Abstract
An observational study and a “clinical trial” seem to prove that rosuvastatin (but not fluvastatin) dramatically increases serum levels of 25-(OH)-D3 (three-fold above starting values). A critical analysis of the two publications, presented below, raises serious concerns. Conclusions from these two studies have already been drawn in the scientific literature.It is argued that claiming or believing in a “novel pleiotropic effect of rosuvastatin” may be misleading and premature.
Introduction
One of us recently summarized data on the relationship between statins, cholesterol and serum 25-(OH)-vitamin D levels.Citation1 Some doubts were raised about two publications (from the same group) dealing with rosuvastatin effects on serum calcidiol levels. If true, findings in the two papers could have consequences on the interpretation of the JUPITER trialCitation2 as recently discussed by others.Citation3-Citation6
In the first observational study, the authors reported almost 3-fold increases of 25-(OH)-D3 levels after 8 weeks of rosuvastatin.Citation7 In the second publication, which reports on a “clinical trial,” they compared rosuvastatin with fluvastatin with almost identical results for the former statin.Citation8 The findings of the two publications are analyzed below.
Results
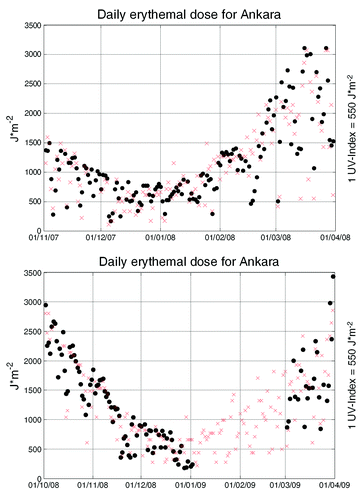
UV-B data are missing and the rise of 25-(OH)-D3 is identical in the two studies. Yavuz et al.Citation7 reported that rosuvastatin (10–20 mg/d) after 8 weeks increased 25-(OH)-D3 from a median of 14.0 ng/ml (~34.9 nmol/l) to 36.3 ng/ml (~90.5 nmol/l). This observational study was performed between November 2007 and March 2008 in Ankara (Turkey) with 91 patients (60% male, 40% female). The authors state that the above months are considered to be winter, with little exposure to UV light. More information is not provided, although UV-B levels have been measured in Ankara since 2007 and can be obtained from local and international agencies, as shown in . Ankara is located at about 39.6 degrees north (similar e.g., to Minneapolis, highest elevation ~300 mts, or Philadelphia, elevation ~9 mts) with an altitude of ~900 m. UV-B irradiance increases 20% per km in low aerosol conditions.Citation9 Thus, given these conditions, inhabitants of Ankara receive by about one fifth more UV-B radiation than e.g., Philadelphians. A control cohort is missing. Confounding factors (vitamin supplementation, physical activity, occupation, vitamin D intake via diet, clothing habits of the female participantsCitation10), are not reported. The increase in the median for 25-(OH)-D3 by 55 nmol/l was accompanied by a reduction of bone specific alkaline phosphatase by about 50%, a small (3.7 pg/ml), but significant increase of calcitriol and no change in osteocalcin levels.
Figure 1. Level 3 OMI erythemal daily dose (left ordinate, black dots) for a 1 × 1 pixel in Ankara. Upper, November 2007 to end of March 2008; Lower, from October 2008 to end of March 2008. Data for January and February 2009 are missing in the database. From http://gdata1.sci.gsfc.nasa.gov/daac-bin/G3/gui.cgi?instance_id=omi, accessed on May 7th, 2010. UV-B index data for Ankara (right ordinate, red crosses). UV-B index data are adjusted to the left ordinate by multiplication with 550.
