gastric cancer cells and blocks their migration and invasion capacity. These results suggest that a far more (1,000 times) potent derivative (s) of LCH-7749944 could have a cancer therapeutic potential in the future.
PAK4 is a promising drug target for cancer, but in order for PAK4 to be a fully effective drug target, a better understanding of the mechanisms by which it causes oncogenesis may be needed. One area of particular importance is the role for PAK4”s catalytic activity. Most drugs that target protein kinases are designed to block kinase activity, but PAK4 and other PAKs may have some kinase independent functions.Citation30,Citation40,Citation91,Citation92 For example, PAK4 has an important role in suppressing apoptosis, which could be directly related to its role in cancer, but under some conditions this occurs completely independently of PAK4”s kinase activity.Citation30,Citation40,Citation41 PAK4 may thus serve not only as a protein kinase, but it may also have other roles, which could include scaffolding roles, and roles in sequestering other regulatory proteins in the cell. The possibility that PAK4 could promote tumorigenesis by a kinase-independent mechanism, or by a combination of kinase-dependent and kinase-independent mechanisms needs to be considered. In this case, drugs designed specifically to block PAK4 kinase activity alone may be incompletely effective in some types of cancer and new strategies for blocking PAK4 should be investigated. Another consideration is that different PAK family members may have different functions in cancer, and may be overexpressed in some of the same types of cancers, as discussed above for PAK1 and PAK4. The possibility should be considered, therefore, that multiple different PAK family members may need to be blocked, in order to combat some types of cancer.
PAK5 and PAK6 in cancer?
Although PAK4 is most strongly linked to cancer among PAK4-6, PAK5 or PAK6 may also been linked to the disease. For example, both PAK4 and PAK5 can protect pancreatic cells from apoptosis.Citation93 There has also been evidence for PAK5 overexpression in some colorectal cancers, and PAK5 plays a role in invasiveness of colorectal cancer cells.Citation94 PAK6 mRNA overexpression can be detected in certain cancer cells,Citation12 and PAK6 protein levels are elevated in some prostate and breast cancer cell lines.Citation95 PAK6 levels increase in prostate tumors that relapsed after androgen deprivation therapy, and it may play a role in motility and stress responses of tumor cells.Citation95 PAK6 may have a role in radiosensitivity in prostate cancer cells, because inhibition of PAK6 combined with irradiation, significantly decreases survival of prostate cancer cells.Citation96 The role for PAK6 in prostate cancer is not entirely clear though, because it was also identified as a gene that is sometimes hypermethylated in prostate cancer, which is more often associated with genes that suppress tumorigenesis.Citation97 Overall, there is no direct or concrete evidence indicating that either PAK5 or PAK6 is essential for the malignant growth as yet.
The Roles of PAK4-6 in Development
Knockout mice have been developed for all of the group B PAKs, in order to study their roles in development. PAK4 null mice are embryonic lethal, which is consistent with high PAK4 expression during embryogenesis, but conditional PAK4 knockout mice have been generated to study its role in specific tissues. PAK5 and PAK6 knockout mice are viable, but the double knockouts have deficits in learning and memory. Some of the key studies regarding the group B Pak knockout mice are described below.
PAK4 and angiogenesis
PAK4 is highly expressed in embryos but PAK4 protein levels are typically low in adult tissues. PAK4 is absolutely required for embryonic development, as PAK4 null embryos die prior to embryonic day E11.5.Citation98 There are multiple possible causes for embryonic death at this early stage. One interesting phenotype exhibited by the PAK4 null embryos is an abnormality in the blood vessels throughout the embryo and extraembryonic tissue. Although some early vessels form, there is almost a complete lack of branching, suggesting a defect in angiogenesis. This may help explain the early death of the PAK4 null embryos,Citation98 and a role for PAK4 in angiogenesis could also play an important part in the oncogenic process.
The role for PAK4 in the heart
PAK4 null embryos have severe abnormalities in the heart. Pak4-/- embryos have a thinning of the myocardial walls of the bulbus cordis and the ventricle, and the sinus venosus region of the heart is dilated and distorted.Citation98 Conditional deletion of PAK4 specifically in the secondary heart field in mice led to a different result. These mice are viable but they exhibit abnormalities in the outflow track of the heart. Deletion of PAK4 in vitro in cardiomyocytes gives some important clues about its role in heart development. PAK4 null cardiomyocytes exhibit severe disruption of the sarcomeric structure.Citation99 Specifically, the sarcomeres have a drastic reduction in F-actin (actin thin filaments), and the normal striated structure of the sarcomere is disrupted. This is consistent with the role for PAK4 in cytoskeletal organization, although it is also somewhat surprising because the cytoskeleton in the sarcomere is much less dynamic than in other cells such as fibroblasts. Other PAKs have also been shown to have functions in the heart. Ablation of PAK1, for example, does not lead to embryonic lethality, but in adult mice it leads to an increase in stress induced cardiac hypertrophy.Citation100 The roles for PAKs in the heart are important, and need to be taken into consideration if they are used as therapeutic targets for drug development. An important consideration for PAK4 is that PAK4 levels were found to be high in the embryonic heart, but nearly undetectable in adult heart.Citation99 This expression pattern suggests that the role for PAK4 in the heart is likely to be a developmental one, and that its role in the adult heart may be negligible. This raises the promising possibility that inhibition of PAK4 may not lead to adverse side effects in the adult heart.
