Abstract
In scientific and public communities, there is an ongoing discussion how to balance between positive and negative effects of solar UV-exposure. On the one hand, solar UV-radiation represents the most important environmental risk factor for the development of non-melanoma skin cancer. Consequently, UV protection is an important measure to prevent these malignancies, especially in risk groups. Otherwise, approximately 90% of all vitamin D needed by the human body has to be formed in the skin through the action of UV-radiation. This dilemma represents a serious problem, for an association of vitamin D-deficiency and multiple independent diseases including various types of cancer, bone diseases, autoimmune diseases, infectious diseases, cardiovascular diseases and hypertension has now been reported in a large number of investigative and epidemiologic studies. As a consequence, it has been assumed that for the general population in the US, Europe and other countries, the net effects of solar UV B-radiation on human health are beneficial at or near current levels. We and others have shown that strict sun protection causes vitamin D-deficiency/insufficiency and that detection and treatment of vitamin D-deficiency in sun deprived risk groups is of high importance. Although further work is necessary to define an adequate vitamin D-status and adequate guidelines for solar and artificial UV-exposure, it is at present mandatory that public health campaigns and sun protection recommendations to prevent skin cancer consider these facts. In this review, we analyze the present literature to help developing well-balanced recommendations on sun protection that ensure an adequate vitamin D-status. These recommendations will hopefully protect us against adverse effects of UV protection without significantly increasing the risk to develop UV-induced skin cancer.
The Janus Faces of UV-Exposure: Photocarcinogenesis and Vitamin D Synthesis
Photocarcinogenesis.
Epidemiological investigations on nonmelanoma skin cancer, malignant melanoma and solar UV-exposure. Historically, the association between solar UV-exposure and non-melanoma skin cancer was first reported by Unna and Dubreuilh at the end of the 19th century.Citation1,Citation2 These physicians recognized actinic keratoses (AK) and squamous cell carcinomas (SCC) in chronically sun-exposed skin areas of sailors and vineyard workers. At present, it is scientifically accepted that solar UV-exposure represents the most important environmental risk factor for the development of non-melanoma skin cancer.Citation3–Citation8 In general, skin cancer includes three major types: SCC,Citation9,Citation10 basal cell carcinoma (BCC),Citation10 and primary cutaneous malignant melanoma (MM).Citation11 It has to be noted that AK are now considered to represent cutaneous SCC in situ.Citation10 While BCC do not and SCC rarely metastasize (with the exception of risk groups that include immunosuppressed patients, e.g., in solid organ transplant recipients), MM is often characterized by aggressive metastatic growth and fatal outcome.
Epidemiological and laboratory data have convincingly shown that sunburns are implicated in the pathogenesis of SCC,Citation12 BCCCitation4,Citation13 and MM.Citation5,Citation14 Today, it is accepted that chronic sun exposure is the most important cause for the formation of SCC,Citation15 but may be less important for the development of BCC.Citation4,Citation16,Citation17 AK are more frequent in men, in sun-sensitive individuals chronically exposed to solar UV, and in individuals who have a history of sunburn.Citation18 Concerning MM, numerous epidemiologic investigations analysing solar UV-exposure parameters have consistently reported an association between the development of MM and short-term intense UV-exposure, particularly burning in childhood. Citation14,Citation19 It has been convincingly demonstrated by many investigators, that the incidence of MM increases with decreasing latitude towards the equator.Citation20,Citation21 However, in contrast to short-term intense exposure, more chronic less intense exposure has not been found to be a risk factor for the development of MM and in fact has been found in several studies to be protective.Citation5,Citation22–Citation24 Grass and Bopp previously have analyzed MM mortality rates in different occupational groups.Citation24 They concluded that indoor working males (including graduates and employees with commercial or technical education) have an increased risk affirming the association between melanoma risk and intermittent solar UV-exposure. In contrast, outdoor workers with chronic solar UV-exposure appeared slightly protected.Citation24 It may be speculated whether these associations may be an explanation for the finding of an increased risk to develop MM after sunscreen use, that was reported previously.Citation25 The hypothesis of an association between sunbed use and cutaneous MM was previously analyzed in a large European case-control study investigating an adult population aged between 18 and 49 years.Citation26 In that study in Belgium, France, The Netherlands, Sweden and the UK, solar UV and sunbed exposure was recorded and analyzed between 1999 and 2001 in 597 newly diagnosed MM cases and 622 controls. 53% of cases and 57% of controls ever used sunbeds. There was a South-to-North gradient with high prevalence of sunbed exposure in northern Europe and lower prevalence in the South (prevalence of use in France 20% compared to 83% in Sweden). The authors concluded that dose and lag-time between first exposure to sunbeds and time of study were not associated with MM risk, neither were sunbathing and sunburns.Citation26
Photocarcinogenesis of non-melanoma skin cancer. The solar UV-spectrum can be divided into several bands that vary in their physical and biological properties, namely UV-C (wavelength below 280 nm), UV-B (280–315 nm) and UV-A (315–400 nm).Citation9 It has to be noted that the predominant part of the short-wave, high-energy and destructive UV-spectrum cannot reach the surface of the earth. This is due to the fact that the ozone layer of the earth’s outer atmosphere absorbs the shorter wavelength up to appr. 310 nm (UV-C and part of UV-B radiation).Citation9 The different layers of human skin absorb UV-radiation in a wavelength-dependent manner. Because UV-B radiation is almost completely absorbed by the epidermis, only 20% of UV-B radiation reach the epidermal basal cell layer or the dermal stratum papillare.Citation9 In contrast, UV-A radiation penetrates deeper into the dermis and deposits 30–50% of its energy in the dermal stratum papillare. These absorption characteristics explain at least in part why UV-B effects (including skin cancer development) have to be expected predominantly in the epidermis and UV-A effects (including skin ageing, solar elastosis) in the dermis.Citation9 It is well known that DNA represents a major epidermal chromophore with an absorption maximum at 260 nm. Both UV-A and UV-B radiation are able to induce structural DNA-damage. UV-B radiation induces molecular rearrangements of the DNA resulting in the characteristic formation of specific photoproducts (most importantly cyclobutane pyrimidine dimers and 6-4 photoproducts), which are known to be mutagenic. The genotoxic potential of UV-A radiation has been clearly shown to be predominantly due to indirect mechanisms