Abstract
In 2007, a group of experts critically analyzed hundreds of publications on bisphenol A (BPA), including the evidence for low dose effects. Here, we have updated these evaluations to determine the strength of the evidence for low dose effects of BPA. Based on the cut-offs for “low doses” established previously (i.e., the lowest observed adverse effect level [LOAEL], or 50 mg/kg/day for mammalian studies), we identified more than 450 low dose studies. Using an integrative approach, we examined five endpoints in depth that had evidence from two or more study types (in vitro, in vivo laboratory animal, and human). Based on all available studies, we are confident that consistent, reproducible, low dose effects have been demonstrated for BPA. We conclude that the doses that reliably produce effects in animals are 1–4 magnitudes of order lower than the current LOAEL of 50 mg/kg/day and many should be considered adverse.
Introduction
Bisphenol A (BPA; CAS# 80-05-7) is a high-volume chemical that is widely used in the production of consumer products.Citation1 BPA has estrogenic properties in vitro and in vivo, binds the membrane-associated estrogen receptor (ER), and may also act via the androgen receptor, thyroid hormone receptor, peroxisome proliferator-activated receptor-γ, and other endocrine-relevant signaling pathways.Citation2 A recent study by the US Environmental Protection Agency (EPA) and US National Toxicology Program (NTP) assigned BPA the third highest Toxicological Priority Index (ToxPi) score of the 309 chemicals examined based on its ability to interact with a number of signaling pathways.Citation3
This review focuses on examining one aspect of the action of BPA, effects at low doses. We provide a brief overview of previous assessments of low dose effects and an updated review of low dose studies published since 2007. Further, we use an integrative biology approach to identify endpoints consistently affected by BPA exposures, examining five endpoints that have been observed in multiple studies from several laboratories, assessing the effects of low doses on multiple levels of biological organization (cells, animals, and human populations).
Defining “Low Dose”
In 2001, an NTP panel of experts assessing low dose effects for a number of endocrine-disrupting chemicals (EDCs) defined “low dose” as any biological change occurring in the range of typical human exposures or at doses lower than those tested in traditional toxicology assessments.Citation4 At that time, the NTP panel concluded that there were low dose effects for a number of EDCs. It should be noted that the NTP’s definitions for low dose (human exposure levels vs. doses used in traditional toxicology assays) produce very different cut-offs for many EDCs, including BPA.Citation5
Defining “low dose” cut-offs for BPA studies
Using the NTP’s definition of doses lower than those tested in traditional toxicology assessments,Citation4 a cut-off for low dose BPA studies was set at 50 mg/kg/day for laboratory animals;Citation6 this was identified as the LOAEL in traditional toxicology studies. This cut-off was selected because no adverse effects should ever be observed below this dose.
Most aquatic animals are exposed to EDCs via the water or soil in which they live. For this reason, the low dose cut-off for aquatic animals was set at 17.2 mg/L, the maximum BPA concentration detected in an environmental sample.Citation7 The low dose cut-off for cell culture experiments was set at 1 × 10−7 M based on calculations of circulating BPA concentrations in animals administered the LOAEL.Citation8 Finally, establishing a low dose cut-off for epidemiology studies is simple, because one definition of “low dose” is a dose in the range of typical human exposures.Citation5 Thus, any study of non-occupationally exposed individuals meets this criterion.
“Low dose effects” make no assumptions about the shape of the dose response curve. Therefore, “low dose” is not synonymous with non-monotonicity. Non-monotonic dose responses have recently received significant attention (for example, ref. Citation5) and will be discussed briefly in this review.
Previous Reviews of the BPA Low Dose Literature
Following the 2001 NTP report on low dose effects for EDCs, several reviews focused on the ability of BPA to cause harmful effects at doses below the US EPA reference dose of 50 μg/kg/day or the LOAEL of 50 mg/kg/day. In 2005, vom Saal and Hughes quantified the number of studies showing harmful effects from low dose BPA.Citation9 In 2006, vom Saal and Welshons identified more than 100 studies that reported significant effects of BPA below the LOAEL; 40 studies reported effects at doses below 50 μg/kg/day.Citation10 In 2007, a NIH-sponsored panel of experts working on EDCs critically analyzed hundreds of publications on BPA, publishing the Chapel Hill consensus statement;Citation11 the accompanying detailed reviews addressed low dose effects in laboratory animals,Citation6 wildlife including amphibians and fish,Citation7 in vitro cell and tissue culture,Citation8 cancer,Citation12 and human populations.Citation1 In 2008, the National Toxicology Program reviewed the entirety of the BPA literature, including studies relevant to low doses.Citation13 Finally, in 2012, Vandenberg and colleagues performed weight-of-evidence evaluations and assessed low dose effects of BPA on the prostate and mammary gland,Citation5 and in 2013, J Rochester published a comprehensive review of all BPA epidemiology studies published to date.Citation14
Importance of Route of Administration?
