Abstract
Background: Maintaining a normal vitamin D status is important for both skeletal and extra-skeletal health. Recent data show that vitamin D deficiency is endemic in women residing in the Arabian Gulf and is common in pregnant women and their newborns. The purpose of this study was to establish the vitamin D and calcium status of children in an urban ambulatory pediatric clinic in Abu Dhabi to determine for the prevalence of vitamin D deficiency in this cohort.
Methods: Patients were recruited prospectively from the pediatric outpatient clinic visits, if they were having blood taken for laboratory investigations other than those related to vitamin D and calcium status. The vitamin D status was compared between 4 age groups (0–0.9 y, 1–1.9 y, 2–7.9 y and 8 -14 y) using the following definitions: deficiency < 25 nmol/l, insufficiency 25–50 nmol/l and sufficiency > 50 nmol/l.
Results: A total of 183 children were included in the study. The percentage of females and males in the deficient range was 21% and 16% respectively, while 32% and 46% of females and males respectively were vitamin D sufficient. The highest prevalence of vitamin D deficiency occurred in the 8–14 y old age group with 31.2% being deficient.
Conclusions: The study highlights that in an ambulatory pediatric clinic population, peri-pubescent children are most at risk of vitamin D deficiency. This age group is often not considered in the discussion for the need for vitamin D supplementation. Serious consideration should be given to including vitamin D supplementation in a school public health program in the UAE.
Introduction
The estimated prevalence of rickets in the Middle East is one hundred fold greater than in western countries, with hypovitaminosis D being reported in Lebanon, Saudi Arabia and Iran in children in the pubescent age.Citation1 Recent data show that vitamin D deficiency is endemic in women residing in the Arabian Gulf including pregnant women and their newborn infants.Citation2-Citation7 Studies in the United Arab Emirates (UAE) have also highlighted that rickets remains prevalent regionally.Citation8,Citation9 We are unaware of any studies in the UAE documenting the vitamin D status in children less than 14 y of age. Vitamin D deficiency possibly has many other effects beside those involving calcium and bone homeostasis, and include a decreased threshold for long-latency diseases such as cancers (including leukemia and colon, prostate and breast cancers), psoriasis, diabetes mellitus, and autoimmune diseases (e.g., multiple sclerosis, rheumatoid arthritis, systemic lupus erythematosis.Citation10 In addition, it is hypothesized that maintaining a normal vitamin D status is important in fetal imprinting.Citation11
The recent American Academy of Pediatrics (AAP) guidelines recommend that vitamin D supplementation should start within the first few days of life and be continued throughout childhood if the risk for vitamin D deficiency persists.Citation10 The demographics of vitamin D deficiency in older children have not been established despite earlier studies in the Middle East and America which pointed to the adolescent female being at high risk.Citation12-Citation15
In both the neonatal and adolescence period, vitamin D deficiency is not an uncommon finding in children in the Middle East. Despite this, there are no reports of studies in children during the peripubescent age and early childhood. The purpose of this study was to establish the vitamin D and calcium status in children in an urban ambulatory pediatric clinic and the prevalence of vitamin D deficiency in this cohort of children, and to determine those children most at-risk.
Results
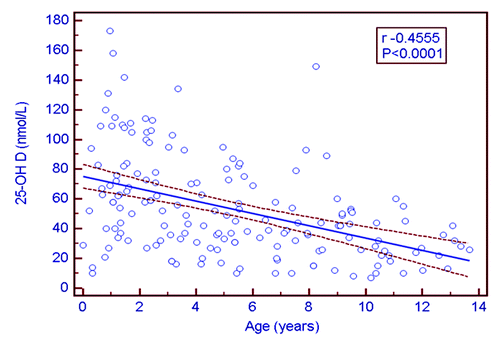
A total of 183 children were included in the study. The mean age (SD) was 5.32 y (3.76). 51.9% of the children were males. depicts the mean (SD) of all biochemical parameters according to the four age groups in 169 patients with complete data measurement. Overall, 17.7% had vitamin D deficiency, and 8.2% and 12.1% were hypocalcemic and hypophosphatemic respectively. Elevated PTH and ALP concentrations were detected in approximately 8% of the sample. The greatest prevalence of vitamin D deficiency, hypocalcemia and hyperparathyroidism was detected in the 8–14 y age group. Serum 25-OHD concentrations were inversely correlated with age (r = -0.45, p < 0.0001) (). 25-OHD concentrations were also correlated with Ca (r = 0.17, p = 0.02), PO4 (r = 0.28, p = 0.002), PTH (r = -0.43, p < 0.0001) and 1,25-(OH)2D (r = 0.29, p = 0003). By multiple regression, the predictors of PTH after backward regression included Ca, vitamin D and age (p < 0.001).
