Abstract
The formation of a permeability barrier between the external environment and the host is essential for survival. To provide this barrier keratinocytes undergo a complex pathway of differentiation, which culminates in keratinocyte cornification and the formation of extracellular lipid enriched lamellar membranes in the stratum corneum. The mechanisms that coordinately regulate the parallel formation of the corneocytes and lamellar membranes are unknown. The extracellular lamellar membranes are derived from the exocytosis of lamellar bodies and to synthesize lamellar bodies the keratinocyte must have abundant quantities of cholesterol, fatty acids and ceramides. These lipids could serve as signaling molecules and thereby coordinately regulate the formation of the stratum corneum. Fatty acids activate PPARs and studies have shown that PPAR activation stimulates keratinocyte differentiation. Cholesterol is converted to oxysterols that activate LXR and studies have shown that LXR activation also stimulates keratinocyte differentation. Additionally, PPAR and LXR activation also facilitates the formation of the lipid enriched lamellar membranes. Ceramides, via a number of mechanisms also stimulate keratinocyte differentiation. Recently, studies have shown that ceramides by increasing PPAR delta also increase the expression of ABCA12, which would facilitate the formation of lamellar bodies. Finally, keratinocytes accumulate a large quantity of cholesterol sulfate, which plays a key role in regulating desquamation. Cholesterol sulfate has also been shown to stimulate keratinocyte differentiation. Thus, cholesterol, cholesterol sulfate, fatty acids, and ceramides all stimulate keratinocyte differentiation and thereby could coordinately regulate the formation of the stratum corneum.
Introduction
A major function of the epidermis is to provide a permeability barrier between the external environment and the organism.Citation1,Citation2 A permeability barrier that prevents the excess loss of water and electrolytes is essential for life on land. To provide this permability barrier, keratinocytes undergo a complex pathway of differentiation, which culminates in keratinocyte cornification and in the formation of extracellular lipid-enriched lamellar membranes in the stratum corneum.Citation3–Citation5 These extracellular lipid-enriched membranes are primarily responsible for the permeability barrier to water and electrolyte transit.Citation1,Citation2 In parallel with lipid matrix formation, keratinocyte cornification occurs, a process characterized by extensive cross-linking of loricrin, involucrin and other structural proteins by transglutaminases, leading to the formation of the cornified envelope.Citation3–Citation5 This structure is essential not only for the mechanical strength of the skin, but it also serves as a scaffold for the deposition of the lamellar membranes.Citation6,Citation7 Thus, the stratum corneum is the end-product of two interdependent mechanisms that occur during keratinocyte differentiation; i.e., those that lead to the formation of the corneocytes (the “bricks”), and those that generate the extracellular lipid-enriched matrix (the “mortar”). Yet, despite its critical importance, the mechanisms that coordinately regulate the parallel formation of the corneocytes and the extracellular lamellar membranes remain largely unknown.