One issue that has received significant attention in the study of BPA is the route of administration, because the diet is considered the major, and sometimes the sole, route of human exposure.Citation15 Because information is still lacking about all of the products containing BPA and non-dietary sources of exposure have been and continue to be identified (reviewed in ref. Citation16), the requirement that animal studies use oral administration is not appropriate. Newborn rodents and monkeys have limited capacity to metabolize BPA and route of administration has little effect on average serum BPA levels.Citation17-Citation20 In addition, in adult animals, the proportion of ingested BPA circulating in serum that remains unconjugated (bioactive) differs dramatically depending on whether absorption occurs via the oral mucosa, which bypasses first-pass metabolism, or only in the gastrointestinal tract (for example, due to gavage administration), which results in a higher proportion of BPA being metabolized.Citation21
New Efforts to Focus on Low Dose
Over the past few years, new attention has focused on addressing the issue of low dose exposures. In 2009, the National Institute of Environmental Health Sciences (NIEHS) launched a program to fill data gaps related to the toxicity of BPA.Citation22 Extramural grants were funded, and a BPA grantees consortium was established; the study of low doses was an important issue that was considered during the design and execution of the funded studies. Additional efforts being undertaken by NIEHS involve a chronic toxicity study of BPA being conducted in collaboration with the US FDA. This guideline compliant study will also include low doses, and will examine endpoints used in traditional toxicology testing as well as hypothesis-driven mechanistic endpoints developed in academic laboratories. The results from this study will be available over the next few years.
This review is focused on summarizing the published results of these studies, as well as other peer-reviewed studies from around the world. Below, we summarize new low dose findings for in vitro, aquatic animal, mammalian laboratory animal, and epidemiology studies. We also examine a few examples in more depth and conclude with consensus statements regarding the strength of the overall evidence for low dose effects of BPA.
Brief Review of New Low Dose Findings
In vitro
We identified more than 100 new in vitro studies that included doses at or below the cut-off of 1 × 10−7 M. Similar to what was reported previously,Citation8 these studies have largely focused on cell lines and primary cells isolated from the male or female reproductive tracts, cells and tissue explants involved in energy balance, neuronal and glial cells, and pituitary cells (Table S1).
Studies of cells isolated from the male reproductive tract identified several tissues that are sensitive to BPA. Effects were observed on cell proliferation, gene expression, and hormone production in human prostate cancer cell lines, as well as spermatogonia, Leydig cells, whole testes, and testicular seminoma cells from a number of species (see refs. Citation23–Citation34 and Table S1). Cells isolated from the female reproductive tract, including ovarian granulosa or theca-interstitial cells, oocyte-cumulous complexes, and placental cytotrophoblasts, have also been studied (see refs. Citation35–Citation44 and Table S1). Low dose effects were observed in many of these cells on endpoints ranging from altered hormone secretionCitation36,Citation39 to altered gene expressionCitation36,Citation40,Citation42 and altered proliferation.Citation43
A large number of studies have also examined the effects of BPA on breast cancer cell lines and primary cells. Although many classical endpoints (i.e., gene expression, cell proliferation)Citation45-Citation50 are affected by low doses of BPA in these cells, newer endpoints have been assessed as well. For example, several studies demonstrate that BPA concentrations below 1 × 10−7 M interfere with chemotherapeutic agents, conferring resistance in cell lines and primary cells isolated from human patients.Citation51-Citation53
In vitro studies have also focused on cells relevant to energy balance. Two studies have shown that low doses affect the release of insulin and ion channel activity in isolated pancreatic islets.Citation54,Citation55 Others show altered release of adiponectin and inflammatory cytokines, changes to the expression of adipocyte proteins, and lipid accumulation in adipose tissue explants or isolated adipocytes and pre-adipocytes.Citation56-Citation59 BPA has also been shown to affect muscle cells, including cardiac myocytes, with low dose effects on arrhythmogenic activities and physiological endpoints related to calcium cycling and contractility.Citation60-Citation62
Overall, these in vitro studies have examined a large number of cell types and biological endpoints that may be relevant to cellular function in vivo. The low dose cut-off of 1 × 10−7 M has been debated and it may be reasonable to shift the low dose cut-off for in vitro studies to 10−9 M or lower. Many studies examining doses at or below 10−9 M report significant biological effects,Citation23,Citation24,Citation26-Citation28,Citation30,Citation33,Citation43,Citation49,Citation52,Citation55,Citation57,Citation60,Citation61,Citation63-Citation78 challenging the hypothesis that BPA only has “weak” endocrine disrupting activity.
