Abstract
We discuss here the variety of approaches that have been taken to inhibit different forms of endocytosis. Typically, both non-specific and specific chemical inhibitors of endocytosis are tried in order to “classify” entry of a new plasma membrane protein into one of the various types of endocytosis. This classification can be confirmed through genetic approaches of protein depletion or overexpression of mutants of known endocytosis machinery components. Although some new compounds have been designed to be selective in biochemical assays, we caution investigators to be alert to the unintended consequences that sometimes arise when these compounds are applied to intact cells.
Endocytosis is a process that cells use to bring extracellular material and plasma membrane into the cell interior. Once internalized, the fluid, membrane proteins and membrane lipids meet different fates by trafficking to different compartments: to late endosomes and lysosomes for degradation, to recycling endosomes for recycling back to the PM, to the trans Golgi network or to other destinations in the cell. Endocytosis is important for the proper signaling and regulation of cell surface receptors, delivery of nutrients into the cell, establishment and maintenance of cell polarity, and the turnover of PM proteins and lipids. Additionally, bacterial toxins and pathogens use endocytosis as a mode of entry to the cell interior. Understanding how this process occurs and how it is regulated is a goal for cell biologists. Complicating this is the fact that endocytosis takes many forms.
Classically, endocytosis can be divided into pinocytosis and phagocytosis (). Pinocytosis involves internalization of fluid while phagocytosis, an actin-dependent process, involves the internalization of large particles such as bacteria. Pinocytosis can be further divided into those that are dependent on the clathrin coat (clathrin-mediated endocytosis, CME) or those that are independent of clathrin (clathrin-independent endocytosis, CIE). CME has been extensively studied for the past 30 years and the machinery involved in selecting the cargo and initiating and completing the process is well understood.Citation1,Citation2 CME requires adaptor proteins that select and concentrate cargo into clathrin-coated pits and depends on the dynamin GTPase to facilitate vesicle scission.Citation2 By contrast, the variety of forms of CIE observed in different cells has presented a complicated picture, making descriptions of these pathways less clear.
Figure 1. Different types of endocytosis. Endocytosis can be broadly classified into pinocytosis and phagocytosis. Phagocytosis involves the internalization of large particles like bacteria whereas macropinocytosis involves the internalization of enlarged fluid-filled endosomes; both processes are driven by actin (shown as hatched lines). Clathrin-mediated endocytosis (CME) is a selective mechanism whereby cell surface proteins containing specific sorting sequences are gathered into membrane depressions by associating with adaptor proteins which recruit clathrin (*). CME endosomes pinch off from the cell surface by recruiting the dynamin GTPase (•) to the bud neck. Clathrin-independent endocytosis (CIE) is shown here as one form, although there are reports of distinct variations of CIE. Most CIE is clathrin- and dynamin-independent and cholesterol-dependent and includes both the CLIC/GEEC and Arf6-associated forms of CIE. In addition other CIE modes (caveolae- and RhoA-dependent) are dynamin-dependent.
