Abstract
Acquired perforating dermatosis (APD) is a rare disorder characterized by transepidermal elimination of contents from dermis with minimal disruption of surrounding structures, believed to be due to altered expression of dermal proteins. Its occurrence in patients with systemic mycosis has never been reported. We report a 60-y gentleman who presented with features of adrenal insufficiency (nausea vomiting, hypotension and increased pigmentation) for 4 mo, multiple hyperpigmented pruritic nodules with central keratinous plug over extensor surface of both lower limbs along with hepatosplenomegaly of one month duration. Investigations revealed low cortisol (2.3 μg/dl; normal: 5–34 μg/dl), elevated ACTH (68 pg/ml; normal: 5–15 pg/ml), enlarged bilateral adrenals with hepatosplenomegaly on CT. Methanamine silver staining of fine needle aspiration from the adrenals and bone marrow aspiration showed numerous oval yeast cells suggestive of histoplasma. Histopathology of biopsy of one of the skin nodules revealed transepidermal elimination process characterized by invagination of epidermis with extrusion of collagen bundles suggestive of APD. Patient improved with hydrocortisone replacement and there was clinical improvement with resolution of skin lesions following amphotericin-B and itraconazole therapy. This is probably the first reported case of APD in a patient with disseminated histoplasmosis who had presented with Addison’s disease.
Introduction
Acquired perforating dermatoses (APD) is a group of rare chronic persisting dermatological disorders with poor treatment outcomes, characterized by transepidermal elimination in which collagen, elastic tissue or necrotic connective tissue from the dermis is extruded to the exterior with minimal disruption of surrounding structures.Citation1-Citation3 It is most commonly seen in patients with diabetes and on hemodialysis. It has also been reported in hypothyroidism, hepatitis, lymphoma, periampullary carcinoma, sclerosing cholangitis, scabies, a variety of malignancies (colon cancer, prostate cancer, hepatocellular carcinoma, breast cancer) and acquired immunodeficiency syndrome (AIDS).Citation2,Citation4
An association of this disease with systemic mycosis has never been reported to the best of our knowledge. We present for the first time the occurrence of APD in a patient of Addison’s disease due to disseminated histoplasmosis, which resolved spontaneously with amphotericin-B and itraconazole therapy.
Case report
A 60 y gentleman presented to Endocrinology clinic with reportts intermittent low grade fever, nausea, vomiting, anorexia, pain abdomen, weight loss and increased pigmentation for 4 mo and multiple, discrete, pigmented, intensely pruritic nodules with history of extrusion of material from these nodules, over extensor aspect of both lower extremities of 1 mo duration (). Examination was significant for low blood pressure (80/60 mm of Hg), tachycardia, oral mucosal hyperpigmentation, hepatosplenomegaly and multiple hyperpigmented nodules with central keratinous plug over the extensor surface of both lower limbs, especially over the thighs. He did not have generalized lymphadenopathy and respiratory examination was normal. Investigations revealed hyponatremia (117 mmol/L; normal: 135–145 mmol/L), hyperkalemia (5.3 mmol/L; normal: 3–5-5.5 mmol/L), normal renal (creatinine-0.8 mg/dl; normal: 0.2–1.2 mg/dl) and hepatic function, low 8 a.m. cortisol (2.3 μg/dl; normal: 5–34 μg/dl) and elevated plasma ACTH (68 pg/ml; normal: 5–15 pg/ml). Contrast enhanced CT (CECT) scan of abdomen revealed mild hepatosplenomegaly with bilateral heterogeneously enhancing enlarged adrenals with areas of necrosis, left adrenal (63 × 36 mm) larger than the right (49 mm × 33 mm) (). Chest X-ray was normal and Mantoux test was negative. Serology was negative for HIV. Immunodeficiency was ruled out by evaluating for T and B cell function. His CD4+ lymphocyte count was normal (1,908 cells/mm3). Serum IgG, IgA and IgM levels were estimated by nephelometry assay (Dade Behring) and were normal [IgG: 1129 mg/dl (normal: 700–1,600 mg/dl); IgA: 232mg/dl (normal: 70–400 mg/dl) and IgM: 218 mg/dl (normal: 40–230 mg/dl)]. USG guided fine needle aspiration (FNA) from the left adrenal mass and bone marrow aspiration from the iliac crest showed plenty of oval cells on methanamine silver staining suggestive of yeasts of Histoplasma capsulatum (). Histopathological examination of punch biopsy specimen of one of the nodules over the left thigh revealed a transepidermal elimination process characterized by invagination of epidermis with extrusion of dermal materials through cup shaped depression (Fig.4A and B). Staining for collagen and elastin revealed collagen bundles extruding from the epidermis (Figs. 5 and 6). A diagnosis of acquired perforating dermatosis, Addison’s disease with disseminated histoplasmosis was made.