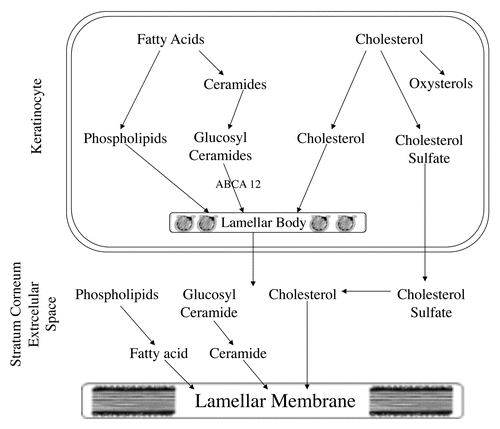
The lipid-enriched extracellular lamellar membranes in the stratum corneum are derived from the exocytosis of lamellar body contents from the outermost stratum granulosum cellsCitation1,Citation2 (). To form lamellar bodies three families of lipids, specifically, cholesterol, phospholipids and glucosylceramides, are synthesized in large quantities within the stratum spinosum and stratum granulosum cells. Studies by our laboratory have demonstrated that not only is the epidermis a very active site of lipid synthesis, but also that inhibition of either cholesterol, fatty acid, ceramide or glucosylceramide synthesis disrupts normal lamellar body formation and subsequently, permeability barrier homeostasis.Citation2 Moreover, cholesterol and fatty acids, along with ceramides and phospholipids, must be generated in the appropriate molar ratio or abnormal lamellar bodies are formed and permeability barrier dysfunction results.Citation2 Additionally, these lipid precursors must be transported into lamellar bodies and recent studies have shown that ABCA12 plays an important role in the transport of glucosylceramides into lamellar bodies.Citation2 The epidermis also produces large quantities of cholesterol sulfate and the catabolism of cholesterol sulfate by steroid sulfatase in the stratum corneum plays a key role in regulating desquamation.Citation8
While the formation of the corneocytes and the extracellular lamellar membranes has been extensively studied as distinct, but independent processes, it is apparent that the formation of a normal stratum corneum that will provide competent permeability barrier function requires the coordinated regulation of both processes. In this review we will discuss the various pathways by which the lipids that are required for lamellar body formation and stratum corneum function could also serve as signaling molecules and thereby coordinately regulate the formation of the stratum corneum ().
Nuclear Hormone Receptors
Differential control of gene expression is essential for tissue differentiation and development. Lipophilic compounds, such as steroid hormones, thyroid hormones, vitamin D and retinoids, are potent regulators of differentiation and development, and their actions have been shown to be mediated by cytoplasmic/nuclear receptors.Citation9 These lipophilic compounds bind to their respective nuclear hormone receptors and then interact with DNA to induce or repress the expression of a large number of different genes. The nuclear hormone receptors are all characterized by a central DNA binding domain which targets the receptor to specific DNA sequences (response elements). The DNA binding domain is composed of two highly conserved zinc fingers that distinguish these nuclear receptors from other DNA binding proteins. The C-terminal portion of the receptor includes the ligand binding domain which recognizes specific hormones, vitamins, retinoids or other lipophilic compounds. The interaction of a ligand with its specific receptor shifts the receptor to a transcriptionally active state which allows the DNA binding domain to regulate gene expression.
Recently, it has been recognized that endogenous intracellular lipids activate certain nuclear hormone receptors (i.e., PPARs, LXR and FXR).Citation10 Whereas PPARs are activated by fatty acids and their metabolic products, LXRs are activated by certain oxysterol metabolites of cholesterol while farnesoid-X receptor (FXR), which is not expressed in keratinocytes, is activated by bile acids. These receptors heterodimerize with RXR and bind to direct repeats separated by a variable number of spacer nucleotides.Citation10,Citation11 The activation of these nuclear hormone receptors regulates the expression of many genes, thereby influencing a variety of cellular functions. The ability of these nuclear hormones to sense cellular lipid levels and subsequently to regulate gene expression has led to their designation as “liposensors.”Citation10
PPARs and LXRs (For detailed information see ref. Citation10–Citation14)
PPARα.
PPARα is expressed in liver, heart, kidney, muscle and brown fat, as well as the epidermis and its appendages. This receptor is activated by unsaturated fatty acids, metabolites of fatty acids and farnesol (an isoprenoid formed during cholesterol synthesis), as well as by the fibrate class of drugs. Clofibrate, gem-fibrozil and fenofibrate are drugs that activate PPARα and are widely used to treat dyslipidemias. PPARα is expressed in keratinocytes and the epidermis and mRNA and protein levels increase during keratinocyte differentiation.
PPARβ/Δ.
PPARβ/Δ is expressed ubiquitously and activated by fatty acids. Drugs that activate PPARβ/Δ (for example GW 1516) increase serum HDL while lowering serum triglyceride in obese rhesus monkeys. In keratinocytes and epidermis, PPARβ/Δ is expressed at high levels, and its expression is not altered by differentiation. Studies have shown that PPARβ/Δ expression in the epidermis is increased by phorbol esters, hair plunking, wounding and epidermal inflammation.
