Abstract
The melanocortin 1-receptor (MC1R) exhibits several variants in form of single nucleotide polymorphisms (SNPs) which are known to differentially regulate melanocyte function. However, whether and how MC1R polymorphisms also affect fibroblast function, has not been investigated so far. Therefore we measured intracellular cyclic adenosine monophosphate (cAMP) concentrations (cAMP-EIA) and cellular proliferation (CellTiter-Blue) upon stimulation with alpha-melanocyte stimulating hormone (α-MSH) in eight different human fibroblast and melanocyte cell lines with wild type and different MC1R SNPs. We found that fibroblasts, as well as melanocytes, show differences in MC1R function depending on the MC1R genotype. MC1R stimulation with α-MSH in wild type (MC1Rwt) melanocytes results in an increase of intracellular cAMP and cellular proliferation. In contrast, MC1Rwt fibroblasts react with a decrease of intracellular cAMP and proliferation. In MC1R polymorphic fibroblasts (R163Q, R151C and V60L) both effects are significantly alleviated. Similar, but inverse effects could be found in MC1R polymorphic melanocytes (R142H and V92M) with a significantly lower cAMP increase and proliferation rate compared to MC1Rwt melanocytes. Our results indicate that the MC1R displays reciprocal growth responses in melanocytes and fibroblasts, depending on the MC1R genotype. Thus, the MC1R seems to be not solely important for the skin pigmentary system, but also for the fibroblast function, and might influence different processes of the dermal compartment like wound healing, fibrosis and keloid formation.
Introduction
The Proopiomelanocortin (POMC) system of the human skin consists of peptide hormones (MSH, Adenocorticotropic Hormone (ACTH), Endorphins, etc.,) that originate from the precursor protein POMC, and the corresponding receptors (Melanocortin receptors, Endorphin receptors). The Melanocortin 1 receptor (MC1R) represents one out of a group of five G-protein coupled receptors (MC1R-MC5R) ubiquitously expressed in all cells of the skin (keratinocytes, melanocytes, fibroblasts, cells of the immune system). MC1R consists of seven transmembrane domains and has a high affinity for POMC derived α-MSH. The activation of the MC1R leads to an increase of intracellular cAMP levels which trigger the activation of protein kinase A (PKA). PKA phosphorylates the cAMP responsive binding protein (CREB), which binds to the cAMP responsive element (CRE) in the promoter region of the microphthalmia transcription factor (MITF). For full activation of the MITF-promoter, CREB needs to bind the CREB binding protein (CBP).Citation1 MITF is a member of the MYC-superfamily of basic-helix-loop-helix (bHLH) zipper-transcription factors and activates genes involved in both skin pigmentation and collagen metabolism.Citation1–Citation3
The MC1R gene is localised on the long arm of Chromosom 16 (16q24.3).Citation4,Citation5 The gene consists of an intronless coding region and is highly polymorphic.Citation6–Citation8 Robbins et al. described the association of some MC1R polymorphisms and receptor functionality modulating the fur colour of mice.Citation9 Based on this discovery, several investigations of the functionality of the MC1R variants were carried out in humans. Population studies showed that several MC1R single nucleotide polymorphisms (SNPs) (D84E, R151C, R160W, D294H, R142H, I155T, V60L, V92M, R163Q) are associated with a poor tanning ability and an augmented risk to develop skin cancer, designating the MC1R a melanoma susceptibility gene (red hair and fair skin (RHC) phenotype).Citation10–Citation16
Interestingly, the alleles differ in their penetrance concerning the RHC phenotype.Citation17,Citation18 The strong RHC alleles comprise the common polymorphisms R151C, R160W and D294H and rare polymorphisms D84E and R142H, which increase odds ratios for red hair 50–120 fold. The weak RHC alleles V60L, V92M and R163Q have odds ratios for red hair between 2 and 6. Polymorphisms R142H, R151C, R160W, D294H and V60L are found in 30% of the individuals of north-european populations and are responsible for 30% of all red haired phenotypes.Citation19
On the molecular level, it has been shown that the aforementioned RHC alleles R142H, R151C, R160W and D294H affect MC1R function.Citation20,Citation21 However, the molecular mechanisms of these effects are still not clarified. One possibility is, according to some recent studies, that the intracellular retention of the receptor and the receptor density on the plasma membrane is different depending on different MC1R variants.Citation20,Citation21 On the other hand the fact that the MC1R-variants differ in their ability to generate cAMP implicates varying degrees in the activation of the involved G-proteins. As heterozygote variants reveal an intermediate functional state between wt and homozygote variants a gene-dose-effect can be assumed.Citation22,Citation23
Because of the important role of MC1R in the pigmentary system of the skin, most investigations of its polymorphisms on a cellular level were done in melanocytes so far.Citation3,Citation22 These studies could demonstrate that MC1R polymorphisms compared to MC1Rwt not only impair melanogenesis, but also affect the UV-response of melanocytes, leading to an augmented growth arrest and apoptosis by ineffective DNA-repair. These findings explain the role or MC1R polymorphisms as a major melanoma risk factor.Citation24–Citation31
In fibroblasts, the MC1R has been shown to regulate fibroblast function by affecting the synthesis and degradation of collagen.Citation32,Citation33 In contrast to melanocytes, the impact of MC1R polymorphisms on receptor function in fibroblasts has not been investigated so far.