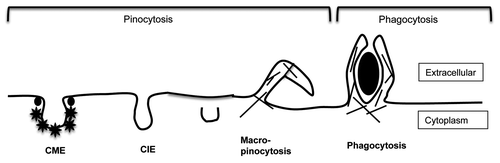
CIE is involved in the internalization of glycolipid-binding toxins, glycosylphosphatidyl inositol-anchored proteins (GPI-AP), and many cell surface proteins (channels, transporters, proteins involved in cell-cell and cell-matrix interactions and in cellular immune function). CIE occurs independently of adaptor proteins and clathrin coats, and mostly does not require dynamin for vesicle scission. CIE pathways are an active area of study. Thus far, it has been shown that the small GTPase Arf6 is associated with the uptake and sorting of many plasma membrane proteinsCitation3 while some lipid-raft associated pathways (called “CLIC/GEEC”)Citation4 are involved in endocytosis of GPI-AP. Rho proteins have been implicated in yet another CIE pathway.Citation5 It is likely that the Arf6 and CLIC-GEEC pathways are closely related since they both are clathrin- and dynamin-independent, cholesterol-dependent and carry GPI-AP into the cell. Finally, macropinocytosis is a stimulated form of CIE where large pinosomes are brought into the cell interior as a consequence of cellular protrusions in an actin-dependent process.Citation6
To better understand the different types of endocytosis, cell biologists have sought ways to block this process through chemical and genetic means. The use of such inhibitors can reveal molecular components required for, and the physiological consequences of blocking, specific forms of endocytosis. These studies contribute to an understanding of the basic mechanism(s) of endocytosis and help define modes of cellular entry for medically relevant components such as signaling receptors, and bacterial and viral pathogens. In this commentary we discuss the approaches that have been taken to block particular forms of endocytosis (see ). These include the use of non-specific chemical inhibitors, the new generation of selective pharmacologic agents, and genetic approaches designed to target a particular form of endocytosis. We will discuss advantages and limitations of each approach.
Table 1. Chemical and genetic endocytosis inhibitors
Classical Chemical Inhibitors of Endocytosis
An attractive feature of chemical inhibitors is that they may be administered acutely to reveal direct inhibition of a particular process. Reversibility of the block can also help support the notion that the effect is specific on the particular mode of endocytosis being examined. Nevertheless, there is always a possibility of indirect effects of the agent influencing another process that will affect that form of endocytosis. For example, inhibitors of actin polymerization, cytochalasin D or latrunculin block both actin-dependent phagocytosis and macropinocytosis, which is not surprising since macropinosomes and phagosomes are coated with actin. Although actin polymerization appears to be required for endocytosis in yeast, this is less clear in mammalian cells.Citation7 Inhibitors of actin polymerization have variable effects on transferrin endocytosis by CME depending on the cell line examined and experimental conditions used.Citation8 Some forms of CIE are not dependent on actin polymerization however recycling of endosomal membrane back to the cell surface is dependent on actin,Citation9,Citation10 which could complicate interpretations of experiments. Thus, care should be taken to ensure that a requirement for a process is a direct and specific one and not a downstream consequence of inhibiting another process.
Over the past 25 years a number of chemical inhibitors have been used with the intent to block CME. In many instances and cell types this is the case; however, these treatments are general cellular perturbants and thus suffer from unknown global effects on the cell (see ). For example, potassium depletion was found to block CME by causing aggregation of clathrin in the cytoplasm thus removing it from functioning at the cell surface.Citation11 However, significant reduction of potassium in the cell leads to a reduction in protein and DNA synthesis.Citation12 Hypertonic sucrose is another treatment used to inhibit CMECitation13 yet it also causes cell shrinkage and may lead to changes in cortical actin cytoskeleton.Citation14 These treatments continue to be popular tools for demonstrating endocytosis through CME despite the fact that both potassium depletion and hypertonic sucrose are reported to impair the formation of both coated and uncoated invaginations at the PM.Citation15 Cytosol acidification blocks CME by freezing clathrin-coated pits at the cell surface;Citation13 however, other effects on the actin cytoskeleton and macropinocytosis have been reported.Citation16,Citation17 Chlorpromazine is a cationic amphipathic drug that causes the assembly of adaptor proteins and clathrin on endosomal membranes thus depleting it from the PM, leading to a block in CME.Citation18 However this drug also causes an inhibition of receptor recyclingCitation18 and inhibits CIE in some cells.Citation19 Indeed, a study testing a number of these inhibitors on four different cell lines revealed that these compounds are not very specific and that the efficacy, even for inhibiting CME, varies in different cell lines.Citation19
GPI-APs and toxins that bind to membrane lipids enter cells via CIE and various manipulations of PM cholesterol have been used to suggest a mechanism requiring cholesterol and glycosphingolipid-enriched membrane or “lipid raft domains.”