that include oxidative damage. Gene mutations that have been shown to be of importance for the pathogenesis of skin cancer include mutations in the p53 gene (AK, SCC), and mutations in the patched (PTCH)/sonic hedgehog pathway (BCC). The UV-induced development of skin carcinomas has been investigated previously using multiple animal and laboratory models. Mutation-associated inactivation of p53 tumor suppressor gene plays a critical role both for stages of initiation and progression of SCC.Citation27 Analysis of data on gene mutations in human premalignant AK lesions, as well as data from UV-induced carcinogenesis experiments in mice have suggested that the first step involves acquisition of UV-induced mutations in the p53 gene by epidermal keratinocytes.Citation27 This defect diminishes sunburn cell formation and enhances cell survival allowing retention of initiated, precancerous keratinocytes.Citation27 Moreover, chronic exposures to solar UV results in the accumulation of p53 mutations in skin, which confer a selective growth advantage to initiated keratinocytes and allow their clonal expansion, leading to formation of AK.Citation27 The expanded cell death-defective clones represent a larger target for additional UV-induced p53 mutations or mutations in other genes, thus enabling progression to carcinomas. Concerning the pathogenesis of BCC, the importance of PTCH, SMOH and TP53 mutations has been demonstrated.Citation28 Suppression of the skin’s immune system has been shown to represent another mechanism by which solar UV-radiation induces and promotes skin cancer growth, even at suberythemogenic doses.Citation29 Immunosuppressive properties have been demonstrated for both UV-B and UV-A.Citation29 Moreover, it has been speculated that UV-B-induced production of vitamin D may be involved in UV-B induced immunosuppression.Citation30
Our present understanding of the synthesis and metabolism of vitamin D-compounds in the skin is demonstrated in . Interestingly, a contribution of the cutaneous vitamin D system to the pathogenesis and prognosis of skin malignancies including MM has been reported.Citation31 We have characterized the expression of key components of the vitamin D endocrine system [vitamin D receptor (VDR), vitamin D-25OHase (CYP27A1), 25(OH) D-1αOHase (CYP27B1), 1,25(OH)2D-24OHase (CYP24A1)] in cutaneous SCC, BCC and MM.Citation32–Citation36 Our findings provide supportive evidence for the concept that endogeneous synthesis and metabolism of vitamin D metabolites as well as VDR expression may regulate growth characteristics of BCC, cutaneous SCC and MM.Citation32–Citation36 An association of Fok 1 restriction fragment length polymorphisms of the VDR with occurrence and outcome of MM, as predicted by tumor (Breslow) thickness, has been reported.Citation37 The same laboratory demonstrated that a polymorphism in the promotor region of VDR (A-1012G, adenine-guanine substitution −1,012 bp relative to the exon 1a transcription start site) is related in MM patients to thicker Breslow thickness groups and to the development of metastasis.Citation38 The authors concluded that polymorphisms of the VDR gene, which can be expected to result in impaired function of biologically active vitamin D metabolites, are associated with susceptibility and prognosis in MM. The importance of VDR polymorphisms for melanoma risk has been systematically reviewed recently in a meta-analysis.Citation39 These authors concluded that current evidence is in favor of an association between 1 VDR gene polymorphism (BsmI) and the risk of developing melanoma, and that this finding indirectly supports the hypothesis that sun exposure may have an antimelanoma effect through activation of the vitamin D system.Citation39
Applying array CGH, amplification of the 1,25(OH)2D-metabolizing enzyme CYP24A1 [1,25(OH)2D-24OHase] was recently detected as a likely target oncogene of the amplification unit 20q13.2 in breast cancer cell lines and tumors.Citation40 It has been speculated that overexpression of CYP24A1 due to gene amplification may abrogate 1,25(OH)2D-mediated growth control. Additionally, amplification of the CYP27B1 [25(OH)D-1αOHase] gene has been reported in human malignant glioma.Citation41 The significance of these findings remains to be investigated. We have analyzed metastases of MM and found no evidence of amplification of CYP27B1 or CYP24A1 genes using Southern analysis.Citation34 However, we detected various splicing variants of the CYP27B1 gene in cutaneous malignancies.Citation41 The clinical significance of this finding remains to be elucidated. Additionally, we have demonstrated that serum 25(OH)D levels are not reduced in MM patients.Citation42
Skin cancer prevention campaigns and recommendations for protection against solar and artificial UV-radiation. It is a major aim of skin cancer prevention campaigns to improve the knowledge of the general population regarding the role of environmental risk factors for the development of skin cancer. While the incidence of skin cancer has dramatically increased during the last decades, it is now accepted that the reasons for this development are multifactoral.Citation7 It has been speculated that besides the age pyramid and other factors, cultural changes that result in increased UV-exposure, may be of particular importance.Citation7 It has been assumed that socio-economical and cultural changes in the behavior of large groups of society may have resulted in an increase of UV-exposure in those individuals. These changes may include more recreational activities and holidays spent in the sun as well as frequent exposure to artificial UV in sunbeds. The wellness-movement with tan representing the current ideal of beauty may have supported this development as well. However, one has to keep in mind that the reported increase in skin cancer incidence may be due to other factors independent from solar UV-radiation. As an example, it has been recently published that the large increase in reported melanoma incidence is likely to be due to a diagnostic drift which classifies benign lesions as stage 1 melanoma.Citation43 In that study, this conclusion could be confirmed by direct histological comparison of contemporary and past histological samples. The distribution of the lesions reported did not correspond to the sites of lesions caused by solar exposure. The authors concluded that these findings should lead to a reconsideration of the treatment of ‘early’ lesions, a search for better diagnostic methods to distinguish them from truly malignant melanomas, re-evaluation of the role of ultraviolet radiation and recommendations for protection from it, as well as the need for a new direction in the search for the cause of melanoma.Citation43