In a second “clinical trial,” rosuvastatin was compared with fluvastatin,Citation8 Patients were “randomized” in a 1:1 ratio to rosuvastatin 10 mg or fluvastatin 80 mg/day for eight weeks and inclusion as well as exclusion parameters were identical to the previous report. Surprisingly, information is missing on how many patients were screened and which method of randomization was employed. Clearly this study was not blinded; raising doubts about observer bias and, of equal concern, there were no drop-outs, similar to the observational study. As before, 25-(OH)-D3 rose under rosuvastatin from 11.8 ng/ml (~29.4 nMol/l) to 35.2 ng/ml (~88 nMol/l). Bone specific alkaline phosphatase decreased by almost 50%, calcitriol increased by 5.7 pg/ml, but osteocalcin did not change. With the exception of a significant but unexplained decrease in bone specific alkaline phosphatase no significant change with respect to the above parameters was observed in the fluvastatin group. Given that the two trials were performed about a year apart, it is surprising that the absolute increase in 25-(OH)-D3 observed with rosuvastatin is more or less the same for both studies. A statistical analysis of the data from the rosuvastatin cohorts in the two trials also shows remarkable similarities for several parameters, though the ratio between men and women was opposite, more diabetics were induced, and only a 10 mg rosuvastatin dose was employed in the second trial ().
Table 1. Demographic data and laboratory values at baseline and after 8 weeks of treatment with rosuvastatin: statistical analysis
Rosuvastatin is Not Metabolized by CYP 3A4/5
In the first publication, the authors offered no explanation for their findings. In the second, they suggested that, “as statins are extensively metabolized by CYP 3A4 and CYP 3A5,” a “common catabolic pathway may be responsible for the increased 25-(OH)-D3 levels.” This hypothesis can be rejected. Rosuvastatin is not metabolized by CYP 3A4/5. It is converted, to a very minor extent, by CYP 2C9, to N-desmethylrosuvastatin and in vivo to the corresponding rosuvastatin lactone.Citation11 Although the lactone form is a weak inhibitor for CYP 2C8, CYP 2C9 and CYP 3A4/5, fluvastatin as lactone is at least as potent.Citation12 As both statins in their lactone form are weak inhibitors for CYP 3A4/5, it seems unlikely that inhibition of this xenobiotic enzyme is the basis for the observed effect, especially since fluvastatin did not increase 25-(OH)-D3.
The reported increase in 25-(OH)-D3 is equivalent to an oral vitamin D3 intake of ~3,000 to 3,800 I.U./d. or a minimal cumulative UV-B dose of around 1 J*cm−2. The magnitude of the observed rise of 25-(OH)-D3 in both studies, which, on average, amounts to ~54 nMol/l after 8 weeks, is surprising. Based on other studies, one is able to obtain an estimate for daily additional oral input of cholecalciferol being equivalent to the average increase in the two studies. Formulas predict that a 1 nMol/l increase in serum 25-(OH)-D3 will require ~70 I.U. of daily cholecalciferol intakeCitation13-Citation15 or a cumulative dose (taking body weight as 75 kg) of around ~170.000 I.U. in 8 weeks. Increases of similar magnitude are observed with cumulative UV-B doses of ~12 J*cm−2 for Caucasian psoriasis patients exposed for 15 d to natural sunlight in Gran Canaria (27°N).Citation16 Cumulative whole-body broad-band UV-B doses (12 times in 4 weeks) between 0.24 and ~1J*cm−2 lead to increases of 25-(OH)-D3 by ~50 nMol/l in human volunteers.Citation17 The latter authors also provide a formula from which to calculate a UV-B dose rate to achieve the desired increase. Using a skin lightness score of 60, a 25-(OH)-D3 increase by 54 nMol/l would require ~82 mJ*cm−2 UV-B whole body exposure 3 times a week for 4 weeks, equivalent to a cumulative dose of ~1 J*cm−2. provides total daily erythemal doses for Ankara from satellite measurements and local UV index data. It should be noted that the satellite algorithm can underestimate UV by a factor of 3.5 compared with Brewer spectrophotometers at a given location, e.g., when snow is present.Citation18 Estimates of a possible vitamin D3 production can be obtained from simulation tools such as http://nadir.nilu.no/cgi-bin/olaeng/VitD_quartMEDandMED.cgi. From this tool, assuming cloudless skies, surface type 2, altitude 0.9 km and skin type IV estimates of 17 min (in mid-February) to 7 min (end of March) are required around midday by exposing one quarter of the body surface to obtain the equivalent of 1000 I.U. of vitamin D3. E.g.: In March 2008 17 d can be identified from the satellite data with 2000 to 3,000 J/m2, most of which is obtained during midday from 11 a.m. to 2 p.m. The time for synthesis of 1000 I.U. vitamin D3 at a midday hourly rate of 600 to 800 J/m2 is 15 to 20 min, provided one quarter of the skin (hands, face and arms) are exposed. Thus, contrary to the assumption by the authors that there was “little exposure to ultra-violet light” for the two studies, this is not at all supported by the satellite or locally measured data.
