Abstract
The p21-activated kinases (PAKs) are downstream effectors of the small G-proteins of the Rac and cdc42 family and have been implicated as essential for cell proliferation and survival. Recent studies have also demonstrated the promise of PAKs as therapeutic targets in various types of cancers. The PAKs are divided into two major groups (group I and II) based on sequence similarities. Although the different roles the PAK groups might play are not well understood, recent efforts have focused on the identification of kinase inhibitors that can discriminate between the two groups. In this review these efforts and newly identified inhibitors will be described and future directions „discussed.
Introduction
The p21-activated kinases (PAKs) are downstream effectors of the Ras-related Rho-family GTPases Rac and Cdc42. As such, the PAKs are involved in the regulation of cell survival, proliferation and migration, making them attractive therapeutic targets for cancer. The biological roles of the PAKs under normal and pathological conditions such as cancer are covered by other reviews in this issue and we will therefore summarize the recent and ongoing research being conducted to develop inhibitors of the PAKs.
The PAK Protein Family and Structure
There are two groups of mammalian p21-activated kinases (PAKs): group I, which is comprised of PAK1, PAK2 and PAK3, and group II consisting of PAK4, PAK5 and PAK6 (). While the groups carry out different functions and have distinct modes of regulation, both groups share a high degree of protein similarity within the kinase domains. The group I PAKs share 93-95% sequence identity within the kinase domain and group II share 75% sequence identity within this domain.Citation1 Comparing group I to II there is an overall 54% sequence identity within this domain.Citation2 Previous studies have indicated that the group I PAKs exist as homodimers that are regulated by an auto-inhibitory domain (AID) from one molecule in the dimer that interacts, in trans with the kinase domain of the other molecule.Citation3 This AID overlaps with a p21-binding domain [PBD, a.k.a. cdc42/Rac interactive binding (CRIB) domain] and upon binding of a GTP-bound form of Rac or cdc42 the auto-inhibition is alleviated (). Recent work from multiple groups now indicates that all PAKs harbor an AID.Citation4 The Kung group has identified an auto-inhibitory fragment at the N-terminal of PAK5 that can inhibit kinase activity in the absence of Cdc42 binding.Citation5 Recently published data showed that PAK5 and PAK6 contain the same related CRIB/AID and that PAK4 contains AID positioned similarly to the PAK I AID ().Citation4 However, in spite of these similarities it appears the mechanisms underlying the activation of the group I and II PAKs are different.
Figure 1. The PAKs and their small-molecule inhibitors. (A) Representation of the domain organization of group I and II PAKs. (B) Molecular structures of small molecule PAK inhibitors.
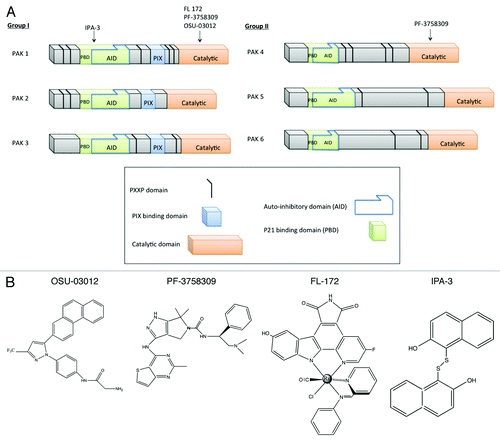
Clearly, one of the major challenges has been subgroup-specific drug design/discovery given the similarity in structure of the PAK-family proteins. Conceptually, a number of approaches can be employed to develop such inhibitors. One possibility would be to develop allosteric inhibitors, which would target regions outside the active site that are divergent between group I and II kinases. An alternative approach would be to take advantage of the sequence differences that do exist between the two groups kinase domains to develop small molecule ATP competitive inhibitors. Such an approach requires a better understanding of the physical structure of the active site of each of the PAKs.