Group B PAKs in neuronal development
PAK4 knockout mice are embryonic lethal, most likely due to defects in the heart, extraembryonic tissue and blood vessels, but abnormalities are also seen in the developing nervous system. In particular, the neuroepithelium around the brain and spinal cord in PAK4 null embryos are especially thin, and motor neurons fail to develop normally.Citation98 To examine the role for PAK4 in neuronal development in more depth, conditional PAK4 knockout mice were generated in which PAK4 was deleted specifically in the nervous system. PAK4 conditional knockout mice are viable but they display growth retardation and die prematurely. The brains show a dramatic decrease in proliferation of cortical and striatal neuronal progenitor cells. In vitro analysis revealed a reduced proliferation and self-renewing capacity of neural progenitor cells isolated from PAK4 knockout brains. The mice also display cortical thinning, impaired neurogenesis, and loss of neuroepithelial adherens junctions. By the time the mice die, by four weeks after birth, they exhibit severe hydrocephalus.Citation101 These results suggest that PAK4 plays a critical role in neural progenitor cell proliferation and establishing the foundation for development of the brain.
PAK5 and PAK6 are highly expressed in the brain.Citation9,Citation102 PAK5 has been shown to be important for filopodia formation in neuroblastoma cells,Citation9 and PAK6 may be important in the response to traumatic brain injury (TBI).Citation103 The guanine nucleotide exchange factor GEFT is highly expressed in the brain, and was shown to lead to increased dendritic spine formation and neurite outgrowth. Interestingly, studies with dominant negative mutants indicate that both PAK1 and PAK5 have essential roles in these processes.Citation104 Surprisingly though, even though PAK5 and PAK6 are highly expressed in the brain, the phenotypes of PAK5 and PAK6 knockout mice are quite subtle, and much less dramatic than the PAK4 knockouts. Knockout of either PAK5 or PAK6 alone does not result in any noticeable phenotype. Double deletion of PAK5/PAK6 results in mice that are viable and fertile. The double knockout mice do not have noticeable gross abnormalities, but when behavioral tests were performed, it became evident that PAK5 and PAK6 are important for certain neuronal functions.Citation105 Specifically, the double knockout mice display a lower activity level than the wild-type mice, and they have a decreased level of aggression. Importantly, behavioral tests revealed specific deficits in learning and memory. This is consistent with the finding that there are high levels of both PAK5 and PAK6 in the cortex, hippocampus and striatum, which are structures that are critically involved in cognitive functions. No histological abnormalities were observed in the PAK5/PAK6 knockout brains, but in vitro, cultured neurons from the knockouts showed abnormally small growth cones and less neurite outgrowth, which could be related to the role for the group B PAKs in cytoskeletal organization. It is interesting that a Drosophila homolog of the group B PAKs, mushroom body tiny (mbt) is involved in neuronal development, specifically of the cells of the mushroom body, a structure also involved in learning and memory.Citation106 These results strongly support an evolutionarily conserved role for group B PAKs in the development of neurons which are necessary for learning and memory. Recently two new PAK5 substrates were identified which may provide some clues as to the mechanism by which group B PAKs affect learning and memory. These two substrates, Pacsin1 and Synaptojanin1, are phosphorylated by PAK5, and PAK5 stimulates their interactions with each other.Citation107 These two proteins regulate synaptic vesicle endocytosis and recycling, and this study raises the intriguing possibility that the cognitive problems exhibited by the PAK5/PAK6 knockout mice could be due to altered endocytosis and vesicle trafficking at the synapse.
The differences between the phenotypes of the PAK4 null mice and the PAK5/PAK6 knockout mice are interesting, but may have more to do with the expression patterns of the different genes as much as differences in their functions. High levels of PAK5 and PAK6 are generally seen later in development in the brain, compared with PAK4, which is higher during embryogenesis. This would support the idea that PAK4 has a role in early neuronal differentiation, whereas PAK5 and PAK6 have roles later in neuronal development or maintenance. It is also intriguing that the PAK5/PAK6 double knockouts have a more dramatic phenotype than either knockout alone, suggesting functional redundancy (compensation) between these two PAKs.
Conclusions
PAK4-6 have important roles in cellular processes including cytoskeletal organization, cell cycle regulation, and cell survival. PAK4 is frequently overexpressed in cancer,Citation12,Citation58,Citation59,Citation66,Citation67 and in some types of cancer the PAK4 gene is amplified.Citation55,Citation69-Citation72 Furthermore, PAK4 is sufficient to lead to cancer in animal models, particularly in a mouse breast cancer model.Citation73 These studies make PAK4 an attractive candidate both as a diagnostic tool for early detection of cancer, and as a drug target for cancer treatment, In addition to its role in cancer, PAK4-6 were shown to have roles in the nervous system.Citation98,Citation103,Citation105,Citation107 While the role for PAK4 in the nervous system is most likely developmental, PAK5 and PAK6 have roles in learning and memory in mouse models. Other PAK family members have also been linked with learning. Blocking the activity of PAKs1-3 in mice impairs spatial memory function.Citation108 Moreover, mutations in PAK3 are linked with nonsyndromatic mental retardationCitation109 and the Drosophila group B PAK, mbt, is involved in neurogenesis in regions that are important for learning.Citation110 A link between PAK5 and PAK6 in human learning and memory disorders has not yet been established, but it will be interesting to determine whether PAK4-6 could serve a diagnostic role in learning disorders. PAK4-6 also have been shown to be involved in neurite outgrowth in cells and mouse models,Citation9,Citation98,Citation101,Citation105 which raises the question of whether activation or overexpression of these PAKs could be valuable for treating neuronal abnormalities or injury. Because of the link between PAK4 in cancer, however, and such treatments would have to be used with caution.
