Abstract
The dermal application of drugs is promising due to the ease of application. In this context nano-scale carrier systems were already evaluated in several studies with respect to the skin interaction and the impact on drug penetration. At the same time the upcoming production of engineered nano-scale materials requires a thorough safety evaluation. Drug delivery as well as risk assessment depends crucially on the ability of such carriers to overcome the skin barrier and reach deeper tissue layers. Therefore, the interaction of nanoparticles with skin and especially skin models is an intriguing field. However, the data obtained do not show a clear image on the effect of nano-carriers. Especially the penetration of such particles is an open and controversially discussed topic. The literature reports different results mainly on pig or murine skin showing strong penetration (pig and mouse) or the opposite. Looking only at the sizes of the particles also no conclusive picture can be obtained. Nevertheless, size is regarded to play an important role for skin penetration. Furthermore, the state of the skin influences penetration (hydration) and the mechanical stress is of outmost importance.
Introduction
Any kind of organism always faced nanoscale environmental compounds interacting with their exterior barrier but a significant interest in these interactions came up not until the broad ascent of artificial nanoparticulate compounds.Citation1 The great potential for future advanced applications in areas such as energy, electronics, automotive, chemistry and life sciences led to a rapidly growing number of nanoparticulate systems, with respect to material composition, size, shape and formulation. Nanomedicines, incorporating drug delivery, diagnostics, implants, cancer therapy, to name just a few, pose an important and appealing area for beneficial application. Regarding final applicability it is essential to investigate the various biological aspects of nanoparticle exposure to the human organism. On one hand health hazards are to be identified and assessed, on the other hand medical and pharmaceutical potentials may be discovered and exploited. The behavior of nanoparticulate substances in biosystems and their physiological effects can, up to now, neither be extrapolated straightforwardly from bulk properties nor can they be predicted from molecular properties of the constituents. The first step of any interaction between an organism and any compound is the uptake of the compound from the environment. Several pathways of absorption exist for human (and most animal) organisms of which the skin is the most obvious and easiest to reach.
The present review focus on skin and its barrier as well as sink function to artificial nanoparticulate compounds. Different classes of nanoparticulate material were studied to gain knowledge on the interaction and possible impact on skin covering bio-resistant particles (e.g., metal oxide, carbon-based) and biodegradable particles (e.g., liposomes, polymeric particles). Furthermore, mainly healthy skin is treated herein. Injured and inflamed skin can be entered even by particles larger than the nanometer scale due to the loss of its barrier function.Citation1 This is also the case for chemically induced irritations of the skin, e.g., by non-polar solvents and strong alkalines. However, particle penetration in combination with deliberately damaged skin or skin under mechanical stress is available and will be discussed.
Pathways for Skin Absorption
Regarding the absorption of any kind of material there can be considered two general pathways: along the skin appendages or through the stratum corneum and the underlying layers. Even though the appendages present only a small portion of the surface they are considered to contribute and might even be addressed for a directed delivery because of the depth they reach. Especially the hair follicles seem to be a promising target regarding nanoparticulate carrier systems.Citation2,Citation3 The invasion of a substance into a skin appendage is not yet an absorption process itself. Compounds inside an appendage are still on the outside of the body by definition. Nevertheless accumulation in such structures may lead to faster and more efficient uptake due to altered barrier morphology in the appendages what will be discussed in detail in a separate paragraph.
Generally, the SC is assumed to be the main barrier for absorption. The hydrophilicity of the absorbent is hereby crucial. The absorption across the stratum corneum offers two possible pathways which are obvious: through the corneocytes (bricks) or along the intercellular spaces along the lipid matrix (mortar) (). The latter pathway seems to be most suited for penetration offering channel-like structures providing higher diffusivity although the pathway is much longer ().Citation4
The full understanding of the processes being responsible for the penetration process is not yet obtained. This holds true even more for particle penetration. For molecular species, approaches relying on a close interaction of theoretic modeling approaches based on experimentally extracted data is necessary and in process.Citation5–Citation7
Overall, a second barrier functionality is achieved by composing layers of different polar character. The SC can be considered to be a lipoidic compartment whereas the underlying living tissue is a more aqueous environment. Hence lipophilic molecules can distribute more easily in the SC and penetration is facilitated. Furthermore, the absorbent needs to fit into the intercellular space and need to move along the lipid phase or the aqueous phase, respectively, restricting the space available and therefore the size of penetrating species influences the absorption behavior. Diffusion into the living tissue, however, is in favor of polar molecules and objects restricting the invasion of too lipophilic compounds.Citation8 As a consequence only substances with partition coefficients (logP) between 1 and 3 are well suited for skin absorption ().
These two aspects of penetration and permeation, the size of an absorbent as well as is partition coefficient, are expressed in the formula of Potts and Guy who found a phenomenological expression based on molecular properties to describe the absorptionCitation9
(6)
Hereby D is the not restricted diffusivity of the permeant in the membrane (skin), h represents the length of the diffusion pathway, MW is the molecular weight (for most molecules the molecular weight correlates well with the molecular volume), P is the octanol/water partition coefficient (accessibility of P is better than the value of the membrane/water coefficient Km), and Kp is the permeability coefficient. Several attempts were made to optimize this relatively crude phenomenological expression but only slight improvements were achieved. Most approaches find the molecular weight to be a strong effector and reliable measure to predict permeabilities and maximum fluxes of solutes through the stratum corneum, as shown by Magnusson et al.Citation10
Empirical findings by Bos et al.Citation11 known as the “500 Dalton rule,” even indicate a molar mass exclusion effect for dermal penetration, which is not predicted by the quantitative penetration models of Potts and Guy and others (Potts,Citation9 MagnussonCitation10). Nevertheless it is shown that diffusion of high-MW corpuscles is drastically impeded in the stratum corneum. As nanoparticles have molar masses on the order of 10Citation6 Dalton and more no significant transport over an intact dermal barrier is to expect for human beings concerning these predictions.
