Abstract
In order to evaluate the role of solar radiation in uveal melanoma etiology, the time and latitude dependency of the incidence rates of this melanoma type were studied in comparison with those of cutaneous malignant melanoma (CMM). Norway and several other countries with Caucasian populations were included. There is a marked north - south gradient of the incidence rates of CMM in Norway, with three times higher rates in the south than in the north. No such gradient is found for uveal melanoma. Similar findings have been published for CMM in other Caucasian populations, with the exception of Europe as a whole. In most populations the ratios of uveal melanoma incidence rates to those of CMM tend to decrease with increasing CMM rates. This is also true for Europe, in spite of the fact that in this region there is an inverse latitude gradient of CMM, with higher rates in the north than in the south.
In Norway the incidence rates of CMM have increased until about 1990 but have been constant, or even decreased (for young people) after that time, indicating constant or decreasing sun exposure. The uveal melanoma rates have been increasing after 1990. In most other populations the incidence rates of CMM have been increasing until recently while those of uveal melanoma have been decreasing. These data generally support the assumption that uveal melanomas are not generated by ultraviolet (UV) radiation and that solar UV, via its role in vitamin D photosynthesis, may have a protective effect.
Introduction
Ultraviolet B (UVB) radiation from the sun is carcinogenic and certainly a main cause of skin cancers, most likely including melanoma.Citation1–Citation7 However, UVB can practically not reach melanocytes in the uveal tract, where melanomas sometimes arise.Citation8–Citation10 Ultraviolet A (UVA) radiation which may be inducing cutaneous malignant melanoma (CMM),Citation11–Citation13 may reach some of the melanocytes in the eye, although at much lower fluence rates than those reaching melanocytes in the skin.Citation9 Thus, while it is almost universally accepted that solar radiation is a cause of CMM,Citation1–Citation6,Citation14–Citation16 this is not likely to be true for uveal melanomas. In fact, epidemiological investigations indicate an inverse latitude gradient for uveal melanomas in the US,Citation10 and choroidal melanomas are not distributed as might be expected if UV played a major role.Citation17,Citation18 However, some studies indicate a positive correlation between sun exposure and ocular melanoma,Citation17,Citation19–Citation25 so the question is not settled yet. For choroidal and ciliary body melanoma (melanoma of the uveal tract) solar radiation may play a role since these localizations can be reached by UV according to some authors.Citation25 However, this is not certain since ciliary body and choroidal melanocytes are covered internally by densely pigmented retinal or ciliary pigment epithelium and externally by nontransparent sclera. Furthermore, UV must pass the cornea, the lens and the vitreous region before reaching the pigment epithelia. It has been stated that only 0.1% UVA can pass an adult lens.Citation26 Thus, only small fluences of even UVA can probably reach the melanocytes in the ciliary body and the choroid.Citation18 Also other authors argue in agreement with this,Citation27,Citation28 so the last word has probably not been said on this matter.
In the present work we followed two possible ways to elucidate this problem further: (1) Comparisons of north-south gradients for cutaneous and uveal melanomas, and (2) Comparisons of time trends of the two melanoma types. Increasing time trends of CMM incidence rates indicate increasing sun exposure. In many populations, including the Norwegian, CMM rates increased with doubling times of about 15–20 years up to 1985–1990.Citation3,Citation16,Citation29 After that time the increase is less marked in many countries,Citation30,Citation31 and in Norway even a decrease has been observed for young people.Citation16,Citation29
Here we show that after 1990 there has been an increase in uveal melanoma rates in Norway, opposite to what is found for CMM. There is no north-south gradient for uveal melanoma in the time period investigated (1993–2004), while there is a prominent north-south gradient for CMM. These data will be compared with data for other populations of Caucasians, and, together with these data, discussed in view of sun exposure, melanoma generation and vitamin D photosynthesis. A protective role of vitamin D may apply for melanomasCitation15,Citation29 as well as for internal cancers.Citation32–Citation37
Results
shows a comparison of epidemiological data of CMM and uveal melanoma in Norway. Unfortunately, we have data for uveal melanoma only from 1993. The incidence rates of CMM for both sexes together, all ages included, have been constant. However, the rates for persons younger than 50 years have decreased in the same period. The rates for uveal melanomas have increased significantly. For CMM there is a strong north-south gradient, while for uveal melanoma there is no such gradient.
