Abstract
Skin aging does not only occur by passing time alone but also by the exposure to different environmental factors. The skin aging process, which is induced by environmental factors, is named premature or extrinsic skin aging process and can be distinguished from the chronologically (intrinsic) skin aging process by characteristic skin aging signs. Well known environmental factors leading to extrinsic skin aging are sun exposure and smoking. Recently, an epidemiological study could further discover an association between air pollution and skin aging. First of all the skin aging inducing effect of sun exposure was discovered and an own term (photoaging) was given to this special field of extrinsic skin aging. Mechanistic studies have further increased our knowledge about the molecular pathways by which environmental factors contribute to extrinsic skin aging. In this regard, profound knowledge how sun exposure leads to extrinsic skin aging were gained in the last years, and additionally there are also indications how smoking and air pollution might contribute to this process. Moreover it was realized that extrinsic skin aging manifests differently between different populations. Thus, in this review we summarize the influence of the different environmental factors: sun exposure, smoking and air pollution on skin aging and further present ethnic-specific manifestations of extrinsic skin aging.
Introduction
Aging of the skin is influenced by two separate processes. The general aging process, which is genetically determined and occurs by passing time alone, is called the intrinsic skin aging process, whereas the skin aging process induced by environmental factors is named extrinsic skin aging process. Each skin aging process leads to characteristic skin aging signs. The intrinsic skin aging process is characterized primarily by functional alterations than by gross morphological changes. The skin appears dry and pale with fine wrinkles displaying a certain degree of laxity and a variety of benign neoplasms. In contrast, the extrinsic skin aging process is characterized by striking morphologic and physiologic changes and in general leads to a premature aging of the skin. Prominent manifestations of the extrinsic skin aging process are coarse wrinkles, solar elastosis and pigment irregularities. These signs superimpose the intrinsic skin aging signs at chronically exposed areas of the body. The rate of extrinsic skin aging varies strikingly among individuals and among ethnic populations, which does not apply for the rate of intrinsic skin aging. On an individual basis the rate of extrinsic skin aging depends on the individual exposure pattern to different environmental factors and also on the individual genetic make-up. Some individuals might be more susceptible regarding skin damages by environmental exposure than other individuals. Furthermore, vast differences in regard to the manifestation of extrinsic skin aging between ethnic groups were observed.Citation1,Citation2 Here, one main difference between the ethnic groups, which might be most likely relevant, is the skin type. But also other genetic differences or habitual differences might be causal factors. The most important environmental factor leading to extrinsically aged skin is solar radiation. The detrimental effect of solar radiation on the facial appearance regarding skin aging was already recognized by the dermatologists Unna and Dubreuilh in the late 19th century, comparing the skin of sailors and farmers to that of indoor workers.Citation3,Citation4 Another important factor, which influences the appearance of the skin, was discovered in 1971 by Harry Daniell.Citation5 He found striking associations between cigarette smoking and wrinkling. Recently, first observations indicate, that also airborne particle exposure leads to a premature skin aging.Citation6
In the following review, we will present the influence of environmental factors like solar radiation, smoking and air pollution on skin aging. Furthermore, we will describe the observed differences in the characteristic manifestation of extrinsic skin aging between different ethnic populations and underlying genetic and environmental factors.