Increased Input of Vitamin D3 or Decreased Clearance of 25-(OH)-D3?
A brief and highly simplified discussion of determinants for the steady-state serum concentration of the biologically important intermediate 25-(OH)-D3 seems appropriate in this context. Input (vitamin D: oral intake and oral bioavailability, skin production via UV-B, release from internal stores) speed of conversion into 25-(OH)-D3 and elimination (via metabolism and excretion) are the main determinants of the steady-state concentration of the prohormone. One can disregard release from internal stores as the best estimate (for both vitamin D3 plus 25-(OH)-D3) is around 15.000 I.U.Citation19 The clearance of 25-(OH)-D3 is governed by (1) glomerular filtration of 25-(OH)-D3 bound to vitamin D binding protein and reabsorption capacity of the megalin-cubulin receptor system in the kidney tubules, (2) metabolic activation via 1α,25 hydroxylase, (3) metabolic inactivation of the prohormone and (preferentially) calcitriol by the 24-hydroxylase and (4) excretion via bile and urine after further hydroxylation and covalent modification (coupling to glucuronic acid or sulfate). An increase of the steady-state concentration by almost a factor of 3 can only be explained by a similar increase in input or decrease in output or any combination of these, according to first principles.
Is There a Possibility of Increased Vitamin D Input by Increased Bioavailability?
Increased oral bioavailability of diet-derived vitamin D3 could be a factor. For 6 weeks patients already had been on the National Cholesterol Education (NCEP) diet, which recommends fish and shellfish (rich in vitamin D), but on average still had insufficient or deficient 25-(OH)-D3 levels. Vitamin D3 in food or applied as oral supplement is taken up together with lipids by the jejunal mucosa. The pre-pro-hormone travels with chylomicrons in the thoracic duct and, once in the arterial circulation, enters liver cells via receptor-mediated uptake (LDL-receptors, remnant receptors).Citation20 It is here, where all statins—which increase liver LDL-receptors by approximately a factor of two—could facilitate uptake and subsequent hydroxylation. It is, however, unlikely that increased liver uptake of vitamin D3 will increase steady-state serum levels of the prohormone when the total amount of the vitamin entering the body is unchanged under rosuvastatin. Furthermore, this should be observed with all statins, which is clearly not the case. However, there exists a finely tuned, reciprocal relationship between endogenous cholesterol synthesis und exogenous cholesterol uptake. One would therefore expect that statins (which have to pass enterocytes, inhibit their 3-hydroxy-3-methylglutaryl-CoA-reductase and decrease intracellular cholesterol) lead to increased activity of the sterol transporter Niemann-Pick C1-like 1 (NPC1L1). The latter, the molecular target of ezitimibe, has been demonstrated to play a significant role in vitamin D uptake.Citation21 Circulating endogenous synthesis cholesterol precursors (e.g., desmosterol and lathosterol) serve as markers for endogenous synthesis and plant-derived campesterol or/and sitosterol for cholesterol absorption, respectively. Changes (e.g., after drug treatment) or differences of the respective ratios (e.g., between populations) are taken as correlates for alterations in the two interdependent systems as shown for pravastatin.Citation22 Indeed, a crossover study in hyperlipidemic men demonstrated an increase in absorption markers and increased NPC1L1 mRNA levels after atorvastatin.Citation23 Thus, the possibility exists that, perhaps depending on the apoliprotein E genotype, see Huebbe et al.,Citation24 statin treatment can lead to an increase in the amount of the vitamin D absorbed but never to the extent the authors reported.
Could Rosuvastatin Interfere With Catabolism and/or Excretion of the 25-(OH)-D3?