The aim of this study was therefore to investigate the functional relevance of MC1R wt compared with MC1R polymorphisms in dermal fibroblasts, and to relate the findings to melanocytes, which are well characterized concerning MC1R, as positive control.
Results
Characterization of MC1R gene in melanocyte and fibroblast cell lines.
MC1R gene was characterised by sequencing several melanocyte and fibroblast cell lines. We found two MC1R wild-type (MC1Rwt) and two MC1R polymorphic melanocyte cell lines (homozygous V92M and heterozygous R142H), and one MC1Rwt and three MC1R polymorphic fibroblast cell lines (heterozygous R163Q, heterozygous V60L and heterozygous R151C) respectively ().
Reciprocal cAMP induction and growth responses in MC1Rwt melanocytes and fibroblasts after α-MSH stimulation.
First, we compared the α-MSH effect on cAMP production and cell proliferation in melanocytes and fibroblasts with MC1Rwt (). MC1Rwt melanocytes showed a significant increase in cellular cAMP concentration of 50% (p = 0.003) following α-MSH (1 µM) treatment (), whereas the cAMP of fibroblasts showed a decrease of 60% (p = 0.007) ().
The proliferation measurements of MC1Rwt melanocytes and MC1Rwt fibroblasts with and without α-MSH stimulation are depicted in and D. Measuring time points were: day 0 (the day when α-MSH was added, see methods), day 2 and in the case of fibroblasts additionally day 4. The additional measuring point (day 4) was added as the effect of α-MSH on fibroblast growth was less pronounced.
Untreated melanocytes showed a proliferation of 46% from day 0 to day 2 whereas the α-MSH stimulated melanocytes revealed a proliferation of 75% for the same time period. The difference of 29% between the non-stimulated and stimulated MC1Rwt melanocytes was highly significant (p < 0.001) ().
The untreated MC1Rwt fibroblasts showed an increase in proliferation from day 0 to day 4 of 484%, whereas the fibroblasts, treated with α-MSH, showed a lower increase of 434%. The growth reduction in fibroblast was significant: 50% from day 0 to day 4 (p < 0.001) ().
In summary, the results in show that α-MSH induces a significant augmentation of both cAMP and cell proliferation in MC1Rwt melanocytes. On the contrary, in MC1Rwt fibroblasts, both cAMP levels and cell growth were significantly reduced.
Functional implications of MC1R polymorphisms on cAMP induction and proliferation.
Since we could demonstrate that α-MSH has inverse biologic effects on MC1Rwt fibroblasts and melanocytes, we intended to examine, if and how MC1R polymorphisms would affect the observed responses to α-MSH.
In and C cAMP measurements in MC1R polymorphic melanocytes treated or untreated with α-MSH, are shown. The increase in cAMP for R142H and V92M cell lines was 2.5% (p = 0.326) and 10.5% (p = 0.32) respectively. The proliferation measurements from the same cell lines stimulated with α-MSH are presented in and D. Hormone stimulation induced an increase in proliferation on day 2 of 23% (p < 0.001) and 12% (p < 0.001) for R142H and V92M cell lines respectively.
The polymorphic fibroblast cell lines underwent similar treatment as the melanocytes. Measurements of intracellular cAMP following α-MSH stimulation in R163Q, R151C and V60L are shown in , C and E respectively. The cell lines displaying MC1R polymorphisms showed no significant activation of the MC1R. We could observe a reduction in cAMP of 26% (p = 0.1) or increase of 0.6% (p = 0.49) and 26% (p = 0.26) for R163Q, R151C and V60L cell lines respectively, which all failed to reach significance.