Sun Exposure and Skin Aging
In the late 19th century the intimation that chronic sun exposure damages the skin leading finally to premature skin aging and skin cancer came from two dermatologists. Paul Gerson Unna observed severe degenerative changes in sun exposed areas of the skin of sailors in his clinic in the German seaport city Hamburg.Citation3 Nearly at the same time William Auguste Dubreuilh observed a striking frequent incidence of keratoses and skin cancers in the vineyard workers in the Bordeaux region of France.Citation4 However, this insight was temporarily lost since Albert M. Kligman published his findings on the structural changes that occur in human skin as a result of sun exposure, which was further distinguishable from the intrinsic skin aging process.Citation7 This finding was strengthened by Lavker who also described profound structural differences between sun exposed and sun protected skin.Citation8 Kligman and Kligman then coined the term “photoaging” in order to separate this special skin aging process from the general intrinsic skin aging process.Citation9 From the whole spectrum of solar radiation it has been convincingly shown that the UV (UV) B (290–320 nm) and A (320–400 nm) fractionCitation10 as well as the infrared (IR) A (770–1400 nm) fractionCitation11-Citation14 are able to induce the extrinsic skin aging process. UVB can penetrate through the upper layer of the skin, the epidermis; UVA even through the subjacent layer, the dermis and IRA is able to penetrate through all three layers of the skin, the epidermis, dermis and subcutis.
UV-induced skin aging process
The UV-induced skin aging process is complex and can be triggered by various pathways including receptor-initiated signaling, mitochondrial damage, protein oxidation, telomere-based DNA damage and arylhydrocarbon receptor (AhR) signaling ().Citation15,Citation16 The receptor-initiated signaling pathway is induced by photoproduction of reactive oxygen species (ROS). ROS activate cell surface receptors of cytokines and growth factors in keratinocytes as well as fibroblasts. Receptor activation then leads to intracellular signaling through stimulation of kinases inducing in a next step the transcription of nuclear transcription factor AP-1 and NF-κB.Citation17,Citation18 Increased AP-1 transcription and activity on the one hand decreases the gene expression of the major dermal collagens I and III in fibroblasts leading to a reduced collagen synthesis.Citation19 On the other side AP-1 triggers the synthesis of matrix metalloproteinases (MMP) in keratinocytes and fibroblasts, which degrade mature dermal collagen. The other UV-induced transcription factor NF-κB stimulates the transcription of inflammatory cytokines and is thus involved in the attraction of neutrophils containing neutrophil collagenases and thus is also involved in collagen degrading.Citation20
Figure 1. Pathways from environmental exposures to premature / extrinsic skin aging. ROS, reactive oxygen species; mtDNA, mitochondrial DNA; AhR, arylhydrocarbon receptor.
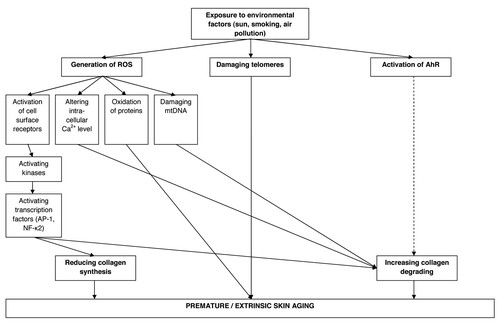
There is also a strong link between mitochondrial damage and photoaging. Mitochondrial damage or more precisely mitochondrial DNA (mtDNA) mutations can be induced in vitro and in vivo by repetitive sublethal UVA irradiation doses and are up to 10-fold more frequent in photoaged skin in comparison to sun-protected skin.Citation21-Citation23 The link to photoaging in regard to collagen destruction becomes even more obvious through the observation that mtDNA mutations are positively associated with MMP-1 levels without concomitant increase of MMP-1 specific tissue inhibitors.Citation24 Mechanistically mtDNA damages are likely to be mediated through ROS. On the one hand, mitochondria continuously generate ROS themself during their main task of energy production (ATP) by consuming oxygen via the respiratory chain. On the other hand, UVA exposure can further increase ROS generation. These ROS can then easily damage the mtDNA because it has only a limited capacity of base excision repair and is located near to the respiratory chain.Citation25
UV radiation is also supposed to be a major contributor to protein oxidation and impairment of proteasomal function in skin,Citation26 which are both hallmarks of aging. Oxidative protein damage may result in loss or gain of function, loss of protein structure and increased or decreased susceptibility for protein degradation.Citation27 Further, the accumulation of oxidized proteins in the cell inhibits proteasomal function meaning the ability of the cell to successfully degrade damaged proteins. This pathway is supposed to be a potential reason for the accelerated dysfunction of skin tissue during agingCitation28 and the maintenance of normal proteasome function is supposed to delay skin aging.Citation29
