Abstract
Background: Although the exact etiology of alopecia areata is still unknown, systemic prednisolone treatment seem to be effective in early stages but significant side effects may occur leading to discontinuation of treatment.
Objective: Evaluation of efficacy and saftety of a short-term medium-dose pulse prednisolone treatment in alopecia areata.
Methods: Monocenter prospective study of intravenous 100 mg intravenous prednisolone pulse therapy on 3 consecutive days at 1-month-intervals for three courses in 23 patients with active alopecia areata rapidly evolving and/or resistant to topical therapies and no contraindication for systemic steroids.
Results: 84% of the patients with multifocal alopecia areata markedly improved after the completion of the three courses. A patient with the ophiasis type only responded after the third course, but relapsed 7 months later. The patients with alopecia areata totalis and universalis did not respond to the treatment. No major side effects were observed.
Conclusion: A series of three monthly courses of medium-dose prednisolone pulse therapy is effective and well tolerated in most patients with active, multifocal alopecia areata. The results are rather disappointing in patients with alopecia areara totalis/universalis.
Introduction
Alopecia areata (AA) is a common form of localized, non-scarring hair loss. At any given time, approximately 0.2% of the population has AA and approximately 1.7% of the population will experience an episode of AA during lifetime.Citation1,Citation2 AA may lead to severe psychological consequences, including high levels of anxiety and depression, even in the sole presence of minor clinical signs.Citation3
Although the exact etiology of AA is still unknown, there is accumulated evidence that supports a hereditary susceptibility and an autoimmune pathogenesis, such as a familial history in 4–27%,Citation4,Citation5 and the increased frequency of other autoimmune diseases, particularly autoimmune thyroid disease, and of organspecific autoantibodies in patients with AACitation6–Citation8 as well as the presence of CD8+ T cells next to the hair follicles.Citation9,Citation10
Among the various immunomodulatory treatments that have been used to induce remission in AA, systemic corticosteroids have been administered in extensive or rapidly progressive AA and in alopecia totalis/universalis.Citation11,Citation12 Systemic oral corticosteroids have been reported to be effective in extensive AA, but relapses occurred when the dosage was reduced.Citation13–Citation19 In order to avoid the side effects of prolonged use of steroids, pulse corticosteroid therapy has been introduced in the treatment of AA by Burton and Shuster in 1975.Citation20 Various doses and schemas of steroid pulse therapy have been tried ever since.
The aim of this study was to prospectively evaluate the efficacy and safety of medium-dose prednisolone pulse therapy in patients with AA resistant to topical therapies or rapidly evolving AA.
Results
In 13 out of 23 patients a response was observed, whereas the progression of AA was stopped already one month after the first corticosteroid pulse.
Of the patients with multifocal AA, response was seen in 8 out of 19 patients (42%) after the first pulse and in 16 patients (84%) after the second and the third pulse. One patient with mulitfocal AA (#10) interrupted the participation at the study after the first pulse, because she observed progression of the hair loss. She was considered as a non-response patient.
The patient with the ophiasis type showed new hair growing only after the third pulse and the patients with AA totalis and universalis showed no response at all.
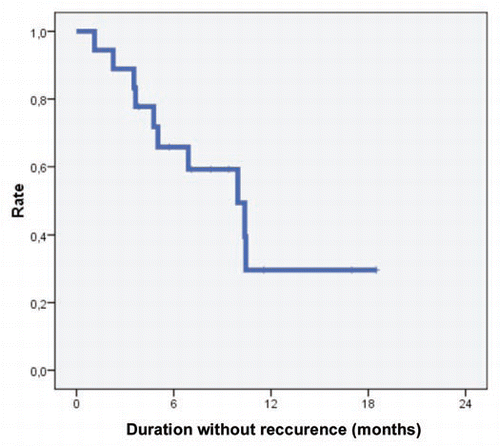
In the responders with multifocal AA (16 out of 19), the following relapse occurred after the third pulse therapy: after two months in 2 patients (12.5%), after 3 months in 1 patient (6%), after 5 months in 1 patient (6%), after 7 months in 1 patient (6%) and after 13 months in 1 patient (6%). Eight patients showed no relapse in the follow-up period and 2 patients (#9 and 13) were not reviewed in the follow-up period. A relapse occurred seven months after the third pulse therapy in the patient with ophiasis. The rate of patients with no relapses is depicted in .