| Abbreviations: | ||
| PAK | = | p21-activated kinases |
| Ser/Thr | = | serine/threonine |
| N terminal | = | amino terminal |
| C terminal | = | carboxyl terminus |
| GBD | = | GTPase binding domain |
| CDK | = | cyclin dependent kinase |
Acknowledgments
A.M. is supported by Busch Biomedical Research Grant.
References
- Daniels RH, Bokoch GM. p21-activated protein kinase: a crucial component of morphological signaling?. Trends Biochem Sci 1999; 24:350 - 5; http://dx.doi.org/10.1016/S0968-0004(99)01442-5; PMID: 10470034
- Knaus UG, Bokoch GM. The p21Rac/Cdc42-activated kinases (PAKs). Int J Biochem Cell Biol 1998; 30:857 - 62; http://dx.doi.org/10.1016/S1357-2725(98)00059-4; PMID: 9744077
- Sells MA, Chernoff J. Emerging from the Pak: the p21-activated protein kinase family. Trends Cell Biol 1997; 7:162 - 7; http://dx.doi.org/10.1016/S0962-8924(97)01003-9; PMID: 17708935
- Bokoch GM, Reilly AM, Daniels RH, King CC, Olivera A, Spiegel S, et al. A GTPase-independent mechanism of p21-activated kinase activation. Regulation by sphingosine and other biologically active lipids. J Biol Chem 1998; 273:8137 - 44; http://dx.doi.org/10.1074/jbc.273.14.8137; PMID: 9525917
- Tang Y, Zhou H, Chen A, Pittman RN, Field J. The Akt proto-oncogene links Ras to Pak and cell survival signals. J Biol Chem 2000; 275:9106 - 9; http://dx.doi.org/10.1074/jbc.275.13.9106; PMID: 10734042
- Abo A, Qu J, Cammarano MS, Dan C, Fritsch A, Baud V, et al. PAK4, a novel effector for Cdc42Hs, is implicated in the reorganization of the actin cytoskeleton and in the formation of filopodia. EMBO J 1998; 17:6527 - 40; http://dx.doi.org/10.1093/emboj/17.22.6527; PMID: 9822598
- Dan C, Kelly A, Bernard O, Minden A. Cytoskeletal changes regulated by the PAK4 serine/threonine kinase are mediated by LIM kinase 1 and cofilin. J Biol Chem 2001; 276:32115 - 21; http://dx.doi.org/10.1074/jbc.M100871200; PMID: 11413130
- Qu J, Cammarano MS, Shi Q, Ha KC, de Lanerolle P, Minden A. Activated PAK4 regulates cell adhesion and anchorage-independent growth. Mol Cell Biol 2001; 21:3523 - 33; http://dx.doi.org/10.1128/MCB.21.10.3523-3533.2001; PMID: 11313478
- Dan C, Nath N, Liberto M, Minden A. PAK5, a new brain-specific kinase, promotes neurite outgrowth in N1E-115 cells. Mol Cell Biol 2002; 22:567 - 77; http://dx.doi.org/10.1128/MCB.22.2.567-577.2002; PMID: 11756552
- Pandey A, Dan I, Kristiansen TZ, Watanabe NM, Voldby J, Kajikawa E, et al. Cloning and characterization of PAK5, a novel member of mammalian p21-activated kinase-II subfamily that is predominantly expressed in brain. Oncogene 2002; 21:3939 - 48; http://dx.doi.org/10.1038/sj.onc.1205478; PMID: 12032833
- Yang F, Li X, Sharma M, Zarnegar M, Lim B, Sun Z. Androgen receptor specifically interacts with a novel p21-activated kinase, PAK6. J Biol Chem 2001; 276:15345 - 53; http://dx.doi.org/10.1074/jbc.M010311200; PMID: 11278661
- Callow MG, Clairvoyant F, Zhu S, Schryver B, Whyte DB, Bischoff JR, et al. Requirement for PAK4 in the anchorage-independent growth of human cancer cell lines. J Biol Chem 2002; 277:550 - 8; http://dx.doi.org/10.1074/jbc.M105732200; PMID: 11668177
- Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, et al. AR and ER interaction with a p21-activated kinase (PAK6). Mol Endocrinol 2002; 16:85 - 99; http://dx.doi.org/10.1210/me.16.1.85; PMID: 11773441
- Schrantz N, da Silva Correia J, Fowler B, Ge Q, Sun Z, Bokoch GM. Mechanism of p21-activated kinase 6-mediated inhibition of androgen receptor signaling. J Biol Chem 2004; 279:1922 - 31; http://dx.doi.org/10.1074/jbc.M311145200; PMID: 14573606
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signalling to actin cytoskeletal dynamics. Nat Cell Biol 1999; 1:253 - 9; http://dx.doi.org/10.1038/12963; PMID: 10559936
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, et al. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature 1998; 393:805 - 9; http://dx.doi.org/10.1038/31729; PMID: 9655397
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, et al. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature 1998; 393:809 - 12; http://dx.doi.org/10.1038/31735; PMID: 9655398