Figure 1. (A) Pigmented nodularlesions seen on both thighs with central keratinous plug. (B) Close up demonstrating nodular lesion with central crater. (C) Picture of both thighs (10 mo after diagnosis) showing complete resolution of lesions with presence of hyperpigmentation.
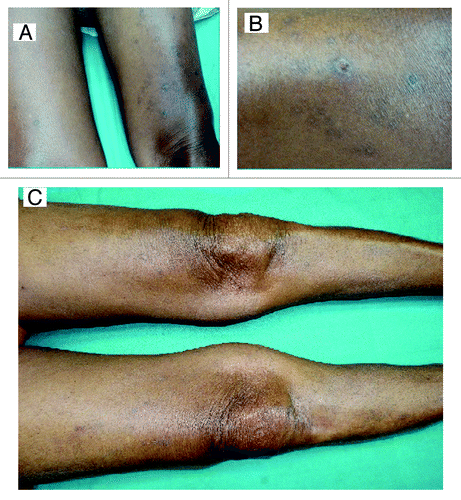
Figure 2. CT abdomen showing enlarged left (63 × 36 mm; black arrow) and right (49 mm × 33 mm; white arrow) adrenals with areas of necrosis.
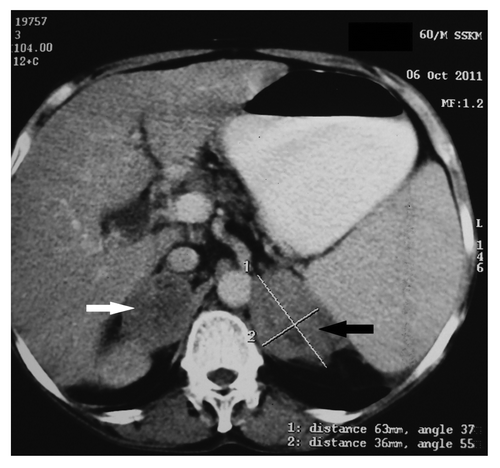
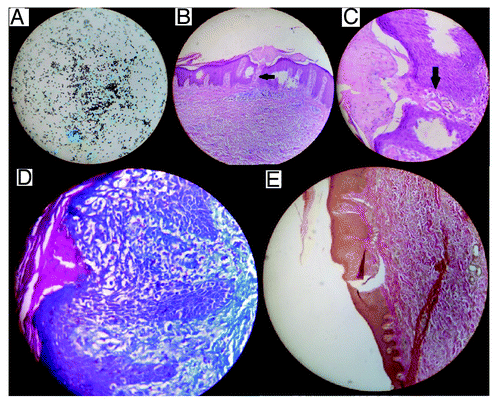
Figure 3. (A) Methanamine silver staining of adrenal fine needle aspiration showing numerous oval yeast cells suggestive of histoplasma capsulatum (×400 magnification). (B) Hematoxylin and eosin staining of skin biopsy showing transepidermal elimination of dermal contents (×100). (C) Higher magnification (×400) showing transepidermal elimination of dermal contents. (D) Masson’s trichrome staining of skin biopsy showing intensely blue staining of collagen within the epidermifigs (×400). (E) Verhoeff van Gieson staining of skin biopsy showing brown staining for elastin sparing the epidermal channel (×100).