The ratio of uveal melanoma cases to CMM cases has increased somewhat for both men and women in the same period ().
For Sweden data for uveal melanoma are available for a longer time, and the time trends from 1960 to 1998 are shown in (A for males and B for females). Until about 1990 the CMM rates increased sharply, as in Norway,Citation29 while the uveal melanoma rates decreased in the same period. Thus, the ratio of uveal melanoma rates to CMM rates decreased strongly (). After 1990, however, the rates of CMM and those of uveal melanoma have been almost constant for both sexes.
The incidence rates of CMM are about three times higher in Australia and New Zealand than in Scandinavia and in The British Isles, while those of uveal melanomas are not much different (). For these countries the ratio of incidence rates of uveal melanomas to CMM are strongly decreasing with increasing CMM rates (). This is true both when UK, Ireland, Scotland and Scandinavia are considered alone, and when also Australia and New Zealand are brought into the picture (). When those European countries, for which relevant data are available, are included, inverse latitudinal gradients are found, both for CMM and for uveal melanoma ( and B). However, the ratio of the rates of uveal melanoma to CMM increases with increasing CMM rates ().
Discussion
Time trends.
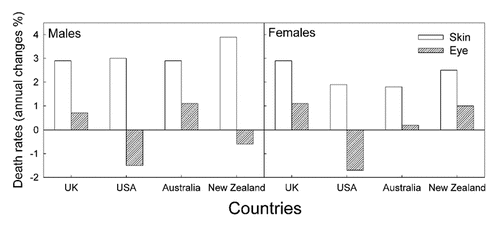
This is, to our knowledge, the first time uveal melanoma trends are analyzed for a population and a period in which CMM rates tend to decrease with time. Surprisingly, an increase in uveal melanoma rates is found. The opposite is observed for populations and periods of increasing CMM incidence trends. This is exemplified by the data for Sweden, where, until 1990, CMM incidence rates increased rapidly, with a doubling time of about 15–20 years,Citation15 as in practically all countries where such investigations have been carried out.Citation1,Citation15 Such increasing trends of CMM rates have been observed for a long time (), and is generally explained by increasing sun exposure, notably in vacations and holydays (intermittent exposures). The rates of conjunctival melanomas in the US have increased in the period 1973–1999, as have those of CMM,Citation40 while the rates of uveal melanomas have remained stable.Citation41 The conjunctiva can be reached even by UVB radiation. For about the same time period even decreasing trends of uveal melanoma rates have been reported for Sweden and some other countries (, reviewed in refs. Citation1, Citation38, Citation39 and Citation42). As will be discussed below, in connection with latitude trends, a possibility exists that the decreasing time trends of uveal melanomas for periods of increasing time trends of CMM can be explained by a protective role of solar radiation on uveal melanomas. This might take place via vitamin D photosynthesis:Citation43 In time periods when CMM rates have been increasing, such as in Sweden before 1990 () and in many other countries with Caucasian populations,Citation1–Citation3,Citation14 the sun exposure of the people have supposedly been increasing, leading to increasing vitamin D synthesis in skin, the uveal rates have generally decreased.Citation1 Exactly opposite trends are found for Norway after 1990 (). These data support the assumption of a protective role of solar radiation in relation for uveal melanomas. According to the CMM rates the sun exposure has increased in Sweden from 1960 to 1998 (). This agrees with the fact that also the incidence of cataract, which is caused by sun exposure also increased in Sweden from 1992 to 2000.Citation44
Latitude trends.