PPARγ.
PPARγ is predominantly expressed in fat storing and synthesizing tissues, such as adipocytes, where it stimulates free fatty acid uptake, lipogenesis and differentiation. It is activated by prostaglandin J2, fatty acids and the thiazolidindione drugs (TZDs). PPARγ is the key transcription factor required for the differentiation of fibroblasts into adipocytes. Drugs, such as troglitazone, pioglitazone and rosiglitazone, reduce insulin resistance in patients with Type 2 diabetes and improve glycemic control. PPARγ is expressed in keratinocytes and epidermis, and keratinocyte/epidermal differentiation result in increases in PPARγ mRNA levels.
LXR.
Two genes encode LXR paralogues, which are differentially expressed (i.e., LXR α is abundantly expressed in liver, kidney, spleen, adrenal and intestine, while LXR-β expression is ubiquitous). Both isoforms are activated by the same oxysterol metabolites, and by nonsterol compounds developed by the pharmaceutical industry (for example GW 3965 or TO-901317). Activation of LXR increases the reverse cholesterol transport pathway (i.e., movement of cholesterol from peripheral cells to the liver for excretion into the bile). Both LXR α and β isoforms are present in human keratinocytes and fetal rat epidermis, but in mouse epidermis only LXR-β was detected.
Activation of PPARs and LXRs Stimulates Keratinocyte Differentiation
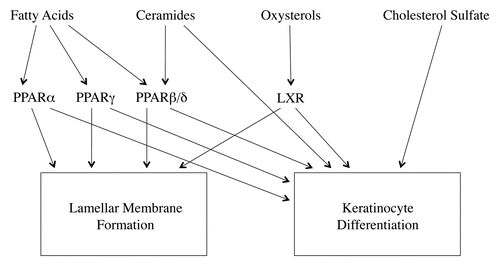
Treatment of cultured keratinocytes with activators of PPARα, PPARβ/Δ, PPARγ or LXR stimulated cornified envelope formation and increased the protein and mRNA levels of markers of keratinocyte differentiation, such as involucrin, filaggrin, loricrin and transglutaminase 1.Citation14–Citation22 This stimulation of keratinocyte differentiation induced by PPAR and LXR activators was seen in both undifferentiated proliferating keratinocytes and in differentiated keratinocytes (i.e., keratinocytes grown under high calcium conditions). Similar to the in vitro results, topical application of PPARα, PPARβ/Δ, PPARγ or LXR activators to mouse skin also stimulated keratinocyte differentiation.Citation14,Citation19,Citation20,Citation23–Citation25 Moreover, in knock-out mice deficient in either PPARα, PPARβ/Δ, PPARγ or LXR, the respective activators did not induce the expression of these differentiation markers indicating that this stimulation was receptor mediated.Citation14,Citation19,Citation20,Citation23–Citation25 Thus, as fatty acid levels increase in keratinocytes to allow for the synthesis of the lipids required for lamellar body formation one can hypothesize that these fatty acids would activate PPARs and thereby also stimulate keratinocyte differentiation.Citation2,Citation14 Similarly, as cholesterol levels increase to provide for lamellar body synthesis one can speculate that this would lead to the increased production of oxysterols that activate LXR thereby also stimulating keratinocyte differentiationCitation2,Citation14 ().
The promoter regions of many of the genes that increase during differentiation have AP-1 binding sites and their expression is known to be enhanced by increased AP-1 binding (for example involucrin, loricrin, transglutaminase 1). Studies have shown that deletions or mutations in the distal AP-1 site of the involucrin promoter prevented PPARα activators from increasing involucrin expression.Citation16,Citation24 Similarly, deletions or mutations in the distal AP-1 site of the involucrin promoter also abolished the ability of LXR activators to increase involucrin expression.Citation17 Moreover, increased AP-1 DNA binding was observed in nuclear extracts from keratinocytes treated with LXR activators and there was an increase in Fra-1 and Jun D mRNA and protein levels.Citation17,Citation26 These results suggest that PPAR/LXR activation of AP-1 transcription maybe one pathway by which PPAR/LXR activators increase the expression of differentiation related proteins.