Proliferation measurements of each particular fibroblast cell line are shown in , D and F. R163Q cells showed a minor decrease of cell growth after treatment with α-MSH of 8% (p = 0.06) from day 0 to day 4 (). The proliferation measurements for the other two polymorphic fibroblast cell lines R151C and V60L showed similar minimal decreases of 7% (p = 0.07) and 6% (p = 0.06) from day 0 to day 4.
The results presented in show that fibroblasts with MC1R polymorphisms, consistent with an impaired receptor function, do not react with a significant decrease of cAMP levels and do not show a significant reduction of growth.
cAMP production and proliferation differ significantly between MC1Rwt and MC1R polymorphic melanocytes and fibroblasts.
Regarding the results presented in – we were interested to see, if the differences between wt and polymorphic cell lines were significant in both cell types. For that purpose we compared the pooled data for cAMP levels in MC1Rwt and MC1R polymorphic melanocytes and fibroblasts stimulated with α-MSH (). Our statistical analysis showed that cAMP levels in MC1Rwt melanocytes are significantly higher than in the polymorphic cell lines (p = 0.013) (), whereas cAMP levels in MC1Rwt fibroblasts are significantly lower than in the fibroblasts with MC1R polymorphisms (p = 0.036) (). The cAMP concentrations in untreated wild-type versus polymorphic cells were not significantly different (p = 0.266 and p = 0.312).
An analogous examination was also performed for the effects of α-MSH on proliferation. In MC1Rwt melanocytes we found a significantly stronger increase in proliferation than in MC1R polymorphic melanocytes (p = 0.008) (), whereas MC1Rwt fibroblasts showed a significantly stronger inhibition of proliferation compared to the MC1R polymorphic fibroblasts (p = 0.003) ().
These findings emphasise the reciprocal response to MC1R stimulation in melanocytes and fibroblasts, and underline the significant inverse effects of MC1R polymorphisms on the receptor function in melanocytes as well as in fibroblasts.
Discussion
As already well known, stimulation of MC1Rwt melanocytes with α-MSH leads to a G-protein coupled induction of cAMP driving the CRE-MITF pathway and promoting melanin synthesis and cell proliferation.Citation1–Citation3 MC1R polymorphisms contribute to a different receptor function, which additionally depends upon whether one or both alleles are affected: gain of function, no change, reduction or loss of function.Citation22 The most important finding of our study is that MC1Rwt melanocytes and MC1Rwt fibroblasts display a reciprocal MC1R function concerning cAMP induction and cell growth. Furthermore, we could show that polymorphisms of MC1R also affect the receptor function in dermal fibroblasts, what has only been shown for human melanocytes so far.
As expected, cAMP did not increase significantly in both polymorphic melanocyte cell lines after α-MSH stimulation and were significantly lower compared to MC1Rwt melanocytes. We were surprised to observe that proliferation in MC1R polymorphic melanocytes seems not to be compromised, as significant differences could be found after stimulation with α-MSH versus unstimulated melanocytes. Given that cAMP levels correlate with the receptor functionality of MC1R, our results suggest that either even minimal MC1R activation suffice to stimulate cellular proliferation in melanocytes irrespective of the polymorphisms investigated here or α-MSH exhibits MC1R-independent yet unknown effects in melanocytes. However, comparing proliferation rates of MC1Rwt and polymorphic MC1R melanocytes the cell growth after stimulation with α-MSH was significantly more pronounced in MC1Rwt melanocytes. These findings are in agreement with previously published reports in reference Citation22, Citation25 and Citation26.
In contrast to melanocytes, MC1Rwt fibroblasts revealed significantly decreased cAMP levels and decelerated cell growth following α-MSH stimulation. Heterozygous MC1R polymorphisms R163Q, V60L, R151C found in our fibroblast cell lines accordingly abolished these inhibitory effects of α-MSH: cAMP levels and fibroblast proliferation were not significantly affected compared to untreated cells, suggesting an impaired receptor function. These observations are additionally supported by the finding that cAMP levels and growth rate after stimulation with α-MSH are significantly lower in fibroblast with MC1Rwt compared to MC1R polymorphic fibroblasts.