Table 1. Summary statistics of biochemical parameters (the values quoted are the reference ranges*; mean ± SD ** and the percent of children with abnormal values***)
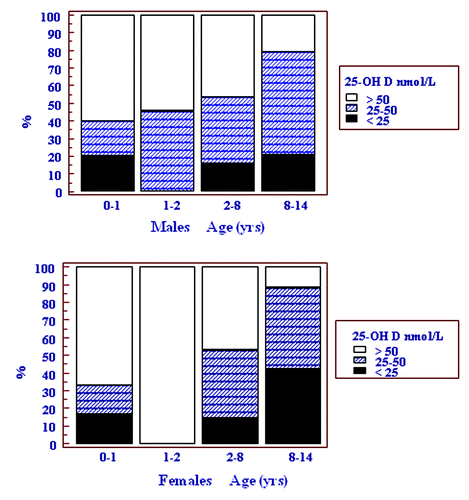
shows the vitamin D status of males and females in the 4 age groups. Vitamin D deficiency was not detected in any child in the age group 1–1.9 y. In addition, the majority of children (> 50%) in both the < 1 and 1–1.9 y age groups had a vitamin D status that was sufficient. The relative risk (RR) for vitamin D deficiency was increased in girls in the 8–14 y compared with 2–7.9 y age groups [RR 2.87 (95% CI 1.14–7.25; p = 0.02)], but not in boys [RR 1.35 (95% CI 0.44–4.08; p = 0.59)]. The overall percentage of females and males in the deficient range was 21% and 16% respectively (p = 0.71). The percent of females and males in the insufficient range was 32% and 46% respectively.
Figure 2. Cumulative frequency graph showing the vitamin D status in both sexes according to four age groups.
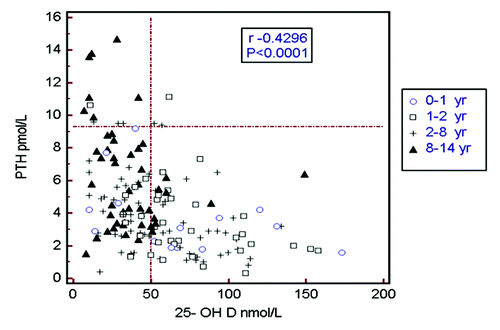
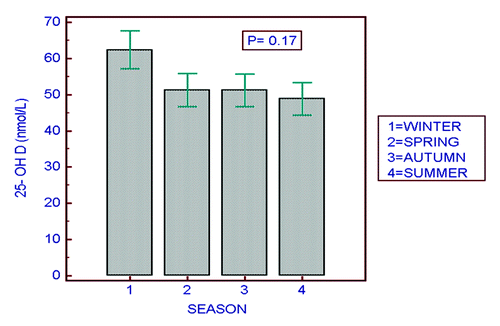
shows the inverse relationship between PTH and 25-OHD concentrations factored by the 4 different age groups (r = 0.42, p < 0.0001 for the whole cohort). A striking observation is the clustering of the triangular shapes (≥ 8 y) to the left of the vertical reference line (25-OHD < 50 nmol/L). The figure highlights the high prevalence of vitamin D insufficiency and hyperparathyroidism in children in the 8–14 y old age group. shows the seasonal variation in 25-OHD concentrations by season. In practical terms there are really only 3 mo of winter and 9 mo of summer. Despite a lack of statistical difference in seasonal variation, there is a trend for values to be higher in winter (p = 0.17).
Discussion
Vitamin D status in our study of children less than 14 y attending a pediatric ambulatory clinic in the UAE has shown a specific distribution and risk profile. The group at highest risk for vitamin D deficiency is females over 8 y. Despite maternal vitamin D deficiency in the UAE, infants in both sexes had a mean vitamin D status in the sufficient range, unlike older children, who had a high proportion that was vitamin D deficient. This study is of importance in mapping the local demographics, as it should increase pediatrician and primary care physician awareness to the at-risk age groups for vitamin D depletion, and improve public health programs to target vitamin D supplementation.