Non-mammalian animal models
We identified more than 75 studies of non-mammalian models that examined doses of BPA below the low dose cut-off of 17.2 mg/L (Table S2). Most examined aquatic animals including invertebrates such as daphnia and mollusks, fish such as medaka, trout and zebrafish, and the amphibian Xenopus laevis.
One well-studied endpoint is the expression of vitellogenin, an egg yolk precursor protein that is expressed in females of many egg-laying species and is a sensitive biomarker for estrogen-like responses. BPA exposures below 12.2 mg/L induced vitellogenin expression in males of various fish species, including fathead minnows,Citation79 goldfish,Citation80 medaka,Citation81 and zebrafish.Citation82 Other studies have focused on the effect of BPA on reproductive endpoints. In some cases, reproductive success was shown to be decreased based on measures of hatchability,Citation83 sperm motility,Citation80 fertilization rates,Citation84 and the cumulative number of offspring produced per animal.Citation85 Mortality is also a common endpoint that has been assessed in both invertebrate and vertebrate species, with significant decreases in survival noted in a number of invertebratesCitation84-Citation89 and fish.Citation87,Citation90-Citation94 Studies have also reported altered gene expression (often of hormone receptors and enzymes, as well as classical liver markers) following low dose BPA exposure in zebrafish,Citation95-Citation97 a well-established genetic model, and in other less well characterized organisms including aquatic midge,Citation98 Atlantic cod,Citation99 carp,Citation100 Kryptolebias marmoratus,Citation101-Citation106 rainbow trout,Citation107 rare minnow,Citation108,Citation109 and Xenopus.Citation110,Citation111
One final study deserves attention due to its use of Organization for Economic Cooperation and Development (OECD) guidelines to test the effects of BPA on histopathological changes in the ovary.Citation112 Zebrafish were exposed to very low doses of BPA for 2 wk starting at 16 wk of age; histological abnormalities were observed at 1, 10, 100, and 1000 µg/L, providing strong evidence for low dose adverse effects on the fish ovary. OECD guidelines examine endpoints that are easily assessed, are reproducible, and focus on adverse effects,Citation113 although there is debate regarding whether they examine appropriate and sensitive endpoints for endocrine disruption.Citation114-Citation116
Mammalian laboratory animals
Previous assessments found evidence for low dose effects of BPA on a number of endpoints, including brain development, behaviors, development of the male reproductive tract, growth and metabolism, and adult male reproductive health using the LOAEL (50 µg/kg body weight/day) as the low dose cut-off.Citation6,Citation13 Additional evidence for low dose effects has continued to accumulate from mammalian studies focused on those endpoints and others (Table S3). In this section, we will focus on systems and endpoints for which strong evidence did not exist in 2007.
Female reproductive system
Prior to 2007, six studies reported effects of early life BPA exposure on gene expression and histology of the female reproductive tract as well as disturbances in chromosome behavior in oocytes.Citation6 Since that time, seven additional studies have revealed low dose effects on abnormalities (including hyperplasias) in the female reproductive tract,Citation117-Citation119 uterine gene expression,Citation120,Citation121 and measures of fertility.Citation122,Citation123 Numerous studies have also shown effects on the formation of ovarian follicles, the presence of polycystic ovaries, follicle growth, and the genetic quality of the egg; these are discussed in a later section of this review.
Fewer studies have examined the effects of adult exposures on female reproduction. These studies have identified some effects on oocyte healthCitation124-Citation126 and uterine gene expression,Citation127,Citation128 but many endpoints have not been replicated (Table S3). Thus, while the evidence for low dose effects on female reproductive endpoints following developmental exposures is strong, the evidence for effects following adult exposures remains inconclusive.
Mammary gland
Since 2007, 11 studies, including one examining the non-human primate,Citation129 have examined the effects of developmental exposures to BPA on the morphology of the mammary glandCitation130-Citation136 and altered responses of this organ to carcinogenic challenges.Citation137-Citation139 Another recent study revealed the presence of carcinomas in the mammary glands of rats exposed to low doses of BPA only during early development in the absence of any additional carcinogen treatment, providing further evidence that the mammary gland is affected by BPA.Citation140 A recent weight-of-evidence analysis found strong support for low dose effects and high levels of consistency between studies for both endpoints;Citation5 low doses were found to alter mammary gland morphology at many life stages, and numerous studies reported that low doses increase the incidence of preneoplastic and neoplastic lesions, as well as increase the response to chemical carcinogens. Some of these studies are discussed in more detail below.