Concerning the inhibitory effects of α-MSH on MC1Rwt fibroblasts our results are contrary to recently published data by Böhm and colleagues, who found an increase of intracellular cAMP in fibroblasts after α-MSH stimulation.Citation33 The fibroblasts investigated in that study were however not further characterized concerning their MC1R gene sequence. Thus, MC1R polymorphisms could be the explanation for this controversy, as we could demonstrate positive, albeit not significant effects of α-MSH on intracellular cAMP in MC1RV60L fibroblasts. The inhibitory effects of α-MSH on MC1Rwt fibroblasts may provide a link to the antifibrotic properties of α-MSH described in the literature.Citation33,Citation34
The different downstream mechanisms after MC1R activation inducing opposite effects of α-MSH in melanocytes and fibroblasts are not defined yet. Possibly, the intrinsic receptor activity and the regulation of MC1R (phosphorylation, internalisation, degradation and recycling) play a role.Citation35,Citation36 However, divergent signalling pathways or different sets of target genes depending on epigenetic mechanisms in different cell types are further considerations. Nevertheless the contrary effects of α-MSH on melanocytes and fibroblasts could be a hint to a key role of the cutaneous POMC system as a complex player in the endocrine adaptation and coordination of skin functions.
Our findings suggest that besides the importance for individual pigmentation,Citation8 MC1R polymorphisms can regulate other physiological processes in the skin. Regarding the fibroblasts, α-MSH delivers antifibrotic effects through modulation of collagen metabolismCitation33,Citation34 and, as shown in this study, by a MC1R mediated inhibition of proliferation of MC1Rwt fibroblasts. This could have significant clinical importance. For instance, in a mouse model of scleroderma α-MSH reduced the bleomycin induced collagen-synthesis and tissue fibrosis.Citation34 Furthermore, there is evidence for a concomitant cytoprotective and anti-apoptotic effect of α-MSH in dermal fibroblasts.Citation37 In addition, certain MC1R polymorphisms have been associated with an increased risk for severe photoaging of facial skinCitation38 while patients having MC1RR160W responded with a significantly increased risk for severe acute radiosensitivity after radiotherapy.Citation39
In conclusion, our work may spark investigating the role of MC1R polymorphisms in skin aging as well as in a variety of fibrosing diseases such as keloids, systemic sclerosis, arterial fibrosclerosis and nephrosclerosis, which are found more often in pigmented races compared to Caucasians.Citation40–Citation43 These studies are ongoing in our laboratory.
Materials and Methods
Cell culture.
Primary human melanocyte and fibroblast cultures were established from neonatal foreskins of healthy individuals with different pigmentation obtained from the Department of Urology, University Hospital of the Saarland. The cells were incubated at 37°C, 5% CO2 and 95% humidity. The next day medium was changed to discard cell debris. To obtain a monoculture of primary human dermal fibroblasts from the isolated cell mix the medium was changed to DMEM (Invitrogen) containing 10% FCS (Promocell) and 1% Penicillin and Streptomycin (Sigma). To establish a mono-culture of primary human dermal melanocytes the isolated cell mix was treated with 400 ng/ml G418. Viability of the fibroblasts was controlled microscopically for three days and medium was changed daily. If on day six vital fibroblasts were still visible the procedure was repeated. The obtained melanocyte monoculture was cultivated in melanocyte growth medium with supplement mix (Promocell) containing 0.4% bovine pituary extract (BPE), 1 ng/ml basic fibroblast growth factor, 5 µg/ml Insulin, 0.5 µg/ml hydrocortisone, 1% streptomycin, penicillin and amphotericin B.
Sequencing of the MC1R gene.
Prior to sequencing the MC1R DNA was amplified by PCR as described: The genomic DNA of the MC1R gene can be amplified directly as the MC1R gene is intronless and polymorphisms are detectable on the gene level.
For DNA purification we used the High Pure PCR Preparation Kit (Roche), DNA concentrations were measured photometrically at 260 nm. For PCR 2 µl DNA (50 ng/µl), 1 µl forward and reverse primer (10 pM), 1 µl dNTPs (10 mM), 0.5 µl TaqPolymerase (5 U/µl), 5 µl 10x Buffer were used per 50 µl reaction. The primer sequences were as follows: primer1forward (P1F) 5′-GTG ACC GGA CAG ACT GG-3′, primer1reverse (P1R) 5′-GCC TGC CTC CTT CCA TCT-3′, P2F 5′-GGT AGA TGC CAG GAG GTG TC-3′, P2R 5′-ACC AGC AGG TCC GAC AAG-3′, P3F 5′-TGC ACT CAC CCA TGT ACT GC-3′, P3R 5′-CCA GCA GAG GAA GAA AAT GC-3′, P4F 5′-CGT GGT CTT CTT CCT GGC TA-3′, P4R 5′-GGA CCA GGG AGG TAA GGA AC-3′. The MC1R DNA was amplified for 35 cycles (primers 1 and 2 forward and reverse 1 min 95°C, 30 sec 94°C, 30 sec 55°C, 45 sec 72°C, 7 min 72°C, primers 3 and 4 forward and reverse 2 min 95°C, 20 sec 94°C, 20 sec 65°C, 45 sec 72°C, 7 min 72°C) in an GeneAmp PCR System 240C (Perkin Elmer). 5 µl of the PCR product was electrophoresed on a 2% agarose Gel. The remaining PCR product was purified either using the Quiaquick PCR Purification Kit or the Quiaquick Gel extraction kit depending on the purity of the obtained PCR product.