Additionally, telomeres, the terminal portions of chromosomes, play an essential role in the general aging process. In the 1960th Leonard Hayflick discovered that human cells can only pass through a certain amount of cell divisions.Citation30 This is due to the fact that telomeres cannot be fully replicated and with each cell division the final 100–200 base pairs of the telomere are lost. When telomeres finally reach a critical length the cell will no longer divide and enters a state of replicative senescence or apoptosis. UV irradiation or other environmental exposures can also lead to telomere damage which induces similar aging processes like telomere shortening leading to a premature aging process.Citation31
Furthermore, in the recent years evidence has increased that the arylhydrocarbon receptor (AhR) is an integral part of the UVB stress response and may have a role in photoaging as the MMP-1 gene is activated via the AhR signaling pathway.Citation16
IRA-induced skin aging process
Accordingly to UV irradiation, IRA irradiation causes an increase in MMP-1 in vitroCitation32 and in vivoCitation14 without a concomitant upregulation of MMP-1 tissue inhibitor. Furthermore, IRA exposure also reduces collagen I expression.Citation33 IRA irradiation of the skin is mainly absorbed by mitochondria and increases here the intra-mitochondrial production of ROS.Citation34,Citation35 These ROS then leave the mitochondria, alter intra-cytoplasmic calcium levels, activate the MAP kinases signaling pathway and lead to elevated MMP-1 expression.Citation36 However, approximately 600 genes are IRA responsiveCitation37 and thus IRA radiation might further induce the extrinsic skin aging process through various other pathways. Up to now it is shown that IRA is able to induce angiogenesisCitation38 and increases the number of mast cells in human skin in vivo,Citation39 effects which are characteristic for photoaging.
Smoking and Skin Aging
In 1969 Harry Daniell recognized that smokers look older than non-smokers.Citation39 Subsequently, he developed a wrinkle scoring system, which allowed him to analyze the association between smoking and wrinkled skin more objectively and with this scoring system he could validate his first impression.Citation5 In the following years the association between smoking and skin wrinkling could be reproduced in various epidemiological studies.Citation40-Citation43 It was further elucidated that smoking is an independent skin aging-inducing environmental factor as the effect of sun exposure and smoking is multiplicative or additive.Citation41,Citation43 Once again the association between smoking and skin aging seems to be mediated through higher expression of MMP-1 and MMP-3 mRNA, but not of their specific inhibitors as well as a decrease of collagen I and III.Citation43,Citation44 Smoking seems also to be associated with increased elastosis and teleangiectasisCitation6,Citation45 indicating further molecular pathways in addition to the induction of MMP-1 expression. Recently, it was suggested that additionally the AhR pathway may play a role in the premature skin aging process of smoking-induced skin aging.Citation46
Air Pollution and Skin Aging
Air pollution represents another environmental threat to which millions of humans worldwide are exposed. Adverse effects of air pollutants on human health are currently a serious concern and have been shown to include a higher risk for cancer, pulmonary and cardiovascular diseases.Citation47,Citation48 The skin is another organ which as outermost barrier is in direct contact with various air pollutants and thus the association between air pollution and skin damaging effects leading to skin aging is most likely. Some studies have already investigated in the effect of ozone on murine cutaneous tissue, which show evidence that ozone as a strong oxidative agent is capable of affecting the integrity of the skin.Citation49,Citation50 Furthermore, ozone was able to induce the expression of MMP-9 in murine skin indicating a role in matrix remodeling.Citation50 A recent epidemiological study discovered a direct link between airborne particulate matter (PM) exposure and the occurrence of prominent skin aging signs especially pigment spots, but also wrinkles.Citation6 One major mechanism by which ambient PM exerts its detrimental effects is through the generation of ROS.Citation51 Furthermore, particles can serve as carriers for organic chemicals and metals that are capable of localizing in mitochondria and generating ROS directly in mitochondriaCitation52 leading to skin aging by mitochondrial damage. A third suggested skin aging inducing pathway might be through polycyclic aromatic hydrocarbons (PAHs) which are adsorbed on the surface of suspended PM in air of urban areasCitation53 and are able to activate the xenobiotic metabolism via the AhR.