- Soosairajah J, Maiti S, Wiggan O, Sarmiere P, Moussi N, Sarcevic B, et al. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J 2005; 24:473 - 86; http://dx.doi.org/10.1038/sj.emboj.7600543; PMID: 15660133
- Li X, Ke Q, Li Y, Liu F, Zhu G, Li F. DGCR6L, a novel PAK4 interaction protein, regulates PAK4-mediated migration of human gastric cancer cell via LIMK1. Int J Biochem Cell Biol 2010; 42:70 - 9; http://dx.doi.org/10.1016/j.biocel.2009.09.008; PMID: 19778628
- Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science 1999; 283:2083 - 5; http://dx.doi.org/10.1126/science.283.5410.2083; PMID: 10092231
- Callow MG, Zozulya S, Gishizky ML, Jallal B, Smeal T. PAK4 mediates morphological changes through the regulation of GEF-H1. J Cell Sci 2005; 118:1861 - 72; http://dx.doi.org/10.1242/jcs.02313; PMID: 15827085
- Wong LE, Reynolds AB, Dissanayaka NT, Minden A. p120-catenin is a binding partner and substrate for Group B Pak kinases. J Cell Biochem 2010; 110:1244 - 54; http://dx.doi.org/10.1002/jcb.22639; PMID: 20564219
- Dohn MR, Brown MV, Reynolds AB. An essential role for p120-catenin in Src- and Rac1-mediated anchorage-independent cell growth. J Cell Biol 2009; 184:437 - 50; http://dx.doi.org/10.1083/jcb.200807096; PMID: 19188496
- Li Z, Zhang H, Lundin L, Thullberg M, Liu Y, Wang Y, et al. p21-activated kinase 4 phosphorylation of integrin beta5 Ser-759 and Ser-762 regulates cell migration. J Biol Chem 2010; 285:23699 - 710; http://dx.doi.org/10.1074/jbc.M110.123497; PMID: 20507994
- King AJ, Sun H, Diaz B, Barnard D, Miao W, Bagrodia S, et al. The protein kinase Pak3 positively regulates Raf-1 activity through phosphorylation of serine 338. Nature 1998; 396:180 - 3; http://dx.doi.org/10.1038/24184; PMID: 9823899
- Chaudhary A, King WG, Mattaliano MD, Frost JA, Diaz B, Morrison DK, et al. Phosphatidylinositol 3-kinase regulates Raf1 through Pak phosphorylation of serine 338. Curr Biol 2000; 10:551 - 4; http://dx.doi.org/10.1016/S0960-9822(00)00475-9; PMID: 10801448
- Frost JA, Steen H, Shapiro P, Lewis T, Ahn N, Shaw PE, et al. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J 1997; 16:6426 - 38; http://dx.doi.org/10.1093/emboj/16.21.6426; PMID: 9351825
- Wu X, Carr HS, Dan I, Ruvolo PP, Frost JA. p21 activated kinase 5 activates Raf-1 and targets it to mitochondria. J Cell Biochem 2008; 105:167 - 75; http://dx.doi.org/10.1002/jcb.21809; PMID: 18465753
- Cammarano MS, Nekrasova T, Noel B, Minden A. Pak4 induces premature senescence via a pathway requiring p16INK4/p19ARF and mitogen-activated protein kinase signaling. Mol Cell Biol 2005; 25:9532 - 42; http://dx.doi.org/10.1128/MCB.25.21.9532-9542.2005; PMID: 16227603
- Gnesutta N, Qu J, Minden A. The serine/threonine kinase PAK4 prevents caspase activation and protects cells from apoptosis. J Biol Chem 2001; 276:14414 - 9; PMID: 11278822
- Schürmann A, Mooney AF, Sanders LC, Sells MA, Wang HG, Reed JC, et al. p21-activated kinase 1 phosphorylates the death agonist bad and protects cells from apoptosis. Mol Cell Biol 2000; 20:453 - 61; http://dx.doi.org/10.1128/MCB.20.2.453-461.2000; PMID: 10611223
- Jakobi R, Moertl E, Koeppel MA. p21-activated protein kinase gamma-PAK suppresses programmed cell death of BALB3T3 fibroblasts. J Biol Chem 2001; 276:16624 - 34; http://dx.doi.org/10.1074/jbc.M007753200; PMID: 11278362
- Bompard G, Rabeharivelo G, Frank M, Cau J, Delsert C, Morin N. Subgroup II PAK-mediated phosphorylation regulates Ran activity during mitosis. J Cell Biol 2010; 190:807 - 22; http://dx.doi.org/10.1083/jcb.200912056; PMID: 20805321
- Eswaran J, Soundararajan M, Kumar R, Knapp S. UnPAKing the class differences among p21-activated kinases. Trends Biochem Sci 2008; 33:394 - 403; http://dx.doi.org/10.1016/j.tibs.2008.06.002; PMID: 18639460
- Ching YP, Leong VY, Wong CM, Kung HF. Identification of an autoinhibitory domain of p21-activated protein kinase 5. J Biol Chem 2003; 278:33621 - 4; http://dx.doi.org/10.1074/jbc.C300234200; PMID: 12860998
- Wells CM, Abo A, Ridley AJ. PAK4 is activated via PI3K in HGF-stimulated epithelial cells. J Cell Sci 2002; 115:3947 - 56; http://dx.doi.org/10.1242/jcs.00080; PMID: 12244132