He initially received injection hydrocortisone 50 mg thrice daily, which was gradually tapered and converted to oral hydrocortisone 15 mg on getting up from sleep in the morning and 10 mg at 3 p.m. He received amphotericin deoxycholate (Fungisome, Lifecare Innovations) 485 mg over 14 d, which was then stopped due to rising creatinine and hypokalemia. Itraconazole (Sporanox, 100 mg capsule, Johnson and Johnson) was started at 200 mg per day. Cetirizine and topical emollients were used to relieve pruritus. Patient showed clinical improvement with cessation of fever, improvement in appetite and weight gain. After 3 mo of itraconazole therapy, the cutaneous lesions over the thighs started regressing. Last evaluated 10 mo after the initial diagnosis, he was doing well on hydrocortisone supplementation and itraconazole 200 mg/day. The lesions over the thighs had resolved completely with presence of residual pigmentation ().
Discussion
APD belongs to the group of disorders termed perforating dermatoses, the others members being Kyrle's disease, perforating folliculitis, elastoses perforans serpiginosa (EPS) and reactive perforating collagenoses (RPC).Citation2,Citation3 They are differentiated by the nature of perforating material: predominantly collagen bundles in RPC, elastin in EPS and keratin in Kyrle’s disease.Citation2 Lesions of APD usually present as pigmented papules, which may become hyperkeratotic, erythematous plaques or umblicated nodules, commonly seen over the arms and legs. However any site can be affected including the trunk and scalp.Citation1-Citation4 Pruritus and koebnerisation are almost universal.Citation2
It is believed that poor blood supply due to microangiopathy in diabetes, accumulation of poorly dialyzable substances in patients on hemodialysis, and trauma (secondary to itching) leads to dermal necrosis, alteration of dermal connective tissue protein expression [increased expression of elastin receptor, transforming growth factor-β (TGF-β), matrix metalloproteinase-1 (MMP-1) and tissue inhibitor of metalloproteinase-1 (TIMP-1)].Citation5,Citation6 This alteration of dermal connective tissue protein expression may play a key role in the development of perforating dermatoses.
APD is a difficult to treat disorder with the outcomes dependent on the underlying disease. Treatment is usually done with either topical corticosteroids or retinoids. There have been reports of successful treatment with oral retinoids, doxycycline, clindamycin and allopurinol. Transcutaneous electrical nerve stimulation, UVB, topical cantharidin (vesicant and keratolytic agent) has also been tried. Spontaneous remission is rare. In some cases treatment of the underlying disease have led to remission.Citation7-Citation9
APD was diagnosed in our patient on the basis of the clinical presentation and histopathologic evidence of extrusion of predominantly collagen bundles. Our patient presented with clinical features of adrenal insufficiency with hyperpigmentation (Addison’s disease), which was confirmed by documentation of low serum cortisol and elevated plasma ACTH. Abdominal imaging revealed bilateral enlarged adrenals and (confirmed) hepatosplenomegaly. Histoplasmosis was diagnosed as the cause of Addison’s disease in view of presence of oval yeast cells on methanamine silver staining of the adrenal FNA ().
Histoplasmosis is predominantly a disease of the reticuloendothelial system caused by Histoplasma capsulatum, a dimorphic fungus, with the yeast form found in the macrophages and phagocytes of humans.Citation10 Most of the infections in immunocompetent individuals are asymptomatic, mild or self-limiting. Disseminated hispoplasmosis (DH) is predominantly a disease of the immunocompromized patients with only 30% occurring in immunocompetent individuals.Citation10 DH was diagnosed in our patient who did not have any immunodeficiency, in view of fever, weight loss, hepatosplenomegaly, adrenomegaly with adrenal insufficiency and histopathologic abundance of oval yeast cells on adrenal FNA and bone marrow aspiration.Citation10 Other forms of histoplasmosis are acute pulmonary histoplasmosis, chronic cavitary pulmonary histoplasmosis and central nervous system histoplasmosis.Citation10 Liposomal amphotericin B is the treatment of choice, and has been observed to be more effective than amphotericin deoxycholate in the treatment of DH in immunocompromized individuals.Citation10 We used deoxycholate preparation due to the prohibitive cost of the liposomal preparation. Also our patient did not have any immunodeficiency and responded well to the combination therapy of amphotericin deoxycholate and itraconazole.