The Norwegian data show a strong north-south gradient of CMM rates (, reviewed in refs. Citation2 and Citation12). No such gradient is found for uveal melanoma (). In the US, as in Norway, the CMM rates increase with decreasing latitude.Citation2,Citation3 The uveal melanoma rates in the US show a significant opposite latitudinal trend,Citation10 even if Hawaii is omitted from the picture. This is interpreted as an indication of a protective role of solar radiation.Citation10 As for Norway, there is no significant latitudinal gradient for uveal melanomas when The British Isles, Scandinavia, Australia and New Zealand are considered (). Clearly, the higher the CMM rates are, the lower are the ratios of the rates of uveal melanoma to CMM (). The same is true for Norway (), and it seems that in these populations solar radiation is generating CMM, but not uveal melanomas. However, in these countries no protective role, as in the US, is indicated. The discrepancy of these data may be related to a possible genetical north south gradient of susceptibility to uveal melanoma, either in the US or in the countries included in and . Such gradients should certainly be taken into account: In Europe CMM rates are higher in the north (Scandinavia) than in the south (). This is likely to be caused by differences in pigmentation. However, even when considering these countries with increasing CMM rates from south to north, the ratio of the incidence rates of uveal melanomas to those of CMM appears to increase with increasing rates of CMM incidence, i.e., with increasing latitude and decreasing annual UV exposure ( and C). This may be related to the observation that blue eyes, which are more prevalent in the north, are risk factors for ocular melanoma.Citation18,Citation45–Citation47 The fact that the relative risk of uveal melanomas can be as much as 19 times smaller for black persons than for white persons in the US,Citation18 probably indicates that melanin itself acts as a protectant.Citation38
The role of solar radiation and vitamin D in uveal melanoma etiology.
UV is likely to be the main carcinogen for CMM, as has been discussed by many of the authors cited above, although some controversies still exist.Citation48 Both UVB and UVA may play roles. The uveal tract, however, cannot be reached by UVB,Citation9 and, since most uveal melanomas arise in the choroid layer,Citation49 to which very little UVA can penetrate,Citation9 solar radiation is unlikely to be significantly involved in the etiology of uveal melanomas. This conclusion is strengthened by the observation that melanomas are evenly distributed on the choroid, while this is not true for solar radiation.Citation17 The time trends discussed above, and partly also the latitudinal trends, suggest that solar radiation is a main carcinogen for CMM, (possibly also for the rare, conjunctival melanomasCitation18 whose changing incidence patterns coincide with those of CMM in the USCitation40), but may act as a protectant against uveal melanomas, possibly through its generation of vitamin D:Citation10 The time trends for uveal melanomas and for CMM are generally opposite. How can we then explain why there in some countries are no latitudinal gradients of uveal melanomas? Two factors should be considered in future investigations of this: Intermittent versus constant UV exposure, and latitudinal gradients of constitutive melanin (genetically determined). Firstly, CMM is more closely related to intermittent UV exposure than to total exposure,Citation1,Citation40,Citation43,Citation48–Citation50 while the vitamin D status is likely to be dependent on total exposure. The genetic impact on CMM rates among Caucasians is demonstrated by , which shows that the incidence rates of CMM decrease with decreasing latitude in Europe. The melanin pigmentation generally increases from north to south in Europe,Citation51 leading to decreasing rates of CMM and of vitamin D photosynthesis.Citation52 Unfortunately, no reliable comparisons of the vitamin D status in north and south Europe are available. The rates of uveal melanomas tend to decrease from north to south in Europe (in contrast to what is found in the US), and so does the ratio of the incidence rates for uveal melanomas to those of CMM (). It has been proposed that melanin by itself has a protective effect against uveal melanomas,Citation53 which may contribute to explain the European data shown in the . A protective role of solar radiation on CMM, in addition to an inductive role as discussed above, is indicated by the following observations: (1) The prognosis of CMM is best for summer-autumn diagnosis.Citation15 (2) The prognosis of CMM appears to be best for tumours arising on skin areas with signs of high UV exposure.Citation54 (3) The CMM incidence rates in the US have increased rapidly in the period 1992–2004, with a doubling time as short as 10 years, whereas the mortality rates have not increased significantly,Citation55 and it was concluded that screening associated diagnosis of thinner melanomas could not explain the increasing incidence rates. A decline of the latitudinal effect of CMM mortality rates and a stabilization of the rates in the US was predicted already in 1997.Citation56 Even earlier it was shown that outdoor work does little to increase the CMM risk.Citation45,Citation57,Citation58 A meta-analysis of the relation between intermittent and chronic UV exposure and uveal melanoma indicates a slightly protective role of outdoor and leisure life.Citation59
Limitations of the work.