- Lu Y, Pan ZZ, Devaux Y, Ray P. p21-activated protein kinase 4 (PAK4) interacts with the keratinocyte growth factor receptor and participates in keratinocyte growth factor-mediated inhibition of oxidant-induced cell death. J Biol Chem 2003; 278:10374 - 80; http://dx.doi.org/10.1074/jbc.M205875200; PMID: 12529371
- Kaur R, Liu X, Gjoerup O, Zhang A, Yuan X, Balk SP, et al. Activation of p21-activated kinase 6 by MAP kinase kinase 6 and p38 MAP kinase. J Biol Chem 2005; 280:3323 - 30; http://dx.doi.org/10.1074/jbc.M406701200; PMID: 15550393
- Eswaran J, Soundararajan M, Knapp S. Targeting group II PAKs in cancer and metastasis. Cancer Metastasis Rev 2009; 28:209 - 17; http://dx.doi.org/10.1007/s10555-008-9181-4; PMID: 19160016
- Gnesutta N, Minden A. Death receptor-induced activation of initiator caspase 8 is antagonized by serine/threonine kinase PAK4. Mol Cell Biol 2003; 23:7838 - 48; http://dx.doi.org/10.1128/MCB.23.21.7838-7848.2003; PMID: 14560027
- Li X, Minden A. PAK4 functions in tumor necrosis factor (TNF) alpha-induced survival pathways by facilitating TRADD binding to the TNF receptor. J Biol Chem 2005; 280:41192 - 200; http://dx.doi.org/10.1074/jbc.M506884200; PMID: 16227624
- Paliouras GN, Naujokas MA, Park M. Pak4, a novel Gab1 binding partner, modulates cell migration and invasion by the Met receptor. Mol Cell Biol 2009; 29:3018 - 32; http://dx.doi.org/10.1128/MCB.01286-08; PMID: 19289496
- Ahmed T, Shea K, Masters JR, Jones GE, Wells CMA. A PAK4-LIMK1 pathway drives prostate cancer cell migration downstream of HGF. Cell Signal 2008; 20:1320 - 8; http://dx.doi.org/10.1016/j.cellsig.2008.02.021; PMID: 18424072
- Gringel A, Walz D, Rosenberger G, Minden A, Kutsche K, Kopp P, et al. PAK4 and alphaPIX determine podosome size and number in macrophages through localized actin regulation. J Cell Physiol 2006; 209:568 - 79; http://dx.doi.org/10.1002/jcp.20777; PMID: 16897755
- Bao W, Thullberg M, Zhang H, Onischenko A, Strömblad S. Cell attachment to the extracellular matrix induces proteasomal degradation of p21(CIP1) via Cdc42/Rac1 signaling. Mol Cell Biol 2002; 22:4587 - 97; http://dx.doi.org/10.1128/MCB.22.13.4587-4597.2002; PMID: 12052868
- Wells CM, Jones GE. The emerging importance of group II PAKs. Biochem J 2010; 425:465 - 73; http://dx.doi.org/10.1042/BJ20091173; PMID: 20070256
- Whale A, Hashim FN, Fram S, Jones GE, Wells CM. Signalling to cancer cell invasion through PAK family kinases. Front Biosci 2011; 16:849 - 64; http://dx.doi.org/10.2741/3724; PMID: 21196207
- Molli PR, Li D-Q, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene 2009; 28:2545 - 55; http://dx.doi.org/10.1038/onc.2009.119; PMID: 19465939
- Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev 2009; 28:51 - 63; http://dx.doi.org/10.1007/s10555-008-9168-1; PMID: 19165420
- Lin R, Bagrodia S, Cerione R, Manor D. A novel Cdc42Hs mutant induces cellular transformation. Curr Biol 1997; 7:794 - 7; http://dx.doi.org/10.1016/S0960-9822(06)00338-1; PMID: 9368762
- Lin R, Cerione RA, Manor D. Specific contributions of the small GTPases Rho, Rac, and Cdc42 to Dbl transformation. J Biol Chem 1999; 274:23633 - 41; http://dx.doi.org/10.1074/jbc.274.33.23633; PMID: 10438546
- Qiu RG, Abo A, McCormick F, Symons M. Cdc42 regulates anchorage-independent growth and is necessary for Ras transformation. Mol Cell Biol 1997; 17:3449 - 58; PMID: 9154844
- Cotteret S, Jaffer ZM, Beeser A, Chernoff J. p21-Activated kinase 5 (Pak5) localizes to mitochondria and inhibits apoptosis by phosphorylating BAD. Mol Cell Biol 2003; 23:5526 - 39; http://dx.doi.org/10.1128/MCB.23.16.5526-5539.2003; PMID: 12897128
- Wang X, Gong W, Qing H, Geng Y, Wang X, Zhang Y, et al. p21-activated kinase 5 inhibits camptothecin-induced apoptosis in colorectal carcinoma cells. Tumour Biol 2010; 31:575 - 82; http://dx.doi.org/10.1007/s13277-010-0071-3; PMID: 20567954
- Kimmelman AC, Hezel AF, Aguirre AJ, Zheng H, Paik JH, Ying H, et al. Genomic alterations link Rho family of GTPases to the highly invasive phenotype of pancreas cancer. Proc Natl Acad Sci U S A 2008; 105:19372 - 7; http://dx.doi.org/10.1073/pnas.0809966105; PMID: 19050074