Based on experiments with radioactively labeled cholecalciferol and 25-(OH)-D3, ~1% of the amount of 25-(OH)-D3 in the body is excreted every day by the kidney and ~2% in the feces via bile after further hydroxylations (e.g., to calcitroic acid) and coupling reactions (glucuronidation).Citation25 Theoretically, it seems possible that kidney clearance of 25-(OH)-D3 and its further hydroxylated metabolites is inhibited by rosuvastatin: ~10% of the dose is actively secreted, whereas fluvastatin has negligible renal clearance.Citation26 A major route of metabolic inactivation of 25-(OH)-D3 is the 24-hydroxylase, where rosuvastatin and/or its lactone could inhibit as shown for synthetic inhibitors.Citation27 It is, however, unlikely, that such a partial „chemical knockout“ would raise 25-(OH)-D3 levels under constant input, but must lead to increased calcitriol levels (as was indeed reported), which are in turn accompanied by faster 25-(OH)-D3 elimination. 24-hydroxylase inhibition can be ruled out as gene mutations in CYP24A1 with complete loss of function led to increased calcitriol, but not 25-(OH)-D levels.Citation28
Interference of Rosuvastatin and/or Its Metabolites With the 25-(OH)-D3 Assay?
The assay used by the authors is selective for the 25-(OH)-D3 and shows little cross-reactivity with 24 R, 25-(OH)2-D3. With immunoassays one can never be sure if natural compounds, heterophile antibodiesCitation29 or drug metabolites can interfere: A recent large trial reported that a 25-(OH)-D3 selective immunoassay had higher values in smokers than in non-smokers, which is contrary to general knowledge. These higher values could not be reproduced by other detection methods and indicate interference in the serum of smokers with either the antibody or the detection system.Citation30 Such interferences, however, were not yet reported for the assay used, but remain a remote explanation.
Conclusion
The results from the two publications,Citation7,Citation8 especially the extreme magnitude of the effect, deserve confirmation by others in well-designed studies, which prospectively also control for UV-B irradiance, oral vitamin D supply and other confounding factors. In addition, unequivocal chemical identification of the major cholecalciferol metabolites by HPLC-MS/MS technology during the entire time course of statin exposure appears mandatory. As possible confirmation of the “novel pleiotropic effect” of rosuvastatin, representative cohorts from its different placebo-controlled trials may be measured for 25-(OH)-D, matched by season, latitude and other factors known to influence prohormone levels.
Nevertheless, serious doubts remain about how the data for these two publications were obtained. One could suspect that retrospective “observations” were converted into a comparative, “prospective” and randomized trial. Examples exist in top medical journals.Citation31 Our doubts are apparently shared by another research team in Ankara (!), who did not observe any increase of 25-(OH)-D3 from basal levels after treatment with atorvastatin or rosuvastatin.Citation32
Acknowledgments
We would like to thank Dr S. Ahmad, NASA GSFC, Greenbelt, MD, USA, for valuable suggestions and Mr Enver Erbas, External Relations Division of the Turkish State Metereological Service for the UV-B Index data. We acknowledge the expert assistance in preparation of this manuscript (including style, figures and references) by Johannes Werner.
Disclosure of Potential Conflicts of Interest
Research of M.B. is partly funded by the Austrian Governmental Department for Environment in the framework of the project “Messungen und Analyse der solaren UV-Strahlung in Österreich.”
References
- Glossmann H H. Vitamin D - an update. Osteologie Forum 2010; (16):23–35.