PAK Inhibitors
Allosteric inhibitors
The first report on the identification and characterization of a highly selective allosteric small molecule inhibitor that targets the auto-regulatory mechanism of group I PAKs was described by the Peterson group. By developing a screen for allosteric inhibitors targeting PAK1 activation they identified the inhibitor IPA-3 (p21-activated kinase inhibitor 3). IPA-3 has an IC50 of 2.5 μM and prevents Cdc42-stimulated PAK1 autophosphorylation.Citation6 IPA-3 is highly selective toward group I PAKs. It showed limited activity against the group II PAKs and against a panel of kinases from which it significantly inhibited (> 50% inhibition at 10 μM) only 9 from 214 kinases tested (4% total). Further studies showed that IPA-3 binds covalently to the PAK1 regulatory domain and thus prevents binding to the GTPase Cdc42. The kinase inhibition by IPA-3 in vitro occurs in a temperature-dependent and irreversible manner. However, pre-activated PAK1 is not inhibited by IPA-3.Citation7 Unfortunately, a structural isomer of IPA-3, PIR-3.5, was found to have no inhibitory activity against PAK1. The primary screen of 33,000 structurally diverse small molecules was performed based on catalytic activity of full-length PAK1. A secondary screen was based on identification of compounds that are non-ATP competitive. From 32 compounds shown to inhibit PAK1 activity at 1 mM ATP, IPA-3 was identified as a lead. IPA-3 is clearly an interesting lead compound and further work is required to overcome issues related to the in vivo stability and oral availability of the compound. In particular, the presence of a disulfide bond suggests that the compound might act through covalent redox modification of PAK1. Nonetheless, the approach taken to identify allosteric inhibitors of the PAKs will prove useful for the study of PAKs biology as well as identification of additional inhibitors.
In addition to the small molecule allosteric inhibitors, a number of other inhibitors have been described. These include peptide inhibitors, such as the PAK1 AID, which unfortunately exhibits PAK1-independent effectsCitation8 and the cell-permeable PAK1-inactivating peptide TAT-PAK18, which has been previously suggested to inhibit PAK activity by disrupting the interaction between PAK1 and PIX and to block the growth of ovarian cancer cell lines.Citation9,Citation10
A potential new therapeutic approach relies on the use of RNA interference, delivered by different approaches.Citation11 The Kissil group has previously shown that shRNA-mediated depletion of PAK1, 2 and 3 inhibits the proliferation and tumorigenicity of NIH3T3/NF2bba cells.Citation12 These data suggest that using RNAi-based inhibitors against the PAKs might be a potential tool in future applications for cancer treatment. Nevertheless, questions regarding the delivery approaches for RNAi based therapeutics as well as stability of gene silencing remain to be addressed.
ATP competitive inhibitors
The challenge in the developing of ATP-competitive inhibitors is the high degree of structural similarity between the ATP binding pocket of the PAKs and other kinases. That is exemplified by compounds such as the natural product staurosporine, a broad-range kinase inhibitor, and its derivative K252a which shows potent, but not selective, inhibition of PAK1.Citation13-Citation15 A subsequent synthetic derivative of K252a, CEP-1347, proved to be a more potent inhibitor of PAK1 but later was shown to be about 100-fold more selective toward MLK3 (mixed lineage kinase 3).Citation16 Another example of a kinase inhibitor exhibiting significant PAK inhibitory capacity was provided by the Heerding group.Citation17 From a series of novel AKT inhibitors containing 2,3,5-trisubstituted pyridines, one compound showed significant potency against PAK1 (IC50 31 nM). However, it was also observed to be a potent inhibitor of many other kinases in the AGC superfamily, such as p70S6K, PDK1, PKA and RSK. Work from the Ringel group reported that OSU-03012 is a direct inhibitor of PAK1.Citation18 OSU-03012 is a derivative of celecoxib that competitively inhibits PDK1 in vitro.Citation19 OSU-03012 unexpectedly reduced levels of phosphorylated PAK with an IC50 value of ~1 µM which is lower than required to block PDK1-mediated AKT phosphorylation.Citation18
An interesting approach was taken by the Meggers group to combine organoruthenium chemistry, small-molecule screening and structure-based design to identify a PAK1 inhibitor (FL172) with an IC50 value of about 100 nM.Citation20 The screen of FL172 (at 3 μΜ) against a panel of 264 kinases revealed that only 15 kinases (5.7% of total) were significantly inhibited by this compound. FL172 also showed isoform selectivity by exhibiting poor inhibitory potency against the group II PAKs and displayed dose-dependent PAK1 inhibitor activity when tested in mammalian cells.Citation20 Recently, the same group has reported the development of octahedral metal-based kinase inhibitors, including a highly specific PAK1 inhibitor (OS-2).Citation21 This study demonstrated that octahedral metal complexes are sophisticated molecules providing versatile scaffolds for developing highly selective and potent kinase inhibitors.