To counteract against the increasing incidence of skin cancer, public health campaigns were developed and introduced, with the aim to improve the knowledge of the general population regarding the role of UV-radiation for the development of skin cancer. However, it has to be noted that positive effects of UV light were not adequately considered in most of these campaigns that in general proposed a strict “no sun policy.”Citation44,Citation45 The first of the campaigns were introduced and established in Australia in the early 1980s, containing neat messages and slogans which were easy to remember, including the “Slip (on a shirt), Slop (on some sunscreen), Slap (on a hat)” initiative. Afterwards several international consensus meetings profited from Australian experiences and renewed similar aims in the primary prevention of skin cancer.Citation46 The World Health Organisation (WHO) started a Global UV Project called INTERSUN (WHO, INTERSUN, The global UV project: a guide and compendium, Geneva 2003) which aimed to encourage countries to take action to reduce UV-induced health risks, additional goals were the development and use of an internationally recognized UV Index (UVI) to fasciltate sun protection messages related to daily UV-intensity and special programmes for schools to teach children and teachers about sun protection.Citation46 Certain intervention programmes were focused especially on children at school. In 2001 the European Society of Skin Cancer Prevention (EUROSKIN) organized an international conference “Children under the sun” in Ovieto, Italy to strengthen the importance of this issue.Citation46 During the last decades, country-specific preventive strategies were developed by several institutions and organisations throughout the world, e.g., Skin Cancer Foundation (SCF) in the US (www.skincancer.org); German Cancer Aid and Association of Dermatological Prevention (ADP) in Germany (www.unserehaut.de).Citation46 At the EUROSKIN conference “Children under the sun”, the ADP announced the “Periods-of-life-Programme” (POLP).Citation46 To achieve an age-accordant education, certain target-groups were defined in an age-dependent manner. Besides dermatologists, general practicioners, gynecologists, midwifes, pediatrics, kindergarten teachers, school teachers and parents were also integrated in this program.Citation46 When POLP started in Germany in 2002, it was first mainly focused on the target group of babies and their parents. Thereafter, kindergarten children (2003) and pupils entering elementary school (2004) were included in close relation to the former “Sun protection programmes in school” of the WHO.Citation46 Depending on individual target-groups, different methods were applied to teach the subject matters adequately (e.g., “sun-songs,” TV-spots) and to identify individuals or groups with specific need for information. Another pursued strategy of identifying risk groups is to classify people according to their individual solar and/or artificial UV-behavior. In this way, certain risk profiles have been established by characterization of typical behavior patterns.
Until today, strict recommendations for protection against artificial and solar UV-radiation still represent a fundamental part of public health campaigns and prevention programmes aimed at reducing UV-radiation-induced skin damage and skin cancer.Citation44,Citation45 These recommendations include the use of sunscreens, protective clothing and avoidance of artificial and solar UV-exposure. Appropriate clothing is extremely effective in absorbing all UV-B radiation thereby preventing any UV-B photons from reaching the skin.Citation47,Citation48 Most sunscreen products combine chemical UV-absorbing sunscreens and physical anorganic sunscreens, which reflect UV, to provide broad spectrum protection. At present, most sunscreen products protect against both UV-B and UV-A radiation.
Still a Long Way to Go: Understanding and Fighting Vitamin D-Deficiency
Vitamin D-deficiency—a serious health problem.
It has been estimated that approximately 1 billion people worldwide have vitamin D-deficiency or -insufficiency.Citation49 Approximately 90% of all requisite vitamin D is formed within the skin through the action of the sun—a serious problem, for a connection between vitamin D-deficiency and various types of cancer (e.g., colon-, prostate and breast cancer) has been confirmed in a large number of studies.Citation50–Citation55 The idea that sunlight and vitamin D inhibit the growth of human cancers is not new.Citation56 When Peller found an apparent deficit of cancer among US Navy personnel, who experienced an excess of skin cancer, he concluded in 1936 that skin cancers induce a relative immunity to other types of cancer.Citation57 Consequently, he advocated the deliberate induction of non-melanoma skin cancers, which were easily to detect and to treat, as a form of vaccination against more life-threatening and less treatable cancers. It was in 1941 when the US pathologist Frank Apperly published geographic data that demonstrated for the first time an inverse correlation between levels of UV-radiation in North America and mortality rates from cancers.Citation58 Apperly concluded that “the presence of skin cancer is really only an occasional accompaniment of a relative cancer immunity in some way related to exposure to ultraviolet radiation.” “A closer study of the action of solar radiation on the body,” he concluded, “might well reveal the nature of cancer immunity.” Since the time of Apperly’s first report, an association between increased risk of dying of various internal malignancies (e.g., breast, colon, prostate and ovarian cancer) and decreasing latitude towards the equator has now been confirmed.Citation53 A correlation of latitudinal association with sun exposure and decreased vitamin D serum levels has been demonstrated.Citation51,Citation53 Notably, black men, who have an increased risk to develop vitamin D deficiency, have also an increased risk of prostate cancer and develop a more aggressive form of the disease. Moreover, it has been reported that sun exposure is associated with a relatively favorable prognosis and increased survival rate in various other malignancies, including malignant melanoma.Citation59 It has been speculated that these findings were related to UV-exposure-induced relatively high serum levels of vitamin D. Berwick et al. recently evaluated the association between measures of skin screening and death from cutaneous melanoma in case subjects (n = 528) from a population-based study of cutaneous melanoma that were followed for an average of more than 5 years.Citation59 They found that sunburn, high intermittent solar UV-exposure, and solar elastosis were statistically significantly inversely associated with death from melanoma and concluded that sun exposure is associated with increased survival from melanoma.Citation59 Animal experiments reported in the literature, as well as epidemiological data from some countries relate survival of various malignancies including colon- and lung cancer with solar UV-exposure, latitude and vitamin D3-synthesis in the skin.Citation60,Citation61 Moreover, laboratory investigations analyzing the importance of the integrity of the vitamin D endocrine system for cancer pathogenesis and progression are in line with the so called vitamin D/cancer hypothesis. AS an example, an increasing body of evidence now demonstrates an association between several VDR polymorphisms and cancer risk and progression.Citation62,Citation63