Assuming that particles penetration and diffusion will follow the intercellular route a “best case” flux of particles over the stratum corneum can be estimated by classical diffusion theory. The particles need to diffuse a distance of some hundred µm (say h′ = 500 µm) via the lipid-filled intercellular space, which has a cross sectional area of about ɛ = 1% of the total surface area.Citation12 Approximating the situation (coarse simplification) by assuming a homogeneous membrane matrix (hence neglecting any size exclusion effects at pores, adsorption at corneocyte surfaces and phase transition steps) of diffusivity D and furthermore no limitation in drug amount (infinite dose) on the outer surface, one may calculate steady state flux parameters over the barrier. The diffusivity D for spherical nanoparticles is approximated by the Stokes-Einstein relation and data from Moghimi et al.Citation12
(7)
(with k: Boltzmann constant, η: dynamic viscosity, r: particle radius, h′: diffusion distance, ɛ: area fraction of intercellular spaces). Obviously, the particle flux drops with the reciprocal of r. A modified maximum particle flux can be defined by assuming a maximum nanoparticle density of 74% (“closest packed”) of the reciprocal of the particle volume:
(8)
Following these assumptions, the maximum particle flux decreases with the fourth power of the particle radius. Some exemplifying results of such estimations are given in . In reality, the first particles will arrive at the inner interface of the barrier not until a lag time elapsed. During this lag time the steady state conditions, i.e., the constant gradient over the barrier, develops. This time also increases with the particle radius. Together with the impeding effects due to the inhomogeneous, porous nature of the stratum corneum as well as the finite dose and exposure time, this strongly limits transport of nanoparticulate material into human skin far below the best case estimation.
Penetration & Permeation Studies of Nanoparticulate Materials
As it is know that nanosized carriers behave differently when interacting with biological barriers, their potential for transdermal drug delivery purposes was addressed in several studies. Applying drugs in pharmaceutical formulations containing nanoparticulate material as drug delivery devices was carefully investigated.Citation13–Citation23 At the same time safety issues gained more and more importance to assess the risk when exposed unintended to these particles.Citation24–Citation26 Here open question for nanoparticulate potential to induce adverse effects based on the nanoparticles properties and their interaction mechanism are of interest. This includes the interaction with proteins which may alter the nanoparticles behavior after adsorption to the surface as well as nanoparticles might impact on the behavior of proteins.Citation27 Furthermore genotoxic effects induced either directly or indirectly (inflammatory processes-mediated) can play a roll as well as oxidative stress due to the formation of active oxygen species. Some nanoparticulate materials might be activated by light and result in photosensitation. Citation27
As already mentioned above, particle penetration is most likely along two possible routes: the intercellular route, following the lipid channels between the corneocytes to deeper skin layers and the appendage route (hair follicles, sweat glands). Both ways have shown considerable interaction with nanoparticulate formulations.Citation2,Citation14,Citation15,Citation28
In this paragraph an overview of penetration and permeation studies on nanoparticulate compounds into human skin and its models (mainly different animal skins) will be given, with an emphasis on skin models and penetration conditions.
This overview will be subdivided according to the particles’ chemical composition, which concomitantly includes a classification according to application foci of the studies: risk assessment for the metal and mineral nanoparticles, pharmaceutical applications for organic nanoscale compounds.
Polymeric particles.
For drug delivery purposes, the most promising and mostly applied carrier technology is based on polymeric materials. Especially on biomaterials that offer an intrinsic biocompatibility and biodegradability. Hence, there are several studies on drug penetration encapsulated in polymer-based particles that show a marked difference between the conventional and nanoparticulate formulations.Citation13–Citation17,Citation29 A crucial question for the investigation of nanoparticulate drug delivery carriers is the site of drug release from the particles, i.e., does the release occur in suspension or on the skin surface leaving the carrier particles outside or do the particles penetrate the skin to release the drug within the tissue? However, when it comes to particle penetration into the skin there is only limited information available. FITC-dextran particles of different sizes (up to 4 µm) were investigated with and without mechanical stress.Citation30 A clear cut-off with respect to the size could be determined (≤1 µm) but only for skin under mechanical stress. Nevertheless, these data are quite surprising regarding the size of the penetrating species. Kohli and Alpar also found latex particles (up to 500 nm)Citation16 to penetrate while applying mechanical stress to the barrier. Shim and co-workers applied polymer-based particles of 40 and 130 nm onto the skin of guinea-pigs and detected clear differences between the penetrated drug amounts. However, working with hairless animals did not show any penetration into the skin clearly indicating that the hair follicles might be an important pathway for skin invasion of particulate materials.Citation29
On the other hand, passive penetration (without mechanical stress) into the skin of polymer particles (d ∼ 300 nm) seemed not to occur for human skin within 6 hours after application but release a drug dummy for cutaneous absorption was observed using two photon and confocal laser scanning microscopy (CLSM).Citation18 The simultaneous investigation of particle movement and load release was enabled by multimodal fluorescence strategy: the particles were covalently labeled with fluorescein and physically loaded with Texas Red as a drug dummy for release. Furthermore dermal structures were visualized by keratinous autofluorescence. The excitation wavelengths were chosen to allow to separately investigating each of the fluorescent compounds. The covalently labeled particles were only distributed in the skin wrinkles and on the skin surface whereas the released Texas Red significantly penetrated the epidermis (). These investigations where carried out on excised human skin stored at −26°C. The freezing procedure preserves the barrier properties of skin, but other relevant characteristics are likely altered (e.g., pH).Citation31,Citation32 Furthermore cellular autofluorescence vanishes from the viable epidermis due to depletion of NADH and flavines.
Protein particles.
These kinds of particles, although already commercially available for oral application, are not yet applied on skin.
Solid lipid nano particles (SLN).
In the early 90th solid lipid nanoparticles were introduced as drug carriers in the pharmaceutical field. In general SLN are composed of physiological solid lipids manufactured by a high pressure homogenization process. Such systems applied onto skin results in higher drug permeation. However, this enhanced drug absorption is not the result of penetrating particles but of the occlusive effect as a result of surface coverage.Citation33 No intact penetration of the SLN is reported to our knowledge.
Liposomes.
Liposomes are composed of a closed bilayer of phospholipids offering a hydrophobic compartment (lipid layer) as well as a hydrophilic compartment (inner volume of the liposome). Their advantage, with respect to pharmaceutical application as drug carriers, is the wide variety of drugs to be incorporated as well as the biocompatibility, inherently connected with natural phospholipids. Hence, liposomes are the biggest group of nanoparticulate carriers used for application in cosmetics or for therapeutic purposes.