Immune system
Five recent studies suggest that developmental BPA exposures alter the response of rodents to infectious agents and allergens.Citation141-Citation145 These studies typically show effects of BPA on some aspects of immune response, but not all (i.e., altered innate but not adaptive immune responses, effects in some organs but not others, etc.). Because these studies used different agents to invoke immune responses and assessed different organs’ responses, there is not yet enough evidence to determine whether the effects of BPA on the immune system are consistent. However, these studies collectively provide weak support for low dose effects of BPA on the response of the immune system to specific agents.
Brain morphology
Since 2007, five studies have demonstrated low dose effects of adult BPA exposures on brain endpoints such as synapse formation and remodeling.Citation146-Citation150 Some of these effects were observed only in response to additional hormone treatments (i.e., BPA attenuates the effects of estrogens on synapse formation/remodeling). Importantly, these studies collectively demonstrate consistent low dose effects on neuronal synapses in rodents and non-human primates.
Metabolism
Finally, although the endpoints examined are variable, three recent studies report effects of adult BPA exposures on metabolic endpoints. Exposures lasting 8 d to 10 wk in length altered blood glucose and insulin levels, glucose and insulin tolerance, food intake, triglyceride levels, and responses to sugar challenges.Citation151-Citation153 The variability in affected endpoints may be evidence of the diverse effects of adult BPA exposures and/or differences in exposure periods.
We used a cut-off of 50 mg/kg/day, the LOAEL for BPA, as a cut-off to determine which studies should be considered “low dose.” This cut-off is consistent with the NTP’s definition of “low dose,”Citation4 and is the same cut-off that was used previously by the Chapel Hill panel.Citation6 Yet, because studies estimate human exposures from dietary sources in the range of 0.1–5 µg/kg body weight/day,Citation154 there have been suggestions that only studies examining exposures in this range should be considered “low dose.”Citation155
Importantly, many significant effects are observed following administration of doses below the EPA reference dose of 50 µg/kg body weight/day, and some of these studies show effects in the range of estimated human exposures (≤ 5 µg/kg body weight/day).Citation118,Citation124,Citation126,Citation130,Citation132,Citation133,Citation135,Citation136,Citation141,Citation143,Citation150,Citation152,Citation156-Citation185 Previous reviews have also noted a relatively large number of studies that demonstrated effects in the range of human exposures from dietary sources.Citation6,Citation9 Studying actual or suspected human exposure levels in rodents may not be appropriate for risk assessment purposes as this leaves no margin of error for differences in metabolism between species or individuals, the presence of vulnerable human subpopulations, or the possibility that animal models are less sensitive than humans. These factors are traditionally accounted for by calculating a “safe” reference dose from the NOAEL using safety factors.Citation5 Thus, if adverse effects are observed in animals at doses ≤ 50 µg/kg/day and these safety factors are applied, the “safe” dose for humans would be calculated as 50 ng/kg/day or lower.
Humans
Since 2007, more than 50 epidemiology studies have examined various health outcomes in relation to BPA exposure. Eight studies examining neurobehavioral outcomes have been published from four prospective US cohorts and a cross-sectional Korean cohort.Citation186-Citation194 These studies focused on pre- and postnatal BPA exposure and neurobehavioral outcomes measured through either parent report or standardized testing. Outcomes included anxious and depressive behavior as well as social and interpersonal problems in children from several age groups. Collectively, these studies raise concern over pre- and postnatal exposure to BPA and negative effects on neurobehavioral outcomes in children.
Fifteen recent studies with various designs have examined the association between BPA and various aspects of metabolic syndrome.Citation195-Citation210 In several, increased urinary BPA concentration was associated with higher odds of increased body mass index, obesity, cardiovascular disease, insulin resistance, and type 2 diabetes (Table S4). Cross-sectional and longitudinal studies have also demonstrated associations between BPA exposure levels and coronary artery disease and peripheral arterial disease.Citation211-Citation213
Other studies have investigated associations between BPA and thyroid dysfunction. This is of particular concern because thyroid hormones play an important role in brain development. Prenatal BPA exposure was associated with decreased serum thyroid stimulating hormone (TSH) in male neonates.Citation214,Citation215 Several cohorts suggest negative associations between urinary BPA and TSH and/or total thyroxine (T4) in adolescents and adults.Citation216,Citation217 Collectively, these studies indicate that BPA may affect thyroid function at different life stages.