Recent work from the Pfizer Oncology Group has identified, through high-throughput screening and structure-based design, the compound PF-3758309, a potent (Kd = 2.7 nM), reversible ATP-competitive, pyrrolopyrazole inhibitor of PAK4.Citation22 PF-3758309 binds the ATP binding site and makes multiple contacts with the hinge region through hydrogen-bond interactions with the pyrrolopyrazole core and the amine linker to the thienopyrimidine ring. PF-3758309 shows similar activity across the group I and II PAKs and displays growth inhibitory activity against a broad range of tumor cell lines. In order to test selectivity, PF-3758309 was screened against a panel of 146 of the 518 known human kinases and was shown to have cellular activity against Src family kinases AMPK, RSK, CHK2 and others. PF-3758309 has been also shown to be a potent anti-tumor agent in human xenograft tumor models, with plasma EC50 value of 0.4 nM.Citation22
PF-3758309 demonstrated oral availability and additional steps have been taken to reduce the efflux of the inhibitor by medicinal chemistry strategies, specifically by lowering the molecular charge. These strategies ultimately lead to the discovery of a number of pyrroloaminopyrazoles as orally bio-available PAK4 inhibitors.Citation23 Two compounds showed good anti- tumor growth activity (52-87%) through oral administration in a xenograft tumor model.
Recently a novel PAK4 inhibitor, LCH-7749944, was developed by the Le group through structure-informed design.Citation24 This ATP-competitive inhibitor was shown to inhibit a number of PAK4 signaling pathways including the PAK4/LIMK1/cofilin and PAK4/MEK-1/ERK1/2/MMP2 pathways as well as EGFR activity.
Future Directions
There is now ample evidence to implicate both PAK families as targets in different types of cancers.Citation25-Citation27 However, a number of questions remain to be answered. First, is there a need for inhibitors that would specifically target the group I or II PAK independently? Second, how difficult would it be to attain this goal?
In regards to the first question, given the different tissue and cell type distributions and substrate specificities of the different PAKs, it is clear that the identification and development of inhibitors that can distinguish between the group I and II PAKs will be instrumental to our understanding of the basic functions of these two groups of proteins, under normal physiological and disease conditions. Moreover, in the context of diseases where evidence exists implicating aberrant activation of PAKs from either of the groups, a major open question is whether inhibiting both groups of PAKs has a confounding effect. In other words, if inhibiting PAK from both groups results in undesired effects, such as reduced tolerability, then clearly inhibitors targeting one group or the other are likely to be beneficial. Further studies using highly selective tool compounds and experimental approaches, such as RNAi-based technologies to knockdown specific PAKs, will no doubt contribute to resolving these questions.
In regards to the second question, the accumulated data on structure and regulation of the two groups of PAKs now provides tools toward the development of selective inhibitors.Citation28 Indeed such efforts have already resulted in competitive inhibitors that display specificity toward one group or the other, as illustrated by a number of examples discussed in this review. Efforts over the next few years using structure-informed design and novel chemistries will likely lead to even more selective and potent inhibitors. In addition, approaches to identify allosteric inhibitors are still in early phases, but will likely prove fruitful, as illustrated by the identification of IPA-3.