First of all uveal melanomas are rare, so in many cases it is not possible to give good 95% confidence intervals. Secondly, the induction time may be different for different types of melanoma. Lentigo maligna melanoma, for instance, occurs late in life and is probably related to accumulated UV dose, in contrast to CMM, which, as stated above, seems to be related more to intermittent exposures. Thirdly, as also stated above, melanin, notably eumelanin, may act as a protectant by itself, i.e., through chemical protection and not only through UV absorption. The north south gradient of CMM in Europe may be related to such protection.
Conclusions.
Comparisons of time trends and latitudinal trends of the incidence rates of CMM and uveal melanoma among Caucasians indicate that solar radiation is likely to act ac a carcinogen for CMM but not for uveal melanoma. For the latter melanoma type, solar radiation may even seem to act in a protective manner: Time trends, as well as latitudinal trends, of CMM and uveal melanoma are generally opposite in agreement with this hypothesis.
The situation is complicated in the central and northern European countries where there is an increase in CMM rates from south to north and a slightly decreasing trend of uveal melanoma. This may be related to an increase in pigmentation, possibly also to an increace in the eumelanin/pheomelanin ratio, from north to south in Europe.
Materials and Methods
In our study we used data from various sources. The incidence data for CMM and uveal melanoma in Norway are obtained from The National Cancer Registry, a population-based registry that since 1953 collects data on cancer incidence and survival.Citation60 In the case of uveal melanoma data are available only after 1993 when this localization was coded separately. The data for uveal melanoma in Sweden have been extracted from the work of Bergman et al.Citation61 while for the European plot the data are from the publication by Virgili et al.Citation38 The annual percent changes of CMM and uveal melanomas death rates are taken from references.Citation1,Citation39 The other epidemiological data used in this work are retrieved from the online data base of the International Agency for Research on Cancer (IARC).Citation62
We performed two main types of analyses: time trends for the two cancer types studied and the correlation between the occurrence of uveal melanoma and the incidence of CMM. Where it was possible we have stratified our data by age (two groups, younger and older than 50 years) and by gender since both of these factors affect the incidence rates of melanomas.
Abbreviations
| CMM | = | cutaneous malignant melanoma (CMM) |
| UV | = | ultraviolet |
| UVA | = | ultraviolet A |
| UVB | = | ultraviolet B |
Figures and Tables
Figure 1 Time trends of the incidence rates (IR) of CMM and uveal melanoma in Norway for the period 1993–2004. For CMM over all data (men and women, age adjusted. Rates for persons below 50 years (all country) are shown separately).
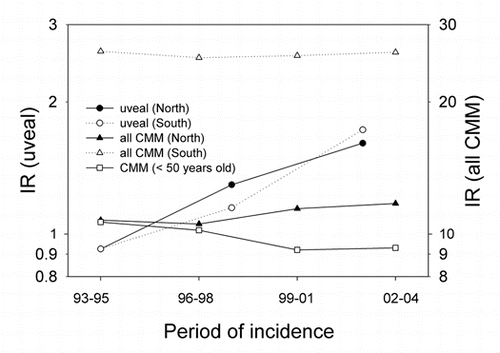
Figure 2 The ratio of the number of uveal melanomas to that of CMM in Norway for three periods. Data shown separately for women and men.