Activation of PPARs and LXRs Stimulates Lamellar Membrane Formation
As discussed in detail in previous reviews the formation of the lipid enriched lamellar membranes in the extracellular space of the stratum corneum is a complex process that requires a number of stepsCitation1,Citation2 (). First, the keratinocyte requires sufficient cholesterol, phospholipids and glucosylceramides to form lamellar bodies (note fatty acids are required for the formation of phospholipids and ceramides and ceramides are required for the formation of glucosylceramide). Second, the lipids must be transported into the lamellar bodies. Third the keratinocyte needs to secrete the lamellar bodies into the extracellular space of the stratum corneum. Finally, the secreted phospholipid must be converted to fatty acids and the secreted glucosylceramide must be metabolized to ceramides in order to form a mature competent lamellar membrane that provides a barrier to the movement of water and electrolytes.
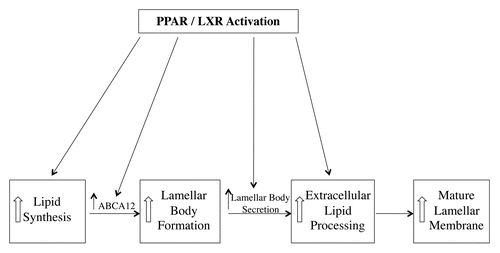
Topical treatment with activators of PPARα, PPARβ/Δ, PPARγ and LXR increased cholesterol, fatty acid and ceramide synthesis in mouse epidermis.Citation27 In human organotypic skin cultures PPARα activators increased the expression of key enzymes required for lipid synthesis including serine-palmitoyltransferase, long chain acyl-CoA synthase and HMG-CoA synthase.Citation28 Additionally, PPARγ, PPARβ/Δ, and LXR activators increase the expression of GPAT3, the initial enzyme in the biosynthesis of triglycerides and phospholipids.Citation29 One would anticipate that an increase in epidermal lipid synthesis would help provide the lipids necessary for keratinocytes to synthesize lamellar bodies.
ABCA12 facilitates the delivery of glucosylceramide into lamellar bodies and is required for lamellar body formation. PPARβ/Δ, PPARγ and LXR activators stimulate ABCA12 expression in human keratinocytes in a dose and time dependent manner.Citation30 This increase in ABCA12 would facilitate the synthesis of lamellar bodies. Recent studies have suggested that ABCG1 may also play a role in the formation of lamellar bodies.Citation31 Moreover, PPAR and LXR activators increase the expression of ABCG1, which may facilitate lamellar body formation.Citation31
Topical treatment of mice with PPARα, PPARβ/Δ and LXR activators enhanced lamellar body secretion in both the basal state and after acute permeability barrier disruption.Citation27 In contrast, topical treatment with activators of PPARγ did not alter lamellar body secretion.Citation27 Moreover, mice deficient in PPARβ/Δ but not other PPARs or LXRs, have a decrease in lamellar body formation and secretion.Citation32 Similarly, RXRα or RXRα/RXR β double knockout mice also demonstrate a defect in the lamellar body secretory system very similar to what is observed in PPARβ/Δ deficient mice.Citation33 Significantly, treatment of the RXR deficient mice with a PPAR β/Δ activator corrected the abnormalities in the lamellar body secretory system.Citation33 These results suggest that PPARβ/Δ may be the key PPAR isoform required for lamellar body formation and secretion. Moreover, these results indicate that activation of PPARs and LXRs could enhance lamellar body secretion.