It has to be noted that the evolution of our understanding of the role of vitamin D in cancer (and additionally in various other diseases that are not related to bone and calcium metabolism including infectious and autoimmune diseases) parallels our understanding of the importance of vitamin D for rickets.Citation48 In both diseases, epidemiologic observations about consequences of solar UV-exposure preceeded laboratory investigations and were subsequently supported by them. Apperly’s enlightening observations on sunlight exposure and cancer, like those of Theobold Palm on the protective effects of solar UV-radiation on rickets a half century earlier,Citation64 were almost unnoticed for many years, only to be rediscovered by epidemiologists decades later. During recent years, great progress has been made in laboratory investigations that searched for the “missing link” between the vitamin D and cancer connection. Of high importance was the discovery that in contrast to earlier assumptions, skin, prostate, colon, breast and many other tissues express the enzyme to convert 25(OH) D to its biologically active form, 1,25(OH)2D.Citation35,Citation56,Citation65,Citation66 Therefore, 1,25(OH)2D is now not exclusively considered as a calciotropic hormone but also as a locally produced potent hormone regulating cell growth.Citation66
In conclusion, the lack of sunlight exposure leads to more than bone disease and an increased risk for cancer—there are multiple other added benefits that include protection against infectious diseases and controlling cholesterol. It has been shown that 1,25(OH)2D represents a direct regulator of antimicrobial innate immune responses.Citation67–Citation70 The innate immune system of mammals provides a rapid response to repel assaults from numerous infectious agents including bacteria, viruses, fungi and parasites. A major component of this system is a diverse combination of cationic antimicrobial peptides (AMPs) that include the α- and β-defensins and cathelicidins.Citation67 Because bacteria have difficulty developing resistance against AMPs and are quickly killed by them, this class of antimicrobial agents is being commercially developed as a source of peptide antibiotics.Citation67 Interestingly, the promoters of the human camp and defensin 2 (defB2) genes contain consensus vitamin D response elements (VDRE) that mediate 1,25(OH)2D-dependent gene expression.Citation68 1,25(OH)2D induces antimicrobial peptide gene expression in isolated human keratinocytes, monocytes and neutrophils, and human cell lines, and 1,25(OH)2D along with LPS synergistically induce camp expression in neutrophils.Citation68 Moreover, 1,25(OH)2D induces corresponding increases in antimicrobial proteins and secretion of antimicrobial activity against pathogens including Pseudomonas aeruginosa.Citation68 Weber et al.Citation69 reported in human keratinocytes an upregulation of CAMP of about one order of magnitude by treatment with 100 nM 1,25(OH)2D or MC 903 (calcipotriol). Surprisingly, 25(OH)D3, the precursor of biologically active 1,25(OH)2D, stimulated CAMP expression at the same magnitude as 1,25(OH)2D or MC 903. In this study, all compounds were active down to levels of 10 nM while the precursor of vitamin D biosynthesis, 7-dehydrocholesterol (7-DHC), was ineffective at all concentrations tested.Citation69 Western blot analysis of independent investigations confirmed that the elevated transcription of CAMP was reflected on the protein level.Citation67,Citation69 The induction of CAMP expression occurred via a consensus VDRE in the CAMP promoter that was bound by the VDR. In conclusion, there is convincing evidence that 1,25(OH)2D and analogues directly regulate antimicrobial peptide gene expression in humans, revealing the potential of these compounds for the treatment of opportunistic infections. It is well known that in innate immune responses, activation of Toll-like receptors (TLRs) triggers direct antimicrobial activity against intracellular bacteria, which in murine, but not human, monocytes and macrophages is mediated principally by nitric oxide.Citation70 It has recently been reported that TLR activation of human macrophages upregulated expression of the vitamin D receptor (VDR) and the vitamin D-1αOHase (CYP27B1) genes, leading to induction of cathelicidin and killing of intracellular Mycobacterium tuberculosis. In that study, it was observed that sera from African- American individuals, known to have increased susceptibility to tuberculosis, had low 25(OH)D3 and were inefficient in supporting cathelicidin messenger RNA induction. These data support a link between TLRs and vitamin D-mediated innate immunity and suggest that differences in ability of human populations to produce vitamin D may contribute to susceptibility to microbial infection.Citation70 It has been reported that vitamin D deficiency predisposes children to respiratory infections and that volunteers inoculated with live attenuated influenza virus are more likely to develop fever and serological evidence of an immune response in the winter.Citation71 Ultraviolet radiation (either from artificial sources or from sunlight) reduces the incidence of viral respiratory infections, as does cod liver oil (which contains vitamin D). An interventional study showed that vitamin D reduces the incidence of respiratory infections in children and it has been concluded that a lack of vitamin D may be of importance for the remarkable seasonality of epidemic influenza (Hope-Simpson’s ‘seasonal stimulus’Citation71). Taken these data together, the effects of solar UV radiation on the immune system are not exclusively immunosuppressive, but may even stimulate distinct immune responses.
Regulating cholesterol levels in the blood may be another effect of the vitamin D endocrine system. It is well known that cholesterol is involved in the pathogenesis of coronary heart disease and is required for synthesis of 1,25(OH)2D and its precursors. It has been shown that, without adequate sun exposure, vitamin D-precursors turn to cholesterol instead of the vitamin. It has been reported that the increased concentration of blood cholesterol during winter months and the fact that outdoor activity (gardening) is associated with lower circulating cholesterol levels in the summer, but not in winter, may explain geographical differences in incidence of coronary heart disease.Citation72,Citation73
Sun protection increases the risk of vitamin D-deficiency.
We recently analyzed whether patients that need to protect themselves for medical reasons from solar and artificial UV-exposure are at an increased risk to become vitamin D-deficient. To address this question, we investigated 25(OH)D-serum levels in renal transplant patients with adequate renal function and in an ageand gender-matched control group at the end of winter.Citation74 Due to their increased risk to develop UV-induced skin cancer, all renal transplant patients had been advised to protect themselves against solar and artificial UV-radiation after transplantation. We found that 25(OH)D-serum levels were significantly lower (p = 0.007) in renal transplant patients [n = 31, geometric mean 10.9 ng/ml (with 95% confidence interval 8.22–4.3)] as compared to age- and gender-matched controls [n = 31, 20.0 ng/ml (15.7–25.5)].Citation74 We made similar findings in another pilot study, where we analyzed basal 25(OH)D-serum levels in a small group of patients with Xeroderma Pigmentosum (XP, n = 3) and basal cell nevus syndrome (BCNS, n = 1).Citation75 At the end of wintertime (February/March), 25(OH)D-levels were markedly decreased in all four patients (mean value: 9.5 ng/ml), as compared to the normal range (15.0–90.0 ng/ml).Citation75 In conclusion, we demonstrate in these two investigations reduced 25(OH)D-serum levels in risk groups that protect themselves against artificial and solar UV-radiation.Citation74,Citation75
How much vitamin D do we need?