Finally, since 2007, multiple studies have examined BPA and human reproduction. Several prospective IVF cohort studies examined associations between BPA and early IVF outcomes in women.Citation218-Citation222 Significant negative associations between BPA and peak ovarian estradiol were observed in multiple cohorts, although other associations were only observed in a single cohort (i.e., oocyte yield, and other downstream IVF outcomes). In one cohort, later reproductive endpoints (i.e., embryo implantation) were negatively associated with urinary BPA concentrations.Citation222
In sum, the epidemiology literature indicates there are reproducible effects of environmental BPA exposures on a number of endpoints including behaviors, metabolic syndrome, and thyroid hormone signaling. Although we did not assess the quality of each of these studies, and some are certainly limited due to their size and/or design, these findings taken together lead to the conclusion that current levels of BPA exposure are related to a number of disease outcomes in humans that are consistent with results from controlled animal studies and mechanistic in vitro studies.
Non-Monotonicity
As mentioned earlier, non-monotonicity is distinct from “low dose.” A number of studies examining low doses of BPA have revealed non-monotonic dose response curves (NMDRCs), which often manifest as U- or inverted U-shaped curves. Non-monotonicity is easily revealed when lower doses induce significant differences from untreated controls and higher doses do not. It is common for these findings to be presented as not showing a dose–response relationship because the relationship is not linear.Citation5
In the in vitro literature, studies with five or more groups are quite common, and thus, a number have demonstrated non-monotonicity ( and ref. Citation223). Fewer in vivo studies have examined the large number of doses necessary to demonstrate non-monotonicity (). Additional analyses of dose response shapes are needed to determine the frequency of NMDRCs for BPA studies, the types of endpoints that manifest NMDRCs, and whether studies of these endpoints in different laboratories produce consistent dose response shapes.Citation224
Table 1. Endpoints demonstrating non-monotonicity in in vitro and in vivo low dose studies of BPA
Table 2. Examples of adverse endpoints in laboratory animals
Integration of Endpoints Across Levels of Biological Organization
This review and the data presented in supplementary tables, coupled with the 2007 reviews of the BPA literature,Citation1,Citation6-Citation8 indicate that there are several hundred studies showing biological effects at or below the low dose cut-offs that were established by the Chapel Hill expert panels; the results of more than 50 epidemiology studies indicate that these effects could be occurring at current levels of human exposure.Citation14 Using the entire body of literature available, we next asked whether similar endpoints are affected across multiple levels of biology and/or in multiple species. Here, we examine five examples where data are available from two or more study types: in vitro, in vivo animals, and human populations. For each example, all published studies (from both pre- and post- Chapel Hill [2007]) were examined, regardless of whether the study in question showed effects of BPA. Details about individual studies are presented in the supplementary tables. Note that we focused on specific endpoints (i.e., insulin regulation), and not all studies in a specific category (i.e., “metabolism”).
These five examples were selected in part based on the expertise available within the BPA grantees consortium. There are a number of additional examples of endpoints that could be examined with an integrative approach. These include conserved effects of BPA on cells and tissues in the male reproductive tract; the response of cultured immune cells and the immune system; measures of adiposity; production of and responses to thyroid hormone; and many others. Due to space constraints, we will not discuss these examples in detail. However, these examples coupled with the five discussed below provide strong evidence for conserved low dose effects.
Example 1: Low dose effects of BPA on liver enzymes
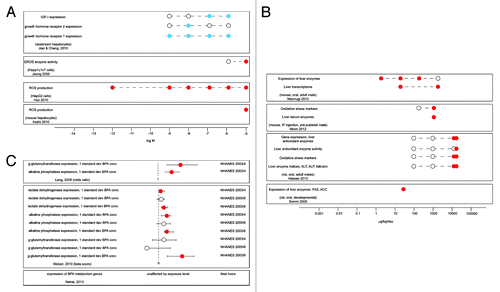
We identified two in vitro studies that have examined the effects of low doses on hepatocytes ().Citation73,Citation225 These studies showed effects on liver enzymes and markers of oxidative stress, potential markers of liver function, at or below 10−9 M; two other in vitro studies found effects of higher doses on similar endpoints.Citation226,Citation227 In laboratory animals, four studies showed low dose effects on the expression of liver enzymes and hepatic gene expression ().Citation152,Citation228-Citation230 This is in contrast to other endpoints such as liver weight and liver histopathology that revealed few low dose effects (Table S3); organ weight is an insensitive measure of pathology and likely reflects only gross toxic effects of an agent. Finally, three human epidemiology studies suggest a relationship between urinary BPA concentrations and changes in liver enzymes ().Citation195,Citation199,Citation231 Collectively, these results indicate that BPA alters hepatic function in multiple species across multiple levels of biological organization; additional analyses are needed to determine whether these alterations should be deemed adverse.