For sequencing 1 µl of the purified PCR product (10 ng/µl), 3.2 µl Primer (forward or reverse) (1 pM), and 2 µl of ready reaction mix (Sigma) and H2O (HPLC-cleaned) were used per 20 µl reaction. 25 cycles were carried out (5 sec 94°C, 10 sec 96°C, 5 sec 50°C, 4 min 60°C). The product obtained from the sequencing PCR was precipitated with 2 µl NaOAc (3 M, pH 4.6) and dried at 90°C. The precipitated probes were measured in an automatic sequencing machine (ABI Prism 310 Genetic analyser, Applied Biosystems) in the Institute of Human Genetics, University Hospital of the Saarland.
The obtained data were analysed with ChromalsPro software.
Measurements of intracellular cAMP concentration.
Biotrac enzyme immunoassay (EIA) (Amersham Biosciences) was used to determine intracellular cAMP levels. The cells were seeded in 96-well-flatbottom plates and incubated for two days at 37°C. For the melanocytes the medium was changed after 24 h to BPE-free melanocyte medium. The melanocytes were seeded at a density of 5 × 105, the fibroblasts at a density of 2 × 104 per well in 100 µl medium. On day three the cells were stimulated for 20 minutes with α-MSH (1 µM) and lysed over night according to the manufacturer's protocol.
Proliferation measurements.
CellTiter-Blue cell viability assay (Promega) was used to measure cellular proliferation. The measurements were carried out in a fluorescent microplate reader (Genius Pro, Tecan).
Initially the melanocytes were seeded in BPE-free medium. The cells were transferred into 12-well plates at a density of 1,000 cells/ml (Fibroblasts) or 1.6 × 104 cells/ml (Melanocytes) in 1 ml medium and were stimulated with 1 µM α-MSH. Untreated melanocytes and fibroblasts served as controls. The day of stimulation was defined as day zero. Further measurements were carried out on day 2 after stimulation for the melanocytes and on days 2 and 4 for the fibroblasts. At the defined time points the proliferation measurement was carried out as follows: After reducing the volume of culture medium from 1 ml to 500 µl per well the cells were incubated for one hour with 100 µl of CellTiter-Blue at standard conditions. 200 µl of the supernatants were transferred into a black 96-well-plate with transparent bottom and the fluorescence was measured at 560 nm excitation and 590 nm emission. The measurements were quantified as RFU (relative fluorescent unit).
Statistics.
The cAMP and RFU values of unstimulated control cells of each experiment were set as 100% and the values of the α-MSH stimulated cells were accordingly related, so that the resulting data could be evaluated independently from the absolute cell counts. Differences between stimulated and unstimulated cells were evaluated using paired student's t-test.
The comparison between MC1Rwt and MC1R polymorphic melanocytes and fibroblasts concerning intracellular cAMP concentrations and proliferation after α-MSH stimulation has been performed by Mann-Whitney-U test. For the proliferation analysis the absolute RFU differences of α-MSH stimulated minus unstimulated cells (d2 for melanocytes and d4 for fibroblasts) were used. For this analysis the data of wild-type and polymorphic MC1R were pooled for melanocytes and fibroblasts respectively. The value distributions are presented as box plots.
All statistical analyses were performed with statistical software PASW Statistics 18 (SPSS, Inc., Chicago, IL); p-values of < 0.05 were considered as significant.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures and Tables
Figure 1 cAMP production and proliferation of MC1Rwt melanocytes and MC1Rwt fibroblasts following α-MSH stimulation. Intracellular cAMP concentration was measured in MC1Rwt melanocytes (A, n = 9) and fibroblasts (C, n = 5) untreated (black bars) or treated with 1 µM α-MSH for 20 min (red). Proliferation of MC1Rwt melanocytes (B, n = 24) and MC1Rwt fibroblasts (D, n = 12) untreated (black) or treated with 1 µM α-MSH (red). Measurements were carried out three hours (day 0), 48 hours (day 2) and 96 hours (day 4) following stimulation with 1 µM α-MSH. Error bars represent standard error of mean (SEM).