At present, there is an ongoing debate on how much vitamin D we need to achieve a protecting effect against cancer and other diseases. From the historical point of view, the US Recommended Dietary Allowance (RDA) of vitamin D from 1989 is 200 IU.Citation76 Yet, investigations in the last decades have shown that taking orally 200 IU vitamin D daily has no effect on bone status.Citation77 In consequence, it has been recommended by some authors that adults may need, at a minimum, five times the RDA, or 1,000 IU, to be adequately protected against bone fractures, some cancers and derive other broad-ranging health benefits.Citation76 In conclusion, the 1989 RDA of 200 IU is antiquated, and the newer 600 IU Daily Reference Intake (DRI) dose for adults older than 70 is still not adequate.Citation76 It has been suggested that even taken daily orally 2,000 IU, that were previously considered the represent the upper tolerable intake (the official safety limit), does not deliver the amounts of vitamin D that may be optimal.Citation76 To evaluate putative risks that may be associated with vitamin D-supplementation, one should first consider the physiological capacity of the human skin to synthesize vitamin D. On a sunny summer day, total body sun exposure produces in the skin approximately more than 10,000 IU vitamin D per day. Considering this fact, concerns about toxic overdose with dietary supplements that exceed 800 IU vitamin D are poorly founded. Moreover, it has been speculated that a person would have to consume almost 67 times more vitamin D than the previously recommended intake for older adults of 600 IU to experience symptoms of overdosage.Citation76 Vieth believes people need 4,000–10,000 IU vitamin D daily and that toxic side effects are not a concern until a 40,000 IU/day dose.Citation76 Reports of other researchers are in line with these findings. It has been suggested by several experts that older adults, sick adults and “perhaps all adults” would need 800–1,000 IU vitamin D daily and it has been indicated that daily doses of 2,400 IU—four times the recommended intake—can be consumed safely.Citation76 According to recent estimations an intake of 1,000 IU daily would bring 25(OH)D serum levels of at least 50% of the population up to advantageous ranges of 30 ng/ml.Citation78 Thus, higher doses of vitamin D are needed as oral supplements, at least for those individuals who do not reach the desired ranges.
The vitamin D-cancer dose-response relations have been investigated in three studies. A meta-analysis of five observational studies of serum 25(OH)D found that it takes about 1,500 IU of vitamin D3 per day to reduce the risk by 50% for colorectal cancer, based on the assumption that 25(OH)D-levels of the population are low.Citation79 In a cohort study of male health professionals, it was found that taking daily 1,500 IU of vitamin D3 should reduce all-cancer mortality rates by approximately 30% for males in the US.Citation80,Citation81 For breast cancer, based on two studies of 25(OH) D-serum levels and breast cancer risk, it was concluded that it takes about 4,000 IU/day for a 50% reduction in risk for breast cancer.Citation82
However, it has to be taken into account that most of the studies outlined above are either epidemiological, ecological or observational. Although ecological studies have been criticized because of inconsistencies with observational intervention studies, they have important advantages, including that they incorporate the effects of diet and lifestyle over a long time period. It has to be noted that these advantages have been previously widely underestimated and are now being increasingly recognized. For it is well known that cancer and other diseases can take several decades to develop and to progress, the advantages outlined above are of high importance for the investigation of these diseases. Additionally, it should be noted that the primary criteria for causality in a biological system established by Hill,Citation83 that are strength of association, reproducibility in different populations, accounting for confounding factors, identification of the mechanisms, and experimental confirmation, are fulfilled when analyzing the role of vitamin D as a risk reduction factor for several types of cancer.Citation84
Beneficial (Vitamin D Photosynthesis) vs. Adverse (Photocarcinogenesis) Effects of Solar UV-Exposure: Time for a Paradigm Shift!
What conclusions do we draw from the findings reported above, most importantly the demonstration of an association between vitamin D-deficiency and the occurrence of numerous independent diseases, including various types of cancer? The important take home message for dermatologists and other clinicians is, that health campaigns promoting strict sun protection procedures to prevent skin cancer may induce the severe health risk of vitamin D-deficiency. There is no doubt that UV-radiation is mutagenic and is the main reason for the development of non-melanoma skin cancer. Therefore, excessive sun exposure has to be avoided, particularly burning in childhood. To reach this goal, the use of sunscreens as well as the wearing of protective clothes and glasses is absolutely important. Additionally, sun exposure around midday should be avoided during the summer in most latitudes. An increase in solar UV-B-radiation reaching the earth’s surface is an important consequence of stratospheric ozone depletion, and is a matter of concern.Citation81,Citation85 Recently however, it has been assumed that the net effects of solar UV B-radiation on human health are beneficial at or near current levels.Citation81,Citation84 Clinicians including dermatologists have to recognize the convincing evidence that the protective effect of less intense solar radiation outweighs its mutagenic effect. In agreement with this assumption, it was concluded that many lives could be prolonged through careful exposure to sunlight or more safely, vitamin D-supplementation, especially