Regarding the penetration behavior of liposomes it is still under discussion if such objects might penetrate (intact) the skin.Citation34–Citation37
However, ultraflexible liposomal structures are assumed to penetrate successfullyCitation38,Citation39 whereas conventional liposomes failed.Citation40,Citation41 As the liposomes are bigger in size (≥50 nm) than the skin openings passive penetration is obstructed and a driving force is required large enough to drag the liposomes through the skin. Cevc and co-author proposed a mechanism to explain the observed dermal penetration based on a hydration gradient driven transport ().Citation42 Thus the different activity of water on the two barrier sides is responsible for aggregate migration.
The water activity gradient in the stratum corneum is reported to be also responsible for the motion of the liposomes along the pores of the intercellular space.
Metallic particles.
Gold nanoparticles (AuNP) were widely used for cellular imaging and gain again more and more attention in recent years. Due to the ease of preparation of different sized particles with comparatively small size distributionsCitation43 and their good contrast for electron microscopy techniques they are as well suited for studying the dermal penetration. There is one study based on rat skin showing the penetration of different sized AuNP (15 nm, 102 nm and 198 nm). Gold could be detected in an acceptor compartment with permeation kinetics linked with the size of the applied particles. The smallest particles were found to aggregate by TEM in deeper skin layers whereas the larger two particles types only reach the viable epidermis and dermis.Citation43
Beryllium nanoparticles (up to 1 µm) were found in the stratum corneum using tape-stripping as well as in deeper skin layers based on TEM imaging.Citation30 In contrast to this, Maghemite (γ-Fe2O3) and iron core shell particles (d ≤ 20 nm) were shown to penetrate into the top layer and eventually could reach the viable epidermis of human skin.Citation44 This study was conducted using electron dispersion spectrometry SEM on vertical skin sections. This technique allows chemical identification of iron particles in the tissue. In addition, these particles were also found in the hair follicles. The authors tried as well to investigate possible mechanism and investigated the barrier properties (resistance) and found that the formulation vehicles (solvent) has already a marked influence on the barrier that can not be identified with the visualization tools. However, the authors hydrated the skin deliberately before applying the nanoparticulate formulations what might significantly alter the barrier properties as a result of swelling. Another study investigated the in vitro penetration and permeation of silver nanoparticles (AgNP) through human skin.Citation45 The polyvinylpyrrolidone coated polydispers particles were found to penetrate into the stratum corneum (TEM analysis) and even permeated into an aqueous acceptor compartment (electrothermal atomic absorption spectroscopy). Particle sizes were distributed between 10 and 50 nm and applied from an ethanolic/synthetic sweat formulation. Even though particles themselves were only imaged in the SC the analytical measurement of Ag in the acceptor indicates permeation. Furthermore, abraded and healthy skin were compared and showed—as expected—marked differences in permeation kinetics and permeated amounts showing higher amounts and faster permeation onsets for the damaged skin samples.
Mineral particles.
Mineral particles are of huge relevance due to their production and usage in large amounts. In 2003/2004 approximately 1,000 t of these materials were fabricated for their usage in sun protection agents with sizes between 50–500 nm.Citation25 Sun-screen-grade nanoparticles are composed of titanium dioxide and (TiO2) and zinc oxide (ZnO).
Regarding the passive diffusion of TiO2 the EU Scientific Committee on Cosmetics and Non-Food Products (SCCNP)Citation46 published a paper based on studies with micro- and nano-sized material. Herein they state that these particles remain on the skin surface or on the outer layer of the stratum corneum and do not penetrate into or through the living skin.Citation47–Citation49 Confirmation was obtained with studies on human, porcine or murine skinCitation50–Citation53 for particles within a size range between 10 and 100 nm. These data were recently confirmed by the outcome of an EU project (NanoDerm). Here, TiO2 are only found in the top layer of the stratum corneum and the openings of the hair follicle.Citation54 Similar results were obtained for ZnO.Citation50,Citation55 Just recently two studies on ZnOCitation56 and TiO2,Citation57 were published; ZnO penetration was investigated in vivo with human volunteers and located the particulated materials only on the skin surface and their accumulation in skin folds and/or hair follicles.Citation56 In vitro measurements of the penetration of TiO2 particles between 20 and 100 nm showed the nanoparticles only in the top 3–5 layers for all skin samples used (porcine skin, healthy human skin and human skin grafted on a severe combined immuno-deficient mouse model).Citation57
Carbon nanotubes (CNT)/fullerenes.
Carbon materials are considered as drug delivery systems due to their inert nature and their volume to be filled with drugs.Citation58 With respect to the interaction of these materials with skin several studies are undertaken looking at the toxic impact of these materials on skin cells (keratinocytes).Citation59–Citation61 To our knowledge only one work investigated the penetration behavior of carbon materials, i.e., fullerenes into skin.Citation62 Rouse et al. coupled a peptide with a fullerene and added a fluorescent marker (3.5–400 nm) to facilitate the observation of the complex. No penetration of the complex in skin was found as long as the skin was untreated. Simulating the mechanical stress of the skin (such as walking)—flexing of the skin—resulted in penetration of the particulate material into skin. In addition, the penetration was dependent on the time the mechanical stress was applied; flexing need to be going on for at least 60 min to allow penetration. This may indicate that the structure of the skin is altered applying mechanical stress.
Semiconductor nanocrystals.
Semiconductor nanocrystals, also known as Quantum dots (QD) are widely used for non-invasive imaging purposes. The QD offer several advantageous properties that offer superior detection and experimental control compared to many other nanoparticulate materials. For all penetration processes the size of the permeant is considered to be one of the main factors. QD are, without additional surface coating available in a very small size range; below 10 nm. Besides the size-dependent fluorescent emission, the QD show typically small size-distributions and favorable optical properties. The inherent fluorescence guarantees a label-free—not altering the chemical composition—material.Citation63 Furthermore, the powerful and convenient fluorescent methods can be non-invasively applied. Typically, most of the experimental data is based on confocal microscopy, sometimes supported with TEM images and quantitative analysis.Citation64
As a result they are applied many-fold in several areas and are used as well for understanding of the interaction of nano-sized materials with skin. There are several publications dealing with QD and the interaction with skin. An important aspect is that none of the present publications deals with human skin but with murine skinCitation64,Citation65 (mouse, rat) and pig skin.Citation21,Citation64 As the different skin types do differ and no information on particle penetration exists care should be taken to generalize the different data.