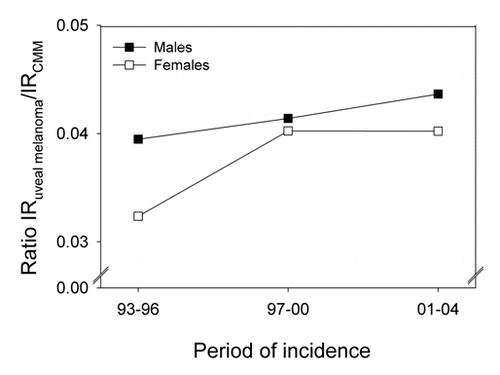
Figure 3 The age adjusted incidence rates (IR) of CMM (per 10,000) and uveal melanoma (per 10,00,000) in Sweden, (A) for men, (B) for women. The ratio of the incidence rates of uveal melanoma to CMM is shown at the bottom of the panels in open triangles.
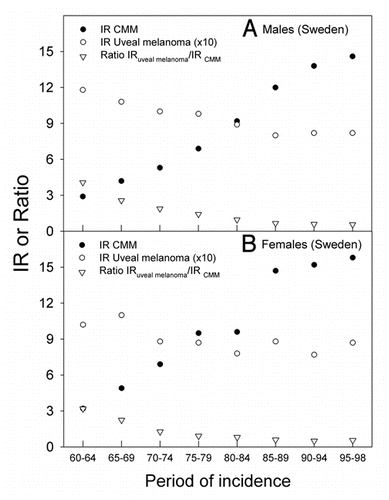
Figure 4 Age adjusted incidence rates (IR) of uveal melanomas as a function of the incidence rates of CMM (A men, B women) for some countries selected on the basis of our earlier CMM work.Citation12
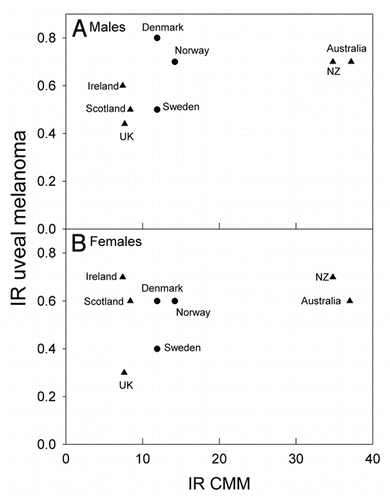
Figure 5 The ratio of the incidence rates (IR) of uveal melanoma to CMM as a function of the incidence rates of CMM for the same countries as those included in .
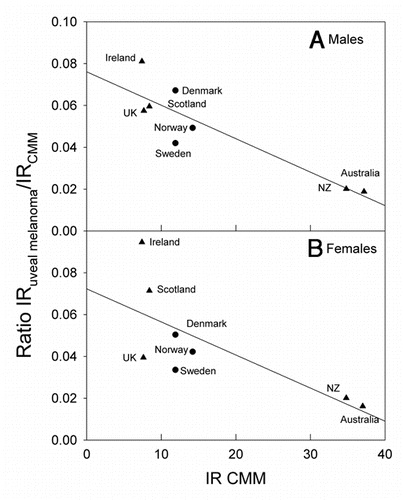
Figure 6 Incidence rates (IR) of CMM per 1,00,000 (A), uveal melanoma per 10,00,000 (B) and the ratio (C) as functions of latitude. Melanoma data were extracted from the work of Virgili et al.Citation38 Data refer to the period 1983–1994 and are from Ragusa (37°C), Tarragona (41°C), Navarra (43°C), Parma (45°C), Geneva (46°C), Slovenia (46°C), Bas Rhin (48°C), Saarland (49°C), CalvadosGen (49°C), Slovakia (49°C), Cracow (50°C), Eindhoven (51°C), West Midlands (53°C), Mersey (53°C), Yorkshire (54°C), Scotland (56°C), Denmark (56°C), Estonia (59°C), Sweden (62°C), Norway (62°C), Finland (64°C) and Iceland (65°C).