Figure 1. Low dose effects of BPA on liver enzymes. (A) In vitro studies examining the effects of BPA on hepatocyte enzyme production and production of reactive oxygen species (ROS). (B) Four in vivo laboratory rodent studies examined the effects of low dose BPA exposures on production of liver enzymes. (C) Summary of epidemiology studies examining effects of BPA on liver enzymes. Open circles indicate applied doses that did not induce significant effects (A and B) or populations that were unaffected (C). Red circles indicate doses that significantly increased the measure of interest (A and B) or populations that were significantly affected (C). Blue circles indicate doses that significantly decreased the measure of interest (A).
Example 2: Low dose effects of BPA on insulin regulation
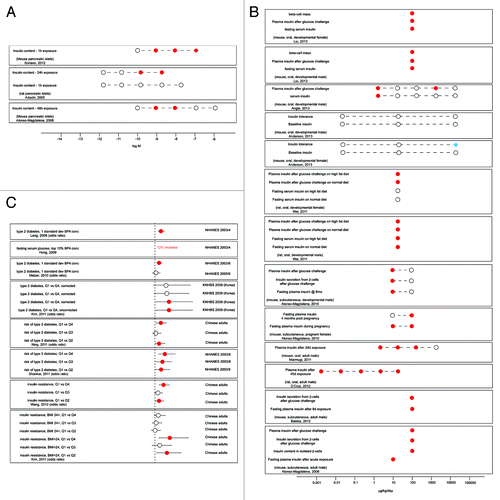
Three studies have reported that isolated pancreatic islets exposed to BPA in vitro have altered insulin responses to glucose ().Citation55,Citation63,Citation232 All three studies reported increases in insulin content following relatively short-term (1–48 h) incubations with low doses of BPA. In rodents, six studies demonstrated that developmental or adult BPA exposures can affect fasting plasma insulin levels as well as insulin levels in response to feeding or glucose challenges ().Citation151,Citation152,Citation168,Citation233-Citation235 In all these studies, plasma insulin levels were increased following BPA exposures, consistent with what was observed in vitro. Finally, as mentioned above, eight epidemiology studies examined relationships between urinary BPA concentrations and type 2 diabetes, a condition that involves abnormal insulin response ().Citation195,Citation199,Citation201,Citation202,Citation204,Citation209,Citation236-Citation238 These studies overwhelmingly report increased odds of diabetes and/or insulin resistance associated with increased BPA exposure metrics. Collectively, these results indicate that low doses of BPA can adversely affect metabolic pathways that are revealed in vitro in cultured pancreatic islets and in vivo in rodents, and suggest associations between BPA exposures and type 2 diabetes in some human populations, an obvious adverse endpoint.
Figure 2. Low dose effects of BPA on insulin signaling. (A) Insulin responses in cultured pancreatic islets. (B) Low dose effects of BPA on insulin levels in lab rodents. (C) Summary of epidemiology studies examining relationships between type II diabetes and urinary concentrations of BPA. Open circles indicate applied doses that did not induce significant effects (A and B) or populations that were unaffected (C). Red circles indicate doses that significantly increased the measure of interest (A and B) or populations that were significantly affected (C). Blue circles indicate doses that significantly decreased the measure of interest (B).
Example 3: Low dose effects of developmental BPA exposures on behavior
Several dozen studies are available examining the effects of low doses of BPA on behaviors in rodents. We focused on studies examining developmental exposures, although the actual exposure periods vary in important ways (Table S3). Examining only those studies that included two specific behavioral tests (the open field test, an assessment for anxiety, and the Morris water maze, a test for spatial learning and memory) reveals the complexity of these behavioral tests which must take into account the sexually dimorphic nature of these behaviors ().Citation169,Citation171,Citation174,Citation239-Citation247 Considering factors such as age, sex, and hormonal status, numerous studies report similar effects in neurobehavioral assessments examining low doses of BPA. Furthermore, previous reports were convincing enough in 2008 for the NTP to conclude that based on experimental data, there was “some concern” that low doses of BPA could affect development of the brain and behaviors in humans.Citation248 Finally, as noted above, eight epidemiology studies have examined relationships between BPA exposure levels—most often measured during gestation—and altered behaviors in children ().Citation186-Citation194 Consistent with a number of rodent studies, several of these studies suggest that low doses of BPA affect behavioral endpoints in children.