In a recent work, healthy skin was compared with damaged skin regarding the QD penetration. Skin was damaged applying tape-stripping to remove the stratum corneum or a sand paper was used to physically damage the skin and facilitate penetration because of a missing or ruptured main barrier. However, no clear penetration was observed.Citation64 Rat skin without stratum corneum as well as healthy rat skin did not show any permeability for the types of QD used in contrast to the results obtained for pig skin with the same particles.Citation21 Only the abraded skin showed some penetration following the authors although this trend is not obvious.Citation64 Surprisingly, following earlier results, even flexing did not facilitate the penetration of the QD into the skin.
In contrast to these data obtained for rat skin, for porcine and mice strong penetration was reported.Citation21,Citation65 The findings in these two papers are really surprising and unexpected and with respect to other data on particle penetration the reliability might be questioned. Ryman-Rasmussen et al. applied two types of QD—spherical and ellipsoidal—with different surface coatings (PEG-amine-, carboxy- and PEG-functionalized) from a borate buffer (pH = 9) onto porcine skin and basically found that all particles do penetrate into skin after 24 hours. Neither the size nor the surface modification had any influence on the QD absorption. Following the penetration of molecules (Potts-Guy equation) this is really surprising for particulate material. Chu and co-workers applied QD in vivo as well as in vitro on mouse skin (which differs from human skin morphology). With fluorescence methods they found penetration in the skin and in addition analyzing the animal organs with inductive coupled plasma atomic emission spectrometry (ICP.AES), the QD were detected in lung, heart, liver and the kidneys. Comparable high levels of the QD were kept over one week. Not proven is the presence of particles in these organs because ICP only detects ionic species not particulate material.
Just recently, another study identified a further condition destroying/reducing the barrier function of skin: ultraviolet radiation (UVR).Citation66 Carboxylated QD applied on mouse skin in a glycerol vehicle showed increased penetration if the skin is exposed to UV radiation (290–400 nm) compared to low level of penetrated QD without illumination. However the overall penetration was low in both cases. The authors could even demonstrate the possible pathway of the intruding QD along the intercellular space using silver enhanced TEM imaging.
Follicular Penetration
The role of the appendages in skin absorption was long considered negligible due to their low surface coverage of only 0.1%. Recently in the case of the follicle orifices this number was corrected to be higher than previously assumed.Citation67 Evaluation of the follicles of several anatomical sites (lateral forehead, upper arm, forearm, thorax, back, thigh, calf ) showed that every body region features its own hair follicle characteristics. A maximum surface coverage was found at the forehead (1.28%) and a minimum on the forearm (0.09%). The available infundibular volume which determines the potential follicular reservoir was found to be highest at the forehead and the calf region and similarly to the estimated reservoir of the stratum corneum.Citation67 However, so far no direct correlation to regional differences in skin absorption of small molecules has been investigated with respect to the hair follicles.
For small molecules the trans-follicular pathway is usually ignored in view of the dominance of especially the lipid pathway, although the matter is little understood. Nonetheless, for several compounds (e.g., caffeine, hydrocortisone, testosteroneCitation68–Citation70) the follicular route seems to promote permeability at the beginning of skin absorption while it is overruled by the other higher capacity pathways at later times in the transport process.Citation68
In the context of the absorption of nanoparticles the follicles have received much higher attention. It is commonly agreed that in non-damaged skin the hair follicles play a central role in the penetration of solid nanoparticles.Citation2 Being invaginations of the epidermis hair follicles may reach deep into the subcutaneous fat (depending for example on the state of development of the follicle). Nonetheless, an efficient barrier, which is similar to the stratum corneum in the upper part of the follicle and features tight junctions in the lower part of the follicleCitation71 prevents particles from easily invading into the living cells.Citation72 Instead, once entered into the follicle openings particles will be stored there until being cleared by hair growth or sebum production. Clearance is much slower than for example for the stratum corneum as inside the follicles particles are to a certain degree protected from natural desquamation, textile contact and washing. This makes the follicles an excellent storage site that can be used for drug release and offering a shortcut to the viable skin layers and the systemic circulation.
Lademann and co-workers reported that only follicles containing growing hair or showing sebum production took up particles.Citation73 Others, “non-active” follicles, were clogged by cellular debris and needed re-opening by washing or light peeling. This was kind of surprising as the direction of hair growth and sebum flow are opposed to the invasion of particles into the follicles suggesting an “active” uptake mechanism transporting nanoparticles deep into the follicle.Citation2 Instead, experiments on excised pig ear skin with and without massage suggested that this is a purely mechanical process. The natural movement of the hair together with the scaly structure of the hair cuticula works like a gear pump which transports particles having an appropriate size into the follicle. The optimum particle size was in the range of 400–700 nmCitation73 and coincided with the size of the scales of the cuticula. In addition material properties did not seem to influence the particle uptake or penetration depth. However, also particles of other sizes were found to penetrate into human follicles such as polystyrene particles of 20 nmCitation14 as well as iron-based particles.Citation44
Several methods were proposed for the investigation of the follicular pathway. Animal studies with hairless and hair bearing species are not appropriate as even hairless species do have follicles. Some assumptions to that rule are newborn hairless rats that develop follicles only within a few days after birth.Citation70 Also, follicle free skin can be artificially created by scarring. However there is a common consensus that murine skin is not an adequate model for the skin absorption in humans. Rat skin for instance has a density of 289 ± 21 hair follicles/cm2 in contrast to human abdominal skin and pig skin from the back showing a density of 11 ± 1 hair follicles/cm2 only.Citation74 Therefore some effort has been taken to develop suitable alternative methods that are based on human skin. Barry and coworkers developed the “SC sandwich” which is composed of an epidermal and a stratum corneum membrane.Citation75 Due to the random distribution of hair follicles on the skin surface there is only negligible chance for a direct connection across both membranes.Citation75 Therefore, if the shunts contribute significantly to the permeation transport through the sandwich will be much reduced compared to what would be expected from simple increase in path length.