Of interest is the finding of a lack of vitamin D deficiency in toddlers aged 1–1.9 y. This could reflect the use of vitamin D fortified milks on weaning of infants, which usually occurs between 12 and 18 mo, vitamin D supplement usage or less skin coverage by clothing and more exposure to sunlight. The high concentrations of vitamin D attained by some infants suggest that vitamin D supplementation takes place in this age group and probably reflects an awareness of both parents and their primary caregivers of the high risk of vitamin D deficiency in this age group in this community (). The prevalence of vitamin D deficiency in the other age and sex groups was very similar (between 15 and 20%), except in females ≥ 8 y of age, in whom the prevalence almost doubled to 42%. This marked increase in vitamin D deficiency in females 8 y and older may be accounted by various factors. Possible causes include a more sedentary lifestyle than males, cultural dress codes which promote the coverage the skin at this age, or a calcium deficient diet which may induce vitamin D deficiency through increased catabolism of 25-OHD.Citation12,Citation13,Citation16 It is likely that as girls approach puberty, they adopt habits similar to adult females in dress code, sunlight exposure and dietary habits that place them in a similar high risk category to their mothers. Maintaining a normal vitamin D status is important for both skeletal as well as extra-skeletal health.Citation17,Citation18 Vitamin D deficiency also contributes to the inability to reach peak bone mass and hence early osteoporosis.Citation19,Citation20 The maintenance of a vitamin D replete state can be achieved by either daily vitamin D supplementation (400 IU)Citation21 or intermittent vitamin D supplementation (25,000 IU monthly).Citation22 One strategy would be to provide intermittent stosstherapy supplementation as part of a public health school based initiative, targeting females > 8 y.
As would be expected with the degree of vitamin D deficiency and insufficiency in the cohort, PTH concentrations correlated inversely with 25-OHD levels. Only 3 children with a vitamin D status in the sufficient category had an elevated PTH concentration, which supports the AAP target of vitamin D > 50 nmol/L as the lower limit of vitamin D sufficiency. These factors together with decreased exposure to sunlight in older children may explain their increase in PTH. In addition, the role of phytate content in the Middle Eastern diet warrants further study as they inhibit calcium absorption.Citation23
The seasonal trend in 25-OHD concentrations is similar to that found in another study in the UAE.Citation2 Due to the inclement heat during the summer months, most people remain indoors, and get more sunlight exposure during the winter months when picnicking and visits to the park are common family activities. This pattern is opposite to that in the more northern Western countries and has implications for supplementation, which in the UAE should be optimized in the non-winter months.
This study has a number of limitations. First, it was conducted in an urban setting and is not generalizable throughout the country, even though SKMC sees patients from all the other emirates. However the UAE has a rapidly transitioning economy and increasing urbanization with an infant mortality of 8/1000 live births and a GDP/capita of US $35,000. Second, the sampling methodology was by convenience non random sampling which may have biased the results. However, all children were ambulatory with minor illnesses that are common in the general population and those suspected clinically of having rickets were excluded. Third the age groups did not include many adolescents who are also at high risk as previously documented.Citation14 Finally we did not determine which patients were on any form of vitamin D supplementation or ascertain their dietary calcium intake.
Conclusion
