Abstract
Since exposure to sunlight is a main factor in the development of non-melanoma skin cancer and there are associations between malignant melanoma and short-term intense ultraviolet (UV) exposure, particularly burning in childhood, strict protection from UV-radiation is recommended. However, up to 90% of all requisite vitamin-D has to be formed within the skin through the action of the sun - a serious problem, for a connection between vitamin-D deficiency, demonstrated in epidemiological studies, and various types of cancer and other diseases has been confirmed. A UVB-triggered skin autonomous vitamin D3 synthesis pathway has recently been described, producing the active Vitamin D metabolite calcitriol. This cutaneous vitamin D3 pathway is unique. Keratinocytes and dendritic cells can convert vitamin D to calcitriol. Cutaneous T cells activated in the presence of calcitriol express the chemokine receptor CCR10 attracting them to the chemokine CCL27 that keratinocytes express selectively in the epidermis, and migrate from dermal layers of the skin to the epidermis under UV radiation. Thus, calcitriol has endocrine roles beyond its calciotropic action, including cell growth and cancer prevention. Therefore, strict sun protection procedures to prevent skin cancer may induce the risk of vitamin-D deficiency. As there is evidence that the protective effect of less intense solar radiation can outweigh its mutagenic effect, better balanced approaches to sun protection should be sought.
UV Filters and Endocrine Disruptors
Endocrine disruptors (EDC) (hormonally active agents) are exogenous substances or mixtures that alter functions of the endocrine system and consequently cause adverse health effects in an intact organism or its progeny (International Programme on Chemical Safety, IPCS). A recent scientific statement by the American Endocrine SocietyCitation1 describes EDC's as a possible health threat: Present in our environment, food and consumer products, EDC's are competent to affect male and female reproduction, brain development and functions, breast development and cancer, prostate cancer, thyroid, metabolism (metabolic syndrome) and cardiovascular system. Convergent results from animal models, from human clinical observations and epidemiological studies identify EDC's as a significant concern to public health.
EDC actions are most deleterious during early developmental periods by disrupting hormone enforced organizational events. Most vulnerable life stages to EDC therefore are the prenatal and early postnatal periods when organ systems are most sensitive. Permanent effects might occur later in life. EDC's may act at concentrations far below threshold of conventional toxicity. In conventional toxicity tests these chemicals remained largely unnoticed, because of complex dose-response curves that involve cellular mechanism originating from multiple signalling pathways. EDC effects may not only impact the exposed individual but may be transmitted to subsequent generations via epigenetic modifications.Citation2
The range of molecules exhibiting EDC—characteristics is broad comprising organochloride pesticides, industrial chemical, plastics and plasticizers, cosmetic ingredients and many more chemicals in widespread use. We identified some of the ultraviolet UV filters used in sun protection (sunscreens) and in diverse cosmetic products as endocrinologically active.Citation3 Two of these substances were identified as reprotoxicants delaying male puberty, causing changes in reproductive organ weights, estrous cycle, female sexual behavior and of estrogen target genes in brain and reproductive organs.Citation4
How Much of Sun Screens do We Really Need?
Assessment of chemical risk requires information on quality and quantity of chemical impact. In a monitoring study on human breast milk we found 6 out of 8 analyzed UV filters in mother/child cohorts from 3 different years. Use of sunscreens or other cosmetic products containing these UV filters was significantly correlated with their presence in human milk. UV filters as cosmetic ingredients in make ups, body lotions, face creams, lip sticks or bubble baths were found to be a relevant source of exposure.Citation4 The link between product use and internal exposure distinguishes UV filters from other EDC's present in human milk such as PCB's, organochlor pesticides and phthalates, other environmental chemicals, where exposure is more general. This makes it possible to reduce exposure during critical vulnerable periods such as pregnancy and nursing. This might protect the infant from additional and unnecessary chemical burden in breast milk. In this context, it becomes important to discuss the optimal amount and method of protection against UV light.
Sun protection and sun exposure should be carefully balanced as UV radiation is a pre-requisite to produce sufficient vitamin D in our skin and body. How much of sun screens do we really need?
Sun Exposure and Skin Cancer
According to Jörg Reichrath there is no doubt that exposure to sunlight is the main reason for the development of non-melanoma skin cancer. In addition, various reports analysing sun exposure parameters have consistently demonstrated an association between the development of malignant melanoma and short-term intense UV-radiation exposure, particularly burning in childhood.Citation5
In consequence, strict protection recommendations from solar and artificial UV-radiation represent a fundamental part of prevention programmes aimed at reducing UV-radiation-induced skin damage and skin cancer. Today, sun protection recommendations include use of broad spectrum protection sunscreens, protective clothing and avoidance of sunlight. Most sunscreen products combine chemical UV-radiation-absorbing sunscreens and physical anorganic sunscreens, which reflect UV-radiation, to provide broad spectrum protection.