Ethnic Differences in the Manifestation of the Extrinsic Skin Aging Process
While the intrinsic skin aging process is rather a general process occurring similarly in all different populations, the extrinsic skin aging process manifests differently between populations regarding the time of development and also regarding prominent skin aging signs. Caucasians have an earlier onset and greater skin wrinkling than other populations and in general increased pigmentary problems are seen in Asians and in African-Americans.Citation1,Citation2,Citation54 One main influencing factor for the ethnic-specific skin aging manifestation is the genetically determined skin type as there is an ethnic variation in melanin content and composition.Citation55 Darker skin types are better protected regarding sun exposure due to the higher melanin content in their skin.Citation56 The influence of the skin type can also be observed within one population. In Caucasians, for example, two extrinsic skin aging manifestation forms are divided regarding their skin type. In fair-skinned persons the skin appears severely atrophic with multiple teleangiectasis and a variety of premalignant lesions such as actinic keratosis, whereas in dark-skinned persons deep furrows and severe solar elastosis occur.Citation57 Other underlying reasons for the ethnic-specific manifestation of skin aging might lay in further genetic variations beyond the skin type and/or in different exposure habits to environmental factors, which influence skin aging. Recently, an epidemiological study comparing the skin aging manifestation in Caucasian women from Düsseldorf in Germany with Japanese women from Nagoya in Japan discovered further underlying reasons for the ethnic-specific manifestation in these two populations. The first reason, which could explain a huge part why Japanese women expressed much less facial wrinkling than German women, was the higher antioxidant level in fasting blood of the Japanese women in comparison to the German women.Citation58 The second reason was a difference in the distribution of a genetic marker in the SLC45A2 gene, which is involved in melanin synthesis. Here, Japanese women had a higher frequency of the allele which was positively associated with the occurrence of pigment spots and thus explained at least a part of the ethnic difference in the occurrence of pigment spots.Citation59
Conclusions
The previously described mechanisms by which the different environmental factors can contribute to premature/extrinsic skin aging are summarized in . These mechanisms mainly lead to two processes, which disrupts the skin collagen matrix: (1) decreased collagen synthesis and (2) increased collagen degradation, leading to the characteristic appearance of extrinsically aged skin. All these mechanisms are also involved in the intrinsic skin aging process but they are increased by environmental exposure. Some of these environmental exposures are preventable by protecting the skin against sun exposure or by quitting smoking, but there are other environmental exposures like air pollution where up to now no protection is available. Here, new therapies have to be based on the molecular mechanism by which air pollutants induce extrinsic skin aging. Furthermore, it is important to investigate the extrinsic skin aging in different populations as environmental exposure lead to ethnic-specific skin aging manifestation. Through this, one can find the best therapy strategy for each population.
| Abbrevations | = | UV, ultraviolet |
| IR | = | infrared |
| ROS | = | reactive oxygen species |
| MMP | = | matrix metalloproteinases |
| mtDNA | = | mitochondrial DNA |
| AhR | = | arylhydrocarbon receptor |
| PM | = | particulate matter |
| PAH | = | polycyclic aromatic hydrocarbons |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Tschachler E, Morizot F. Ethnic differences in skin aging. In: Gilchrest BA, Krutmann J (eds). Skin Aging. Springer, Heidelberg-Berlin, 2006.
- Halder RM, Ara CJ. Skin cancer and photoaging in ethnic skin. Dermatol Clin 2003; 21:725 - 32, x; http://dx.doi.org/10.1016/S0733-8635(03)00085-8; PMID: 14717413
- Unna P. Histopathologie der Hautkrankheiten. Berlin, August Hirschwald, 1894.