Otberg et al. chose a more work intensive approach by closing each hair follicle opening with varnish wax under microscopic observation.Citation68 At the control skin site the varnish wax was applied directly adjacent to each follicle in order to compensate for the area reduction at the test site. The same group also suggested a differential stripping technique for selective sampling of the follicular content.Citation76 In a first step tape-stripping removes substances remaining on the skin surface or having penetrated into the stratum corneum. Substances (or particles) inside the follicles are protected and are sampled in a second step by a cyanoacrylatebiopsy that removes the content of the hair follicles.
Conclusion
First of all, one can state that the penetration of particulate materials into skin is a very complex issue. Ab initio predictions are therefore not (yet) a practical approach to describe the penetration processes. For this reason, up to now only experimental data for particle penetration is available and will be indispensible also in the future. However, the experiments can not be transferred straightforwardly form the non-particulate situation, but has to take into account the particulate nature of the analyte and not only the material. Furthermore, practical and ethical considerations limit the freedom of the experimental design. In addition, the choice of the experimental parameters as well as the following interpretation of the data obtained strongly depends on the field of the research and the particular problem investigated. Therefore, it is of outmost importance when looking at the available data to take the experimental settings into account. Furthermore, care has to be taken when transferring data from one model system to another and hence far-reaching conclusions are made for instance regarding safety hazards.
Looking at , problematic data might be the results on the penetration and permeation of Quantum dots. For mouse skin as well as for porcine skin penetration was reported whereas on rat skin only for abraded skin permeation into deeper skin layers was observed. In any case, the data available yields a heterogeneous picture.
In contrast, for human skin only 2 publications report passive permeation of particles beyond the stratum corneum. In both cases small primary particles are used even though at least two of the particle types were described to form bigger agglomerates. As a consequence only small amounts were found in the viable part of the skin.
Another important issue is the mechanical stress applied to the skin when investigating the penetration behavior. There seems to be a tremendous influence on the kind of mechanical stimulus as well as on the time that stimulation is present. Particles as big as 2 µm were reported to penetrate into human skin.Citation30 In contrast to that for much smaller particles such as the surface-modified fullerenes flexation of more than 60 min was necessary to improve penetration into deeper layers of porcine skin.Citation62 However, it is not clear if the possible agglomerates (∼250 nm) penetrate or if the primary particles penetrate.
As an overall consequence a standardized operation procedure is not only desirable but essential to be able to investigate and eventually predict the behavior of nano-scale materials with skin. A well established protocol need to be defined to make the results comparable with each other. Slight differences in the treatment of the skin samples or the set-up might already contribute to altered penetration behavior. Pretreatment, way of application, cleaning/rinsing procedures, hydration state, and detection methods will influence the results and need to be carefully controlled.
Nevertheless, the growing number of nanoparticle occurrence in manufacturing and application need a better understanding of the relevant mechanisms of nanoparticulate penetration into skin, not least for an adequate risk assessment.
An issue not being studied so far is the influence of the nanoparticulate formulations on biochemical processes in the skin. This might be an intriguing field for future investigations.
Abbreviations
| MPA | = | 3-mercaptopropionic acid |
| TMAOH | = | tetramethylammonium hydroxide |
| AOT | = | sodium bis(2-ethylhexyl) sulfosuccinate |
| Baa-Lys-(FITC)-NLS | = | phenylalanine-based fullerene [bucky amino acid] (fluorescein isothiocyanate) nuclear localisation sequence; Cit, citrate |
| SEM | = | scanning electron microscopy |
| TEM | = | transmission electron microscopy |
| AuNP | = | gold nanoparticles |
| AgNP | = | silver nanoparticles |
Figures and Tables
Figure 1 Color-coded simulations of three diffusing compounds in a 3D stratum corneum model, calculated for different diffusivity ratios of the compounds between corneocytes (bricks) and in the intercellular space (mortar). (Top view; red indicates high concentration). For nanoparticulate compounds a strictly intercellular route (right case) will be due. (Courtesy of M. Heisig, IWR, Ruprecht-Karls-Universität Heidelberg, Germany).Citation77
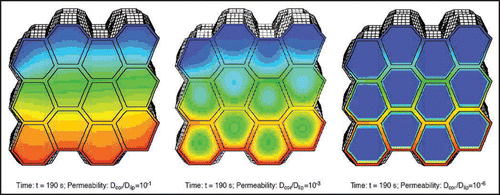
Figure 2 Sketch of the morphology of human stratum corneum: The stratum corneum is composed out of rigid corneocytes (CC), dead cells densely packed with keratinous filaments in a matrix of connecting proteins and confined by the cornified envelope (CE), separated by the intercellular space (IS) filled with lipid compounds (fatty acids, ceramides etc.,) and aqueous films to form lamellar lipid bilayers (LLB) (published with permission from ref. 4).
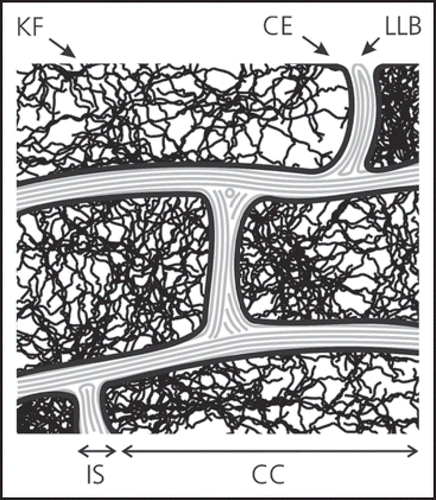
Figure 3 Relation between absorption and lipophilicity. Optimal absorption conditions are for 1 ≤ log (P) ≤ 3 (P being the octanol/water partition coefficient).