Cyclodextrins such as methyl-β-cyclodextrin have been used to deplete cells of PM cholesterol, leading to a block in endocytosis of various toxins and GPI-APs.Citation20 Although this treatment is effective and can be reversed by cholesterol repletion, it results in profound changes in cell surface structure and can affect other endocytic pathways including macropinocytosis and CME.Citation21,Citation22 As an alternative to extracting cholesterol from cells, the cholesterol-binding agent filipin can be used to bind to cell surface cholesterol and rapidly inhibit endocytosis of GPI-APs.Citation23,Citation24 Although free PM cholesterol is required for endocytosis of GPI-APs and lipid-binding toxins, it is also necessary for entry of a number of PM proteins (MHCI, Glut1, CD44, CD98 and CD147) that are not lipid-raft partitioning proteins but enter cells by CIE.Citation25,Citation26 At higher concentrations of filipin CME can also be inhibited, thus demonstrating that most, if not all, forms of endocytosis are dependent upon PM cholesterol.Citation22 One compound that consistently blocks macropinocytosis is amiloride, which acts to inhibit Na+/H+ exchange at the cell surface.Citation27,Citation28 Although this compound appears to be fairly specific for blocking this process, macropinocytosis is generally easy to identify, even without the use of inhibitors.
Taken together, many, if not all, of the aforementioned inhibitors are agents that act in a non-specific way and thus caution is in order when interpreting the findings. Nevertheless, the effects of these widely available inhibitors on endocytosis of a particular cargo protein in comparison to other cargo proteins can help characterize or place that cargo as entering cells via a particular class of endocytosis.
Targeted Chemical Inhibitors of Endocytosis
In an attempt to develop potent and specific pharmacological inhibitors of endocytosis, chemical libraries have been screened for their ability to inhibit the GTPase activity of dynamin. Dynasore was the first compound identified to block dynamin’s GTPase activity in a biochemical assay and it has been shown to rapidly and reversibly block CME in cells.Citation29 Dynasore has been widely used and has spawned the development of related compounds, the dynols and dyngoes, with different characteristics and affinities for dynamins 1 and 2.Citation30 The development of these compounds was assisted by knowledge of the biochemistry and structure of dynamin. Overall, the advantage of these dynamin-targeted drugs is their rapid time of action and reversibility. Interestingly, dynasore treatment was shown to affect cortical actin,Citation31 reinforcing earlier studies reporting roles for dynamin in the actin cytoskeleton.Citation32 Hence the idea that dynasore acts solely as a CME inhibitor is misleading. Rather, the effects of dynasore may reveal other roles of dynamin.
Success with the chemical screen for dynamin inhibitors stimulated a search for compounds that would specifically block the formation of clathrin-coated vesicles at the cell surface. The N-terminal domain of clathrin heavy chain interacts with many cellular proteins, including amphiphysinCitation33 and these interactions are critical for clathrin function. A chemical screen was performed to identify compounds that would block the binding of amphiphysin to the N-terminal domain of clathrin. The screen identified “pitstops” 1 and 2 as compounds that blocked this interaction in biochemical assays and also blocked CME in cells.Citation34 The authors showed that endocytosis of shiga toxin was not affected by pitstop treatment, implying that the CIE mode of entry was not affected.Citation34 Although this compound was a potent inhibitor of CME and presumably was working in part through this specific effect on blocking the binding of proteins to the N-terminal domain of clathrin, pitstop 2 has other unexpected targets in cells.
We found that pitstop 2 also inhibits CIE, and its ability to block CIE is still observed in cells where clathrin had been depleted by siRNACitation35 suggesting that this compound was affecting other cellular targets. Indeed, we found that treatment of cells with pitstop 2 results in a severe decrease in lateral mobility of cell surface proteins. Both integral membrane proteins and peripheral cytosolic proteins were essentially made immobile after addition of pitstop 2 to the cells.Citation35 Ironically, cellular entry of shiga toxin, which von Kleist et al. used as a representative CIE cargo, could still occur in the presence of pitstop, probably due to the ability of shiga toxin to cross-link lipids and force entry into cells.Citation36 Thus, despite the initial characterization of pitstop that indicated target specificity, in cells these new compounds appear to have additional targets that lead to severe changes in the cell surface.