One has to keep in mind that up to 90% of all requisite vitamin D has to be formed within the skin through the action of the sun—a serious problem, for a connection between vitamin D deficiency and various types of cancer (e.g., colon-, prostate- and breast cancer) and other diseases (including bone diseases, cardio-vascular diseases, infectious diseases) has now been confirmed in a large number of studies.Citation6–Citation10
An Open Debate among Dermatologists
As a consequence, this association between vitamin D deficiency and various diseases including internal malignancies has now opened a debate among dermatologists, whether UV-radiation protection recommendations to prevent skin cancer should be moderated. What is the rationale that vitamin D deficiency may be associated with an increased risk of various diseases including certain types of cancer? The idea that sunlight and vitamin D inhibit the growth of human cancers is not new (reviewed in ref. Citation10). Based on an apparent deficit of non-skin cancer among US Navy personnel, who experienced an excess of skin cancer, Peller concluded in 1936 that skin cancers induce a relative immunity to other types of cancer. In consequence, he advocated the deliberate induction of skin cancers, which were easily to detect and to treat, as a form of vaccination against more life-threatening and less treatable cancers. Over the 20th century the field of vitamin D and cancer has changed considerably. By now a negative association has been confirmedCitation6 between increased risk of dying of various internal malignancies (e.g., breast, colon, prostate and ovarian cancer) and decreasing latitude towards the equator (increased sunlight levels). Additionally, a correlation of this latitudinal association with decreased vitamin D serum levels was demonstrated.Citation7 Black men, who have an increased risk to develop vitamin D deficiency, have also an increased risk of prostate cancer and develop a more aggressive form of the disease. In conclusion, the evolution of our understanding of the role of vitamin D in cancer parallels our understanding of the importance of vitamin D for rickets. In both diseases, epidemiologic observations about consequences of sun exposure preceeded experimental observations and were subsequently validated by them. Apperly's insightful observations on sunlight exposure and cancer, like those of Theobold Palm on the protective effects of UV-radiation on rickets half a century earlier, passed virtually unnoticed by his contemporaries, only to be re-discovered by epidemiologists decades later.
Specifics of the Cutaneous Vitamin D3 Pathway
Bodo Lehman describes a skin autonomous vitamin D3 synthesis pathway.
Vitamin D3, also known as cholecalciferol or calciol, is produced from cutaneous 7-dehydrocholesterol (7-DHC, provitamin D3) by an UVB-triggered photochemical reaction.Citation11 The production of vitamin D3 in the skin strongly depends on the wavelength, the dose of the UVB and most probably the ratio of UVB (wavelength range: 280–320 nm) to UVA (wavelength range: 320–380 nm) in the solar spectrum.Citation12 The efficacy of UVB radiation on the vitamin D3 synthesis dramatically decreases by 80% within a very small wavelength range from 300 nm to 310 nm. A very low conversion rate of only 0.6% has been found at 320 nm.Citation12 These different conversion rates are most probably attributed to the fact that four photo-reversible reactions and one non-reversible phototransformation with overall nine different action spectra are involved in the synthesis of previtamin D3, which is the direct precursor of vitamin D3. The epidermal milieu including lipid layers, multibilayers, skin pigment and the concentration of 7-DHC is most probably responsible for selectivity, efficiency but also interindividual variations of the previtamin D3 photosynthesis in human skin. Vitamin D3 should be better described as an biologically inert prohormone which is then activated to the hormone 1α, 25-dihydroxyvitamin D3 (calcitriol) by two subsequent hydroxylations in the liver and kidney.Citation13–Citation15 It is interesting that enzymatic formation of calcitriol also occurs in many non-renal tissues including prostate, breast, intestine, lung and other tissues.Citation16–Citation18 Calcitriol contributes not only to the maintenance of the calcium and phosphate homeostasis but also acts as a regulator of various biological processes including cell growth, differentiation, apoptosis, cancer prevention, immunological processes, defense, photoprotection and of other cell functions.Citation19–Citation21 Genomic effects of calcitriol are mediated by the vitamin D receptor (VDR). By contrast, rapid non-genomic effects are mediated by a putative membrane associated vitamin D receptor (mVDR).