in non-summer months.Citation84 Therefore, it is time for a paradigm shift and recommendations of health campaigns on sun protection should be moderated, representing a more balanced view of positive and negative effects of solar UV-exposure. As Michael Holick reported previously,Citation49,Citation86 we have learned that at most latitudes such as Boston, USA, very short and limited solar UV-exposure is sufficient to obtain “adequate” vitamin D-levels. Exposure of the body in a bathing suit to one minimal erythemal dose (MED) of sunlight is equivalent to ingesting at least about 10,000 IU of vitamin D and it has been reported that exposure of less than 18% of the body surface (hands, arms and face) two to three times a week to a third to a half of an MED; (about 5 min for skin-type-2 adult in Boston at noon in July) in the spring, summer and autumn is more than adequate. Anyone intending to stay exposed to sunlight longer than recommended above should apply a sunscreen with a sufficient sun protection factor to prevent sunburn and the damaging effects of excessive exposure to sunlight. Although further work is necessary to define the influence of vitamin D-deficiency on the occurrence of melanoma and non-melanoma skin cancer, it is at present mandatory that especially dermatologists strengthen the importance of an adequate vitamin D-status if sun exposure is seriously curtailed. It has to be emphasized that in groups that are at high risk of developing vitamin D-deficiency (e.g., nursing home residents; patients with skin type I or patients under immunosuppressive therapy that must be protected from solar UV-exposure), vitamin D-status needs to be monitored subsequently. As a consequence of the severe health risks that are associated with vitamin D-deficiency, vitamin D-deficiency has to be treated, e.g., by giving vitamin D orally as recommended previously.Citation76,Citation86 It has been shown that a single dose of 50,000 IU vitamin D once a week for 8 weeks is efficient and safe to treat vitamin D-deficiency.Citation76 Another means of guaranteeing vitamin D-sufficiency, especially in nursing home residents, is to give 50,000 IU of vitamin D once a month. An alternative to prevent vitamin D-deficiency would be the use of vitamin D-containing ointments. However, it should be noted that vitamin D-containing ointments are, at least in Europe, not allowed as cosmetics. These antiquated laws are the result of the fear of vitamin D-intoxication that was evident in Europe in the 1950sCitation87 and need to be reevaluated, for they do not reflect our present scientific knowledge. If we follow the guidelines discussed above carefully, they will ensure an adequate vitamin D-status, thereby protecting us against adverse effects of strict solar UV protection recommendations. Most importantly, these measures will protect us sufficiently against the influence of vitamin D-deficiency on the development of various malignancies and other diseases without increasing our risk to develop UV-radiation-induced skin cancer. To reach this goal it is important that this information is transferred to every clinician, especially to dermatologists. Otherwise dermatologists will not be prepared for the moderation of recommendations for protection against artificial and solar UV-radiation, that is necessary to protect us against vitamin D-deficiency, cancer and other diseases. As an addendum, it should be noted that the International Agency for Research on Cancer (IARC) recently released a report, questioning the relevance of vitamin D for cancer.Citation88 However this report has been criticized by well recommended experts in the field due to many deficiencies in the interpretation of the data.Citation89,Citation90
Figures and Tables
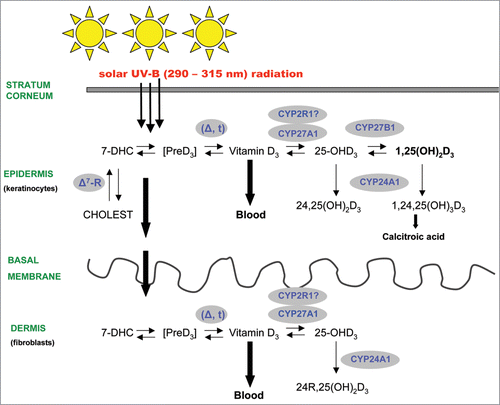
Figure 1 Schematic illustration of the cutaneous vitamin D endocrine system in human skin. Please note that the skin represents an unique tissue in the human body’s vitamin D endocrine system, producing various vitamin D metabolites for endocrine, paracrine and autocrine signalling pathways. Importantly, vitamin D is photosynthesized in the skin (epidermis and dermis) by solar or artificial UV-B-radiation (before it is transferred to the blood for endocrine signalling to cover the body’s needs in vitamin D), and biologically active 1,25-dihydroxyvitamin D3 is synthesized in many skin cells, where it acts locally and regulates a broad variety of independent cellular functions via autocrine/paracrine pathways.
References
- Unna PG. Die Histopathologie der Hautkrankheiten. Orth J: Lehrbuch der speziellen pathologischen Anatomie 1894; Berlin August Hirschwald 839 - 842
- Dubreuilh W. Des hyperkeratoses circumscriptes. Ann Derm et Syph 1896; 7:1158 - 1204
- Preston DS, Stern RS. Nonmelanoma cancers of the skin. N Engl J Med 1992; 327:1649 - 1662
- Kricker A, Armstrong BK, English DR. Sun exposure and non-melanocytic skin cancer. Cancer Causes Control 1994; 5:367 - 392
- Elwood JM, Jopson J. Melanoma and sun exposure: An overview of published studies. Int J Cancer 1997; 73:198 - 203
- Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol 2001; 63:8 - 18
- Tilgen W, Rass K, Reichrath J. 30 Jahre dermatologische Onkologie. Akt Dermatol 2005; 31:79 - 88
- Wang H, Diepgen TL. Reichrath J. The Epidemiology of basal cell and squamous cell carcinoma. Molecular Mechanisms of Basal Cell and Squamous Cell Carcinomas 2006; Berlin Springer
- Rass K. Reichrath J. UV-damage and DNA-repair in basal and squamous cell carcinomas. Molecular mechanisms of basal cell and squamous cell carcinomas 2006; Berlin Springer Molecular Mechanisms of Basal Cell and Squamous Cell Carcinomas Austin: Landes Bioscience Medical Intelligence Unit
- Reichrath J, Querings K. Reichrath J. Histology of epithelial skin tumors. Molecular Mechanisms of Basal Cell and Squamous Cell Carcinomas 2006; Berlin Springer Austin: Landes Bioscience Medical Intelligence Unit