- Zhang HJ, Siu MK, Yeung MC, Jiang LL, Mak VC, Ngan HY, et al. Overexpressed PAK4 promotes proliferation, migration and invasion of choriocarcinoma. Carcinogenesis 2011; 32:765 - 71; http://dx.doi.org/10.1093/carcin/bgr033; PMID: 21325635
- Wells CM, Whale AD, Parsons M, Masters JRW, Jones GE. PAK4: a pluripotent kinase that regulates prostate cancer cell adhesion. J Cell Sci 2010; 123:1663 - 73; http://dx.doi.org/10.1242/jcs.055707; PMID: 20406887
- Liu Y, Xiao H, Tian Y, Nekrasova T, Hao X, Lee HJ, et al. The pak4 protein kinase plays a key role in cell survival and tumorigenesis in athymic mice. Mol Cancer Res 2008; 6:1215 - 24; http://dx.doi.org/10.1158/1541-7786.MCR-08-0087; PMID: 18644984
- Ahn HK, Jang J, Lee J, Se Hoon P, Park JO, Park YS, et al. P21-activated kinase 4 overexpression in metastatic gastric cancer patients. Transl Oncol 2011; 4:345 - 9; PMID: 22190998
- Jiang H, Luo S, Li H. Cdk5 activator-binding protein C53 regulates apoptosis induced by genotoxic stress via modulating the G2/M DNA damage checkpoint. J Biol Chem 2005; 280:20651 - 9; http://dx.doi.org/10.1074/jbc.M413431200; PMID: 15790566
- Wang J, An H, Mayo MW, Baldwin AS, Yarbrough WG. LZAP, a putative tumor suppressor, selectively inhibits NF-kappaB. Cancer Cell 2007; 12:239 - 51; http://dx.doi.org/10.1016/j.ccr.2007.07.002; PMID: 17785205
- Stav D, Bar I, Sandbank J. Usefulness of CDK5RAP3, CCNB2, and RAGE genes for the diagnosis of lung adenocarcinoma. Int J Biol Markers 2007; 22:108 - 13; PMID: 17549666
- Raidl M, Pirker C, Schulte-Hermann R, Aubele M, Kandioler-Eckersberger D, Wrba F, et al. Multiple chromosomal abnormalities in human liver (pre)neoplasia. J Hepatol 2004; 40:660 - 8; http://dx.doi.org/10.1016/j.jhep.2003.12.020; PMID: 15030983
- Mak GW, Chan MM, Leong VY, Lee JM, Yau TO, Ng IO, et al. Overexpression of a novel activator of PAK4, the CDK5 kinase-associated protein CDK5RAP3, promotes hepatocellular carcinoma metastasis. Cancer Res 2011; 71:2949 - 58; http://dx.doi.org/10.1158/0008-5472.CAN-10-4046; PMID: 21385901
- Hou J, Lin L, Zhou W, Wang Z, Ding G, Dong Q, et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011; 19:232 - 43; http://dx.doi.org/10.1016/j.ccr.2011.01.001; PMID: 21316602
- Siu MKY, Chan HY, Kong DS, Wong ESY, Wong OGW, Ngan HYS, et al. p21-activated kinase 4 regulates ovarian cancer cell proliferation, migration, and invasion and contributes to poor prognosis in patients. Proc Natl Acad Sci U S A 2010; 107:18622 - 7; http://dx.doi.org/10.1073/pnas.0907481107; PMID: 20926745
- Kim JH, Kim HN, Lee KT, Lee JK, Choi SH, Paik SW, et al. Gene expression profiles in gallbladder cancer: the close genetic similarity seen for early and advanced gallbladder cancers may explain the poor prognosis. Tumour Biol 2008; 29:41 - 9; http://dx.doi.org/10.1159/000132570; PMID: 18497548
- Parsons DW, Wang TL, Samuels Y, Bardelli A, Cummins JM, DeLong L, et al. Colorectal cancer: mutations in a signalling pathway. Nature 2005; 436:792; http://dx.doi.org/10.1038/436792a; PMID: 16094359
- Chen S, Auletta T, Dovirak O, Hutter C, Kuntz K, El-ftesi S, et al. Copy number alterations in pancreatic cancer identify recurrent PAK4 amplification. Cancer Biol Ther 2008; 7:1793 - 802; http://dx.doi.org/10.4161/cbt.7.11.6840; PMID: 18836286
- Mahlamäki EH, Kauraniemi P, Monni O, Wolf M, Hautaniemi S, Kallioniemi A. High-resolution genomic and expression profiling reveals 105 putative amplification target genes in pancreatic cancer. Neoplasia 2004; 6:432 - 9; http://dx.doi.org/10.1593/neo.04130; PMID: 15548351
- Begum A, Imoto I, Kozaki K, Tsuda H, Suzuki E, Amagasa T, et al. Identification of PAK4 as a putative target gene for amplification within 19q13.12-q13.2 in oral squamous-cell carcinoma. Cancer Sci 2009; 100:1908 - 16; http://dx.doi.org/10.1111/j.1349-7006.2009.01252.x; PMID: 19594544
- Yu W, Kanaan Y, Bae YK, Gabrielson E. Chromosomal changes in aggressive breast cancers with basal-like features. Cancer Genet Cytogenet 2009; 193:29 - 37; http://dx.doi.org/10.1016/j.cancergencyto.2009.03.017; PMID: 19602461