Previously, Bodo Lehmann's group demonstrated that UVB irradiation of cultured neonatal keratinocytes supplemented with 7-DHC at about 300 nmCitation22–Citation25 and of human skin in vivoCitation25 activates the metabolism of 7-dehydrocholesterol to hormonally active calcitriol. The enzymatic conversion of vitamin D3 to calcitriol in keratinocytes is mainly catalyzed by the mixed function oxidases CYP27A1 and CYP27B1.Citation12–Citation14 The synthesis rate of calcitriol is positively correlated to the substrate concentration of vitamin D3.Citation22 In addition, the activity of both anabolic and catabolic vitamin D hydroxylases determines the concentration of calcitriol in keratinocytes.Citation26 Calcitriol regulates a number of genes in keratinocytes, and can act in an autocrine and/or paracrine manner.Citation27
Recently, it has been demonstrated that epidermal keratinocytes and dermal fibroblasts are metabolically different with regard to the hydroxylation of vitamin D3.Citation28 Calcitriol is derived from keratinocytes which have the complete enzyme machinery to produce and degrade this metabolite. By contrast, fibroblasts can only produce the direct precursor of calcitriol, 25-hydroxyvitamin D3 (calcidiol, 25OHD3). Calcidiol from fibroblasts can diffuse to basal keratinocytes and to the blood circulation. This dermal—epidermal substrate transfer of calcidiol may result in higher synthesis rate of calcitriol in keratinocytes compared to epidermal keratinocytes alone.Citation28
This cutaneous vitamin D3 pathway is unique, but its relevance for healthy and diseased skin is widely unknown as of yet. On the other hand, it is well known that both calcitriol and UVB radiation exert potent antipsoriatic and other beneficial effects in human skin.Citation29 We hypothesize from our findings that the antipsoriatic effect of UVB radiation is attributed at least in part to UVB-triggered cutaneous synthesis of calcitriol.
It is worth mentioning that photosynthesized vitamin D3 can also be sequentially hydroxylated by the CYP11A1 to 20,22-dihydroxyvitamin D3 and other unknown trihydroxylated vitamin D3 metabolites in cultured keratinocytes.Citation30 The pathophysiological role of this pathway is unknown yet.
It has been known for a long time that formation of singlet oxygen is induced by UVA radiation within the skin, and this reactive oxygen species is seemingly involved in cell damaging and tumor promoting action of UVA on skin.Citation31 Of note, vitamin D3 is photo-oxidized by singlet oxygen to 6R- and 6S-epidioxy-vitamin D3 in vitro. In particular the 6R-epidioxy-vitamin D3 develops significant biological activity in rats.Citation32 The physiological role of these endoperoxides for humans remains to be ascertained.
Physiological and pathophysiological roles of vitamin D3 metabolites produced by enzymatic and photochemical reactions in the skin are widely unknown and need to be clarified. It can be postulated from these findings that biological effects of UV radiation on human skin are partly and indirectly mediated by biologically active vitamin D3 metabolites.
Vitamin D, Dendritic Cells and T Cells in Skin
T-cell mediated skin diseases and skin problems affect millions of people every day. Resulting skin lesions can be difficult to treat and it is important to develop local and more targeted treatments. Recent data on the function of microenvironmental factors may help develop such therapies. T cells and their recirculation are important for immune surveillance and in response to infections. The infiltration of T cells into lymphoid tissues and inflammatory sites is a multistep process that is tightly regulated by the expression of adhesion molecules, chemokine receptors and the interaction between the T cells and vascular endothelium.Citation33 To target T cells to different tissues, the immune system takes cues from various sources, including vitamins from food and sunlight. Dendritic cells (DCs) are important in this process. They use signals in their local environment to induce the expression of tissue-specific molecules on the T cells. It is known that DCs in lymph nodes draining the skin preferentially induce the expression of skin-homing receptors on T cells, while dendritic cells in lymph nodes draining the gut induce the expression of gut-homing receptors.Citation34 Although receptors and molecules involved in lymphocyte trafficking have been studied extensively, it is not clear what induces or regulates the expression of these tissue-specific molecules. How is this specificity determined? Vitamins A and D and their metabolites are known to affect both innate and adaptive immune responses, and have been shown to suppress inflammation and lymphocyte function, and they can induce the generation of regulatory cells in the skin. Recently, they have also been shown to affect the expression of receptors involved in trafficking of T cells into the gut and the skin.