No major side effects were registered. One patient developed herpes labialis during the pulse therapy and another patient developed a bronchial asthma attack two days after the pulse therapy.
Discussion
Although spontaneous remission can be observed in the majority of cases of multifocal AA with limited hair loss (possibly, up to approximately 80 percent within one year), most patients experience recurrences at some stage.Citation21 Extensive AA, particularly alopecia totalis and universalis, and ophiasis tend to be recalcitrant. Less than 10% of patients with alopecia totalis and universalis recover spontaneously. An early remission can be induced by steroid therapy through disruption of the dynamic of the inflammatory reaction in the hair follicle.
To avoid the long-term side effects of prolonged steroid therapy, pulsed steroid therapy was first introduced in the treatment of AA by Burton and Shuster in 1975.Citation20 They used a single intravenous dose of 2 g methyl prednisolone in 22 patients. Forteen patients (63%) showed no response, 5 (23%) showed a poor and 3 (14%) showed a good response. The disappointing results were due to the selection of the patients; about half of the patients had alopecia totalis of several years' duration.
Several scientists followed the example of Burton and Shuster and administered pulsed steroid therapy in various doses and schemas in patients with AA. Perriand-Wolfensberger et al.Citation22 administered 250 mg intravenous methyl prednisolone twice daily on three consecutive days in 9 patients. In 8 patients the course of the ongoing episode of AA was stopped and a regrowth on 80–100% of the bald surface was observed in 6 patients at the 6-month follow-up. Kiesch et al.Citation23 treated 7 children with severe, rapidly evolving AA with 5 mg/kg intravenous methyl prednisolone twice daily for three days. Complete regrowth occurred in 5 patients at the 12-month follow-up. Friedli et al.Citation24 administered 250 mg intravenous methyl prednisolone twice daily on three successive days in 45 patients with rapid and extensive hair loss for less than a year. Patients with multifocal AA showed the best response rate, with 9 (45%), 12 (60%), 13 (65%) and 12 (60%) showing 100% or 50% to 100% regrowth at 1, 3, 6 and 12 months, respectively. In patients with ophiasis AA and AA totalis and universalis the results were less encouraging. Seiter et al.Citation25 treated 30 patients with 8 mg/kg intravenous methyl prednisolone on three consecutive days at 4-week intervals for at least three courses, 67% of patients with multifocal AA showed more than 50% hair regrowth. None of the patients with AA totalis or universalis and only one patient with ophiasis responded to therapy. Tsai et al.Citation26 administered to children younger than 12 years of age 5 mg/kg oral prednisolone in three divided doses and to adults, 500 mg intravenous methyl prednisolone. He treated 14 patients, patients with multifocal AA exhibited the most favorable response, with more than 75% hair regrowth. Assouly et al.Citation27 administered 500 mg intravenous methyl prednisolone daily on three consecutive days or 5 mg/kg intravenous methyl prednisolone twice daily on three consecutive days in children. The pulses were repeated after 4 and 8 weeks and a second series was carried out or not according to each case. He treated 66 patients, patients with the ophiasis type did not respond to treatment. A quarter of patients with alopecia universalis, half of the patients with alopecia totalis and 63.8% of the patients with multifocal alopecia areata presented a good response. Luggen and HunzigerCitation28 treated 25 patients with severe AA with 500 mg intravenous methyl prednisolone on 3 consecutive days. Four out of 10 patients with multifocal AA and 3 out of 9 patients with ophiasis had full hair regrowth, whereas all 6 patients with alopecia totalis/universalis failed to respond. The first controlled study with 43 patients, who received 200 mg oral prednisolone pulse therapy weekly against placebo over 6 months, detected a significant hair regrowth obtained in 8 patients in the prednisolone-treated group and none in the placebo group.Citation19 Two of the responders experienced a relapse during the observation period of 3 months. However, an intention-to-treat re-analysis found the response not to be significant.Citation29
In order to minimize side effects of the pulse steroid therapy and to accomplish a good response with a lower steroid dose, we decided to treat our patients with AA resistant to topical therapies or rapidly evolving AA with medium-dose intravenous prednisolone (100 mg) on 3 consecutive days at 1-month intervals for three courses. The only patients who did not respond were the patients with alopecia totalis and universalis. These patients are generally recalcitrant to therapy, even with much higher doses of pulse steroid therapy. The ophiasis type patient responded only after the third course of pulse therapy, but relapsed 7 months later. The patients with multifocal AA showed a response of 84% after the completion of the three courses. A relapse occurred two to 13 months after the third pulse therapy in 6 out of 14 patients with follow-up (43%), 8 patients (57%) showed no relapse in the follow-up period.