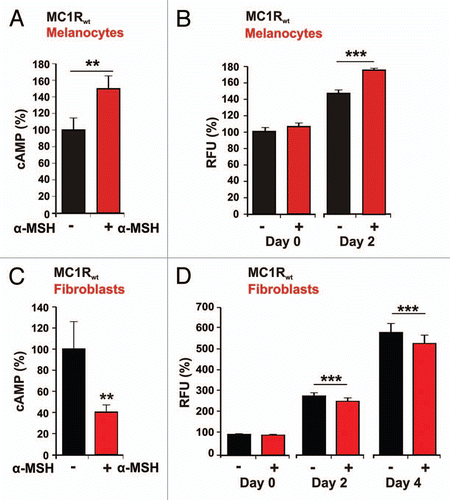
Figure 2 cAMP production and proliferation of melanocytes with MC1R polymorphisms following α-MSH stimulation. Intracellular cAMP concentration was measured in melanocytes with MC1R polymorphisms (A, n = 5 and C, n = 5) untreated (black bars) or treated with 1 µM α-MSH for 20 min (red). Proliferation of melanocytes with MC1R polymorphisms (B, n = 12 and D, n = 12) untreated (black) or treated with 1 µM α-MSH (red). Measurements were carried out three hours (day 0) and 48 hours (day 2) following stimulation with 1 µM α-MSH. Error bars represent SEM. The MC1R polymorphisms are described in .
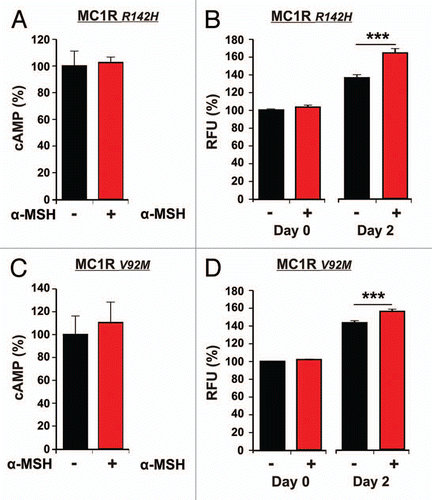
Figure 3 cAMP production and proliferation of fibroblasts with MC1R polymorphisms following α-MSH stimulation. Intracellular cAMP concentration was measured in fibroblasts with MC1R polymorphisms (A, n = 3, C, n = 3 and E, n = 3) untreated (black bars) or treated with 1 µM α-MSH for 20 min (red). Proliferation of fibroblasts with MC1R polymorphisms (B, n = 12, D, n = 12 and F, n = 12) untreated (black) or treated with 1 µM α-MSH (red). Measurements were carried out three hours (day 0) and 48 hours (day 2) following stimulation with 1 µM α-MSH. Error bars represent SEM. The polymorphisms are described in .
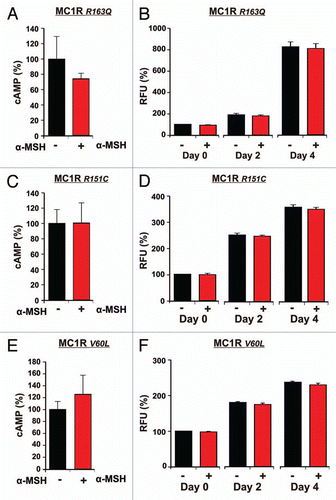
Figure 4 Comparison of cAMP levels and proliferation rate in MC1Rwt and MC1R polymorphic melanocytes and fibroblasts. Intracellular cAMP levels after stimulation with 1 µM α-MSH were compared in MC1Rwt (grey) and MC1R polymorphic (MC1RSNP) (blue) melanocytes (A) and fibroblasts (C). The differences in cell proliferation with and without α-MSH treatment (RFU after α-MSH stimulation—RFU of untreated cells) were compared in MC1Rwt and MC1RSNP melanocytes (B) and fibroblasts (D). The value distributions are presented as box plots.