The lipids in lamellar bodies are metabolized in the extracellular spaces of the stratum corneum to form mature lamellar membranes.Citation1,Citation2 Of particular note glucosylceramide is converted to ceramide by the enzyme β-glucocerebrosidase. Topical treatment of mouse epidermis with activators of PPARs and LXRs increase the activity of β-glucocerebrosidase.Citation27 Additional, treatment of human organotypic cultures have shown that PPARα activators increased β-glucocerebrosidase expression.Citation28 This increase in enzyme activity would facilitate the extracellular lipid processing and enhance lamellar membrane formation.
Given the above results it is not surprising that topical treatment with PPAR and LXR activators improves permeability barrier homeostasis. In the basal state where permeability barrier function is already ideal PPAR and LXR activators do not result in a reduction in transepidermal water loss, a sensitive measure of permeability barrier function.Citation27 However, following acute permeability barrier disruption the restoration of permeability barrier function was accelerated by PPAR and LXR activators.Citation27 As discussed above there are a number of key steps in the formation of the competent lamellar membranes that mediate permeability barrier function and treatment with PPAR and LXR activators enhances these steps (). Thus, in addition to stimulating the formation of corneocytes (the “bricks”), activation of PPARs and LXRs also stimulates the formation of the extracellular lipid matrix (the “mortar”).
Cholesterol Sulfate
Cholesterol sulfate is very abundant in the epidermis with the highest levels in the granular layer and a progressive decline from inner to outer stratum corneum.Citation2,Citation8 A key role for cholesterol sulfate in desquamation has been demonstrated in X-linked ichthyosis, where a defect in the steroid sulfatase gene allows cholesterol sulfate levels to reach levels ten-fold higher than normal, resulting in abnormal corneocyte retention and a thickened stratum corneum.Citation8 In addition to its role in desquamation, cholesterol sulfate stimulates keratinocyte differentiation.Citation34,Citation35 Incubation of keratinocytes with cholesterol sulfate has been shown to increase cornified envelope formation and stimulate the expression of filaggrin, loricrin, involucrin and transglutaminases 1. Cholesterol sulfate stimulates keratinocyte differentiation via a number of different mechanisms, including the activation of protein kinase C and directly by affecting gene transcription.Citation34,Citation35 Thus, the increase in cholesterol sulfate levels in the epidermis could be a positive signal to further enhance keratinocyte differentiation.
Cholesterol sulfate is synthesized in the epidermis by the cytosolic enzyme cholesterol sulfotransferase. Recent studies have shown that cholesterol sulfotransferase type 2B isoform 1b (SULT2B1b) is the isozyme that catalyzes the synthesis of cholesterol sulfate in the epidermis/keratinocytes.Citation36 SULT2B1b expression increases during calcium induced differentiation in parallel with an increase in cholesterol sulfotransferase activity.Citation36,Citation37 Moreover, PPAR and LXR activators increase SULT2B1b expression in both undifferentiated and differentiated keratinocytes.Citation37 Thus, increases in fatty acids and/or cholesterol via oxysterols could activate PPARs and LXRs and stimulate the expression of SULT2B1b leading to increased synthesis of cholesterol sulfate, which would then further enhance keratinocyte differentiation ().
Ceramides
As keratinocytes differentiate ceramide production increases to provide the building blocks for lamellar body formation.Citation1,Citation2 Wakita and colleagues were the first to demonstrate that ceramides stimulate keratinocyte differentiation and inhibit proliferation.Citation38 Cornified envelope formation, involucrin expression and transglutaminase activity are increased in keratinocytes treated with cell permeable ceramides.Citation38–Citation40 The mechanisms by which ceramides enhance keratinocyte differentiation are not fully understood but ceramides increase AP-1 activityCitation39,Citation41 and as noted earlier in this review many of the genes whose expression is increased during keratinocyte differentiation contain AP-1 response elements in their promoters and their expression is stimulated by AP-1. Thus, increasing ceramide levels in differentiating keratinocytes could provide another signaling system to link lamellar membrane formation (the “mortar”) with corneocyte formation (the “bricks”) ().
