Abstract
Background: Vitiligo has been associated with multiple endocrine and immune conditions. Several laboratory tests have been assessed in this disease with controversial results.
Objective: The aim of this study is to analyze the levels autoantibodies, basal glycaemia, vitamin B12, folic acid and thyroid function and its association with the diagnosis and outcome of vitiligo patients through a case-control study.
Material and methods: This case-control study was performed on 196 consecutive patients with vitiligo referred to our Dermatology Department. As a control group, 160 healthy individuals without vitiligo or known history of immunologic/endocrine disease were included. Data were analyzed using the SPSS 17.0 statistical software package.
Results: Clinical, analytical and demographic data have been recorded. Our results showed that anti-thyroid peroxidase antibody and anti-parietal gastric cell antibody could be useful laboratory markers in a subpopulation of vitiligo patients. However, testing anti-nuclear antibody, anti-thyroglobulin antibody, folic acid and vitamin B12 seems to have limited clinical implication and diagnostic relevance in our routine clinical practice.
Limitations: This study addressed a selected population of vitiligo patients in Spain and may not generalize to different clinical settings or regions. The study of a wider sample would confirm these findings and allow a detailed analysis of the above factors as a function of the clinical subtype of vitiligo.
Conclusion: We have determined the more efficient serological markers to order in vitiligo patients. Our findings suggest that anti-thyroid peroxidase antibody and anti-parietal gastric cell could be useful tests for the characterization of specific subpopulations of vitiligo patients in terms of severity and co-morbidity, so their determination could have a prognostic value.
Introduction
Vitiligo is the most common pigmentary disorder, with a reported prevalence of 0.1–4% worlwideCitation1,Citation2 Vitiligo has been associated with multiple endocrine and immune conditions, such us diabetes, thyroid disease or pernicious anemia ().Citation3-Citation14 Its etiology is still unknown, although multiple hypotheses have been considered. Three main pathogenesis mechanisms have been purposed: self-destruction, neural and autoimmune.Citation2-Citation10 Nevertheless, vitiligo is nowadays widely considered as an autoimmune disorder.Citation9,Citation10 However, mechanism of melanocyte disappearance has never been clearly understood and cellular and humoral autoimmune phenomena as primary events remains still unproven.Citation3,Citation8-Citation10
Table 1. More frequently associated diseases in our vitiligo patients (n = 196)
Several laboratory tests have been described to be altered in this disease, although results are controversial.Citation3-Citation7 Based on suggested associations described in literature, we decided to evaluate the presence of Anti-nuclear antibody (ANA), anti-thyroid peroxidase antibody (ATPO), anti-parietal gastric cell antibody (APGC), Anti-thyroglobulin antibody (ATG), glycaemia, vitamin B12, folic acid, TSH (Thyroid-stimulating hormone) and FT4 (Free T4) in patients and controls.
Objective
The aim of this study was to determine the significance of specific serological findings such as organ and non-organ specific autoantibodies, basal glycaemia, vitamin B12, folic acid and thyroid function in diagnosis and outcome of vitiligo patients.
Material and Methods
One hundred and 90 six consecutive patients with vitiligo referred to the Dermatology Department, University of La Laguna from September 2003 to September 2007 were recruited for this study. 160 healthy individuals were included as a control group, All recruitment and clinical assessments were conducted with written informed consent, and with the explicit approval of our Institutional Ethics Review Board. Patients were diagnosed both clinically and by Wood’s lamp by a dermatologist and depigmenting disorders other than vitiligo were excluded. They were not under systemic steroid treatment when they were included in our study. This study was done prior the consensus report of the Vitiligo European Task Force,Citation3 so clinical subtyping could not reflect the currently accepted morphological definition. However, classification of vitiligo used in this study was: segmental and non-segmental (vulgaris, achrofacial, focal and universal, ). Data were analyzed using the SPSS 17.0 statistical software package. Continuous variables were analyzed with the T-test, Chi square, ANOVA or nonparametric test appropriately. p < 0.05 was considered statistically significant.