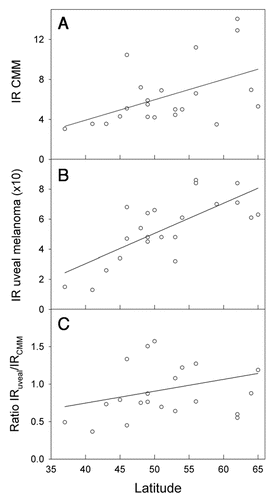
Figure 7 The annual percent changes of CMM and uveal melanomas death rates as taken from references.Citation1,Citation39 Average values for the time period from 1951 to 1978.
Acknowledgements
The present work was supported by the Research Council of Norway (Norges forskningsråd) and the Norwegian Cancer Society (Kreftforeningen).
References
- Lee JA. Melanoma and exposure to sunlight. Epidemiol Rev 1982; 4:110 - 136
- Moan J, Dahlback A. Young AR, Young A, Bjorn LO, Moan J, Nultsch W. Ultraviolet Radiation and Skin Cancer: Epidemiological Data from Scandinavia. Environmental UV Photobiology 1993; New York Plenum Press 255 - 293
- Weinstock MA. Young AR, Young A, Bjorn LO, Moan J, Nultsch W. Ultraviolet Radiation and Skin Cancer: Epidemiological Data from the United States and Canada. Environmental UV Photobiology 1993; New York Plenum Press 295 - 344
- Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer 1997; 73:198 - 203
- Gandini S, Sera F, Cattaruzza MS, Pasquini P, Picconi O, Boyle P, Melchi CF. Meta-analysis of risk factors for cutaneous melanoma: II. Sun exposure. Eur J Cancer 2005; 41:45 - 60
- Pleasance ED, Cheetham RK, Stephens PJ, McBride DJ, Humphray SJ, Greenman CD, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 2010; 463:191 - 196
- Situm M, Buljan M, Bulic SO, Simic D. The mechanisms of UV radiation in the development of malignant melanoma. Coll Antropol 2007; 31:13 - 16
- Egan KM, Seddon JM, Glynn RJ, Gragoudas ES, Albert DM. Epidemiologic aspects of uveal melanoma. Surv Ophthalmol 1988; 32:239 - 251
- World Health Organization. The effects of solar UV radiation on the eye 1994; Geneva World Health Organization 1 - 56
- Yu GP, Hu DN, McCormick SA. Latitude and incidence of ocular melanoma. Photochem Photobiol 2006; 82:1621 - 1626
- Setlow RB, Grist E, Thompson K, Woodhead AD. Wavelengths effective in induction of malignant melanoma. Proc Natl Acad Sci USA 1993; 90:6666 - 6670
- Moan J, Dahlback A, Setlow RB. Epidemiological support for an hypothesis for melanoma induction indicating a role for UVA radiation. Photochem Photobiol 1999; 70:243 - 247
- Wang SQ, Setlow R, Berwick M, Polsky D, Marghoob AA, Kopf AW, Bart RS. Ultraviolet A and melanoma: a review. J Am Acad Dermatol 2001; 44:837 - 846
- Muir CS. Magnus K. Malignant melanoma of skin. Trends in Cancer Incidence 1982; Washington New York London Hemisphere Publishing Corporation 363 - 385
- Moan J, Porojnicu AC, Dahlback A. Ringborg U, Brandberg Y, Breitbart EW, Greinert R. Epidemiology of cutaneous malignant melanoma. Skin cancer prevention 2007; New York Informa Healthcare 179 - 201
- Moan J, Porojnicu AC, Dahlback A. Ultraviolet radiation and malignant melanoma. Adv Exp Med Biol 2008; 624:104 - 116
- Schwartz LH, Ferrand R, Boelle PY, Maylin C, D'Hermies F, Virmont J. Lack of correlation between the location of choroidal melanoma and ultraviolet-radiation dose distribution. Radiat Res 1997; 147:451 - 456
- Hu DN. Photobiology of ocular melanocytes and melanoma. Photochem Photobiol 2005; 81:506 - 509
- Tucker MA, Shields JA, Hartge P, Augsburger J, Hoover RN, Fraumeni JF Jr. Sunlight exposure as risk factor for intraocular malignant melanoma. N Engl J Med 1985; 313:789 - 792