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM, Kastelein JJP, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195 - 207; http://dx.doi.org/10.1056/NEJMoa0807646; PMID: 18997196
- Zittermann A, Gummert JF, Börgermann J. The role of vitamin d in dyslipidemia and cardiovascular disease. Curr Pharm Des 2011; 17:933 - 42; http://dx.doi.org/10.2174/138161211795428786; PMID: 21418036
- Goldstein MR, Mascitelli L, Pezzetta F. Rosuvastatin and vitamin D: might there be hypovitaminosis D on JUPITER?. Int J Cardiol 2010; 145:556 - 7; http://dx.doi.org/10.1016/j.ijcard.2010.05.022; PMID: 20557956
- Gupta A, Thompson PD. The relationship of vitamin D deficiency to statin myopathy. Atherosclerosis 2011; 215:23 - 9; http://dx.doi.org/10.1016/j.atherosclerosis.2010.11.039; PMID: 21185021
- Ware WR. The JUPITER lipid lowering trial and vitamin D: Is there a connection?. Dermatoendocrinol 2010; 2:50 - 4; http://dx.doi.org/10.4161/derm.2.2.13235; PMID: 21547097
- Yavuz B, Ertugrul DT, Cil H, Ata N, Akin KO, Yalcin AA, et al. Increased levels of 25 hydroxyvitamin D and 1,25-dihydroxyvitamin D after rosuvastatin treatment: a novel pleiotropic effect of statins?. Cardiovasc Drugs Ther 2009; 23:295 - 9; http://dx.doi.org/10.1007/s10557-009-6181-8; PMID: 19543962
- Ertugrul DT, Yavuz B, Cil H, Ata N, Akin KO, Kucukazman M, et al. STATIN-D Study: Comparison of the Influences of Rosuvastatin and Fluvastatin Treatment on the Levels of 25 Hydroxyvitamin D. Cardiovasc Ther 2010; In press PMID: 20370794
- Gröbner J, Albold A, Blumthaler M, Cabot T, de La Casiniere A, Lenoble J, et al. Variability of spectral solar ultraviolet irradiance in an Alpine environment. J Geophys Res 2000; 105:D22 26991 - 7003; http://dx.doi.org/10.1029/2000JD900395
- Lips P. Vitamin D status and nutrition in Europe and Asia. J Steroid Biochem Mol Biol 2007; 103:620 - 5; http://dx.doi.org/10.1016/j.jsbmb.2006.12.076; PMID: 17287117
- Martin PD, Warwick MJ, Dane AL, Hill SJ, Giles PB, Phillips PJ, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther 2003; 25:2822 - 35; http://dx.doi.org/10.1016/S0149-2918(03)80336-3; PMID: 14693307
- Sakaeda T, Fujino H, Komoto C, Kakumoto M, Jin J, Iwaki K, et al. Effects of acid and lactone forms of eight HMG-CoA reductase inhibitors on CYP-mediated metabolism and MDR1-mediated transport. Pharm Res 2006; 23:506 - 12; http://dx.doi.org/10.1007/s11095-005-9371-5; PMID: 16388406
- Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 2003; 77:204 - 10; PMID: 12499343
- van Groningen L, Opdenoordt S, van Sorge A, Telting D, Giesen A, de Boer H. Cholecalciferol loading dose guideline for vitamin D-deficient adults. Eur J Endocrinol 2010; 162:805 - 11; http://dx.doi.org/10.1530/EJE-09-0932; PMID: 20139241
- Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, et al. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab 2011; 96:981 - 8; http://dx.doi.org/10.1210/jc.2010-0015; PMID: 21289249
- Osmancevic A, Nilsen LT, Landin-Wilhelmsen K, Søyland E, Abusdal Torjesen P, Hagve TA, et al. Effect of climate therapy at Gran Canaria on vitamin D production, blood glucose and lipids in patients with psoriasis. J Eur Acad Dermatol Venereol 2009; 23:1133 - 40; http://dx.doi.org/10.1111/j.1468-3083.2009.03245.x; PMID: 19453805
- Armas LAG, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol 2007; 57:588 - 93; http://dx.doi.org/10.1016/j.jaad.2007.03.004; PMID: 17637484
- Fioletov VE, McArthur LJB, Mathews TW, Marrett L. Estimated ultraviolet exposure levels for a sufficient vitamin D status in North America. J Photochem Photobiol B 2010; 100:57 - 66; http://dx.doi.org/10.1016/j.jphotobiol.2010.05.002; PMID: 20554218