Recent studies have further shown that ceramides also increase ABCA12 expression in keratinocytes.Citation42 Interestingly this stimulation of ABCA12 expression by ceramides is mediated via PPARΔ. Ceramides increased the expression of PPARΔ but not other PPARs or LXRs.Citation42 This increase in PPARΔ expression is mediated by increased AP-1 activity.Citation41,Citation42 Moreover, PPARΔ knockdown with siRNA attenuated the ability of ceramides to increase ABCA12 expression.Citation42 Of note, PPARΔ knockdown did not affect the ability of ceramides to stimulate involucrin expression,Citation42 which is likely accounted for by the ability of AP-1 to directly stimulate involucrin expression. One can view the ceramide induced increase in ABCA12 expression as a feed forward effect. As the keratinocyte produces increasing ceramides, which will be converted to glucosylceramides, the ceramides provide a signal to increase ABCA12 expression to facilitate the transport of glucosylceramides into lamellar bodies.
Conclusion
The formation of a normal functional stratum corneum requires both extracellular lipid membranes and corneocytes (“bricks and mortar”). In this review we describe a number of pathways by which the increasing quantities of lipids required for lamellar body synthesis and lamellar membrane formation could regulate corneocyte formation. In addition, we describe how the accumulation of lipids could also regulate lipid metabolism to facilitate the formation of lamellar bodies. The formation of a normal stratum corneum is a complex process that must be precisely coordinated and it is likely that lipids play an important role in orchestrating this process.
Figures and Tables
Acknowledgements
The authors appreciate the helpful discussions and insights provided by members of the Dermatology Service at the San Francisco VA Medical Center, particularly Peter Elias, Walter Holleran, Yoshikazu Uchida, Mao-Qiang Man and Theodora Mauro. We also appreciate the constructive comments of Carl Grunfeld. We would also like to thank Arthur Moser for creating the figures. This work was supported by grants from the NIH and the Research Service of the Department of Veterans Affairs.
References
- Elias PM. Stratum corneum defensive functions: an integrated view. J Invest Dermatol 2005; 125:183 - 200
- Feingold KR. Thematic review series: skin lipids. The role of epidermal lipids in cutaneous permeability barrier homeostasis. J Lipid Res 2007; 48:2531 - 2546
- Eckert RL, Crish JF, Robinson NA. The epidermal keratinocyte as a model for the study of gene regulation and cell differentiation. Physiol Rev 1997; 77:397 - 424
- Fuchs E. Epidermal differentiation. Curr Opin Cell Biol 1990; 2:1028 - 1035
- Kalinin AE, Kajava AV, Steinert PM. Epithelial barrier function: assembly and structural features of the cornified cell envelope. Bioessays 2002; 24:789 - 800
- Elias PM, Schmuth M, Uchida Y, Rice RH, Behne M, Crumrine D, et al. Basis for the permeability barrier abnormality in lamellar ichthyosis. Exper Dermatol 2002; 11:248 - 256
- Schmuth M, Fluhr JW, Crumrine DC, Uchida Y, Hachem JP, Behne M, et al. Structural and functional consequences of loricrin mutations in human loricrin keratoderma (Vohwinkel syndrome with ichthyosis). J Invest Dermatol 2004; 122:909 - 922