- Swerdlow AJ, Storm HH, Sasieni PD. Risks of second primary malignancy in patients with cutaneous and ocular melanoma in Denmark 1943–1989. Int J Cancer 1995; 61:773 - 779
- Graham S, Marshall J, Haughey B, Stoll H, Zielezny M, Brasure J, West D. An inquiry into the epidemiology of melanoma. Am J Epidemiol 1985; 122:606 - 619
- Dolin PJ, Johnson GJ. Solar ultraviolet radiation and ocular disease: a review of the epidemiological and experimental evidence. Ophthalmic Epidemiol 1994; 1:155 - 164
- Dolin PJ, Foss AJ, Hungerford JL. Uveal melanoma: is solar ultraviolet radiation a risk factor?. Ophthalmic Epidemiol 1994; 1:27 - 30
- Seddon JM, Gragoudas ES, Glynn RJ, Egan KM, Albert DM, Blitzer PH. Host factors, UV radiation and risk of uveal melanoma. A case-control study. Arch Ophthalmol 1990; 108:1274 - 1280
- Vajdic CM, Kricker A, Giblin M, McKenzie J, Aitken J, Giles GG, Armstrong BK. Sun exposure predicts risk of ocular melanoma in Australia. Int J Cancer 2002; 101:175 - 182
- Boettner EA, Walter JR. Transmission of the ocular media. Invest Ophthalmol Vis Sci 1962; 1:776 - 783
- Li W, Judge H, Gragoudas ES, Seddon JM, Egan KM. Patterns of tumor initiation in choroidal melanoma. Cancer Res 2000; 60:3757 - 3760
- Horn EP, Hartge P, Shields JA, Tucker MA. Sunlight and risk of uveal melanoma. J Natl Cancer Inst 1994; 86:1476 - 1478
- Moan J, Porojnicu AC, Dahlback A, Setlow RB. Addressing the health benefits and risks, involving vitamin D or skin cancer, of increased sun exposure. Proc Natl Acad Sci USA 2008; 105:668 - 673
- Gaudette LA, Gao RN. Changing trends in melanoma incidence and mortality. Health Rep 1998; 10:29 - 41
- Lens MB, Dawes M. Global perspectives of contemporary epidemiological trends of cutaneous malignant melanoma. Br J Dermatol 2004; 150:179 - 185
- Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast-, colon- and prostate cancer (Norway). Cancer Causes Control 2004; 15:149 - 158
- Moan J, Porojnicu A, Lagunova Z, Berg JP, Dahlback A. Colon cancer: prognosis for different latitudes, age groups and seasons in Norway. J Photochem Photobiol B 2007; 89:148 - 155
- Porojnicu AC, Lagunova Z, Robsahm TE, Berg JP, Dahlback A, Moan J. Changes in risk of death from breast cancer with season and latitude: sun exposure and breast cancer survival in Norway. Breast Cancer Res Treat 2007; 102:323 - 328
- Porojnicu AC, Dahlback A, Moan J. Sun exposure and cancer survival in Norway: changes in the risk of death with season of diagnosis and latitude. Adv Exp Med Biol 2008; 624:43 - 54
- Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer 2007; 7:684 - 700
- Giovannucci E. Vitamin D status and cancer incidence and mortality. Adv Exp Med Biol 2008; 624:31 - 42
- Virgili G, Gatta G, Ciccolallo L, Capocaccia R, Biggeri A, Crocetti E, et al. Incidence of uveal melanoma in Europe. Ophthalmology 2007; 114:2309 - 2315
- Strickland D, Lee JA. Melanomas of eye: stability of rates. Am J Epidemiol 1981; 113:700 - 702
- Yu GP, Hu DN, McCormick S, Finger PT. Conjunctival melanoma: is it increasing in the United States?. Am J Ophthalmol 2003; 135:800 - 806