Genetic Approaches to Endocytosis Inhibitors
To avoid the problem of non-specific effects of chemical inhibitors, genetic approaches have been used to inhibit endocytosis, in particular CME, by altering the expression of specific proteins. These have included the expression of mutant forms of critical proteins involved in endocytosis and siRNA-mediated depletion of these proteins. One of the first genetic approaches used was the expression of a mutant form of dynamin, K44A, patterned after a temperature-sensitive mutant in Drosophila. Expression of dynamin 2 K44A inhibits CME and it has been widely used to demonstrate that an endocytic event requires functional dynamin for fission.Citation37 However, Damke et al. also noted that as a result of the block in CME invoked by Dyn2K44A the rate of clathrin-independent fluid endocytosis increased.Citation38 Interestingly, recent reports have shown a more general role for dynamin in regulating cortical actin structure.Citation31,Citation32,Citation39 Additional studies have targeted other regulatory proteins of CME such as the clathrin-associated proteins AP180 and Eps15. Expression of the carboxyl-terminal clathrin-binding domain of AP180 (AP180C)Citation40 or a truncated form of Eps15 lacking the epsin homology (EH) domainCitation41 can effectively block the formation of clathrin-coated pits. Expression of the C-terminal (Hub) region of clathrin also effectively blocks CME.Citation42
Concerns have been raised that the overexpression of wild type and dominant negative forms of proteins might lead to many indirect effects and consequently investigators turned to methods to block CME by silencing the expression of players of the pathway such as clathrin heavy chain and the μ2 subunit of the AP2 adaptor complex. Knocking down these proteins by siRNA or shRNA can clearly indicate whether endocytosis of a particular type of PM protein requires clathrin and which adaptor protein.Citation43,Citation44 A drawback of this approach is that the time it takes to deplete a cell of these proteins can be considerable (3–7 d) and during this time the cell may adapt and even alter gene expression such that one cannot be assured that only CME is impacted. Also, loss of a protein like clathrin impairs trafficking at the TGN and to and from the lysosome,Citation45,Citation46 which might lead to defects in trafficking and lysosomal function. One clever approach to circumvent this drawback has been the use of the “knock-sideways” clathrin depletion scheme developed by Robinson and colleagues. Stable cell lines depleted of endogenous clathrin heavy chain and expressing a tagged form can be treated with rapamyacin, causing the clathrin to be translocated to the mitochondria thus acutely depleting the cell of clathrin.Citation47 The knock-sideways approach is acute and comes closest to a specific genetic inhibition similar to that imposed by the original temperature-sensitive mutant of dynamin in Drosophila. Drawbacks of this approach include the time required to prepare the cell lines expressing the two tagged proteins, the concern that during the translocation of clathrin other proteins may also be diverted and the possible cellular effects of inhibiting the mTOR pathway in cells.
Perspective
In conclusion, as our understanding of the different forms of endocytosis increases, it becomes more difficult to embrace one particular inhibitor as being diagnostic for a particular mode of endocytosis. Thus, results with endocytosis inhibitors, whether identified from a screen of a general endocytic process or from a screen of inhibitors for a specific biochemical activity, need to be interpreted carefully. The use of targeted pharmacological inhibitors (dynasore) appear to offer better choices than the non-selective chemical inhibitors (potassium depletion and cytosol acidification) but once again these experiments should be supported with corroborating evidence from the expression of mutant proteins and depletion of cellular proteins. The problems encountered with the “old” inhibitors, should not deter us from discovering “new” inhibitors. The identification and use of inhibitors that act quickly, specifically and reversibly will be invaluable as we set out to understand the physiological functions and mechanisms of different endocytic modes of cellular entry.