Physicians within the Middle East should assiduously screen all children for risk factors for vitamin D insufficiency or deficiency. This could be through a careful history for sunlight exposure, dietary factors or by determining their serum vitamin D status. This study shows that this screening would be especially prudent in girls greater than 8 y of age. Vitamin D deficiency poses a major global and local public health challenge, yet the treatment is relatively cheap, rewarding and easily attained through vitamin D supplementation or a change in lifestyle. As expressed by the AAP, “pediatricians and other health care professionals should strive to make vitamin D supplements readily available to all children within their community, especially for those children most at risk.”Citation10
Subjects and Methods
This study was conducted at Sheikh Khalifa Medical City (SKMC) from 2005 to 2008. This is an urban tertiary care referral hospital situated in Abu Dhabi (24,28 °N) the capital of the UAE. Children < 14 y of age were recruited prospectively from the pediatric outpatient clinic by convenience sampling. The primary diagnoses included conditions such as atopic dermatitis, asthma, enuresis and constipation, or other minor medical condition. Eligibility criterion was any child presenting for an outpatient clinic visit who was having bloods drawn for reasons related to their primary diagnosis. Parents were asked permission to add a “vitamin D profile” which in most instances included the following biochemical parameters: 25-hydroxyvitamin D (25-OHD), 1,25-dihydroxyvitamin D [1,25-(OH)2D], PTH, alkaline phosphatase (ALP), calcium (Ca) and inorganic phosphate (PO4). Exclusion criteria were a clinical suspicion of rickets, children with chronic disease (e.g., renal failure, cancer, cystic fibrosis etc) or recent hospitalization, and children on antiepileptic medication. The definitions used to define their vitamin D status were as follows: deficiency, 25-OHD < 25 nmol/l, insufficiency, 25–50 nmol/l and sufficiency, > 50 nmol/l.Citation10,Citation24 Vitamin D and biochemical testing were performed in house by methods previously described.Citation25 25-OHD was measured at SKMC by Waters HPLC 2695 separation module with UV detection using Chromsystems kits. This HPLC assay includes the measurement of both 25-OHD2 and 25-OHD3 separately and the reported values are the combined concentrations. The intra-assay coefficient of variation was 4% and the inter-assay coefficient of variation was 5.8%. Measurement of 1,25-(OH)2D was done using Diasorin RIA at Biomnis Laboratories. The inter-assay coefficient of variation was 11.3% and the intra-assay coefficient of variation was 11.2%. Two-site chemiluminescent immunoassay (IMMULITE 2000) was used to measure intact PTH. Ca and PO4 were measured by colorimetric assay (Beckman Synchron DXC800). Approval for the study was attained form the Institutional Review Board at our hospital, SKMC.
Statistical analyses were performed using MedCalc for Windows, version 11.3.3.0 (MedCalc Software, Mariakerke, Belgium). Univariate analyses (age and vitamin D) are described as mean and standard deviation. Correlation analysis for normally distributed data was tested by the Pearson correlation coefficient. The vitamin D status was compared for four age groups (0–0.9 y, 1–1.9 y, 2–7.9 y and ≥ 8 y) by ANOVA. The difference in the vitamin D status between sexes was compared by the Student t test. Multiple regression analysis was used to determine the predictors for PTH and included vitamin D, age, sex and Ca. All hypotheses tested were 2 tailed and a p value < 0.05 was regarded as statistically significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- El-Hajj Fuleihan G. Vitamin D Deficiency in the Middle East and its Health Consequences for Children and Adults. Clinic Rev Bone Miner Metab 2009; 7:77 - 93; http://dx.doi.org/10.1007/s12018-009-9027-9
- Saadi HF, Nagelkerke N, Benedict S, Qazaq HS, Zilahi E, Mohamadiyeh MK, et al. Predictors and relationships of serum 25 hydroxyvitamin D concentration with bone turnover markers, bone mineral density, and vitamin D receptor genotype in Emirati women. Bone 2006; 39:1136 - 43; http://dx.doi.org/10.1016/j.bone.2006.05.010; PMID: 16814623
- Dawodu A, Wagner CL. Mother-child vitamin D deficiency: an international perspective. Arch Dis Child 2007; 92:737 - 40; http://dx.doi.org/10.1136/adc.2007.122689; PMID: 17715433
- Thandrayen K, Pettifor JM. Maternal Vitamin D Status: Implications for the Development of Infantile Nutritional Rickets. Endocrinol Metab Clin North Am 2010; 39:303 - 20; http://dx.doi.org/10.1016/j.ecl.2010.02.006; PMID: 20511053