- Singh AD, Topham A. Incidence of uveal melanoma in the United States: 1973–1997. Ophthalmology 2003; 110:956 - 961
- Stang A, Parkin DM, Ferlay J, Jockel KH. International uveal melanoma incidence trends in view of a decreasing proportion of morphological verification. Int J Cancer 2005; 114:114 - 123
- Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relevant to malignant melanoma?. Br J Dermatol 2002; 147:197 - 213
- Lundstrom M, Stenevi U, Thorburn W. The Swedish National Cataract Register: A 9-year review. Acta Ophthalmol Scand 2002; 80:248 - 257
- Gallagher RP, Elwood JM, Rootman J, Spinelli JJ, Hill GB, Threlfall WJ, Birdsell JM. Risk factors for ocular melanoma: Western Canada Melanoma Study. J Natl Cancer Inst 1985; 74:775 - 778
- Holly EA, Aston DA, Char DH, Kristiansen JJ, Ahn DK. Uveal melanoma in relation to ultraviolet light exposure and host factors. Cancer Res 1990; 50:5773 - 5777
- Hu DN, Yu GP, McCormick SA, Schneider S, Finger PT. Population-based incidence of uveal melanoma in various races and ethnic groups. Am J Ophthalmol 2005; 140:612 - 617
- Shuster S. Is sun exposure a major cause of melanoma?. No. BMJ 2008; 337:764
- Grin JM, Grant-Kels JM, Grin CM, Berke A, Kels BD. Ocular melanomas and melanocytic lesions of the eye. J Am Acad Dermatol 1998; 38:716 - 730
- Oliveria SA, Saraiya M, Geller AC, Heneghan MK, Jorgensen C. Sun exposure and risk of melanoma. Arch Dis Child 2006; 91:131 - 138
- Jablonski NG, Chaplin G. The evolution of human skin coloration. J Hum Evol 2000; 39:57 - 106
- Zittermann A, Schleithoff SS, Koerfer R. Putting cardiovascular disease and vitamin D insufficiency into perspective. Br J Nutr 2005; 94:483 - 492
- Hu DN, Simon JD, Sarna T. Role of ocular melanin in ophthalmic physiology and pathology. Photochem Photobiol 2008; 84:639 - 644
- Berwick M, Lachiewicz A, Pestak C, Thomas N. Solar UV exposure and mortality from skin tumors. Adv Exp Med Biol 2008; 624:117 - 124
- Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing Burden of Melanoma in the United States. J Invest Dermatol 2009;
- Lee JA. Declining effect of latitude on melanoma mortality rates in the United States. A preliminary study. Am J Epidemiol 1997; 146:413 - 417
- Goodman KJ, Bible ML, London S, Mack TM. Proportional melanoma incidence and occupation among white males in Los Angeles County (California, United States). Cancer Causes Control 1995; 6:451 - 459
- Armstrong BK. Epidemiology of malignant melanoma: intermittent or total accumulated exposure to the sun?. J Dermatol Surg Oncol 1988; 14:835 - 849
- Shah CP, Weis E, Lajous M, Shields JA, Shields CL. Intermittent and chronic ultraviolet light exposure and uveal melanoma: a meta-analysis. Ophthalmology 2005; 112:1599 - 1607
- Moller B, Aagnes B. Bray F. Cancer in Norway 2005. Cancer incidence, mortality, survival and prevalence in Norway 2006; Oslo, Norway Cancer Registry of Norway
- Bergman L, Seregard S, Nilsson B, Ringborg U, Lundell G, Ragnarsson-Olding B. Incidence of uveal melanoma in Sweden from 1960 to 1998. Invest Ophthalmol Vis Sci 2002; 43:2579 - 2583
- The International Agency for Research on Cancer (IARC). Cancer Incidence in Five Continents Volumes I to IX 2010; January 2010 http://ci5.iarc.fr/CI5i-ix/ci5i-ix.htm