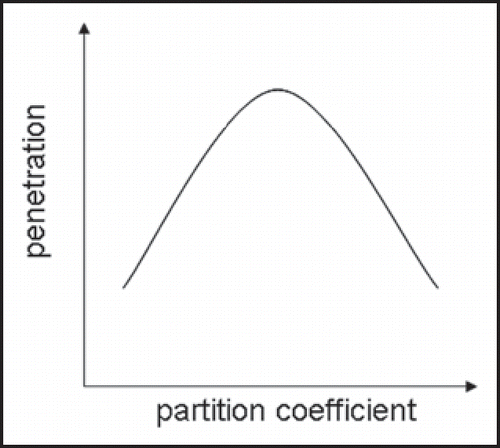
Figure 4 PLGA nanoparticles in human skin furrows 5 hours after administration. (a) A two photon micrograph at z = 15 µm subsurface depth showing superficial keratin fluorescence and clearly resolved single particles. (b) A pseudo-color overlay of two photon and confocal images at z = 28 µm revealing the release of a drug dummy from the particles and its cutaneous uptake. The two photon channel (green) shows keratinous layers and single particles, the 488 nm-excited confocal channel (blue) addresses the fluorescein-labeled particles solely and the 543 nm-excited confocal channel (red) exclusively shows the dummy compound (Texas Red) (published with permission from ref. 4).
Figure 5 Scheme of mechanism of ultradeformable vesicle transport through a stratum corneum pore. The vesicle will penetrate into the pore if the force from the osmotic pressure exceeds the deformation resistance of the vesicle (published with permission from ref. 4).
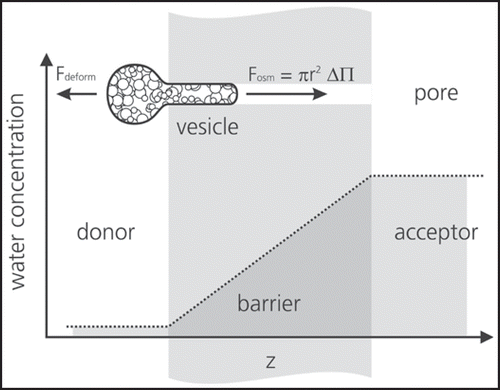
Table 1 “Best case” transport parameters of nanoparticles over a typical stratum corneum barrier
Table 2 Overview on particle penetration experiments performed with different types of skin and different particles
References
- Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: An emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives 2005; 113:823 - 839
- Lademann J, Richter H, Teichmann A, Otberg N, Blume-Peytavi U, Luengo J, et al. Nanoparticles—An efficient carrier for drug delivery into the hair follicles. European Journal of Pharmaceutics and Biopharmaceutics 2007; 66:159 - 164
- Lademann J, Richter H, Schaefer UF, Blume-Peytavi U, Teichmann A, Otberg N, Sterry W. Hair Follicles—A Long-Term Reservoir for Drug Delivery. Skin Pharmacology and Physiology 2006; 19:232 - 236
- Sattler KD. Handbook of Nanophysics 2010; CRC Press
- Naegel A, Hansen S, Neumann D, Lehr CM, Schaefer UF, Wittum G, Heisig M. Erratum to “In-silico model of skin penetration based on experimentally determined input parameters. Part II: Mathematical modelling of in-vitro diffusion experiments. Identification of critical input parameters” [Eur J Pharm Biopharm 2008; 68:368–79] (DOI:10.1016/j.ejpb.2007.05.018). European Journal of Pharmaceutics and Biopharmaceutics 2008; 68:846
- Naegel A, Hansen S, Neumann D, Lehr CM, Schaefer UF, Wittum G, Heisig M. In-silico model of skin penetration based on experimentally determined input parameters. Part II: Mathematical modelling of in-vitro diffusion experiments. Identification of critical input parameters. European Journal of Pharmaceutics and Biopharmaceutics 2008; 68:368 - 379
- Hansen S, Henning A, Naegel A, Heisig M, Wittum G, Neumann D, et al. In-silico model of skin penetration based on experimentally determined input parameters. Part I: Experimental determination of partition and diffusion coefficients. European Journal of Pharmaceutics and Biopharmaceutics 2008; 68:352 - 367
- Moghimi HR, Barry BW, Williams AC. Bronaugh RL, Maibach HI. Stratum corneum and barrier performance: A model lamellar structural approach. Drugs— Cosmetics—Mechanisms—Methodology 1999; New York, Basel, Hong Kong Marcel Dekker 515 - 553
- Potts RO, Guy RH. Predicting skin permeability. Pharmaceutical Research 1992; 9:663 - 669
- Magnusson BM, Anissimov YG, Cross SE, Roberts MS. Molecular size as the main determinant of solute maximum flux across the skin. Journal of Investigative Dermatology 2004; 122:993 - 999
- Bos JD, Meinardi MMHM. The 500 Dalton rule for the skin penetration of chemical compounds and drugs. Experimental Dermatology 2000; 9:165 - 169
- Moghimi HR, Williams AC, Barry BW. A lamellar matrix model for stratum corneum intercellular lipids II. Effect of geometry of the stratum corneum on permeation of model drugs 5-fluorouracil and oestradiol. International Journal of Pharmaceutics 1996; 131:117 - 129
- Alvarez-Roman R, Naik A, Kalia YN, Guy RH, Fessi H. Enhancement of topical delivery from biodegradable nanoparticles. Pharmaceutical Research 2004; 21:1818 - 1825
- Alvarez-Roman R, Naik A, Kalia Y, Guy RH, Fessi H. Skin penetration and distribution of polymeric nanoparticles. J Control Release 2004; 99:53 - 62
- Toll R, Jacobi U, Richter H, Lademann J, Schaefer H, Blume-Peytavi U. Penetration profile of microspheres in follicular targeting of terminal hair follicles. Journal of Investigative Dermatology 2004; 123:168 - 176
- Kohli AK, Alpar HO. Potential use of nanoparticles for transcutaneous vaccine delivery: Effect of particle size and charge. International Journal of Pharmaceutics 2004; 275:13 - 17
- Luengo J, Weiss B, Schneider M, Ehlers A, Stracke F, König K, et al. Influence of Nanoencapsulation on Human Skin Transport of Flufenamic Acid. Skin Pharmacology and Physiology 2006; 19:190 - 197
- Stracke F, Weiss B, Lehr C-M, König K, Schaefer UF, Schneider M. Multiphoton microscopy for the investigation of dermal penetration of nanoparticle-borne drugs. Journal of Investigative Dermatology 2006; 126:2224 - 2233
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Variables influencing interactions of untargeted quantum dot nanoparticles with skin cells and identification of biochemical modulators. Nano Letters 2007; 7:1344 - 1348
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. Journal of Investigative Dermatology 2007; 127:143 - 153
- Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Penetration of intact skin by quantum dots with diverse physicochemical properties. Toxicological Sciences 2006; 91:159 - 165
- Joo HH, Lee HY, Kim JC, Lee HJ, Kang HY. Integrities and skin permeation-enhancing characteristics of behenic acid nanoparticles. Journal of Dispersion Science and Technology 2009; 30:148 - 153
- Küchler S, Radowski MR, Blaschke T, Dathe M, Plendl J, Haag R, et al. Nanoparticles for skin penetration enhancement—A comparison of a dendritic core-multishellnanotransporter and solid lipid nanoparticles. European Journal of Pharmaceutics and Biopharmaceutics 2009; 71:243 - 250
- Brayner R. The toxicological impact of nanoparticles. Nano Today 2008; 3:48 - 55
- Nohynek GJ, Dufour EK, Roberts MS. Nanotechnology, cosmetics and the skin: Is there a health risk?. Skin Pharmacology and Physiology 2008; 21:136 - 149
- Vega-Villa KR, Takemoto JK, Yánez JA, Remsberg CM, Forrest ML, Davies NM. Clinical toxicities of nanocarrier systems. Advanced Drug Delivery Reviews 2008; 60:929 - 938
- SCENIHR SCoEaN-IHR. Risk Assessment of Products of Nanotechnologies. Health and Consumer Protetcion 2009;
- Alvarez-Roman R, Naik A, Kalia YN, Fessi H, Guy RH. Visualization of skin penetration using confocal laser scanning microscopy. European Journal of Pharmaceutics and Biopharmaceutics 2004; 58:301 - 316
- Shim J, Kang HS, Park WS, Han SH, Kim J, Chang IS. Transdermal delivery of mixnoxidil with block copolymer nanoparticles. J Control Release 2004; 97:477 - 484
- Tinkle SS, Antonini JM, Rich BA, Roberts JR, Salmen R, DePree K, Adkins EJ. Skin as a route of exposure and sensitization in chronic beryllium disease. Environmental Health Perspectives 2003; 111:1202 - 1208
- Schaefer U, Szayna M, Kuhn WP, Loth H. NMR-microscopy of human skin in vivo and in vitro. Proceedings of the Controlled Release Society 1995; 626 - 627
- Swarbrick J, Lee G, Brom J. Drug permeation through human skin: I. Effect of storage conditions of skin. Journal of Investigative Dermatology 1982; 78:63 - 66
- Souto EB, Almeida AJ, Müller RH. Lipid nanoparticles (SLN®, NLC®) for cutaneous drug delivery: Structure, protection and skin effects. Journal of Biomedical Nanotechnology 2007; 3:317 - 331
- Jung S, Otberg N, Thiede G, Richter H, Sterry W, Panzner S, Lademann J. Innovative liposomes as a transfollicular drug delivery system: penetration into porcine hair follicles. J Invest Dermatol 2006; 126:1728 - 1732
- Honeywell-Nguyen PL, Wouter Groenink HW, de Graaff AM, Bouwstra JA. The in vivo transport of elastic vesicles into human skin: effects of occlusion, volume and duration of application. J Control Release 2003; 90:243 - 255
- Honeywell-Nguyen PL, de Graaff AM, Groenink HW, Bouwstra JA. The in vivo and in vitro interactions of elastic and rigid vesicles with human skin. Biochim Biophys Acta 2002; 1573:130 - 140
- Verma DD, Verma S, Blume G, Fahr A. Liposomes increase skin penetration of entrapped and non-entrapped hydrophilic substances into human skin: a skin penetration and confocal laser scanning microscopy study. Eur J Pharm Biopharm 2003; 55:271 - 277
- Cevc G. Transfersomes, liposomes and other lipid suspensions on the skin: Permeation enhancement, vesicle penetration and transdermal drug delivery. Critical Reviews in Therapeutic Drug Carrier Systems 1996; 13:257 - 388
- Van Den Bergh BAI, Vroom J, Gerritsen H, Junginger HE, Bouwstra JA. Interactions of elastic and rigid vesicles with human skin in vitro: Electron microscopy and twophoton excitation microscopy. Biochimica et Biophysica Acta—Biomembranes 1999; 1461:155 - 173
- Van Kuijk-Meuwissen MEMJ, Junginger HE, Bouwstra JA. Interactions between liposomes and human skin in vitro, a confocal laser scanning microscopy study. Biochimica et Biophysica Acta—Biomembranes 1998; 1371:31 - 39
- Van Kuijk-Meuwissen MEMJ, Mougin L, Junginger HE, Bouwstra JA. Application of vesicles to rat skin in vivo: A confocal laser scanning microscopy study. J Control Release 1998; 56:189 - 196
- Cevc G, Gebauer D. Hydration-driven transport of deformable lipid vesicles through fine pores and the skin barrier. Biophysical Journal 2003; 84:1010 - 1024
- Sonavane G, Tomoda K, Sano A, Ohshima H, Terada H, Makino K. In vitro permeation of gold nanoparticles through rat skin and rat intestine: Effect of particle size. Colloids and Surfaces B: Biointerfaces 2008; 65:1 - 10
- Baroli B, Ennas MG, Loffredo F, Isola M, Pinna R, Lopez-Quintela MA. Penetration of metallic nanoparticles in human full-thickness skin. Journal of Investigative Dermatology 2007; 127:1701 - 1712
- Larese FF, D’Agostin F, Crosera M, Adami G, Renzi N, Bovenzi M, Maina G. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology 2009; 255:33 - 37
- SCCNFP. Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumer Conerning Titanium Dioxide 2000; Brussels European Commission
- Lademann J, Weigmann HJ, Rickmeyer C, Barthelmes H, Schaefer H, Mueller G, Sterry W. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacology and Applied Skin Physiology 1999; 12:247 - 256
- Tan MH, Commens CA, Burnett L, Snitch PJ. A pilot study on the percutaneous absorption of microfine titanium dioxide from sunscreens. Australasian Journal of Dermatology 1996; 37:185 - 187
- Dussert AS, Gooris E, Hemmerle J. Characterization of the mineral content of a physical sunscreen emulsion and its distribution onto human stratum corneum. International Journal of Cosmetic Science 1997; 19:119 - 129