- Dubreuilh W. Des Hyperkeratoses circonscrites. Ann Derm Syph 1896; 7:1158 - 204
- Daniell HW. Smoker’s wrinkles. A study in the epidemiology of “crow’s feet”. Ann Intern Med 1971; 75:873 - 80; PMID: 5134897
- Vierkötter A, Schikowski T, Ranft U, Sugiri D, Matsui M, Krämer U, et al. Airborne particle exposure and extrinsic skin aging. J Invest Dermatol 2010; 130:2719 - 26; http://dx.doi.org/10.1038/jid.2010.204; PMID: 20664556
- Kligman AM. Early destructive effect of sunlight on human skin. JAMA 1969; 210:2377 - 80; http://dx.doi.org/10.1001/jama.1969.03160390039008; PMID: 5395389
- Lavker RM. Structural alterations in exposed and unexposed aged skin. J Invest Dermatol 1979; 73:59 - 66; http://dx.doi.org/10.1111/1523-1747.ep12532763; PMID: 448178
- Kligman LH, Kligman AM. The nature of photoaging: its prevention and repair. Photodermatol 1986; 3:215 - 27; PMID: 3534809
- Kligman LH. The ultraviolet-irradiated hairless mouse: a model for photoaging. J Am Acad Dermatol 1989; 21:623 - 31; http://dx.doi.org/10.1016/S0190-9622(89)70229-2; PMID: 2778126
- Kligman LH. Intensification of ultraviolet-induced dermal damage by infrared radiation. Arch Dermatol Res 1982; 272:229 - 38; http://dx.doi.org/10.1007/BF00509050; PMID: 7165330
- Schieke SM, Schroeder P, Krutmann J. Cutaneous effects of infrared radiation: from clinical observations to molecular response mechanisms. Photodermatol Photoimmunol Photomed 2003; 19:228 - 34; http://dx.doi.org/10.1034/j.1600-0781.2003.00054.x; PMID: 14535893
- Schroeder P, Haendeler J, Krutmann J. The role of near infrared radiation in photoaging of the skin. Exp Gerontol 2008; 43:629 - 32; http://dx.doi.org/10.1016/j.exger.2008.04.010; PMID: 18534799
- Schroeder P, Calles C, Benesova T, Macaluso F, Krutmann J. Photoprotection beyond ultraviolet radiation--effective sun protection has to include protection against infrared A radiation-induced skin damage. Skin Pharmacol Physiol 2010; 23:15 - 7; http://dx.doi.org/10.1159/000257259; PMID: 20090404
- Yaar M, Gilchrest BA. Photoageing: mechanism, prevention and therapy. Br J Dermatol 2007; 157:874 - 87; http://dx.doi.org/10.1111/j.1365-2133.2007.08108.x; PMID: 17711532
- Krutmann J, Morita A, Chung JH. Sun exposure: what molecular photodermatology tells us about its good and bad sides. J Invest Dermatol 2012; 132:976 - 84; http://dx.doi.org/10.1038/jid.2011.394; PMID: 22170486
- Fisher GJ, Voorhees JJ. Molecular mechanisms of photoaging and its prevention by retinoic acid: ultraviolet irradiation induces MAP kinase signal transduction cascades that induce Ap-1-regulated matrix metalloproteinases that degrade human skin in vivo. J Investig Dermatol Symp Proc 1998; 3:61 - 8; PMID: 9732061
- Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996; 379:335 - 9; http://dx.doi.org/10.1038/379335a0; PMID: 8552187
- Fisher GJ, Datta S, Wang Z, Li XY, Quan T, Chung JH, et al. c-Jun-dependent inhibition of cutaneous procollagen transcription following ultraviolet irradiation is reversed by all-trans retinoic acid. J Clin Invest 2000; 106:663 - 70; http://dx.doi.org/10.1172/JCI9362; PMID: 10974019