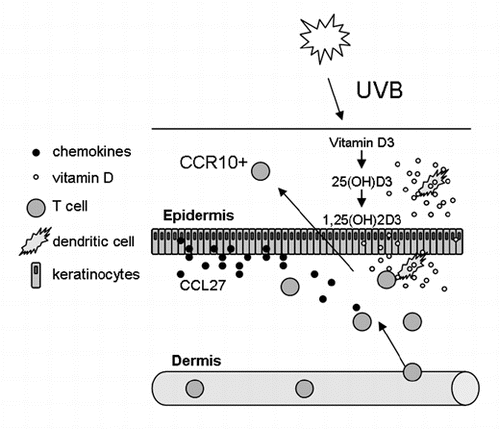
Vitamin A can be metabolized by DCs and has been shown to induce the expression of gut-specific receptors on T cells and promote their migration to the gut.Citation35 A similar mechanism also applies for skin-associated vitamins, namely vitamin D. During UVB exposure from the sun, a signal can be generated through local formation of vitamin D3, which imprints T cells with skin-specific receptors. Vitamin D3 is relatively inactive and is mainly metabolized in the liver and kidneys. However, DCs have the enzymes needed to convert inactive vitamin D to its most active metabolite, 1,25(OH)2D3.Citation36 When T cells were activated in the presence of 1,25(OH)2D3, the expression of the chemokine receptor CCR10 was induced on the T cells. This receptor attracts T cells to the chemokine CCL27, which is selectively expressed by keratinocytes of the epidermis () and mobilizes the T cells within the skin by promoting migration from dermal layers of the skin to the epidermis.Citation37 At the same time, the expression of gut-specific receptors was decreased on the T cells.Citation36 Thus, UVB-induced increase of skin-mobilizing T cells could be a way of enhancing T-cell immune responses in the skin when needed. These could be both effector T cells as well as regulatory T cells.Citation38 The effective concentrations of vitamin D3 for inducing skin specificity on T cells can be found in skin, thus, this mechanism may be occurring locally in the skin. In contrast, the dietary form of vitamin D was not as effective as the sunlight-induced form at inducing CCR10 expression.Citation36 Other factors that are present in the skin may also have effects on trafficking receptors associated with migration in the skin. They may be acting locally in skin, and/or possibly taken up by dendritic cells that transfer them to the draining lymph nodes, where they are present during T-cell activation. Several such factors are under investigation. Combinatorial treatment where the presence or absence of such factors is manipulated may prove to be a beneficial therapeutic approach to develop targeted local treatments of skin diseases.
1,25(OH)2D is not Exclusively Considered as a Calciotropic Hormone
In contrast to earlier assumptions, not only skin, but prostate, colon, breast, and many other tissues express the enzyme to convert 25(OH)D to its active form, 1,25(OH)2D.Citation39–Citation41 Therefore 1,25(OH)2D is now not exclusively considered as a calciotropic hormone but also as a locally produced potent hormone regulating various cellular functions including cell growth.Citation10,Citation40 In consequence, a whole number of recently published studies point at a protective effect of locally produced vitamin D in the pathogenesis of various malignancies.Citation10,Citation41 Interestingly, new findings demonstrate a contribution of the skin vitamin D system to the pathogenesis of malignant skin tumors including melanoma.Citation41 In contrast to the internal cancers discussed above, many studies have shown that the incidence of malignant melanoma increases with decreasing latitude towards the equator.Citation42 However, in contrast to short-term intense exposure, more chronic less intense exposure has not been found to be a risk factor for the development of malignant melanoma and in fact has been found in some studies to be protective.Citation41–Citation43 It may be speculated whether these connections may be an explanation for the finding of an increased risk to develop melanoma after sunscreen use, that was reported recently.Citation44
How Much Vitamin D do We Need to Achieve a Protecting Effect Against Cancer and Other Diseases?
The US Recommended Dietary Allowance (RDA) of vitamin D from 1989 is 200 IU.Citation45 Yet, studies have shown that 200 IU/day has no effect on bone status.Citation42 It has been recommended that adults may need, at a minimum, five times the RDA, or 1,000 IU, to adequately prevent bone fractures, protect against some cancers and derive other broad-ranging health benefits.Citation45 In conclusion, the 1989 RDA of 200 IU is antiquated, and the newer 600 IU Daily Reference Intake (DRI) dose for adults older than 70 is still not adequate.Citation45 It has been suggested that even the 2,000 IU upper tolerable intake, the official safety limit, does not deliver the amounts of vitamin D that may be optimal.Citation45 On a sunny summer day, total body sun exposure produces approximately 10,000 IU vitamin D per day. As a result, concerns about toxic overdose with dietary supplements that exceed 800 IU are poorly founded. It has been speculated that a person would have to consume almost 67 times more vitamin D than the current 600 IU recommended intake for older adults to experience symptoms of overdosage.Citation45 Vieth believes people need 4,000–10,000 IU vitamin D daily and that toxic side effects are not a concern until a 40,000 IU/day dose.Citation45 Other researchers agree with these findings. They suggest that older adults, sick adults, and “perhaps all adults” need 800–1,000 IU daily. They indicate that daily doses of 2,400 IU—four times the recommended intake—can be consumed safely.Citation43
What conclusions do we draw from these findings, most importantly the demonstration of an association between vitamin D deficiency and the occurrence of various types of cancer? The most important take home message, especially for dermatologists, is that strict sun protection procedures to prevent skin cancer may induce the severe health risk of vitamin D deficiency. There is no doubt that UV radiation is mutagenic and is the main reason for the development of non-melanoma skin cancer. Therefore, excessive sun exposure has to be avoided, particularly burning in childhood. To reach this goal, the use of sunscreens as well as the wearing of protective clothes and glasses is absolutely important. Additionally, sun exposure around mid-day should be avoided during the summer in most latitudes. However, the dermatological community has to recognize that there is evidence that the protective effect of less intense solar radiation outweighs its mutagenic effect. In consequence, many lives could be prolonged through careful exposure to sunlight or more safely, vitamin D supplementation, especially in non-summer months. Therefore, recommendations of dermatologists on sun protection should be moderated.