- Elias PM, Williams ML, Holleran WM, Jiang YJ, Schmuth M. Pathogenesis of permeability barrier abnormalities in the ichthyoses: inherited disorders of lipid metabolism. J Lipid Res 2008; 49:697 - 714
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, et al. The nuclear receptor superfamily: the second decade. Cell 1995; 83:835 - 839
- Chawla A, Repa JJ, Evans RM, Mangelsdorf DJ. Nuclear receptors and lipid physiology: opening the X-files. Science 2001; 294:1866 - 1870
- Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell 1995; 83:841 - 850
- Kalaany NY, Mangelsdorf DJ. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Ann Rev Physiol 2006; 68:159 - 191
- Kliewer SA, Xu HE, Lambert MH, Willson TM. Peroxisome proliferator-activated receptors: from genes to physiology. Recent Prog Horm Res 2001; 56:239 - 263
- Schmuth M, Jiang YJ, Dubrac S, Elias PM, Feingold KR. Thematic review series: skin lipids. Peroxisome proliferator-activated receptors and liver X receptors in epidermal biology. J Lipid Res 2008; 49:499 - 509
- Hanley K, Jiang Y, He SS, Friedman M, Elias PM, Bikle DD, et al. Keratinocyte differentiation is stimulated by activators of the nuclear hormone receptor PPARalpha. J Invest Dermatol 1998; 110:368 - 375
- Hanley K, Komuves LG, Ng DC, Schoonjans K, He SS, Lau P, et al. Farnesol stimulates differentiation in epidermal keratinocytes via PPARalpha. J Biol Chem 2000; 275:11484 - 11491
- Hanley K, Ng DC, He SS, Lau P, Min K, Elias PM, et al. Oxysterols induce differentiation in human keratinocytes and increase Ap-1-dependent involucrin transcription. J Invest Dermatol 2000; 114:545 - 553
- Kim S, Hong I, Hwang JS, Choi JK, Rho HS, Kim DH, et al. Phytosphingosine stimulates the differentiation of human keratinocytes and inhibits TPA-induced inflammatory epidermal hyperplasia in hairless mouse skin. Mol Med 2006; 12:17 - 24
- Mao-Qiang M, Fowler AJ, Schmuth M, Lau P, Chang S, Brown BE, et al. Peroxisome-proliferator-activated receptor (PPAR)gamma activation stimulates keratinocyte differentiation. J Invest Dermatol 2004; 123:305 - 312
- Schmuth M, Haqq CM, Cairns WJ, Holder JC, Dorsam S, Chang S, et al. Peroxisome proliferator-activated receptor (PPAR)beta/delta stimulates differentiation and lipid accumulation in keratinocytes. J Invest Dermatol 2004; 122:971 - 983
- Thuillier P, Brash AR, Kehrer JP, Stimmel JB, Leesnitzer LM, Yang P, et al. Inhibition of peroxisome proliferator-activated receptor (PPAR)-mediated keratinocyte differentiation by lipoxygenase inhibitors. Biochem J 2002; 366:901 - 910
- Westergaard M, Henningsen J, Svendsen ML, Johansen C, Jensen UB, Schroder HD, et al. Modulation of keratinocyte gene expression and differentiation by PPAR-selective ligands and tetradecylthioacetic acid. J Invest Dermatol 2001; 116:702 - 712
- Kim DJ, Bility MT, Billin AN, Willson TM, Gonzalez FJ, Peters JM. PPARbeta/delta selectively induces differentiation and inhibits cell proliferation. Cell Death Differ 2006; 13:53 - 60
- Komuves LG, Hanley K, Lefebvre AM, Man MQ, Ng DC, Bikle DD, et al. Stimulation of PPARalpha promotes epidermal keratinocyte differentiation in vivo. J Invest Dermatol 2000; 115:353 - 360
- Komuves LG, Schmuth M, Fowler AJ, Elias PM, Hanley K, Man MQ, et al. Oxysterol stimulation of epidermal differentiation is mediated by liver X receptor-beta in murine epidermis. J Invest Dermatol 2002; 118:25 - 34
- Schmuth M, Elias PM, Hanley K, Lau P, Moser A, Willson TM, et al. The effect of LXR activators on AP-1 proteins in keratinocytes. J Invest Dermatol 2004; 123:41 - 48