Table 1 Characterisation of the MC1R variants in melanocyte and fibroblast cell lines
Acknowledgments
We are grateful to Prof. Dr. M. Hoth, Drs. A.S. Wenning, E. Schwarz, I. Bogeski and C. Kummerow for their help and suggestions. We thank A. Stark, A. Weinhold and H. Palm for their technical assistance.
References
- Wikberg JE, Muceniece R, Mandrika I, et al. New aspects on the melanocortins and their receptors. Pharmacol Res 2000; 42:393 - 420
- Hodgkinson CA, Moore KJ, Nakayama A, et al. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basichelix-loop-helix-zipper protein. Cell 1993; 74:395 - 404
- Kadekaro AL, Kavanagh R, Kanto H, et al. alpha-Melanocortin and endothelin-1 activate antiapoptotic pathways and reduce DNA damage in human melanocytes. Cancer Res 2005; 65:4292 - 4299
- Gantz I, Yamada T, Tashiro T, et al. Mapping of the gene encoding the melanocortin-1 (alpha-melanocyte stimulating hormone) receptor (MC1R) to human chromosome 16q24.3 by Fluorescence in situ hybridization. Genomics 1994; 19:394 - 395
- Magenis RE, Smith L, Nadeau JH, et al. Mapping of the ACTH MSH, and neural (MC3 and MC4) melanocortin receptors in the mouse and human Mamm. Genome 1994; 5:503 - 508
- Makova KD, Ramsay M, Jenkins T, Li WH. Human DNA sequence variation in a 6.6-kb region containing the melanocortin 1 receptor promoter. Genetics 2001; 158:1253 - 1268
- Moro O, Ideta R, Ifuku O. Characterization of the promoter region of the human melanocortin-1 receptor (MC1R) gene. Biochem Biophys Res Commun 1999; 262:452 - 460
- Garcia-Borron JC, Sanchez-Laorden BL, Jimenez-Cervantes C. Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res 2005; 18:393 - 410
- Robbins LS, Nadeau JH, Johnson KR, et al. Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 1993; 72:827 - 834
- Scherer D, Nagore E, Bermejo JL, et al. Melanocortin receptor 1 variants and melanoma risk: a study of 2 European populations. Int J Cancer 2009; 125:1868 - 1875
- Smith R, Healy E, Siddiqui S, et al. Melanocortin 1 receptor variants in an Irish population. J Invest Dermatol 1998; 111:119 - 122
- Healy E, Flannagan N, Ray A, et al. Melanocortin-1-receptor gene and sun sensitivity in individuals without red hair. Lancet 2000; 355:1072 - 1073
- Valverde P, Healy E, Jackson I, Rees JL, Thody AJ. Variants of the melanocyte-stimulating hormone receptor gene are associated with red hair and fair skin in humans. Nat Genet 1995; 11:328 - 330
- Box NF, Wyeth JR, O'Gorman LE, Martin NG, Sturm RA. Characterization of melanocyte stimulating hormone receptor variant alleles in twins with red hair. Hum Mol Genet 1997; 6:1891 - 1897
- Figl A, Thirumaran RK, Ugurel S, et al. Multiple melanomas after treatment for Hodgkin lymphoma in a non-Dutch p16-Leiden mutation carrier with 2 MC1R high-risk variants. Arch Dermatol 2007; 143:495 - 499
- Raimondi S, Sera F, Gandini S, et al. MC1R variants, melanoma and red hair color phenotype: a metaanalysis. Int J Cancer 2008; 122:2753 - 2760
- Sturm RA. Skin colour and skin cancer—MC1R, the genetic link. Melanoma Res 2002; 12:405 - 416
- Duffy DL, Box NF, Chen W, et al. Interactive effects of MC1R and OCA2 on melanoma risk phenotypes. Hum Mol Genet 2004; 13:447 - 461
- Healy E, Jordan SA, Budd PS, et al. Functional variation of MC1R alleles from red-haired individuals. Hum Mol Genet 2001; 10:2397 - 2402
- Beaumont KA, Newton RA, Smit DJ, et al. Altered cell surface expression of human MC1R variant receptor alleles associated with red hair and skin cancer risk. Hum Mol Genet 2005; 14:2145 - 2154
- Sanchez-Laorden BL, Sanchez-Mas J, Martinez-Alonso E, et al. Dimerization of the human melanocortin 1 receptor: functional consequences and dominant-negative effects. J Invest Dermatol 2006; 126:172 - 181
- Scott MC, Wakamatsu K, Ito S, et al. Human melanocortin 1 receptor variants, receptor function and melanocyte response to UV radiation. J Cell Sci 2002; 115:2349 - 2355