Acknowledgments
We thank L. Maldonado-Baez, D. Karabasheva, and J. Caviston for comments. This work was supported by the Intramural Research Program of the National Heart, Lung and Blood Institute, NIH.
References
- Conner SD, Schmid SL. Regulated portals of entry into the cell. Nature 2003; 422:37 - 44; http://dx.doi.org/10.1038/nature01451; PMID: 12621426
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 2011; 12:517 - 33; http://dx.doi.org/10.1038/nrm3151; PMID: 21779028
- Naslavsky N, Weigert R, Donaldson JG. Convergence of non-clathrin- and clathrin-derived endosomes involves Arf6 inactivation and changes in phosphoinositides. Mol Biol Cell 2003; 14:417 - 31; http://dx.doi.org/10.1091/mbc.02-04-0053; PMID: 12589044
- Sabharanjak S, Sharma P, Parton RG, Mayor S. GPI-anchored proteins are delivered to recycling endosomes via a distinct cdc42-regulated, clathrin-independent pinocytic pathway. Dev Cell 2002; 2:411 - 23; http://dx.doi.org/10.1016/S1534-5807(02)00145-4; PMID: 11970892
- Grassart A, Dujeancourt A, Lazarow PB, Dautry-Varsat A, Sauvonnet N. Clathrin-independent endocytosis used by the IL-2 receptor is regulated by Rac1, Pak1 and Pak2. EMBO Rep 2008; 9:356 - 62; http://dx.doi.org/10.1038/embor.2008.28; PMID: 18344974
- Swanson JA. Shaping cups into phagosomes and macropinosomes. Nat Rev Mol Cell Biol 2008; 9:639 - 49; http://dx.doi.org/10.1038/nrm2447; PMID: 18612320
- Mooren OL, Galletta BJ, Cooper JA. Roles for actin assembly in endocytosis. Annu Rev Biochem 2012; 81:661 - 86; http://dx.doi.org/10.1146/annurev-biochem-060910-094416; PMID: 22663081
- Fujimoto LM, Roth R, Heuser JE, Schmid SL. Actin assembly plays a variable, but not obligatory role in receptor-mediated endocytosis in mammalian cells. Traffic 2000; 1:161 - 71; http://dx.doi.org/10.1034/j.1600-0854.2000.010208.x; PMID: 11208096
- Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol 1997; 139:49 - 61; http://dx.doi.org/10.1083/jcb.139.1.49; PMID: 9314528
- Weigert R, Yeung AC, Li J, Donaldson JG. Rab22a regulates the recycling of membrane proteins internalized independently of clathrin. Mol Biol Cell 2004; 15:3758 - 70; http://dx.doi.org/10.1091/mbc.E04-04-0342; PMID: 15181155
- Larkin JM, Brown MS, Goldstein JL, Anderson RG. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell 1983; 33:273 - 85; http://dx.doi.org/10.1016/0092-8674(83)90356-2; PMID: 6147196
- Ledbetter ML, Lubin M. Control of protein synthesis in human fibroblasts by intracellular potassium. Exp Cell Res 1977; 105:223 - 36; http://dx.doi.org/10.1016/0014-4827(77)90120-3; PMID: 844499
- Hansen SH, Sandvig K, van Deurs B. Molecules internalized by clathrin-independent endocytosis are delivered to endosomes containing transferrin receptors. J Cell Biol 1993; 123:89 - 97; http://dx.doi.org/10.1083/jcb.123.1.89; PMID: 8408209
- Malek AM, Xu C, Kim ES, Alper SL. Hypertonicity triggers RhoA-dependent assembly of myosin-containing striated polygonal actin networks in endothelial cells. Am J Physiol Cell Physiol 2007; 292:C1645 - 59; http://dx.doi.org/10.1152/ajpcell.00533.2006; PMID: 17192281