- Heaney RP, Horst RL, Cullen DM, Armas LAG. Vitamin D3 distribution and status in the body. J Am Coll Nutr 2009; 28:252 - 6; PMID: 20150598
- Haddad JG, Matsuoka LY, Hollis BW, Hu YZ, Wortsman J. Human plasma transport of vitamin D after its endogenous synthesis. J Clin Invest 1993; 91:2552 - 5; http://dx.doi.org/10.1172/JCI116492; PMID: 8390483
- Reboul E, Goncalves A, Comera C, Bott R, Nowicki M, Landrier J, et al. Vitamin D intestinal absorption is not a simple passive diffusion: evidences for involvement of cholesterol transporters. Mol Nutr Food Res 2011; 55:691 - 702; http://dx.doi.org/10.1002/mnfr.201000553; PMID: 21280209
- Matthan NR, Resteghini N, Robertson M, Ford I, Shepherd J, Packard C, et al. Cholesterol absorption and synthesis markers in individuals with and without a CHD event during pravastatin therapy: insights from the PROSPER trial. J Lipid Res 2010; 51:202 - 9; http://dx.doi.org/10.1194/jlr.M900032-JLR200; PMID: 19578163
- Tremblay AJ, Lamarche B, Lemelin V, Hoos L, Benjannet S, Seidah NG, et al. Atorvastatin increases intestinal expression of NPC1L1 in hyperlipidemic men. J Lipid Res 2011; 52:558 - 65; http://dx.doi.org/10.1194/jlr.M011080; PMID: 21123766
- Huebbe P, Nebel A, Siegert S, Moehring J, Boesch-Saadatmandi C, Most E, et al. APOE {varepsilon}4 is associated with higher vitamin D levels in targeted replacement mice and humans. FASEB J 2011; 25:3262 - 70; http://dx.doi.org/10.1096/fj.11-180935; PMID: 21659554
- Gray RW, Weber HP, Dominguez JH, Lemann J. The metabolism of vitamin D3 and 25-hydroxyvitamin D3 in normal and anephric humans. J Clin Endocrinol Metab 1974; 39:1045 - 56; http://dx.doi.org/10.1210/jcem-39-6-1045; PMID: 4372244
- Launay-Vacher V, Izzedine H, Deray G. Statins' dosage in patients with renal failure and cyclosporine drug-drug interactions in transplant recipient patients. Int J Cardiol 2005; 101:9 - 17; http://dx.doi.org/10.1016/j.ijcard.2004.04.005; PMID: 15860377
- Schuster I, Egger H, Bikle D, Herzig G, Reddy GS, Stuetz A, et al. Selective inhibition of vitamin D hydroxylases in human keratinocytes. Steroids 2001; 66:409 - 22; http://dx.doi.org/10.1016/S0039-128X(00)00159-8; PMID: 11179750
- Schlingmann KP, Kaufmann M, Weber S, Irwin A, Goos C, John U, et al. Mutations in CYP24A1 and Idiopathic Infantile Hypercalcemia. N Engl J Med 2011; 365:410 - 21; http://dx.doi.org/10.1056/NEJMoa1103864; PMID: 21675912
- Holmes EW, Garbincius J, McKenna KM. Non-linear analytical recovery in the DiaSorin Liaison immunoassay for 25-hydroxy vitamin D. Clin Chim Acta 2011; 412:2355 - 6; http://dx.doi.org/10.1016/j.cca.2011.09.003; PMID: 21925154
- Grimnes G, Almaas B, Eggen AE, Emaus N, Figenschau Y, Hopstock LA, et al. Effect of smoking on the serum levels of 25-hydroxyvitamin D depends on the assay employed. Eur J Endocrinol 2010; 163:339 - 48; http://dx.doi.org/10.1530/EJE-09-1041; PMID: 20479012
- Kleinert S, Horton R. Retraction–autologous myoblasts and fibroblasts versus collagen [corrected] for treatment of stress urinary incontinence in women: a [corrected] randomised controlled trial. Lancet 2008; 372:789 - 90; http://dx.doi.org/10.1016/S0140-6736(08)61320-3; PMID: 18774408
- Demir CC, Mousa U, Anil C, Bozkus Y. Effects of atorvastatin and rosuvastatin therapy on serum 25-hydroxyvitamin D levels: a comparative study [European Congress of Endocrinology 2011, Rotterdam, April 30–May 4 - European Society of Endocrinology]. Endocrine Abstracts; 2011. (vol 26).