Table 2. clinical subtypes of vitiligo in the sample
Results
Patients and control characteristics
Our study group included 196 patients (55.5% female, n = 109; 44.4% male, n = 87) with vitiligo. Female to male ration was 1.2:1. Patients were aged from 3 to 74 y and the mean age was 36.18. Ethnicity of the patients was recorded as follows: 98.4% were Caucasian, 1.0% were Hispanic and 0.5% were Indian. 109 patients (55.5%) showed active progression of vitiligo over the last year. The mean duration of the disease was 9.18 y (Range: 1 mo-52 y). The age of onset of the disease ranged from 1 to 70 y, with a mean age of 27.20 ± 17.48 y for females and 26.29 ± 18.06 y for males. In 81 cases (41.3%) the onset was at 20 y or earlier. Clinical subtypes of vitiligo presented in the sample are recorded in . Differences in segmental and non-segmental vitiligo patients in our sample are shown in .
Table 3. Clinical and analytical differences between segmental/non segmental subtypes
Vitiligo extended less than 25% of body surface area (BSA) in 149 patients (76%) and more than 50% in 8 patients (4%). Köebner phenomenon was observed in 100 patients (51,1%). Positive family history (first or second degree affected relatives) was found in 42.9% (n = 84) associated to an earlier age of onset (p < 0.05). Additional dermatological and systemic conditions of the patients are showed in and .
Control group included 160 healthy individuals (103 females and 57 males). Mean age of the patients was 39.9 y (Range: 6–89).
Laboratory findings
No significant difference in thyroid hormones between patients and controls could be found (p = 0.26). However, thyroid alterations were more frequently seen among patients compared with controls ( and ).
Figure 1. Frequency of serologic altered values in patients (n = 196) and controls (n = 160). Comparison of serologic alterations between patient and control groups (p values are shown in the table at the top of the bars; asterisks are shown when values are < 0.05).
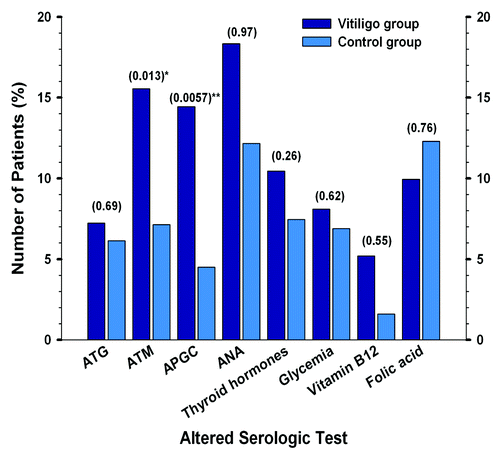
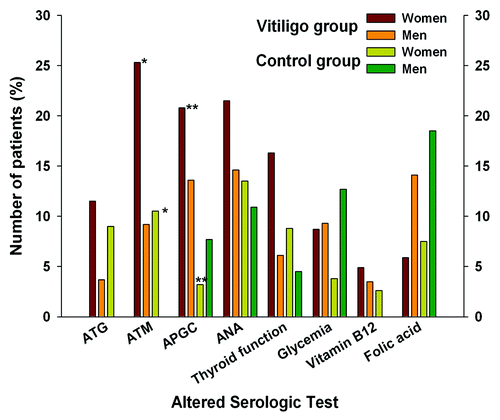
Figure 2. Frequency of altered parameters in case (n = 196) and control group (n = 160) compared by sex. Statistically significant p values (< 0,05) are shown with asterisk at the top of the bars: ATM and APGC levels between women belonging to vitiligo or control group showed significant differences (*p = 0,014, **p = 0.002 respectively).
Elevated basal glycaemia was found in 16 patients (8%) and decreased serum levels of vitamin B12 and folic acid were seen in 5.1% (n = 10) and 9.7% (n = 19) of patients respectively. No differences between patients and controls were observed in these laboratory tests. However vitamin B12 serum levels were lower in vitiligo group. No correlation between severity of vitiligo and basal glucose, thyroid function, vitamin B12 and folic acid levels could be observed in our study (p > 0.05).