- Bassir M, Laborie S, Lapillonne A, Claris O, Chappuis M, Salle B. Vitamin D deficiency in Iranian mothers and their neonates: a pilot study. Acta Paediatr 2001; 90:577 - 9; http://dx.doi.org/10.1111/j.1651-2227.2001.tb00802.x; PMID: 11430721
- Elidrissy AT, Sedrani SH, Lawson DE. Vitamin D deficiency in mothers of rachitic infants. Calcif Tissue Int 1984; 36:266 - 8; http://dx.doi.org/10.1007/BF02405328; PMID: 6432290
- Dawodu A, Agarwal M, Hossain M, Kochiyil J, Zayed R. Hypovitaminosis D and vitamin D deficiency in exclusively breast-feeding infants and their mothers in summer: a justification for vitamin D supplementation of breast-feeding infants. J Pediatr 2003; 142:169 - 73; http://dx.doi.org/10.1067/mpd.2003.63; PMID: 12584539
- Rajah J, Jubeh JA, Haq A, Shalash A, Parsons H. Nutritional rickets and z scores for height in the United Arab Emirates: To D or not to D?. Pediatr Int 2008; 50:424 - 8; http://dx.doi.org/10.1111/j.1442-200X.2008.02700.x; PMID: 18937749
- Dawodu A, Agarwal M, Sankarankutty M, Hardy D, Kochiyil J, Badrinath P. Higher Prevalence of Vitamin D Deficiency in Mothers of Rachitic Than Nonrachitic Children. J Pediatr 2005; 147:109 - 11; http://dx.doi.org/10.1016/j.jpeds.2005.03.001; PMID: 16027707
- Wagner CL, Greer FR. American Academy of Pediatrics Section on Breastfeeding, American Academy of Pediatrics Committee on Nutrition: Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 2008; 122:1142 - 52; http://dx.doi.org/10.1542/peds.2008-1862; PMID: 18977996
- McGrath J. Does ‘imprinting’ with low prenatal vitamin D contribute to the risk of various adult disorders?. Med Hypotheses 2001; 56:367 - 71; http://dx.doi.org/10.1054/mehy.2000.1226; PMID: 11359362
- Abdullah MA, Salhi HS, Bakry LA, Okamoto E, Abomelha AM, Stevens B, et al. Adolescent rickets in Saudi Arabia: a rich and sunny country. J Pediatr Endocrinol Metab 2002; 15:1017 - 25; PMID: 12199329
- Narchi H, El Jamil M, Kulaylat N. Symptomatic rickets in adolescence. Arch Dis Child 2001; 84:501 - 3; http://dx.doi.org/10.1136/adc.84.6.501; PMID: 11369569
- Schnadower D, Agarwal C, Oberfield SE, Fennoy I, Pusic M. Hypocalcemic Seizures and Secondary Bilateral Femoral Fractures in an Adolescent With Primary Vitamin D Deficiency. Pediatrics 2006; 118:2226 - 30; http://dx.doi.org/10.1542/peds.2006-1170; PMID: 17079597
- Al-Jurayyan NA, El-Desouki ME, Al-Herbish AS, Al-Mazyad AS, Al-Qhtani MM. Nutritional rickets and osteomalacia in school children and adolescents. Saudi Med J 2002; 23:182 - 5; PMID: 11938395
- Pettifor JM. Rickets and Vitamin D Deficiency in Children and Adolescents. Endocrinol Metab Clin North Am 2005; 34:537 - 53; http://dx.doi.org/10.1016/j.ecl.2005.04.002; PMID: 16085158
- Holick MF, Vitamin D. A millenium perspective. J Cell Biochem 2003; 88:296 - 307; http://dx.doi.org/10.1002/jcb.10338; PMID: 12520530
- Prentice A, Goldberg GR, Schoenmakers I. Vitamin D across the lifecycle: physiology and biomarkers. Am J Clin Nutr 2008; 88:500S - 506; PMID: 18689390
- Prentice A, Schoenmakers I, Ann Laskey M, de Bono S, Ginty F, Goldberg GR. Nutrition and bone growth and development. Proc Nutr Soc 2006; 65:348 - 60; http://dx.doi.org/10.1079/PNS2006519; PMID: 17181901
- El-Hajj Fuleihan G, Vieth R. Vitamin D insufficiency and musculoskeletal health in children and adolescents. Int Congr Ser 2007; 1297:91 - 108; http://dx.doi.org/10.1016/j.ics.2006.09.019
- IOM (Institute of Medicine). 2011 Dietary Reference Intakes for Calcium and Vitamin D.Washington, DC: The National Academies Press.
- Hochberg Z, ed. Vitamin D and Rickets: Consensus Development for the Supplementation of Vitamin D in Childhood and Adolescence. Endocr Dev Basel, Karger, 2003 6:259-281.
- Pettifor JM. Nutritional rickets: deficiency of vitamin D, calcium, or both?. Am J Clin Nutr 2004; 80:Suppl 1725S - 9S; PMID: 15585795
- Lips P. Relative value of 25(OH)D and 1,25(OH)2D measurements. J Bone Miner Res 2007; 22:1668 - 71; http://dx.doi.org/10.1359/jbmr.070716; PMID: 17645404
- Rajah J, Abdel-Wareth L, Haq A. Failure of alphacalcidol (1α-hydroxyvitamin D3) in treating nutritional rickets and the biochemical response to ergocalciferol. J Steroid Biochem Mol Biol 2010; 121:273 - 6; http://dx.doi.org/10.1016/j.jsbmb.2010.03.075; PMID: 20398760