- Man MQ, Choi EH, Schmuth M, Crumrine D, Uchida Y, Elias PM, et al. Basis for improved permeability barrier homeostasis induced by PPAR and LXR activators: liposensors stimulate lipid synthesis, lamellar body secretion and post-secretory lipid processing. J Invest Dermatol 2006; 126:386 - 392
- Rivier M, Castiel I, Safonova I, Ailhaud G, Michel S. Peroxisome proliferator-activated receptor-alpha enhances lipid metabolism in a skin equivalent model. J Invest Dermatol 2000; 114:681 - 687
- Lu B, Jiang YJ, Kim P, Moser A, Elias PM, Grunfeld C, Feingold KR. Expression and regulation of GPAT isoforms in cultured human keratinocytes and rodent epidermis. J Lipid Res 2010; 51:3207 - 3216
- Jiang YJ, Lu B, Kim P, Paragh G, Schmitz G, Elias PM, Feingold KR. PPAR and LXR activators regulate ABCA12 expression in human keratinocytes. J Invest Dermatol 2008; 128:104 - 109
- Jiang YJ, Lu B, Tarling EJ, Kim P, Man MQ, Crumrine D, et al. Regulation of ABCG1 expression in human keratinocytes and murine epidermis. J Lipid Res 2010; 51:3185 - 3195
- Man MQ, Barish GD, Schmuth M, Crumrine D, Barak Y, Chang S, et al. Deficiency of PPARbeta/delta in the epidermis results in defective cutaneous permeability barrier homeostasis and increased inflammation. J Invest Dermatol 2008; 128:370 - 377
- Calleja C, Messaddeq N, Chapellier B, Yang H, Krezel W, Li M, et al. Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev 2006; 20:1525 - 1538
- Denning MF, Kazanietz MG, Blumberg PM, Yuspa SH. Cholesterol sulfate activates multiple protein kinase C isoenzymes and induces granular cell differentiation in cultured murine keratinocytes. Cell Growth Differ 1995; 6:1619 - 1626
- Hanley K, Wood L, Ng DC, He SS, Lau P, Moser A, et al. Cholesterol sulfate stimulates involucrin transcription in keratinocytes by increasing Fra-1, Fra-2 and Jun D. J Lipid Res 2001; 42:390 - 398
- Higashi Y, Fuda H, Yanai H, Lee Y, Fukushige T, Kanzaki T, Strott CA. Expression of cholesterol sulfotransferase (SULT2B1b) in human skin and primary cultures of human epidermal keratinocytes. The J Invest Dermatol 2004; 122:1207 - 1213
- Jiang YJ, Kim P, Elias PM, Feingold KR. LXR and PPAR activators stimulate cholesterol sulfotransferase type 2 isoform 1b in human keratinocytes. J Lipid Res 2005; 46:2657 - 2666
- Wakita H, Tokura Y, Yagi H, Nishimura K, Furukawa F, Takigawa M. Keratinocyte differentiation is induced by cell-permeant ceramides and its proliferation is promoted by sphingosine. Arch Dermatol Res 1994; 286:350 - 354
- Geilen CC, Barz S, Bektas M. Sphingolipid signaling in epidermal homeostasis. Current knowledge and new therapeutic approaches in dermatology. Skin Pharmacol Appl Skin Physiol 2001; 14:261 - 271
- Pillai S, Cho S, Mahajan M, Frew L, Rawlings AV. Synergy between vitamin D precursor 25-hydroxyvitamin D and short chain ceramides on keratinocyte proliferation and differentiation. J Investig Dermatol Symp Proc 1996; 1:39 - 43
- Tan NS, Michalik L, Noy N, Yasmin R, Pacot C, Heim M, et al. Critical roles of PPARbeta/delta in keratinocyte response to inflammation. Genes Dev 2001; 15:3263 - 3277
- Jiang YJ, Uchida Y, Lu B, Kim P, Mao C, Akiyama M, et al. Ceramide stimulates ABCA12 expression via peroxisome proliferator-activated receptor {delta} in human keratinocytes. J Biol Chem 2009; 284:18942 - 18952