- Gilchrest BA, Eller MS, Geller AC, Yaar M. The pathogenesis of melanoma induced by ultraviolet radiation. N Engl J Med 1999; 340:1341 - 1348
- Green A, Battistutta D. Incidence and determinants of skin cancer in a high-risk Australian population. Int J Cancer 1990; 46:356 - 361
- Neale RE, Davis M, Pandeya N, Whiteman DC, Green AC. Basal cell carcinoma on the trunk is associated with excessive sun exposure. J Am Acad Dermatol 2007; 56:380 - 386
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer 2005; 41:45 - 60
- Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med 2001; 344:975 - 983
- Gallagher RP, Hill GB, Bajdik CD, Coldman AJ, Fincham S, McLean DI, et al. Sunlight exposure, pigmentation factors and risk of nonmelanocytic skin cancer II. Squamous cell carcinoma. Arch Dermatol 1995; 131:164 - 169
- Gallagher RP, Spinelli JJ, Lee TK. Tanning beds, sunlamps and risk of cutaneous malignant melanoma. Cancer Epidemiol Biomarkers Prev 2005; 14:562 - 566
- Frost CA, Green AC. Epidemiology of solar keratoses. Br J Dermatol 1994; 131:455 - 464
- Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. II Importance of UV-light exposure. Int J Cancer 1988; 42:319 - 324
- Green A, Siskind V. Geographical distribution of cutaneous melanoma in Queensland. Med J Aust 1983; 1:407 - 410
- Eide MJ, Weinstock MA. Association of UV index, latitude and melanoma incidence in nonwhite populations—US Surveillance, Epidemiology and End Results (SEER) Program, 1992 to 2001. Arch Dermatol 2005; 141:477 - 481
- Elwood JM, Gallagher RP, Hill GB, Pearson JC. Cutaneous melanoma in relation to intermittent and constant sun exposure—the western Canada Melanoma Study. Int J Cancer 1985; 35:427 - 433
- Kennedy C, Bajdik CD, Willemze R, de Gruijl FR, Bouwes Bavinck JN. The Influence of Painful Sunburns and Lifetime Sun Exposure on the Risk of Actinic Keratoses, Seborrheic Warts, Melanocytic Nevi, Atypical Nevi and Skin Cancer. J Invest Dermatol 2003; 120:1087 - 1093
- Gass R, Bopp M. Mortality from malignant melanoma: epidemiological trends in Switzerland. Schweiz Rundsch Med Prax 2005; 94:1295 - 1300
- Westerdahl J, Olsson H, Mosback A, et al. Is the use of sunscreensa risk factor for melanoma?. Melanoma Res 1995; 5:59 - 65
- Bataille V, Boniol M, De Vries E, Severi G, Brandberg Y, Sasieni P, et al. A multicentre epidemiological study on sunbed use and cutaneous melanoma in Europe. Eur J Cancer 2005; 41:2141 - 2149
- Melnikova VO, Ananthaswamy HN. Reichrath J. p53 protein and non-melanoma skin cancer. Molecular Mechanisms of Basal Cell and Squamous Cell Carcinomas 2006; Berlin Springer Austin: Landes Bioscience Medical Intelligence Unit
- Reifenberger J, Wolter M, Knobbe CB, Kohler B, Schonicke A, Scharwachter C, et al. Somatic mutations in the PTCH, SMOH, SUFUH and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol 2005; 152:43 - 51
- Baron ED. Reichrath J. The immune system and nonmelanoma skin cancers. Molecular Mechanisms of Basal Cell and Squamous Cell Carcinomas 2006; Berlin Springer Austin: Landes Bioscience Medical Intelligence Unit
- Reichrath J, Rappl G. Ultraviolet (UV)-induced immunosuppression: is Vitamin D the missing link?. J Cell Biochem 2003; 89:6 - 8
- Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relavant to malignant melanoma?. Br J Dermatol 2002; 147:197 - 213
- Reichrath J, Kamradt J, Zhu XH, Kong Xf, Tilgen W, Holick MF. Analysis of 1,25-dihydroxyvitamin D3 receptors in basal cell carcinomas. Am J Pathol 1999; 155:583 - 589
- Reichrath J, Rafi L, Rech M, Mitschele T, Meineke V, Gärtner BC, et al. Analysis of the vitamin D system in cutaneous squamous cell carcinomas (SCC). J Cut Pathol 2004; 31:224 - 231
- Reichrath J, Rafi L, Rech M, Meineke V, Tilgen W, Seifert M. No evidence for amplification of 25-hydroxyvitamin D-1α-OHase (1α-OHase) or 1,25-dihydroxyvitamin D-24-OHase (24-OHase) genes in malignant melanoma (MM). J Steroid Biochem Mol Biol 2004; 90:163 - 166
- Mitschele T, Diesel B, Friedrich M, Meineke V, Maas RM, Gärtner BC, et al. Analysis of the Vitamin D System in Basal Cell Carcinomas (BCCs). Lab Invest 2004; 84:693 - 702
- Seifert M, Rech M, Meineke V, Tilgen W, Reichrath J. Differential biological effects of 1,25-dihydroxyvitamin D3 on melanoma cell lines in vitro. J Steroid Biochem Mol Biol 2004; 90:375 - 379
- Hutchinson PE, Osborne JE, Lear JT, Smith AG, Bowers PW, Morris PN, et al. Vitamin D receptor polymorphisms are associated with altered prognosis in patients with malignant melanoma. Clin Cancer Res 2000; 6:498 - 504
- Halsall JA, Osborne JE, Potter L, Pringle JH, HUtchinson PE. A novel polymorphism in the 1A promoter region of the vitamin D receptor is associated with altered susceptibilty and prognosis in malignant melanoma. Br J Cancer 2004; 91:765 - 770
- Mocellin S, Nitti D. Vitamin D receptor polymorphisms and the risk of cutaneous melanoma: a systematic review and meta-analysis. Cancer 2008; 113:2398 - 2407
- Albertson DG, Ylstra B, Segraves R, Collins C, Dairkee SH, Kowbel D, et al. Quantitative mapping of amplicon structure by array CGH identifies CYP24 as a candidate oncogene. Nat Genet 2000; 25:144 - 146
- Diesel B, Radermacher J, Bureik M, Bernhardt R, Seifert M, Reichrath J, et al. Vitamin D3 Metabolism in Human Glioblastoma Multiforme: Functionality of CYP27B1 Splice Variants, Metabolism of Calcidiol, and Effect of Calcitriol. Clin Cancer Res 2005; 11:5370 - 5380
- Reichrath J, Querings K. No evidence for reduced 25-hydroxyvitamin D serum levels in melanoma patients. Cancer Causes Control 2004; 15:97 - 98
- Levell NJ, Beattie CC, Shuster S, Greenberg DC. Melanoma epidemic: a midsummer night’s dream?. Br J Dermatol 2009; 161:630 - 634
- Reichrath J. The challenge resulting from positive and negative effects of sunlight: how much solar UV-exposure is appropriate to balance between risks of vitamin D deficiency and skin cancer?. Prog Biophys Mol Biol 2006; 92:9 - 16
- Reichrath J. Vitamin D and the skin: An ancient friend, revisited. Exp Dermatol 2007; 16:618 - 625