- Carpentier JL, Sawano F, Geiger D, Gorden P, Perrelet A, Orci L. Potassium depletion and hypertonic medium reduce “non-coated” and clathrin-coated pit formation, as well as endocytosis through these two gates. J Cell Physiol 1989; 138:519 - 26; http://dx.doi.org/10.1002/jcp.1041380311; PMID: 2466853
- Cosson P, de Curtis I, Pouysségur J, Griffiths G, Davoust J. Low cytoplasmic pH inhibits endocytosis and transport from the trans-Golgi network to the cell surface. J Cell Biol 1989; 108:377 - 87; http://dx.doi.org/10.1083/jcb.108.2.377; PMID: 2918022
- Suzuki K, Namiki H. Cytoplasmic pH-dependent spreading of polymorphonuclear leukocytes: regulation by pH of PKC subcellular distribution and F-actin assembly. Cell Biol Int 2007; 31:279 - 88; http://dx.doi.org/10.1016/j.cellbi.2006.11.005; PMID: 17188004
- Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol 1993; 123:1107 - 17; http://dx.doi.org/10.1083/jcb.123.5.1107; PMID: 8245121
- Vercauteren D, Vandenbroucke RE, Jones AT, Rejman J, Demeester J, De Smedt SC, et al. The use of inhibitors to study endocytic pathways of gene carriers: optimization and pitfalls. Mol Ther 2010; 18:561 - 9; http://dx.doi.org/10.1038/mt.2009.281; PMID: 20010917
- Kilsdonk EP, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, et al. Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem 1995; 270:17250 - 6; http://dx.doi.org/10.1074/jbc.270.29.17250; PMID: 7615524
- Liu NQ, Lossinsky AS, Popik W, Li X, Gujuluva C, Kriederman B, et al. Human immunodeficiency virus type 1 enters brain microvascular endothelia by macropinocytosis dependent on lipid rafts and the mitogen-activated protein kinase signaling pathway. J Virol 2002; 76:6689 - 700; http://dx.doi.org/10.1128/JVI.76.13.6689-6700.2002; PMID: 12050382
- Rodal SK, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol Biol Cell 1999; 10:961 - 74; PMID: 10198050
- Rothberg KG, Ying YS, Kamen BA, Anderson RG. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol 1990; 111:2931 - 8; http://dx.doi.org/10.1083/jcb.111.6.2931; PMID: 2148564
- Orlandi PA, Fishman PH. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J Cell Biol 1998; 141:905 - 15; http://dx.doi.org/10.1083/jcb.141.4.905; PMID: 9585410
- Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, et al. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic 2009; 10:590 - 9; http://dx.doi.org/10.1111/j.1600-0854.2009.00894.x; PMID: 19302270
- Naslavsky N, Weigert R, Donaldson JG. Characterization of a nonclathrin endocytic pathway: membrane cargo and lipid requirements. Mol Biol Cell 2004; 15:3542 - 52; http://dx.doi.org/10.1091/mbc.E04-02-0151; PMID: 15146059
- Grinstein S, Rotin D, Mason MJ. Na+/H+ exchange and growth factor-induced cytosolic pH changes. Role in cellular proliferation. Biochim Biophys Acta 1989; 988:73 - 97; http://dx.doi.org/10.1016/0304-4157(89)90004-X; PMID: 2535787
- West MA, Bretscher MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A431 cells. J Cell Biol 1989; 109:2731 - 9; http://dx.doi.org/10.1083/jcb.109.6.2731; PMID: 2556406
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell 2006; 10:839 - 50; http://dx.doi.org/10.1016/j.devcel.2006.04.002; PMID: 16740485