ANA were positive in 18.3% of patients followed by ATPO (15.5%) and APGC (14.4%). ATG was increased in 7.2%. Positive auto-antibodies were more frequently observed among females. No differences between ANA and ATG positivity was seen in both groups (p > 0.05). Presence of ATPO and APGC antibodies was significantly higher among vitiligo group (p = 0.013 and 0.0057, respectively). Increased titers of ATPO and APGC were related to severity of disease. Odds ratio for presenting increased titers of ATPO in patients was 2.91 compared with controls [OR: 2.91; CI 95% (1.22–7.16)]. Odds ratio for presenting APGC positivity in patients was 4.48 compared with controls [OR: 4.48; CI 95% (1.44–15.49)]. A higher mean age and a later onset of the disease was found in patients with positive APGC (p < 0.05). More than 75% affected BSA was seen in 7.4% of patients with elevated APGC compared with 2.1% in patients with negative APGC (p = 0.019).
An increased frequency of diabetes among male patients with elevated APGC titers compared with females with the same characteristics was found. This finding was also observed in males with positive ATPO.
Discussion
Vitiligo is an acquired disorder characterized by progressive, patchy loss of pigmentation from skin, overlying hair and mucosa.Citation1,Citation2 Several theories on vitiligo etiopathogenesis have been purposed. Stress, accumulation of toxic compounds, infection, autoimmunity, mutations, altered cellular environment and impaired melanocyte migration and/or proliferation may all contribute to vitiligo etiopathogenesis in varying proportions.Citation1-Citation10 Positive family history has been reported in the literature in 8–36%.Citation11-Citation14 In addition, it is considered as a poor prognostic factor. In our study, 42.8% of patients reported a family history of vitiligo but no correlation with the extent of the disease could be observed (p = 0.26). However, an earlier age of onset was demonstrated in patients with affected relatives. To our knowledge, this is the highest rate of positive family history published so far. It could be explained by the different ethnic backgrounds of the assessed population and geographical isolation of our region.
Consistent with literature, a high frequency of thyroid disease, diabetes, psoriasis, alopecia areata and pernicious anemia was found in our patients (). This finding may support an immunological basis for vitiligo.
Several reports showing an increased frequency of organ-specific antibodies in vitiligo patients when compared with controls have been reported.Citation9-Citation14 Although relationship between vitiligo and thyroid autoimmunity has already been studied, there still exists a debate about this association.Citation9,Citation10,Citation14 Consistent with literature, thyroid alterations were more frequently seen in our patients than in controls. However no significant difference was observed. Very few studies evaluating blood levels of folic acid and vitamin B12 in patients with vitiligo have been reported.Citation2,Citation3 In this study, no differences in vitamin levels between patients and controls could be found. Relationship between basal glucose levels, thyroid function, vitamin B12 and folic acid and the severity of vitiligo (measured by BSA) was not significant in our study (p > 0.05). However, thyroid function screening has been recommended in vitiligo patients in recent studies.Citation9
Clinical differences between our patients with high and normal titers of ATPO and ACPG (higher prevalence of diabetes mellitus, more severe disease) could indicate that vitiligo may not be a single disease with a single underlying mechanism, but most likely could be a group of conditions with similar clinical presentation. This is further substantiated by the existence of different forms of vitiligo and its relation with several autoimmune/endocrinologic conditions.Citation4-Citation14
Our data also suggest that ATPO and APGC may be linked to the pathogenesis of vitiligo-associated autoimmune process, although the role in autoimmunity remains unclear. Clinically, these laboratory tests may be helpful to identify patients who display a more severe course of the disease. Furthermore, our results showed that the assessment of ATPO and APGC might be helpful to identify those male patients at risk of developing diabetes mellitus. However, serum levels of folic acid, vitamin B12, ANA, ATG and thyroid function do not appear to play a role in the pathogenesis of vitiligo. For this reason, sub-typing of ANA, ATG and these vitamins seems to have limited clinical implication in our routine clinical practice.
These data not only support an autoimmune mechanism in the parthenogenesis of vitiligo, like other recent works,Citation9,Citation10 but also highlight the important genetic component of the condition in this specific Spanish population. An optimal approach to vitiligo patients should rely on an understanding of the pathophysiology and an early recognition of underlying disorders. However, the question still remains whether autoimmunity might arise as a secondary phenomenon following melanocyte destruction in this condition.