- Leonard JH, Marks LH, Chen W, et al. Screening of human primary melanocytes of defined melanocortin-1 receptor genotype: pigmentation marker, ultrastructural and UV-survival studies. Pigment Cell Res 2003; 16:198 - 207
- Suzuki I, Tada A, Ollmann MM, et al. Agouti signaling protein inhibits melanogenesis and the response of human melanocytes to alpha-melanotropin. J Invest Dermatol 1997; 108:838 - 842
- Abdel-Malek Z, Swope VB, Suzuki I, et al. Mitogenic and melanogenic stimulation of normal human melanocytes by melanotropic peptides. Proc Natl Acad Sci USA 1995; 92:1789 - 1793
- Suzuki I, Cone RD, Im S, Nordlund J, Abdel-Malek ZA. Binding of melanotropic hormones to the melanocortin receptor MC1R on human melanocytes stimulates proliferation and melanogenesis. Endocrinology 1996; 137:1627 - 1633
- Hunt G. Melanocyte-stimulating hormone: a regulator of human melanocyte physiology. Pathobiology 1995; 63:12 - 21
- Nordlund JJB, Raymond E, Hearing, Vincent J, King, Richard A. The Pigmentary System: Physiology and Pathophysiology 1998; New York Oxford Univ Press
- Hadley M. Endocrinology 1996; Englewood Cliffs, NJ Prentice Hall
- Hirobe T. Melanocyte stimulating hormone induces the differentiation of mouse epidermal melanocytes in serum-free culture. J Cell Physiol 1992; 152:337 - 345
- Kadekaro AL, Leachman S, Kavanagh RJ, Swope V, Cassidy P, Supp D, et al. Melanocortin 1 receptor genotype: an important determinant of the damage response of melanocytes to ultraviolet radiation. FASEB J 2010; 24:3850 - 3860
- Böhm M, Luger TA. Melanocortins in fibroblast biology—current update and future perspective for dermatology. Exp Dermatol 2004; 13:16 - 21
- Böhm M, Raghunath M, Sunderkotter C, et al. Collagen metabolism is a novel target of the neuropeptide alpha-melanocyte-stimulating hormone. J Biol Chem 2004; 279:6959 - 6966
- Kokot A, Sindrilaru A, Schiller M, et al. alpha-melanocyte-stimulating hormone suppresses bleomycin-induced collagen synthesis and reduces tissue fibrosis in a mouse model of scleroderma: melanocortin peptides as a novel treatment strategy for scleroderma?. Arthritis Rheum 2009; 60:592 - 603
- Sanchez-Laorden BL, Herraiz C, Valencia JC, et al. Aberrant trafficking of human melanocortin 1 receptor variants associated with red hair and skin cancer: Steady-state retention of mutant forms in the proximal Golgi. Journal of Cellular Physiology 2009; 220:640 - 654
- Scott G, Cassidy L, Abdel-Malek Z. α-melanocyte-stimulating hormone and endothelin-1 have opposing effects on melanocyte adhesion, migration and pp125(FAK) phosphorylation. Experimental Cell Research 1997; 237:19 - 28
- Hill RP, Wheeler P, MacNeil S, Haycock JW. Alpha-melanocyte stimulating hormone cytoprotective biology in human dermal fibroblast cells. Peptides 2005; 26:1150 - 1158
- Elfakir A, Ezzedine K, Latreille J, et al. Functional MC1R-gene variants are associated with increased risk for severe photoaging of facial skin. J Invest Dermatol 130:1107 - 1115
- Fogarty GB, Muddle R, Sprung CN, et al. Unexpectedly severe acute radiotherapy side effects are associated with single nucleotide polymorphisms of the melanocortin-1 receptor. International Journal of Radiation Oncology Biology Physics 2010; 77:1486 - 1492
- Beall AD, Nietert PJ, Taylor MH, et al. Ethnic disparities among patients with pulmonary hypertension associated with systemic sclerosis. The Journal of Rheumatology 2007; 34:1277 - 1282
- Rostand SG, Kirk KA, Rutsky EA, Pate BA. Racial differences in the incidence of treatment for end-stage renal disease. N Engl J Med 1982; 306:1276 - 1279
- Datubo-Brown DD. Keloids: a review of the literature. Br J Plast Surg 1990; 43:70 - 77
- Dustan HP. Does keloid pathogenesis hold the key to understanding black/white differences in hypertension severity?. Hypertension 1995; 26:858 - 862