- Fisher GJ, Choi HC, Bata-Csorgo Z, Shao Y, Datta S, Wang ZQ, et al. Ultraviolet irradiation increases matrix metalloproteinase-8 protein in human skin in vivo. J Invest Dermatol 2001; 117:219 - 26; http://dx.doi.org/10.1046/j.0022-202x.2001.01432.x; PMID: 11511297
- Berneburg M, Gattermann N, Stege H, Grewe M, Vogelsang K, Ruzicka T, et al. Chronically ultraviolet-exposed human skin shows a higher mutation frequency of mitochondrial DNA as compared to unexposed skin and the hematopoietic system. Photochem Photobiol 1997; 66:271 - 5; http://dx.doi.org/10.1111/j.1751-1097.1997.tb08654.x; PMID: 9277148
- Berneburg M, Krutmann J. Mitochondrial DNA deletions in human skin reflect photo- rather than chronologic aging. J Invest Dermatol 1998; 111:709 - 10; http://dx.doi.org/10.1046/j.1523-1747.1998.00337.x; PMID: 9764861
- Berneburg M, Plettenberg H, Medve-König K, Pfahlberg A, Gers-Barlag H, Gefeller O, et al. Induction of the photoaging-associated mitochondrial common deletion in vivo in normal human skin. J Invest Dermatol 2004; 122:1277 - 83; http://dx.doi.org/10.1111/j.0022-202X.2004.22502.x; PMID: 15140232
- Berneburg M, Plettenberg H, Krutmann J. Photoaging of human skin. Photodermatol Photoimmunol Photomed 2000; 16:239 - 44; http://dx.doi.org/10.1034/j.1600-0781.2000.160601.x; PMID: 11132125
- Ballard JW, Dean MD. The mitochondrial genome: mutation, selection and recombination. Curr Opin Genet Dev 2001; 11:667 - 72; http://dx.doi.org/10.1016/S0959-437X(00)00251-3; PMID: 11682311
- Bulteau AL, Moreau M, Nizard C, Friguet B. Impairment of proteasome function upon UVA- and UVB-irradiation of human keratinocytes. Free Radic Biol Med 2002; 32:1157 - 70; http://dx.doi.org/10.1016/S0891-5849(02)00816-X; PMID: 12031900
- Shacter E. Protein oxidative damage. Methods Enzymol 2000; 319:428 - 36; http://dx.doi.org/10.1016/S0076-6879(00)19040-8; PMID: 10907531
- Widmer R, Ziaja I, Grune T. Protein oxidation and degradation during aging: role in skin aging and neurodegeneration. Free Radic Res 2006; 40:1259 - 68; http://dx.doi.org/10.1080/10715760600911154; PMID: 17090415
- Hwang JS, Hwang JS, Chang I, Kim S. Age-associated decrease in proteasome content and activities in human dermal fibroblasts: restoration of normal level of proteasome subunits reduces aging markers in fibroblasts from elderly persons. J Gerontol A Biol Sci Med Sci 2007; 62:490 - 9; http://dx.doi.org/10.1093/gerona/62.5.490; PMID: 17522352
- Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965; 37:614 - 36; http://dx.doi.org/10.1016/0014-4827(65)90211-9; PMID: 14315085
- Kosmadaki MG, Gilchrest BA. The role of telomeres in skin aging/photoaging. Micron 2004; 35:155 - 9; http://dx.doi.org/10.1016/j.micron.2003.11.002; PMID: 15036269
- Schieke S, Stege H, Kürten V, Grether-Beck S, Sies H, Krutmann J. Infrared-A radiation-induced matrix metalloproteinase 1 expression is mediated through extracellular signal-regulated kinase 1/2 activation in human dermal fibroblasts. J Invest Dermatol 2002; 119:1323 - 9; http://dx.doi.org/10.1046/j.1523-1747.2002.19630.x; PMID: 12485435