References
- Rodríguez-Martín M, García Bustínduy M, Sáez Rodríguez M, Noda Cabrera A. Randomized, double-blind clinical trial to evaluate the efficacy of topical tacalcitol and sunlight exposure in the treatment of adult nonsegmental vitiligo. Br J Dermatol 2009; 160:409 - 14; http://dx.doi.org/10.1111/j.1365-2133.2008.08906.x; PMID: 19016706
- Balci DD, Yonden Z, Yenin JZ, Okumus N. Serum homocysteine, folic acid and vitamin B12 levels in vitiligo. Eur J Dermatol 2009; 19:382 - 3; PMID: 19451048
- Taïeb A, Picardo M, VETF Members. The definition and assessment of vitiligo: a consensus report of the Vitiligo European Task Force. Pigment Cell Res 2007; 20:27 - 35; http://dx.doi.org/10.1111/j.1600-0749.2006.00355.x; PMID: 17250545
- Tjioe M, Gerritsen MJ, Juhlin L, van de Kerkhof PC. Treatment of vitiligo vulgaris with narrow band UVB (311 nm) for one year and the effect of addition of folic acid and vitamin B12. Acta Derm Venereol 2002; 82:369 - 72; http://dx.doi.org/10.1080/000155502320624113; PMID: 12430737
- Kemp EH, Gawkrodger DJ, Watson PF, Weetman AP. Immunoprecipitation of melanogenic enzyme autoantigens with vitiligo sera: evidence for cross-reactive autoantibodies to tyrosinase and tyrosinase-related protein-2 (TRP-2). Clin Exp Immunol 1997; 109:495 - 500; http://dx.doi.org/10.1046/j.1365-2249.1997.4781381.x; PMID: 9328128
- Cui J, Arita Y, Bystryn JC. Cytolytic antibodies to melanocytes in vitiligo. J Invest Dermatol 1993; 100:812 - 5; http://dx.doi.org/10.1111/1523-1747.ep12476636; PMID: 8496621
- Harning R, Cui J, Bystryn JC. Relation between the incidence and level of pigment cell antibodies and disease activity in vitiligo. J Invest Dermatol 1991; 97:1078 - 80; http://dx.doi.org/10.1111/1523-1747.ep12492607; PMID: 1748818
- Koga M, Tango T. Clinical features and course of type A and type B vitiligo. Br J Dermatol 1988; 118:223 - 8; http://dx.doi.org/10.1111/j.1365-2133.1988.tb01778.x; PMID: 3348967
- Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, et al, Therapy Guidelines and Audit Subcommittee, British Association of Dermatologists, Clinical Standards Department, Royal College of Physicians of London, Cochrane Skin Group, Vitiligo Society. Guideline for the diagnosis and management of vitiligo. Br J Dermatol 2008; 159:1051 - 76; http://dx.doi.org/10.1111/j.1365-2133.2008.08881.x; PMID: 19036036
- Spritz RA. The genetics of generalized vitiligo and associated autoimmune diseases. J Dermatol Sci 2006; 41:3 - 10; http://dx.doi.org/10.1016/j.jdermsci.2005.10.001; PMID: 16289692
- Grunnet I, Howitz J, Reymann F, Schwartz M. Vitiligo and pernicious anemia. Arch Dermatol 1970; 101:82 - 5; http://dx.doi.org/10.1001/archderm.1970.04000010084015; PMID: 5416800
- Gauthier Y, Cario Andre M, Taïeb A. A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy?. Pigment Cell Res 2003; 16:322 - 32; http://dx.doi.org/10.1034/j.1600-0749.2003.00070.x; PMID: 12859615
- Handa S, Kaur I. Vitiligo: clinical findings in 1436 patients. J Dermatol 1999; 26:653 - 7; PMID: 10554431
- Kurtev A, Dourmishev AL. Thyroid function and autoimmunity in children and adolescents with vitiligo. J Eur Acad Dermatol Venereol 2004; 18:109 - 11; http://dx.doi.org/10.1111/j.1468-3083.2004.00728.x; PMID: 14678552