Occurrence of APD in the background of Addison’s disease due to disseminated histoplasmosis has not been reported. Temporal relationship of the occurrence of APD with the clinical course and its complete resolution with the treatment of histoplasmosis establishes the likely causal relationship between disseminated histoplasmosis and APD in our patient. Our patient highlights the observation that treatment of the underlying disease often leads to resolution of APD. It may be hypothesized that altered structural protein in the dermis as a consequence of disseminated histoplasmosis may have had some role in the development of APD in our patient. This is most likely an immunological phenomenon in the absence of definitive demonstration of histoplasma yeast cells in the skin biopsy. One of the limitations of our report is the lack of immunohistochemistry to look for altered protein expression in the dermis of the skin biopsy specimen due to institutional non-availability.
To summarize, this is probably the first reported case of the occurrence of APD in a patient of disseminated histoplasmosis who had presented with adrenal insufficiency, and responded well to hydrocortisone supplementation and amphotericin-B with itraconazole therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Morton CA, Henderson IS, Jones MC, Lowe JG. Acquired perforating dermatosis in a British dialysis population. Br J Dermatol 1996; 135:671 - 7; http://dx.doi.org/10.1111/j.1365-2133.1996.tb03873.x; PMID: 8977664
- Saray Y, Seçkin D, Bilezikçi B. Acquired perforating dermatosis: clinicopathological features in twenty-two cases. J Eur Acad Dermatol Venereol 2006; 20:679 - 88; http://dx.doi.org/10.1111/j.1468-3083.2006.01571.x; PMID: 16836495
- Rapini RP, Herbert AA, Drucker CR. Acquired perforating dermatosis. Evidence for combined transepidermal elimination of both collagen and elastic fibers. Arch Dermatol 1989; 125:1074 - 8; http://dx.doi.org/10.1001/archderm.1989.01670200050007; PMID: 2757403
- Jeon H, Sarantopoulos GP, Gharavi NM, Taylor E, Chiu MW. Acquired perforating dermatosis associated with metastatic colon cancer. Dermatol Online J 2011; 17:7; PMID: 22136863
- Fujimoto N, Akagi A, Tajima S, Ishibashi A, Nomura K, Matsushita A, et al. Expression of the 67-kDa elastin receptor in perforating skin disorders. Br J Dermatol 2002; 146:74 - 9; http://dx.doi.org/10.1046/j.1365-2133.2002.04550.x; PMID: 11841369
- Gambichler T, Birkner L, Stücker M, Othlinghaus N, Altmeyer P, Kreuter A. Up-regulation of transforming growth factor-beta3 and extracellular matrix proteins in acquired reactive perforating collagenosis. J Am Acad Dermatol 2009; 60:463 - 9; http://dx.doi.org/10.1016/j.jaad.2008.06.006; PMID: 19231643
- Gönül M, Cakmak SK, Gül U, Kiliç A, Ergül G. Two cases of acquired perforating dermatosis treated with doxycycline therapy. Int J Dermatol 2006; 45:1461 - 3; http://dx.doi.org/10.1111/j.1365-4632.2006.02947.x; PMID: 17184266
- Gambichler T, Altmeyer P, Kreuter A. Treatment of acquired perforating dermatosis with narrowband ultraviolet B. J Am Acad Dermatol 2005; 52:363 - 4; http://dx.doi.org/10.1016/j.jaad.2004.08.018; PMID: 15692489
- Wong J, Phelps R, Levitt J. Treatment of acquired perforating dermatosis with cantharidin. Arch Dermatol 2012; 148:160 - 2; http://dx.doi.org/10.1001/archdermatol.2011.350; PMID: 22351815
- Hage CA, Wheat LJ. Histoplasmosis. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo J editors. Harrison’s principles of internal medicine.18th edition, Mc Graw Hill 2012; 1:1641-2.