- Kim MS, Kim YK, Cho KH, Chung JH. Infrared exposure induces an angiogenic switch in human skin that is partially mediated by heat. Br J Dermatol 2006; 155:1131 - 8; http://dx.doi.org/10.1111/j.1365-2133.2006.07510.x; PMID: 17107379
- Schroeder P, Pohl C, Calles C, Marks C, Wild S, Krutmann J. Cellular response to infrared radiation involves retrograde mitochondrial signaling. Free Radic Biol Med 2007; 43:128 - 35; http://dx.doi.org/10.1016/j.freeradbiomed.2007.04.002; PMID: 17561101
- Darvin ME, Haag S, Meinke M, Zastrow L, Sterry W, Lademann J. Radical production by infrared A irradiation in human tissue. Skin Pharmacol Physiol 2010; 23:40 - 6; http://dx.doi.org/10.1159/000257262; PMID: 20090407
- Krutmann J, Schroeder P. Role of mitochondria in photoaging of human skin: the defective powerhouse model. J Investig Dermatol Symp Proc 2009; 14:44 - 9; http://dx.doi.org/10.1038/jidsymp.2009.1; PMID: 19675552
- Calles C, Schneider M, Macaluso F, Benesova T, Krutmann J, Schroeder P. Infrared A radiation influences the skin fibroblast transcriptome: mechanisms and consequences. J Invest Dermatol 2010; 130:1524 - 36; http://dx.doi.org/10.1038/jid.2010.9; PMID: 20130591
- Chung JH, Eun HC. Angiogenesis in skin aging and photoaging. J Dermatol 2007; 34:593 - 600; http://dx.doi.org/10.1111/j.1346-8138.2007.00341.x; PMID: 17727362
- Daniell HW. Smooth tobacco and wrinkled skin. N Engl J Med 1969; 280:53; http://dx.doi.org/10.1056/NEJM196901022800129; PMID: 5761724
- Kadunce DP, Burr R, Gress R, Kanner R, Lyon JL, Zone JJ. Cigarette smoking: risk factor for premature facial wrinkling. Ann Intern Med 1991; 114:840 - 4; PMID: 2014944
- Ernster VL, Grady D, Miike R, Black D, Selby J, Kerlikowske K. Facial wrinkling in men and women, by smoking status. Am J Public Health 1995; 85:78 - 82; http://dx.doi.org/10.2105/AJPH.85.1.78; PMID: 7832266
- Aizen E, Gilhar A. Smoking effect on skin wrinkling in the aged population. Int J Dermatol 2001; 40:431 - 3; http://dx.doi.org/10.1046/j.1365-4362.2001.01238.x; PMID: 11678995
- Yin L, Morita A, Tsuji T. Skin aging induced by ultraviolet exposure and tobacco smoking: evidence from epidemiological and molecular studies. Photodermatol Photoimmunol Photomed 2001; 17:178 - 83; http://dx.doi.org/10.1034/j.1600-0781.2001.170407.x; PMID: 11499540
- Yin L, Morita A, Tsuji T. Alterations of extracellular matrix induced by tobacco smoke extract. Arch Dermatol Res 2000; 292:188 - 94; http://dx.doi.org/10.1007/s004030050476; PMID: 10836612
- Kennedy C, Bastiaens MT, Bajdik CD, Willemze R, Westendorp RG, Bouwes Bavinck JN, Leiden Skin Cancer Study. Effect of smoking and sun on the aging skin. J Invest Dermatol 2003; 120:548 - 54; http://dx.doi.org/10.1046/j.1523-1747.2003.12092.x; PMID: 12648216
- Morita A, Torii K, Maeda A, Yamaguchi Y. Molecular basis of tobacco smoke-induced premature skin aging. J Investig Dermatol Symp Proc 2009; 14:53 - 5; http://dx.doi.org/10.1038/jidsymp.2009.13; PMID: 19675554
- Castaño-Vinyals G, Cantor KP, Malats N, Tardon A, Garcia-Closas R, Serra C, et al. Air pollution and risk of urinary bladder cancer in a case-control study in Spain. Occup Environ Med 2008; 65:56 - 60; http://dx.doi.org/10.1136/oem.2007.034348; PMID: 17634245