As Michael Holick reported previously,Citation46 we have learned that at most latitudes such as Boston, MA very short and limited solar exposure is sufficient to achieve “adequate” vitamin D levels. Exposure of the body in a bathing suit to one minimal erythemal dose (MED) of sunlight is equivalent to ingesting about 10,000 IU of vitamin D and it has been reported that exposure of less than 18% of the body surface (hands, arms and face) two to three times a week to a third to between a third and a half of an MED; (about 5 min for skin-type-2 adult in Boston at noon in July) in the spring, summer and autumn is more than adequate.Citation46 Anyone intending to stay exposed to sunlight longer than recommended above should apply a sunscreen with a sufficient sun-protection factor to prevent sunburn and the damaging effects of excessive exposure to sunlight. Although further work is necessary to define the influence of vitamin D deficiency on the occurrence of melanoma and non-melanoma skin cancer, it is at present mandatory that especially dermatologists strengthen the importance of an adequate vitamin D status if sun exposure is seriously curtailed. It has to be emphasized that in groups that are at high risk of developing vitamin D deficiency (e.g., nursing-home residents; patients with skin type I or patients under immunosuppressive therapy that must be protected from sun exposure), vitamin D status should be monitored subsequently. Vitamin D deficiency should be treated, e.g., by giving vitamin D orally as recommended previously.Citation46 It has been shown that a single dose of 50,000 IU vitamin D once a week for 8 weeks is efficient and safe to treat vitamin D deficiency.Citation47 In a meta analysis of randomized controlled trials supplemental vitamin D in a dose of 700–1,000 IU prevented the risk of falling in older individuals by 19%.Citation49 Another means of guaranteeing vitamin D sufficiency, especially in nursing-home residents, is to give 50,000 IU of vitamin D once a month. An alternative to prevent vitamin D deficiency would be the use of vitamin D containing ointments. However, it should be noted that vitamin D containing ointments are, at least in Europe, not allowed as cosmetics. These antiquated laws are the result of the fear of vitamin D intoxication that was evident in Europe in the 1950sCitation48 and should be re-evaluated, for they do not reflect our present scientific knowledge. If we follow the guidelines discussed above carefully, they will ensure an adequate vitamin D status, thereby protecting us against adverse effects of strict sun protection recommendations. Most importantly, these measures will protect us sufficiently against the influence of vitamin D deficiency on the occurrence of various malignancies without increasing our risk to develop UV-induced skin cancer. To reach this goal it is of high importance that this information is transferred to every clinician, especially to dermatologists. Otherwise dermatologists will not be prepared for the moderation of sun protection recommendations, that is necessary to protect us against vitamin D deficiency, cancer and other diseases.