These results are encouraging, because they show the possibility of treating patients with AA resistant to topical therapies or rapidly evolving AA with medium-dose intravenous prednisolone, without any serious side effects and good response rates. The best results are obtained in patients with multifocal AA. The treatment of patients with ophiasis may also be effective, while no response was recorded in patients with alopecia totalis or universalis.
Patients and Methods
Twenty-three consecutive patients, 10 male and 13 female, 12–78 years old, who presented at the Departments of Dermatology, Venereology, Allergology and Immunology in Dessau Medical Center during the study period (inclusion: November 2005 to April 2008) were evaluated in this study.
Inclusion criteria were (1) active, rapidly evolving AA, (2) active AA not responding to topical therapies (topical corticosteroids for all patients and topical minoxidil, dithranol for some patients) and (3) no contraindication for systemic corticosteroids (peptic ulcer, diabetes mellitus, severe arterial hypertension, cardiac failure, acute and chronic infection, nephropathy, endocrine disorder, psychosis).
From the 23 patients included in the study, 19 patients presented with multifocal AA, 1 with the ophiasis type, 1 with AA totalis and 2 with AA universalis.
Complete blood count, serum chemistry, inflammation parameters and thyroid autoantibodies were analyzed before initiating the pulse steroid therapy. A general physical examination was also performed. Subsequently, the patients were admitted to the hospital and received prednisolone 100 mg intravenously on three consecutive days. Ranitidine 150–300 mg/d p.o. was also administered during the 3 days of pulse steroid therapy. The same procedure was repeated after 4 and after 8 weeks. Since the primary target of the procedure was a registered patients' treatment institutional review board and human subject committee approvals were waived.
Treatment success (response) was based on observation of discontinuation of hair loss and/or new hair growing in the bald lesions or decrease of the lesional size. Treatment failure (non-response) was based on observation of a missing change of the lesions or the appearance of new bald patches or a continuation of hair fall.
A summary of the patient data is presented in .