- Beelen R, Hoek G, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, et al. Long-term exposure to traffic-related air pollution and lung cancer risk. Epidemiology 2008; 19:702 - 10; http://dx.doi.org/10.1097/EDE.0b013e318181b3ca; PMID: 18633326
- Thiele JJ, Traber MG, Polefka TG, Cross CE, Packer L. Ozone-exposure depletes vitamin E and induces lipid peroxidation in murine stratum corneum. J Invest Dermatol 1997; 108:753 - 7; http://dx.doi.org/10.1111/1523-1747.ep12292144; PMID: 9129228
- Valacchi G, Pagnin E, Okamoto T, Corbacho AM, Olano E, Davis PA, et al. Induction of stress proteins and MMP-9 by 0.8 ppm of ozone in murine skin. Biochem Biophys Res Commun 2003; 305:741 - 6; http://dx.doi.org/10.1016/S0006-291X(03)00812-X; PMID: 12763055
- Donaldson K, Tran L, Jimenez LA, Duffin R, Newby DE, Mills N, et al. Combustion-derived nanoparticles: a review of their toxicology following inhalation exposure. Part Fibre Toxicol 2005; 2:10; http://dx.doi.org/10.1186/1743-8977-2-10; PMID: 16242040
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, et al. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environ Health Perspect 2003; 111:455 - 60; http://dx.doi.org/10.1289/ehp.6000; PMID: 12676598
- Menichini E. Urban air pollution by polycyclic aromatic hydrocarbons: levels and sources of variability. Sci Total Environ 1992; 116:109 - 35; http://dx.doi.org/10.1016/0048-9697(92)90368-3; PMID: 1411492
- Nouveau-Richard S, Yang Z, Mac-Mary S, Li L, Bastien P, Tardy I, et al. Skin ageing: a comparison between Chinese and European populations. A pilot study. J Dermatol Sci 2005; 40:187 - 93; http://dx.doi.org/10.1016/j.jdermsci.2005.06.006; PMID: 16154324
- Alaluf S, Heath A, Carter N, Atkins D, Mahalingam H, Barrett K, et al. Variation in melanin content and composition in type V and VI photoexposed and photoprotected human skin: the dominant role of DHI. Pigment Cell Res 2001; 14:337 - 47; http://dx.doi.org/10.1034/j.1600-0749.2001.140505.x; PMID: 11601655
- Rijken F, Bruijnzeel PLB, van Weelden H, Kiekens RCM. Responses of black and white skin to solar-simulating radiation: differences in DNA photodamage, infiltrating neutrophils, proteolytic enzymes induced, keratinocyte activation, and IL-10 expression. J Invest Dermatol 2004; 122:1448 - 55; http://dx.doi.org/10.1111/j.0022-202X.2004.22609.x; PMID: 15175036
- Lober CW, Fenske NA. Photoaging and the skin: differentiation and clinical response. Geriatrics 1990; 45:36 - 40, 42; PMID: 2318416
- Perner D, Vierkötter A, Sugiri D, Matsui M, Ranft U, Esser C, et al. Association between sun-exposure, smoking behaviour and plasma antioxidant levels with the different manifestation of skin ageing signs between Japanese and German women--a pilot study. J Dermatol Sci 2011; 62:138 - 40; http://dx.doi.org/10.1016/j.jdermsci.2011.02.010; PMID: 21458244
- Vierkötter A, Krämer U, Sugiri D, Morita A, Yamamoto A, Kaneko N, et al. Development of Lentigines in German and Japanese Women Correlates with Variants in the SLC45A2 Gene. J Invest Dermatol 2012; 132:733 - 6; http://dx.doi.org/10.1038/jid.2011.350; PMID: 22071478