- Hill TA, Gordon CP, McGeachie AB, Venn-Brown B, Odell LR, Chau N, et al. Inhibition of dynamin mediated endocytosis by the dynoles--synthesis and functional activity of a family of indoles. J Med Chem 2009; 52:3762 - 73; http://dx.doi.org/10.1021/jm900036m; PMID: 19459681
- Gu C, Yaddanapudi S, Weins A, Osborn T, Reiser J, Pollak M, et al. Direct dynamin-actin interactions regulate the actin cytoskeleton. EMBO J 2010; 29:3593 - 606; http://dx.doi.org/10.1038/emboj.2010.249; PMID: 20935625
- Krueger EW, Orth JD, Cao H, McNiven MA. A dynamin-cortactin-Arp2/3 complex mediates actin reorganization in growth factor-stimulated cells. Mol Biol Cell 2003; 14:1085 - 96; http://dx.doi.org/10.1091/mbc.E02-08-0466; PMID: 12631725
- Lemmon SK, Traub LM. Getting in touch with the clathrin terminal domain. Traffic 2012; 13:511 - 9; http://dx.doi.org/10.1111/j.1600-0854.2011.01321.x; PMID: 22239657
- von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, et al. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell 2011; 146:471 - 84; http://dx.doi.org/10.1016/j.cell.2011.06.025; PMID: 21816279
- Dutta D, Williamson CD, Cole NB, Donaldson JG. Pitstop 2 is a potent inhibitor of clathrin-independent endocytosis. PLoS One 2012; 7:e45799; http://dx.doi.org/10.1371/journal.pone.0045799; PMID: 23029248
- Römer W, Berland L, Chambon V, Gaus K, Windschiegl B, Tenza D, et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 2007; 450:670 - 5; http://dx.doi.org/10.1038/nature05996; PMID: 18046403
- van der Bliek AM, Redelmeier TE, Damke H, Tisdale EJ, Meyerowitz EM, Schmid SL. Mutations in human dynamin block an intermediate stage in coated vesicle formation. J Cell Biol 1993; 122:553 - 63; http://dx.doi.org/10.1083/jcb.122.3.553; PMID: 8101525
- Damke H, Baba T, Warnock DE, Schmid SL. Induction of mutant dynamin specifically blocks endocytic coated vesicle formation. J Cell Biol 1994; 127:915 - 34; http://dx.doi.org/10.1083/jcb.127.4.915; PMID: 7962076
- Unsworth KE, Mazurkiewicz P, Senf F, Zettl M, McNiven M, Way M, et al. Dynamin is required for F-actin assembly and pedestal formation by enteropathogenic Escherichia coli (EPEC). Cell Microbiol 2007; 9:438 - 49; http://dx.doi.org/10.1111/j.1462-5822.2006.00801.x; PMID: 16965516
- Zhao XH, Greener T, Al-Hasani H, Cushman SW, Eisenberg E, Greene LE. Expression of auxilin or AP180 inhibits endocytosis by mislocalizing clathrin: evidence for formation of nascent pits containing AP1 or AP2 but not clathrin. J Cell Sci 2001; 114:353 - 65; PMID: 11148137
- Benmerah A, Lamaze C, Bègue B, Schmid SL, Dautry-Varsat A, Cerf-Bensussan N. AP-2/Eps15 interaction is required for receptor-mediated endocytosis. J Cell Biol 1998; 140:1055 - 62; http://dx.doi.org/10.1083/jcb.140.5.1055; PMID: 9490719
- Liu SH, Marks MS, Brodsky FM. A dominant-negative clathrin mutant differentially affects trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway. J Cell Biol 1998; 140:1023 - 37; http://dx.doi.org/10.1083/jcb.140.5.1023; PMID: 9490717
- Hinrichsen L, Harborth J, Andrees L, Weber K, Ungewickell EJ. Effect of clathrin heavy chain- and alpha-adaptin-specific small inhibitory RNAs on endocytic accessory proteins and receptor trafficking in HeLa cells. J Biol Chem 2003; 278:45160 - 70; http://dx.doi.org/10.1074/jbc.M307290200; PMID: 12960147
- Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol 2003; 162:909 - 18; http://dx.doi.org/10.1083/jcb.200305145; PMID: 12952941
- Deborde S, Perret E, Gravotta D, Deora A, Salvarezza S, Schreiner R, et al. Clathrin is a key regulator of basolateral polarity. Nature 2008; 452:719 - 23; http://dx.doi.org/10.1038/nature06828; PMID: 18401403
- Janvier K, Bonifacino JS. Role of the endocytic machinery in the sorting of lysosome-associated membrane proteins. Mol Biol Cell 2005; 16:4231 - 42; http://dx.doi.org/10.1091/mbc.E05-03-0213; PMID: 15987739
- Robinson MS, Sahlender DA, Foster SD. Rapid inactivation of proteins by rapamycin-induced rerouting to mitochondria. Dev Cell 2010; 18:324 - 31; http://dx.doi.org/10.1016/j.devcel.2009.12.015; PMID: 20159602
- Altankov G, Grinnell F. Depletion of intracellular potassium disrupts coated pits and reversibly inhibits cell polarization during fibroblast spreading. J Cell Biol 1993; 120:1449 - 59; http://dx.doi.org/10.1083/jcb.120.6.1449; PMID: 8449988
- Schlegel R, Dickson RB, Willingham MC, Pastan IH. Amantadine and dansylcadaverine inhibit vesicular stomatitis virus uptake and receptor-mediated endocytosis of alpha 2-macroglobulin. Proc Natl Acad Sci U S A 1982; 79:2291 - 5; http://dx.doi.org/10.1073/pnas.79.7.2291; PMID: 6179094
- Kang SJ, Shin KS, Song WK, Ha DB, Chung CH, Kang MS. Involvement of transglutaminase in myofibril assembly of chick embryonic myoblasts in culture. J Cell Biol 1995; 130:1127 - 36; http://dx.doi.org/10.1083/jcb.130.5.1127; PMID: 7657697
- Gibson AE, Noel RJ, Herlihy JT, Ward WF. Phenylarsine oxide inhibition of endocytosis: effects on asialofetuin internalization. Am J Physiol 1989; 257:C182 - 4; PMID: 2475026
- Frost SC, Lane MD, Gibbs EM. Effect of phenylarsine oxide on fluid phase endocytosis: further evidence for activation of the glucose transporter. J Cell Physiol 1989; 141:467 - 74; http://dx.doi.org/10.1002/jcp.1041410304; PMID: 2687296
- Massol P, Montcourrier P, Guillemot JC, Chavrier P. Fc receptor-mediated phagocytosis requires CDC42 and Rac1. EMBO J 1998; 17:6219 - 29; http://dx.doi.org/10.1093/emboj/17.21.6219; PMID: 9799231
- Dickson RB, Willingham MC, Pastan IH. Receptor-mediated endocytosis of alpha 2-macroglobulin: inhibition by ionophores and stimulation by Na+ and HCO3(-). Ann N Y Acad Sci 1982; 401:38 - 49; http://dx.doi.org/10.1111/j.1749-6632.1982.tb25705.x; PMID: 6188403
- Horwitz SB, Chia GH, Harracksingh C, Orlow S, Pifko-Hirst S, Schneck J, et al. Trifluoperazine inhibits phagocytosis in a macrophagelike cultured cell line. J Cell Biol 1981; 91:798 - 802; http://dx.doi.org/10.1083/jcb.91.3.798; PMID: 6120173
- Kuratomi Y, Akiyama S, Ono M, Shiraishi N, Shimada T, Ohkuma S, et al. Thioridazine enhances lysosomal accumulation of epidermal growth factor and toxicity of conjugates of epidermal growth factor with Pseudomonas exotoxin. Exp Cell Res 1986; 162:436 - 48; http://dx.doi.org/10.1016/0014-4827(86)90348-4; PMID: 3484705
- Lagana A, Vadnais J, Le PU, Nguyen TN, Laprade R, Nabi IR, et al. Regulation of the formation of tumor cell pseudopodia by the Na(+)/H(+) exchanger NHE1. J Cell Sci 2000; 113:3649 - 62; PMID: 11017880