- Liu Y, Chen N, Cui X, Zheng X, Deng L, Price S, et al. The protein kinase Pak4 disrupts mammary acinar architecture and promotes mammary tumorigenesis. Oncogene 2010; 29:5883 - 94; http://dx.doi.org/10.1038/onc.2010.329; PMID: 20697354
- Karantza-Wadsworth V, White E. A mouse mammary epithelial cell model to identify molecular mechanisms regulating breast cancer progression. Methods Enzymol 2008; 446:61 - 76; http://dx.doi.org/10.1016/S0076-6879(08)01604-2; PMID: 18603116
- Debnath J, Mills KR, Collins NL, Reginato MJ, Muthuswamy SK, Brugge JS. The role of apoptosis in creating and maintaining luminal space within normal and oncogene-expressing mammary acini. Cell 2002; 111:29 - 40; http://dx.doi.org/10.1016/S0092-8674(02)01001-2; PMID: 12372298
- Karantza-Wadsworth V, Patel S, Kravchuk O, Chen G, Mathew R, Jin S, et al. Autophagy mitigates metabolic stress and genome damage in mammary tumorigenesis. Genes Dev 2007; 21:1621 - 35; http://dx.doi.org/10.1101/gad.1565707; PMID: 17606641
- Wallace SW, Durgan J, Jin D, Hall A. Cdc42 regulates apical junction formation in human bronchial epithelial cells through PAK4 and Par6B. Mol Biol Cell 2010; 21:2996 - 3006; http://dx.doi.org/10.1091/mbc.E10-05-0429; PMID: 20631255
- Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene 2008; 27:6958 - 69; http://dx.doi.org/10.1038/onc.2008.346; PMID: 19029937
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci 2006; 119:979 - 87; http://dx.doi.org/10.1242/jcs.02898; PMID: 16525119
- Wang RA, Zhang H, Balasenthil S, Medina D, Kumar R. PAK1 hyperactivation is sufficient for mammary gland tumor formation. Oncogene 2006; 25:2931 - 6; http://dx.doi.org/10.1038/sj.onc.1209309; PMID: 16331248
- Arias-Romero LE, Villamar-Cruz O, Pacheco A, Kosoff R, Huang M, Muthuswamy SK, et al. A Rac-Pak signaling pathway is essential for ErbB2-mediated transformation of human breast epithelial cancer cells. Oncogene 2010; 29:5839 - 49; http://dx.doi.org/10.1038/onc.2010.318; PMID: 20711231
- Soule HD, Maloney TM, Wolman SR, Peterson WD Jr., Brenz R, McGrath CM, et al. Isolation and characterization of a spontaneously immortalized human breast epithelial cell line, MCF-10. Cancer Res 1990; 50:6075 - 86; PMID: 1975513
- Miller FR, Santner SJ, Tait L, Dawson PJ. MCF10DCIS.com xenograft model of human comedo ductal carcinoma in situ. J Natl Cancer Inst 2000; 92:1185 - 6; http://dx.doi.org/10.1093/jnci/92.14.1185A; PMID: 10904098
- Basolo F, Elliott J, Tait L, Chen XQ, Maloney T, Russo IH, et al. Transformation of human breast epithelial cells by c-Ha-ras oncogene. Mol Carcinog 1991; 4:25 - 35; http://dx.doi.org/10.1002/mc.2940040106; PMID: 2009132
- Dawson PJ, Wolman SR, Tait L, Heppner GH, Miller FR. MCF10AT: a model for the evolution of cancer from proliferative breast disease. Am J Pathol 1996; 148:313 - 9; PMID: 8546221
- So JY, Lee HJ, Kramata P, Minden A, Suh N. Differential expression of key signaling proteins in MCF10 cell lines, a human breast cancer progression model. Mol Cell Pharmacol 2012; 4:31 - 40
- Li Q, Mullins SR, Sloane BF, Mattingly RR. p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer. Neoplasia 2008; 10:314 - 29; PMID: 18392133
- Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A 2010; 107:9446 - 51; http://dx.doi.org/10.1073/pnas.0911863107; PMID: 20439741
- Zhao ZS, Manser E. Do PAKs make good drug targets?. F1000 Biol Rep 2010; 2:70 - 3; PMID: 21173843
- Zhang J, Wang J, Guo Q, Wang Y, Zhou Y, Peng H, et al. LCH-7749944, a novel and potent p21-activated kinase 4 inhibitor, suppresses proliferation and invasion in human gastric cancer cells. Cancer Lett 2012; 317:24 - 32; http://dx.doi.org/10.1016/j.canlet.2011.11.007; PMID: 22085492
- Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J 1998; 17:754 - 64; http://dx.doi.org/10.1093/emboj/17.3.754; PMID: 9451000
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol 1997; 7:202 - 10; http://dx.doi.org/10.1016/S0960-9822(97)70091-5; PMID: 9395435
- Giroux V, Iovanna J, Dagorn JC. Probing the human kinome for kinases involved in pancreatic cancer cell survival and gemcitabine resistance. FASEB J 2006; 20:1982 - 91; http://dx.doi.org/10.1096/fj.06-6239com; PMID: 17012250