Intentional UV Exposition Does Not Correct a Vitamin D Deficiency
Laurence Feldmeyer agrees with the idea that the recommended daily vitamin D intake of 800 IU cannot be obtained through diet only and requires oral supplementation.Citation50 Although ultraviolet phototherapy has been shown to increase serum 25-hydroxyvitamin D concentrations in several studies, usually no detailed dose or treatment frequency required for a particular response, or the quantitative effect of basic skin pigmentation were mentioned in the respective publications.Citation45,Citation51 Moreover, most of the studies were based on very small collectives and were of short duration. In a case series of 24 postmenopausal women with active plaque psoriasis treatment for 8–12 weeks, UVB broadband (UVBbb) increased serum levels of 25(OH)D3 1.62-folds from basal level.Citation52 Almost all studies were performed with UVBbb. Recently, two studies investigated the influence of UVB narrowband (UVBnb) on vitamin D in serum.Citation53,Citation54 They showed that low-dose UVBnb treatment induces a significant increase of the vitamin D status in persons with low initial levels of 25(OH) D3Citation53, and that UVBnb is less efficient for the production of vitamin D than UVBbb, as expected from the UV spectrum.Citation54
Only two studies investigated the vitamin D plasma increase under psoralen-UVA (PUVA) therapy.Citation55,Citation56 Although the action spectrum for the conversion of 7-dehydrocholesterol to vitamin D3 is thought to be within the UVB range (280–310 nm), the results of Rogers et al. suggest that the higher wavelengths of UVA (310–400 nm) may have an effect, too, at least in the presence of psoralen. Nevertheless, the possibility that the small amounts of UVB emitted by the lamps used may explain the increase either alone or in combination with UVA or psoralen, or both has not been ruled out. The stimulation of vitamin D synthesis by PUVA was short-lived. It had returned almost to pre-treatment levels by the third irradiation. Another study showed no effect of PUVA on serum vitamin D.Citation56 Krause et al. measured blood pressure in nine patients under UVBbb and nine patients under UVA during 6 weeks, vitamin D in the UVA group remain unchanged.Citation57 We could not find any studies on the effect of UVA1 (340–400 nm) on serum vitamin D in the medical literature. Contrary to UVA, UVA1 has almost no overlap with the UVB spectrum.
Feldmeyer et al. investigated the serum elevation of 25(OH) D (the major circulating metabolite, used to determine vitamin D status in a patient) under UVBnb and UVA1 therapy in patients undergoing phototherapy for a skin disease, in order to determine the effect of these nowadays frequently used wavelengths for phototherapy on vitamin D plasma levels, and particularly to test the dogma, that UVA has no effect on serum vitamin D. 25(OH)D was determined before the start of light therapy, as well as 1 week after the start of therapy and after completion of light therapy at 12 weeks.
The first preliminary results show that, as expected, serum vitamin D increases under UVBnb therapy. Under UVA/UVB we also have an increase in vitamin D, however, less clear than with UVBnb. Under UVA therapy, no increase in vitamin D was measured.
In conclusion, as UVA exposure does not increase vitamin D synthesis, and the UVBnb-induced increase in vitamin D synthesis is linked to a higher risk of skin cancer, the optimum wavelength for production of previtamin D3 corresponding to maximal DNA damage, intentional UV exposition is not an appropriate way to correct a vitamin D deficiency. As discussed above, there is a controversy about the correlation and causality of vitamin D and cancer overall and skin cancer in particular.Citation58 While more data is collected, Feldmeyer et al. firmly believe that dermatological recommendations for sun protection by behaviour, clothing and sunscreen application should not be reconsidered at this point in time. This position does not agree with the view of the other symposium speakers.
Figures and Tables
Figure 1 UVB rays from sunlight generates vitamin D3 from its precursor in the skin. Vitamin D3 is not very active and can be converted to both 25(OH)D3 and 1,25(OH)2D3 by dendritic cells. Dendritic cells come in close contact with T cells, and in the presence of vitamin D3, the CCR10 receptor is induced on the T cells. This attracts the T cells to CCL27, which is selectively produced and secreted by keratinocytes in the epidermis. Thus, once T cells have infiltrated the dermal layers of the skin from the cutaneous blood vessel, they can be mobilized within the skin and migrate to the epidermis. This way, T cells could be drawn to the epidermis when they are needed.