References
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell 2000; 103:239 - 52; http://dx.doi.org/10.1016/S0092-8674(00)00116-1; PMID: 11057897
- Jaffer ZM, Chernoff J. p21-activated kinases: three more join the Pak. Int J Biochem Cell Biol 2002; 34:713 - 7; http://dx.doi.org/10.1016/S1357-2725(01)00158-3; PMID: 11950587
- Pirruccello M, Sondermann H, Pelton JG, Pellicena P, Hoelz A, Chernoff J, et al. A dimeric kinase assembly underlying autophosphorylation in the p21 activated kinases. J Mol Biol 2006; 361:312 - 26; http://dx.doi.org/10.1016/j.jmb.2006.06.017; PMID: 16837009
- Baskaran Y, Ng YW, Selamat W, Ling FT, Manser E. Group I and II mammalian PAKs have different modes of activation by Cdc42. EMBO Rep 2012; 13:653 - 9; http://dx.doi.org/10.1038/embor.2012.75; PMID: 22653441
- Ching YP, Leong VY, Wong CM, Kung HF. Identification of an autoinhibitory domain of p21-activated protein kinase 5. J Biol Chem 2003; 278:33621 - 4; http://dx.doi.org/10.1074/jbc.C300234200; PMID: 12860998
- Deacon SW, Beeser A, Fukui JA, Rennefahrt UE, Myers C, Chernoff J, et al. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem Biol 2008; 15:322 - 31; http://dx.doi.org/10.1016/j.chembiol.2008.03.005; PMID: 18420139
- Viaud J, Peterson JR. An allosteric kinase inhibitor binds the p21-activated kinase autoregulatory domain covalently. Mol Cancer Ther 2009; 8:2559 - 65; http://dx.doi.org/10.1158/1535-7163.MCT-09-0102; PMID: 19723886
- Zhao ZS, Manser E, Chen XQ, Chong C, Leung T, Lim L. A conserved negative regulatory region in alphaPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol 1998; 18:2153 - 63; PMID: 9528787
- Hashimoto H, Sudo T, Maruta H, Nishimura R. The direct PAK1 inhibitor, TAT-PAK18, blocks preferentially the growth of human ovarian cancer cell lines in which PAK1 is abnormally activated by autophosphorylation at Thr 423. Drug Discov Ther 2010; 4:1 - 4; PMID: 22491145
- Maruta H, He H, Tikoo A, Nur-e-Kamal M. Cytoskeletal tumor suppressors that block oncogenic RAS signaling. Ann N Y Acad Sci 1999; 886:48 - 57; http://dx.doi.org/10.1111/j.1749-6632.1999.tb09399.x; PMID: 10667202
- Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. RNA interference in the clinic: challenges and future directions. Nat Rev Cancer 2011; 11:59 - 67; http://dx.doi.org/10.1038/nrc2966; PMID: 21160526
- Yi C, Wilker EW, Yaffe MB, Stemmer-Rachamimov A, Kissil JL. Validation of the p21-activated kinases as targets for inhibition in neurofibromatosis type 2. Cancer Res 2008; 68:7932 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-08-0866; PMID: 18829550
- Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J 2007; 408:297 - 315; http://dx.doi.org/10.1042/BJ20070797; PMID: 17850214
- Kaneko M, Saito Y, Saito H, Matsumoto T, Matsuda Y, Vaught JL, et al. Neurotrophic 3,9-bis[(alkylthio)methyl]-and-bis(alkoxymethyl)-K-252a derivatives. J Med Chem 1997; 40:1863 - 9; http://dx.doi.org/10.1021/jm970031d; PMID: 9191963
- Ghoreschi K, Laurence A, O'Shea JJ. Selectivity and therapeutic inhibition of kinases: to be or not to be?. Nat Immunol 2009; 10:356 - 60; http://dx.doi.org/10.1038/ni.1701; PMID: 19295632