- Greinert R, Breitbart EW, Mohr P, Volkmer B. Health Initiatives for the Prevention of Skin Cancer. Adv Exp Med Biol 2008; 624:125 - 136
- Matsuoka LY, Wortsman J, Dannenberg MJ, Hollis BW, Lu Z, Holick MF. Clothing prevents ultraviolet- B radiation-dependent photosynthesis of vitamin D3. J Clin Endocrinol Metab 1992; 75:1099 - 1103
- Holick MF. Evolution and function of vitamin D. Recent Results Cancer Res 2003; 164:3 - 28
- Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266 - 281
- Gorham ED, Garland FC, Garland CF. Sunlight and breast cancer incidence in the USSR. Int J Epidemiol 1990; 19:614 - 622
- Garland CF, Comstock GW, Garland FC, et al. Serum 25-hydroxyvitamin D and colon cancer: eight year prospective study. Lancet 1989; 2:1176 - 1178
- Garland CF, Garland FC, Gorham ED. Can colon cancer incidence and death rates be reduced with calcium and vitamin D?. Am J Clin Nutr 1991; 54:193 - 201
- Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002; 94:1867 - 1875
- Garland CF, Gorham ED, Mohr SB, et al. Vitamin D and prevention of breast cancer: Pooled analysis. J Steroid Biochem Mol Biol 2007; 103:708 - 711
- Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin d status for colorectal cancer prevention a quantitative meta analysis. Am J Prev Med 2007; 32:210 - 216
- Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev 1998; 7:391 - 395
- Peller S. Carcinogenesis as a means of reducing cancer mortality. Lancet 1936; 2:552 - 556
- Apperly FL. The relation of solar radiation to cancer mortality in North America. Cancer Res 1941; 1:191 - 195
- Berwick M, Armstrong BK, Ben-Porat L, Fine J, Kricker A, Eberle C, Barnhill R. Sun exposure and mortality from melanoma. J Natl Cancer Inst 2005; 97:195 - 199
- Moan J, Porojnicu AC, Robsahm TE, Dahlback A, Juzeniene A, Tretli S, Grant W. Solar radiation, vitamin D and survival rate of colon cancer in Norway. J Photochem Photobiol B 2005; 78:189 - 193
- Porojnicu AC, Robsahm TE, Dahlback A, Berg JP, Christiani D, Bruland OS, Moan J. Seasonal and geographical variations in lung cancer prognosis in Norway. Does Vitamin D from the sun play a role?. Lung cancer 2007; 55:263 - 270
- Köstner K, Denzer N, Müller CSL, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a metaanalysis of the literature. Anticancer Res in press
- Raimondi S, Johansson H, Maisonneuve P, Gandini S. Review and meta-analysis on vitamin D receptor polymorphisms and cancer risk. Carcinogenesis 2009; 30:1170 - 1180 Epub 2009
- Palm TA. The geographical distribution and etiology of rickets. Practitioner 1890; 45:270 - 279
- Reichrath J. Will analogs of 1,25-dihydroxyvitamin D3 (calcitriol) open a new era in cancer therapy?. Onkologie 2001; 24:128 - 133
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol 2004; 13:11 - 15
- Gombard HF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly upregulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J 2005; 19:1067 - 1177
- Wang T-T, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol 2004; 173:2909 - 2912
- Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjö A, Törmä H, Ståhle M. Vitamin D Induces the Antimicrobial Protein hCAP18 in Human Skin. J Invest Dermatol 2005; 124:1080 - 1082
- Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006; 311:1770 - 1773
- Cannell JJ, Vieth R, Umhau JC, Holick MF, Grant WB, Madronich S, et al. Epidemic influenza and vitamin D. Epidemiol Infect 2006; 7:1 - 12
- Grimes DS, et al. Sunlight, cholesterol and coronary heart disease. Q J Med 1996; 89:579 - 589
- Wolf HI. Cholesterol and vitamin D. Ugeskr Laeger 2007; 169:1592
- Querings K, Girndt M, Geisel J, Georg T, Tilgen W, Reichrath J. 25-Hydroxyvitamin D-deficiency in renal transplant recipients: an underrecognized health problem. J Clin Endocrinol Metab 2006; 91:526 - 529
- Querings K, Reichrath J. A plea for detection and treatment of vitamin D deficiency in patients under photoprotection, including patients with xeroderma pigmentosum and basal cell nevus syndrome. Cancer Causes Control 2004; 15:219
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999; 69:842 - 856
- Dawson-Hughes B, et al. Rates of bone loss in postmenopausal women randomly assigned to one of two dosages of vitamin D. Am J Clin Nutr 1995; 61:1140 - 1145
- Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol 2008; 624:55 - 71
- Gorham ED, Garland CF, Garland FC, Grant WB, Mohr SB, Lipkin M, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med 2007; 32:210 - 216
- Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WH. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst 2006; 98:451 - 459
- Grant WB, Moan J, Reichrath J. Comment on “The effects on human health from stratospheric ozone depletion and its interactions with climate change” by Norval M, Cullen AP, de Gruijl FR, Longstreth J, Takizawa Y, Lucas RM, et al.. Photochem Photobiol Sci 2007; 6:232 Photochem Photobiol Sci 2007; 6:912–5
- Garland CF, Gorham ED, Mohr SB, Grant WB, Giovannucci EL, Lipkin M, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol 2007; 103:708 - 711
- Hill AB. The environment and disease: association or causation?. Proc R Soc Med 1965; 58:295 - 300
- Grant WB. Solar ultraviolet irradiance and cancer incidence and mortality. Adv Exp Med Biol 2008; 624:16 - 30
- Norval M, Cullen AP, de Gruijl FR, Longstreth J, Takizawa Y, Lucas RM, et al. The effects on human health from stratospheric ozone depletion and its interactions with climate change. Photochem Photobiol Sci 2007; 6:232
- Holick MF. Sunlight “D” ilemma: risk of skin cancer or bone disease and muscle weakness. Lancet 2001; 357:961
- British Pediatric Association. Hypercalcemia in infants and vitamin D. BMJ 1956; 2:149
- IARC. Vitamin D and cancer. IARC working group reports 2008; Lyon, France International Agency for Research on Cancer
- Holick MF. Shining light on the vitamin D. Dermato-Endocrinology 2009; 1:4 - 6
- Grant WB. A critical review of Vitamin D and Cancer. Dermato-Endocrinology 2009; 1:25 - 33