- Gong W, An Z, Wang Y, Pan X, Fang W, Jiang B, et al. P21-activated kinase 5 is overexpressed during colorectal cancer progression and regulates colorectal carcinoma cell adhesion and migration. Int J Cancer 2009; 125:548 - 55; http://dx.doi.org/10.1002/ijc.24428; PMID: 19415746
- Kaur R, Yuan X, Lu ML, Balk SP. Increased PAK6 expression in prostate cancer and identification of PAK6 associated proteins. Prostate 2008; 68:1510 - 6; http://dx.doi.org/10.1002/pros.20787; PMID: 18642328
- Zhang M, Siedow M, Saia G, Chakravarti A. Inhibition of p21-activated kinase 6 (PAK6) increases radiosensitivity of prostate cancer cells. Prostate 2010; 70:807 - 16; PMID: 20054820
- Wang Y, Yu Q, Cho AH, Rondeau G, Welsh J, Adamson E, et al. Survey of differentially methylated promoters in prostate cancer cell lines. Neoplasia 2005; 7:748 - 60; http://dx.doi.org/10.1593/neo.05289; PMID: 16207477
- Qu J, Li X, Novitch BG, Zheng Y, Kohn M, Xie JM, et al. PAK4 kinase is essential for embryonic viability and for proper neuronal development. Mol Cell Biol 2003; 23:7122 - 33; http://dx.doi.org/10.1128/MCB.23.20.7122-7133.2003; PMID: 14517283
- Nekrasova T, Minden A. Role for p21-activated kinase PAK4 in development of the mammalian heart. Transgenic Res 2012; 21:797 - 811; http://dx.doi.org/10.1007/s11248-011-9578-7; PMID: 22173944
- Taglieri DM, Monasky MM, Knezevic I, Sheehan KA, Lei M, Wang X, et al. Ablation of p21-activated kinase-1 in mice promotes isoproterenol-induced cardiac hypertrophy in association with activation of Erk1/2 and inhibition of protein phosphatase 2A. J Mol Cell Cardiol 2011; 51:988 - 96; http://dx.doi.org/10.1016/j.yjmcc.2011.09.016; PMID: 21971074
- Tian Y, Lei L, Minden A. A key role for Pak4 in proliferation and differentiation of neural progenitor cells. Dev Biol 2011; 353:206 - 16; http://dx.doi.org/10.1016/j.ydbio.2011.02.026; PMID: 21382368
- Lee SR, Ramos SM, Ko A, Masiello D, Swanson KD, Lu ML, et al. AR and ER interaction with a p21-activated kinase (PAK6). Mol Endocrinol 2002; 16:85 - 99; http://dx.doi.org/10.1210/me.16.1.85; PMID: 11773441
- Zhao W, Yang J, Shi W, Wu X, Shao B, Wu Q, et al. Upregulation of p21-activated Kinase 6 in rat brain cortex after traumatic brain injury. J Mol Histol 2011; 42:195 - 203; http://dx.doi.org/10.1007/s10735-011-9324-8; PMID: 21541790
- Bryan B, Kumar V, Stafford LJ, Cai Y, Wu G, Liu M. GEFT, a Rho family guanine nucleotide exchange factor, regulates neurite outgrowth and dendritic spine formation. J Biol Chem 2004; 279:45824 - 32; http://dx.doi.org/10.1074/jbc.M406216200; PMID: 15322108
- Nekrasova T, Jobes ML, Ting JH, Wagner GC, Minden A. Targeted disruption of the Pak5 and Pak6 genes in mice leads to deficits in learning and locomotion. Dev Biol 2008; 322:95 - 108; http://dx.doi.org/10.1016/j.ydbio.2008.07.006; PMID: 18675265
- Melzig J, Rein KH, Schäfer U, Pfister H, Jäckle H, Heisenberg M, et al. A protein related to p21-activated kinase (PAK) that is involved in neurogenesis in the Drosophila adult central nervous system. Curr Biol 1998; 8:1223 - 6; http://dx.doi.org/10.1016/S0960-9822(07)00514-3; PMID: 9811608
- Strochlic TI, Concilio S, Viaud J, Eberwine RA, Wong LE, Minden A, et al. Identification of neuronal substrates implicates Pak5 in synaptic vesicle trafficking. Proc Natl Acad Sci U S A 2012; 109:4116 - 21; PMID: 22371566
- Hayashi ML, Choi SY, Rao BS, Jung HY, Lee HK, Zhang D, et al. Altered cortical synaptic morphology and impaired memory consolidation in forebrain- specific dominant-negative PAK transgenic mice. Neuron 2004; 42:773 - 87; http://dx.doi.org/10.1016/j.neuron.2004.05.003; PMID: 15182717
- Bienvenu T, des Portes V, McDonell N, Carrieá A, Zemni R, Couvert P, et al. Missense mutation in PAK3, R67C, causes X-linked nonspecific mental retardation. Am J Med Genet 2000; 93:294 - 8; http://dx.doi.org/10.1002/1096-8628(20000814)93:4<294::AID-AJMG8>3.0.CO;2-F; PMID: 10946356
- Melton DW. Gene targeting in the mouse. Bioessays 1994; 16:633 - 8; http://dx.doi.org/10.1002/bies.950160907; PMID: 7980488