References
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr Rev 2009; 30:293 - 342
- Gore AC, Heindel JJ, Zoeller T. Endocrine disruptors for endocrinologists (and others). Endocrinology 2006; 147:1 - 3
- Schlumpf M, Schmid P, Durrer S, Conscience M, Maerkel K, Henseler M, et al. Endocrine activity and developmental toxicity of cosmetic UV filters—an update. Toxicology 2004; 205:113 - 122
- Schlumpf M, Kypke K, Vökt C, Birchler M, Durrer S, Faass O, et al. Endocrine active UV filters: developmental toxicity and exposure through breast milk. Chimia 2008; 62:345 - 361
- Osterlind A, Tucker MA, Stone BJ, Jensen OM. The Danish case-control study of cutaneous malignant melanoma. II Importance of UV-light exposure. Int J Cancer 1988; 42:319 - 324
- Gorham ED, Garland FC, Garland CF. Sunlight and breast cancer incidence in the USSR. Int J Epidemiol 1990; 19:614 - 622
- Garland CF, Comstock GW, Garland FC, et al. Serum 25-hydroxyvitamin D and colon cancer: eight year prospective study. Lancet 1989; 1176 - 1178
- Garland CF, Garland FC, Gorham ED. Can colon cancer incidence and death rates be reduced with calcium and vitamin D?. Am J Clin Nutr 1991; 54:193 - 201
- Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet-B radiation. Cancer 2002; 94:1867 - 1875
- Holick MF. Vitamin D deficiency. N Engl J Med 2007; 357:266 - 281
- Holick MF, Frommer JE, McNeal SC, Richtand NM, et al. Photometabolism of 7-dehydrocholesterol to previtamin D3 in skin. Biochem Biophys Res Commun 1977; 76:107 - 115
- MacLaughlin JA, Anderson RR, Holick MF. Spectral character of sunlight modulates photosynthesis of previtamin D3 and its photoisomers in human skin. Science 1982; 216:1001 - 1003
- Holick MF, MacLaughlin JA, Clark MB, Holick SA, et al. Photosynthesis of previtamin D3 in human skin and the physiologic consequences. Science 1980; 210:203 - 205
- Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 2004; 29:664 - 673
- Ohyama Y, Yamasaki T. Eight cytochrome P450S catalyze vitamin D metabolism. Front Biosci 2005; 10:608 - 619
- Zehnder D, Bland R, Stewart PM, Hewison M. Analysis of the tissue distribution of 1α-hydroxylase identifies novel extra-renal sites for the synthesis of 1,25-dihydroxyvitamin D3. J Endocrinol 2000; 164:1
- Hewison M, Burke F, Evans KN, Lammas DA, et al. Extrarenal 25-hydroxyvitamin D3-1α-hydroxylase in human health and disease. J Steroid Biochem Mol Biol 2007; 103:316 - 321
- Lehmann B, Meurer M. Reichrath J, Friedrich M, Tilgen W. Extrarenal sites of calcitriol synthesis: the particular role of the skin. Recent Results in Cancer Research 2003; 164:Springer Verlag 135 - 145 Vitamin D Analogs in Cancer Prevention and Therapy
- Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 2006; 81:353 - 373
- Lehmann B, Querings K, Reichrath J. Vitamin D and skin: new aspects for dermatology. Exp Dermatol 2004; 13:11 - 15
- Reichrath J, Lehmann B, Carlberg C, Varani J, et al. Vitamins as hormones. Horm Metab Res 2007; 39:71 - 84
- Lehmann B, Genehr T, Knuschke P, Pietzsch J, et al. UVB-induced conversion of 7-dehydrocholesterol to 1α,25-dihydroxyvitamin D3 in an in vitro human skin equivalent model. J Invest Dermatol 2001; 117:1179 - 1185
- Schuessler M, Astecker N, Herzig G, Vorisek G, et al. Skin is an autonomous organ in synthesis, two-step activation and degradation of vitamin D3: CYP27 in epidermis completes the set of essential vitamin D3-hydroxylases. Steroids 2001; 66:399 - 408
- Vantieghem K, Kissmeyer AM, De Haes P, Bouillon R, et al. UVB-induced production of 1,25(OH)2D3 and vitamin D activity in human keratinocytes pretreated with a sterol delta 7 reductase inhibitor. J Cell Biochem 2006; 98:81 - 92
- Lehmann B, Sauter W, Knuschke P, Dreßler S, et al. Demonstration of UVB-induced synthesis of 1α,25-dihydroxyvitamin D3 (calcitriol) in human skin by microdialysis. Arch Dermatol Res 2003; 295:24 - 28
- Bär M, Domaschke D, Meye A, Lehmann B, et al. Wavelength-dependent induction of CYP24A1-mRNA after UVB-triggered calcitriol synthesis in cultured human keratinocytes. J Invest Dermatol 2007; 127:206 - 213
- Segaert S, Bouillon R. A Norma W, Bouillon R, Thomasset M. Epidermal keratinocytes as source and target cells for vitamin D. Vitamin D endocrine system: structural, biological, genetic and clinical aspects 2000; Proceedings of the Eleventh Workshop on Vitamin D May 27–June 1, 2000 Nashville TN, USA Riverside Printing and Reprographics University of California 583 - 590
- Vantieghem K, De Haes P, Bouillon R, Segaert S. Dermal fibroblasts pretreated with a sterol delta7-reductase inhibitor produce 25-hydroxyvitamin D3 upon UVB irradiation. J Photochem Photobiol 2006; 85:72 - 78