Figure 3. Low dose effects of BPA on select neurobehaviors. (A) Effects of BPA on anxiety behaviors in the open field test. (B) Effects of BPA on spatial learning and memory in rodents in the Morris water maze. (C) Epidemiology studies examining relationships between maternal urinary BPA concentrations and neurodevelopmental parameters in children. Open circles indicate applied doses that did not induce significant effects (A and B) or populations that were unaffected (C). Red circles indicate doses that significantly increased the measure of interest (A) or populations that were significantly affected (C). Blue circles indicate doses that significantly decreased the measure of interest (A and B).

Example 4: Low dose effects of BPA on the response of the mammary gland to subsequent environmental challenges
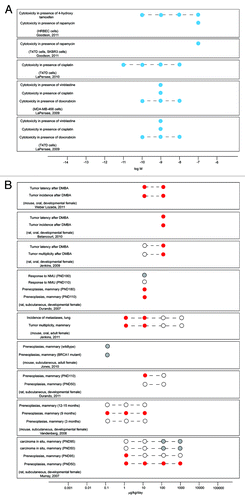
Three studies have examined the response of cultured mammary cancer cells to chemotherapeutic agents in the presence of low doses of BPA ().Citation51-Citation53 These studies indicate that BPA alters the response of mammary epithelial cells to environmental challenges. In rodents, five studies have shown that BPA induces preneoplastic or neoplastic lesions,Citation132,Citation134,Citation157,Citation163,Citation249 and four additional studies have demonstrated that low dose BPA exposures increase the response of the mammary gland to carcinogenic challenges administered later in life ().Citation137-Citation139,Citation250 The consistency of these results across laboratories is indicative of a highly reproducible low dose effect and provides strong evidence for adverse effects of BPA on this organ.
Figure 4. Low dose BPA alters the response of the mammary gland to environmental challenges. (A) In vitro studies reveal that BPA exposures confer chemotherapeutic resistance in mammary cells. (B) Low doses of BPA induce preneoplastic and neoplastic lesions in the rodent mammary gland, and alter adult challenges with carcinogenic agents. Open circles indicate applied doses that did not induce significant effects (A and B). Blue circles indicate doses that significantly decreased the measure of interest (A). Red circles indicate doses that significantly increased the measure of interest (B). Gray circles indicate doses that induced the adverse effect noted, but statistics were not performed.
Example 5: Low dose effects of BPA on the ovary
Oogenesis begins in the fetal ovary with the entry of germ cells into meiosis and the subsequent formation of follicles (an oocyte surrounded by somatic cells). In laboratory mammals, 11 studies have shown effects of low dose BPA exposures during ovarian development on three types of endpoints: (1) BPA exposures at the onset of meiosis affect meiotic prophase, increasing the likelihood that the exposed adult will ovulate chromosomally abnormal eggs,Citation251,Citation252 (2) exposures occurring during follicle formation result in an increase in multi-oocyte follicles and a reduction in the total pool of oocytes,Citation251,Citation253-Citation256 and (3) exposures throughout gestation affect the adult ovary, leading to polycystic changes ();Citation118,Citation119,Citation122,Citation257,Citation258 these effects have been observed in numerous mammalian species including mouse, rat, sheep, and monkey as well as in nematodesCitation259 and in human fetal ovaries exposed in vitro.Citation260 Taken together, these studies suggest that exposure during ovary development can adversely affect several different aspects of early oogenesis.
Figure 5. Low dose effects of BPA on the ovary. (A) Effects of developmental exposures on ovarian endpoints, including adverse effects that manifest in adulthood. (B) Summary of in vitro studies showing effects of BPA on hormone production and enzyme activity in ovarian cells. (C) Effects of adult exposures on ovarian endpoints in rodents. (D) Summary of epidemiology studies examining relationships between BPA exposure metrics and ovarian response in women undergoing IVF. Open circles indicate applied doses that did not induce significant effects (A–C) or populations that were unaffected (D). Red circles indicate doses that significantly increased the measure of interest (A–C) or populations that were significantly affected (D). Blue circles indicate doses that significantly decreased the measure of interest (B). Gray circles indicate doses that induced the adverse effect noted, but statistics were not performed.