- Maroney AC, Finn JP, Connors TJ, Durkin JT, Angeles T, Gessner G, et al. Cep-1347 (KT7515), a semisynthetic inhibitor of the mixed lineage kinase family. J Biol Chem 2001; 276:25302 - 8; http://dx.doi.org/10.1074/jbc.M011601200; PMID: 11325962
- Lin H, Yamashita DS, Xie R, Zeng J, Wang W, Leber J, et al. Tetrasubstituted pyridines as potent and selective AKT inhibitors: Reduced CYP450 and hERG inhibition of aminopyridines. Bioorg Med Chem Lett 2010; 20:684 - 8; http://dx.doi.org/10.1016/j.bmcl.2009.11.061; PMID: 20006500
- Porchia LM, Guerra M, Wang YC, Zhang Y, Espinosa AV, Shinohara M, et al. 2-amino-N-4-[5-(2-phenanthrenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]-phenyl acetamide (OSU-03012), a celecoxib derivative, directly targets p21-activated kinase. Mol Pharmacol 2007; 72:1124 - 31; http://dx.doi.org/10.1124/mol.107.037556; PMID: 17673571
- Zhu J, Huang JW, Tseng PH, Yang YT, Fowble J, Shiau CW, et al. From the cyclooxygenase-2 inhibitor celecoxib to a novel class of 3-phosphoinositide-dependent protein kinase-1 inhibitors. Cancer Res 2004; 64:4309 - 18; http://dx.doi.org/10.1158/0008-5472.CAN-03-4063; PMID: 15205346
- Maksimoska J, Feng L, Harms K, Yi C, Kissil J, Marmorstein R, et al. Targeting large kinase active site with rigid, bulky octahedral ruthenium complexes. J Am Chem Soc 2008; 130:15764 - 5; http://dx.doi.org/10.1021/ja805555a; PMID: 18973295
- Feng L, Geisselbrecht Y, Blanck S, Wilbuer A, Atilla-Gokcumen GE, Filippakopoulos P, et al. Structurally sophisticated octahedral metal complexes as highly selective protein kinase inhibitors. J Am Chem Soc 2011; 133:5976 - 86; http://dx.doi.org/10.1021/ja1112996; PMID: 21446733
- Murray BW, Guo C, Piraino J, Westwick JK, Zhang C, Lamerdin J, et al. Small-molecule p21-activated kinase inhibitor PF-3758309 is a potent inhibitor of oncogenic signaling and tumor growth. Proc Natl Acad Sci U S A 2010; 107:9446 - 51; http://dx.doi.org/10.1073/pnas.0911863107; PMID: 20439741
- Guo C, McAlpine I, Zhang J, Knighton DD, Kephart S, Johnson MC, et al. Discovery of Pyrroloaminopyrazoles as novel PAK inhibitors. J Med Chem 2012; 55:4728 - 39; http://dx.doi.org/10.1021/jm300204j; PMID: 22554206
- Zhang J, Wang J, Guo Q, Wang Y, Zhou Y, Peng H, et al. LCH-7749944, a novel and potent p21-activated kinase 4 inhibitor, suppresses proliferation and invasion in human gastric cancer cells. Cancer Lett 2012; 317:24 - 32; http://dx.doi.org/10.1016/j.canlet.2011.11.007; PMID: 22085492
- Dummler B, Ohshiro K, Kumar R, Field J. Pak protein kinases and their role in cancer. Cancer Metastasis Rev 2009; 28:51 - 63; http://dx.doi.org/10.1007/s10555-008-9168-1; PMID: 19165420
- Ong CC, Jubb AM, Zhou W, Haverty PM, Harris AL, Belvin M, et al. p21-activated kinase 1: PAK'ed with potential. Oncotarget 2011; 2:491 - 6; PMID: 21653999
- Molli PR, Li DQ, Murray BW, Rayala SK, Kumar R. PAK signaling in oncogenesis. Oncogene 2009; 28:2545 - 55; http://dx.doi.org/10.1038/onc.2009.119; PMID: 19465939
- Knight ZA, Shokat KM. Features of selective kinase inhibitors. Chem Biol 2005; 12:621 - 37; http://dx.doi.org/10.1016/j.chembiol.2005.04.011; PMID: 15975507