References
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol 1992; 128:702
- Price VH. Alopecia areata: Clinical aspects. J Invest Dermatol 1991; 96:68
- Hunt N, McHale S. The psychological impact of alopecia. BMJ 2005; 331:951 - 953
- De Waard-van der Spek FB, Oranje AP, De Raeymaecker DM. Juvenile versus maturity-onset alopecia areata—A comparative retrospective clinical study. Clin Exp Dermatol 1989; 14:429 - 436
- Friedmann PS. Alopecia areata and autoimmunity. Br J Dermatol 1981; 105:153 - 157
- Muller SA, Winkelman RK. Alopecia areata: An evaluation of 736 patients. Arch Dermatol 1963; 88:290 - 297
- Gilhar A, Kalish RS. Alopecia areata: A tissue specific autoimmune disease of the hair follicle. Autoimmun Rev 2006; 5:64 - 69
- Cunliffe WJ, Hall R, Stevenson CJ, Weightman D. Alopecia areata, thyroid disease and autoimmunity. Br J Dermatol 1969; 81:877 - 881
- Gilhar A, Krueger GG. Hair growth in scalp grafts from patients with alopecia areata and alopecia universalis grafted onto nude mice. Arch Dermatol 1987; 123:44 - 50
- Gilhar A, Ullmann Y, Berkutzki T, Assy B, Kalish RS. Autoimmune hair loss (alopecia areata) transferred by T lymphocytes to human scalp explants on SCID mice. J Clin Invest 1998; 101:62 - 67
- Ross EK, Shapiro J. Management of hair loss. Dermatol Clin 2005; 23:227 - 243
- MacDonald Hull SP, Wood ML, Hutchinson PE, Sladden M, Messenger AG. British Association of Dermatologists. Guidelines for the management of alopecia areata. Br J Dermatol 2003; 149:692 - 699
- Winter RJ, Kern F, Blizzard RM. Prednisone therapy for alopecia areata: a follow-up report. Arch Dermatol 1976; 112:1549 - 1552
- Dillaha CJ, Rothman S. Therapeutic experiments in alopecia areata with orally administered cortisone. JAMA 1952; 150:546 - 550
- Kern F, Hoffmann WH, Hambrick GW Jr, Blizzard RM. Alopecia areata: immunologic studies and treatment with prednisone. Arch Dermatol 1973; 107:407 - 412
- Fisher DA. Systemic steroids for treatment of alopecia areata. Arch Dermatol 1977; 113:1731 - 1732
- Unger WP, Schemmer RJ. Corticosteroids in the treatment of alopecia totalis: systemic effects. Arch Dermatol 1978; 114:1486 - 1490
- Olsen EA, Carson SC, Turney EA. Systemic steroids with or without 2% topical minoxidil in the treatment of alopecia areata. Arch Dermatol 1992; 128:1467 - 1473
- Kar BR, Handa S, Dogra S, Kumar B. Placebo-controlled oral pulse prednisolone therapy in alopecia areata. J Am Acad Dermatol 2005; 52:287 - 290
- Burton JL, Shuster S. Large doses of glucocorticoid in the treatment of alopecia areata. Acta Derm Venereol 1975; 55:493 - 496
- Ikeda T. A new classification of alopecia areata. Dermatologica 1965; 131:421
- Perriand-Wolfensberger J, Pasche-Koo F, Mainetti C, Labarthe MP, Salomon D, Saurat JH. Pulse of methylprednisolon in alopecia areata. Dermatology 1993; 187:282 - 285
- Kiesch N, Stene JJ, Goens J, Vanhooteghem O, Song M. Pulse steroid therapy for children's severe alopecia areata?. Dermatology 1997; 194:395 - 397
- Friedli A, Labarthe MP, Engelhardt E, Feldmann R, Salomon D, Saurat JH. Pulse methylprednisolon therapy for severe alopecia areata: An open prospective study of 45 patients. J Am Acad Dermatol 1998; 39:597 - 602
- Seiter S, Ugurel S, Tilgen W, Reinhold U. High-dose pulse corticosteroid therapy in the treatment of severe alopecia areata. Dermatology 2002; 101:223 - 226
- Tsai YM, Chen W, Hsu ML, Lin TK. High-dose steroid pulse therapy for the treatment of severe alopecia areata. J Formos Med Assoc 2001; 101:223 - 226
- Assouly P, Reygagne P, Jouanique C, Matard B, Marechal E, Reynert P, et al. Intravenous pulse methylprednisolone therapy for severe alopecia areata: an open study of 66 patients. Ann Dermatol Venereol 2003; 326 - 330
- Luggen P, Hunziker T. High-dose intravenous corticosteroid pulse therapy in alopecia areata: Own experience compared with the literature. J Dtsch Dermatol Ges 2008; 6:375 - 378
- Sladden MJ, Hutchinson PE. Is oral pulsed prednisolone useful in alopecia areata? Critical appraisal of a randomized trial. J Am Acad Dermatol 2005; 53:1100 - 1101