- Gamer AO, Leibold E, Van Ravenzwaay B. The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicology in Vitro 2006; 20:301 - 307
- Mavon A, Miquel C, Lejeune O, Payre B, Moretto P. In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharmacology and Physiology 2007; 20:10 - 20
- Schulz J, Hohenberg H, Pflücker F, Gärtner E, Will T, Pfeiffer S, et al. Distribution of sunscreens on skin. Advanced Drug Delivery Reviews 2002; 54
- Pflücker F, Wendel V, Hohenberg H, Gärtner E, Will T, Pfeiffer S, et al. The human stratum corneum layer: An effective barrier against dermal uptake of different forms of topically applied micronised titanium dioxide. Skin Pharmacology and Applied Skin Physiology 2001; 14:92 - 97
- Lekki J, Stachura Z, Dabros W, Stachura J, Menzel F, Reinert T, et al. On the follicular pathway of percutaneous uptake of nanoparticles: Ion microscopy and autoradiography studies. Nuclear Instruments and Methods in Physics Research, Section B: Beam Interactions with Materials and Atoms 2007; 260:174 - 177
- Cross SE, Innes B, Roberts MS, Tsuzuki T, Robertson TA, McCormick P. Human skin penetration of sunscreen nanoparticles: In-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacology and Physiology 2007; 20:148 - 154
- Zvyagin AV, Zhao X, Gierden A, Sanchez W, Ross JA, Roberts MS. Imaging of zinc oxide nanoparticle penetration in human skin in vitro and in vivo. Journal of Biomedical Optics 2008; 13:064031
- Gontier E, Ynsa MD, Bíró T, Hunyadi J, Kiss B, Gáspár K, et al. Is there penetration of titania nanoparticles in sunscreens through skin? A comparative electron and ion microscopy study. Nanotoxicology 2008; 2:218 - 231
- Lacerda L, Bianco A, Prato M, Kostarelos K. Carbon nanotubes as nanomedicines: From toxicology to pharmacology. Advanced Drug Delivery Reviews 2006; 58:1460 - 1470
- Rouse JG, Yang J, Barron AR, Monteiro-Riviere NA. Fullerene-based amino acid nanoparticle interactions with human epidermal keratinocytes. Toxicology in Vitro 2006; 20:1313 - 1320
- Lam CW, James JT, McCluskey R, Arepalli S, Hunter RL. A review of carbon nanotube toxicity and assessment of potential occupational and environmental health risks. Critical Reviews in Toxicology 2006; 36:189 - 217
- Monteiro-Riviere NA, Nemanich RJ, Inman AO, Wang YY, Riviere JE. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicology Letters 2005; 155:377 - 384
- Rouse JG, Yang J, Ryman-Rasmussen JP, Barron AR, Monteiro-Riviere NA. Effects of mechanical flexion on the penetration of fullerene amino acid-derivatized peptide nanoparticles through skin. Nano Letters 2007; 7:155 - 160
- Hild WA, Breunig M, Goepferich A. Quantum dots—Nano-sized probes for the exploration of cellular and intracellular targeting. European Journal of Pharmaceutics and Biopharmaceutics 2008; 68:153 - 168
- Zhang LW, Monteiro-Riviere NA. Assessment of quantum dot penetration into intact, tape-stripped, abraded and flexed rat skin. Skin Pharmacology and Physiology 2008; 21:166 - 180
- Chu M, Wu Q, Wang J, Hou S, Miao Y, Peng J, Sun Y. In vitro and in vivo transdermal delivery capacity of quantum dots through mouse skin. Nanotechnology 2007; 18
- Mortensen LJ, Oberdörster G, Pentland AP, DeLouise LA. In Vivo Skin Penetration of Quantum Dot Nanoparticles in the Murine Model: The Effect of UVR. Nano Letters 2008; 8:2779 - 2787
- Otberg N, Richter H, Schaefer H, Blume-Peytavi U, Sterry W, Lademann J. Variations of hair follicle size and distribution in different body sites. J Invest Dermatol 2004; 122:14 - 19
- Otberg N, Patzelt A, Rasulev U, Hagemeister T, Linscheid M, Sinkgraven R, et al. The role of hair follicles in the percutaneous absorption of caffeine. Br J Clin Pharmacol 2008; 65:488 - 492
- Hueber F, Wepierre J, Schaefer H. Role of transepidermal and transfollicular routes in percutaneous absorption of hydrocortisone and testosterone: in vivo study in the hairless rat. Skin Pharmacol 1992; 5:99 - 107
- Illel B, Schaefer H, Wepierre J, Doucet O. Follicles play an important role in percutaneous absorption. J Pharm Sci 1991; 80:424 - 427
- Langbein L, Grund C, Kuhn C, Praetzel S, Kartenbeck J, Brandner JM, et al. Tight junctions and compositionally related junctional structures in mammalian stratified epithelia and cell cultures derived therefrom. Eur J Cell Biol 2002; 81:419 - 435
- Nohynek GJ, Lademann J, Ribaud C, Roberts MS. Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Crit Rev Toxicol 2007; 37:251 - 277
- Lademann J, Meinke M, Sterry W, Patzelt A. How safe are nanoparticles?. Wie sicher sind Nanopartikel? 2009; 60:305 - 309
- Bronaugh RL, Stewart RF, Congdon ER. Methods for in vitro percutaneous absorption studies II. Animal models for human skin. Toxicology and Applied Pharmacology 1982; 62:481 - 488
- Barry BW. Drug delivery routes in skin: a novel approach. Adv Drug Deliv Rev 2002; 54:31 - 40
- Teichmann A, Jacobi U, Ossadnik M, Richter H, Koch S, Sterry W, Lademann J. Differential stripping: determination of the amount of topically applied substances penetrated into the hair follicles. J Invest Dermatol 2005; 125:264 - 269
- Feuchter D, Heisig M, Wittum G. A geometry model for the simulation of drug diffusion through the stratum corneum. Computing and Visualization in Science 2006; 9:117 - 130
- Potts RO, Guy RH. Gurney R, Teubner A. The prediction of percutaneous penetration: a mechanistic model. Dermal and Transdermal Drug Delivery New Insights and Perspectives 1993; Stuttgart Wissenschaftliche Verlagsgesellschaft mbH 153 - 160