- Prystowsky JH, Muzio PJ, Sevran S, Clemens TL. Effect of UVB phototherapy and oral calcitriol (1,25-dihydroxyvitamin D3) on vitamin D photosynthesis in patients with psoriasis. J Am Acad Dermatol 1996; 35:690 - 695
- Guryev O, Cavalho RA, Usanov S, Gilep A, et al. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc Natl Acad Sci 2003; 100:14754 - 14759
- Albro P, Bilski P, Corbett JT, Schroeder JL, et al. Photochemical reactions and phototoxicity of sterols: novel self-perpetuating mechanism for lipid photooxydation. Photochem Photobiol 1997; 66:316 - 325
- Moriuchi S, Tsuruki F, Otawara Y, Hosoya N, et al. Biological Activity of the endoperoxides derived from vitamin D derivatives by dye-sensitized photo-oxidation. J Nutr Sci Vitaminol 1979; 25:455 - 458
- Johnston B, Butcher EC. Chemokines in rapid leukocyte adhesion triggering and migration. Seminars in Immunology 2002; 14:83 - 92
- Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol 2008; 8:685 - 698
- Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic Acid Imprints Gut-Homing Specificity on T Cells. Immunity 2004; 21:527 - 538
- Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol 2007; 8:285 - 293
- Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, et al. Cutting Edge: The Orphan Chemokine Receptor G Protein-Coupled Receptor-2 (GPR-2, CCR10) Binds the Skin-Associated Chemokine CCL27 (CTACK/ALP/ILC). J Immunol 2000; 164:3465 - 3470
- Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T Cells Induced by 1{alpha},25-Dihydroxyvitamin D3 and Mycophenolate Mofetil Treatment Mediate Transplantation Tolerance. J Immunol 2001; 167:1945 - 1953
- Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF. Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev 1998; 7:391 - 395
- Reichrath J. Will analogs of 1,25-dihydroxyvitamin D3 (calcitriol) open a new era in cancer therapy?. Onkologie 2001; 24:128 - 133
- Osborne JE, Hutchinson PE. Vitamin D and systemic cancer: is this relavant to malignant melanoma?. Br J Dermatol 2002; 147:197 - 213
- Green A, Siskind V. Geographical distribution of cutaneous melanoma in Queensland. Med J Aust 1983; 1:407 - 410
- Elwood JM, Gallagher RP, Hill GB, Pearson JC. Cutaneous melanoma in relation to intermittent and constant sun exposure—the western Canada Melanoma Study. Int J Cancer 1985; 35:427 - 433
- Westerdahl J, Olsson H, Mosback A, et al. Is the use of sunscreensa risk factor for melanoma?. Melanoma Res 1995; 5:59 - 65
- Vieth R. Vitamin D supplementation, 25-hydroxyvitamin D concentrations, and safety. Am J Clin Nutr 1999; 69:842 - 856
- Holick MF. Sunlight “D” ilemma: risk of skin cancer or bone disease and muscle weakness. Lancet 2001; 357:961
- Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet 1998; 351:805 - 806
- British Pediatric Association. Hypercalcemia in infants and vitamin D. Brit Med J 1956; 2:149
- Bischoff-Ferrari HA, Dawson-Huges B, Staehelin HB, Orav E, Stuck AE, Theiler R, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomized controlled trials. Brit Med J 2009; 339:3692; http://dx.doi.org/10.1136/bmj.b3692
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 2006; 84:18 - 28
- Armas LA, Dowell S, Akhter M, Duthuluru S, Huerter C, Hollis BW, et al. Ultraviolet-B radiation increases serum 25-hydroxyvitamin D levels: the effect of UVB dose and skin color. J Am Acad Dermatol 2007; 57:588 - 593
- Osmancevic A, Landin-Wilhelmsen K, Larko O, Mellstrom D, Wennberg AM, Hulthen L, et al. UVB therapy increases 25(OH) vitamin D syntheses in postmenopausal women with psoriasis. Photodermatol Photoimmunol Photomed 2007; 23:172 - 178
- Cicarma E, Mork C, Porojnicu AC, Juzeniene A, Tam TT, Dahlback A, et al. Influence of narrowband UVB phototherapy on vitamin D and folate status. Exp Dermatol 2009; In press
- Osmancevic A, Landin-Wilhelmsen K, Larko O, Wennberg AM, Krogstad AL. Vitamin D production in psoriasis patients increases less with narrowband than with broadband ultraviolet B phototherapy. Photodermatol Photoimmunol Photomed 2009; 25:119 - 123
- Rogers S, Marks J, Shuster S, Hillyard CJ. Effect of PUVA on serum 25-OH vitamin D in psoriatics. Br Med J 1979; 2:833 - 834
- Guilhou JJ, Colette C, Monpoint S, Lancrenon E, Guillot B, Monnier L. Vitamin D metabolism in psoriasis before and after phototherapy. Acta Derm Venereol 1990; 70:351 - 354
- Krause R, Buhring M, Hopfenmuller W, Holick MF, Sharma AM. Ultraviolet B and blood pressure. Lancet 1998; 352:709 - 710
- Reddy KK, Gilchrest BA. What is all this Commotion about Vitamin D?. J Invest Dermatol 2010; 130:321 - 326