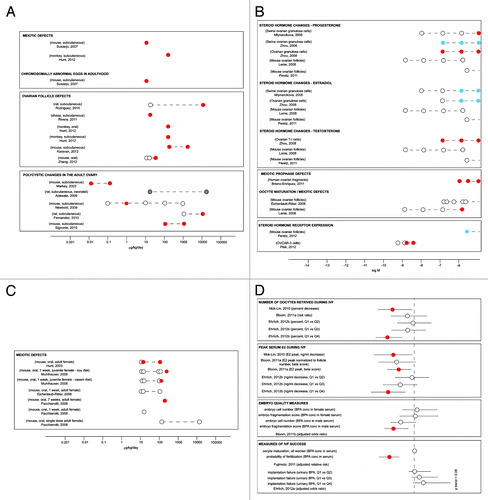
In the sexually mature female, groups of ovarian follicles initiate a complex growth process during which the oocyte undergoes growth and maturation and the theca and granulosa cells mature and provide an appropriate endocrine and paracrine environment. Each cell type plays an integral part in ovarian health and fertility. In vitro studies have shown effects of BPA on hormone production and enzyme expression/activity in granulosa and theca cells, but these effects were generally observed at doses above 10−8 M; lower doses were not typically examined ().Citation36,Citation37,Citation43,Citation260-Citation264 As mentioned, BPA alters zebrafish ovarian histopathology including the incidence of atretic follicles.Citation112 Four mammalian studies have also examined the effects of adult BPA exposure on meiotic maturation of the oocyte with variable results ().Citation124,Citation125,Citation263,Citation265 However, other factors like dietary phytoestrogens are thought to influence the response to BPA at this stage.Citation266 A number of studies of women undergoing assisted reproduction are consistent with the conclusion that BPA exposure is associated with poorer oocyte and embryo quality ().Citation218-Citation222,Citation267 Thus, collectively, these studies suggest that low dose BPA exposure exerts adverse effects on growing follicles in the adult ovary.
Mechanisms of Low Dose Effects
Hormones are known to have effects at extremely low doses due to high affinity receptor–ligand interactions, the non-linear relationship between hormone concentration and number of bound receptors, and the non-linear relationship between the number of bound receptors and biological effects.Citation5,Citation114,Citation268 EDCs that interact with hormone receptors have been shown to follow these same “rules.” Low dose effects should also be considered in the context of circulating endogenous hormones (as well as phytoestrogens from the diet); thus, some low dose effects of EDCs may involve additive or synergistic effects.Citation269,Citation270 Finally, it should be noted that many low dose effects can be attributed to the period of exposure. Exposures to hormones and EDCs during critical periods can irreversibly affect differentiation and organogenesisCitation271 and some developmental exposures have been shown to alter the epigenome, suggesting altered epigenetics may be one mechanism of BPA action.Citation272-Citation274
Current State of Evidence
Several recent reviews have concluded that there are reproducible low dose effects for a number of EDCs, including BPA.Citation5,Citation11,Citation114,Citation275 Additionally, in 2008, the NTP determined there was “some concern” about the effects of human exposure levels on prostate development, brain development, and some behaviors,Citation248 suggesting that this group also concluded that low dose effects were reproducible for these endpoints. Thus, the question of whether low dose effects “exist” is no longer the correct one to ask; clearly they have been reported consistently for a number of endpoints, and the list of these endpoints continues to grow. In our analysis, the low dose cut-off for mammalian animal studies was set at 50 mg/kg/day, a dose identified in previous toxicology studies, consistent with the NTP’s definition of a low dose;Citation4 this dose was also used by the Chapel Hill expert panel.Citation6 Because of the number of studies reporting effects below this cut-off, these previous reviews and our current analysis challenge the setting of the LOAEL at 50 mg/kg/day as well as the “safe” reference dose of 50 µg/kg/day.
Are these low dose effects adverse? There are two widely accepted definitions for “adverse effect”: (1) the US EPA’s definition is “a biochemical change, functional impairment, or pathologic lesion that affects the performance of the whole organism, or reduces an organism's ability to respond to an additional environmental challenge.”Citation276 (2) the OECD definition is “a change in morphology, physiology, growth, development, or lifespan of an organism, which results in impairment of functional capacity or impairment of capacity to compensate for additional stress or increase in susceptibility to the harmful effects of other environmental influences.”Citation277 Based on these definitions, we consider many of the endpoints affected by BPA to be adverse (). In sum, it is clear that consistent, reproducible, low dose effects are observed in several mammalian species and fall under the definition of adverse effects.
As for the issue of reproducibility, there are historical examples of specific endpoints shown to be sensitive to low doses of BPA by some scientists but not reproduced by other groups (reviewed in ref. Citation4). Subsequent analyses showed that many of the studies that were unable to detect low dose effects had problems including lack of concurrent positive controls, lack of a response in the positive control, or the requirement for inappropriately high doses of the positive control, as well as other experimental issues. Weight-of-evidence analyses using principles of endocrinology indicate that low dose effects of BPA are highly reproducible and consistent between many different studies.Citation5,Citation275 The fact that there are any studies showing low dose effects of BPA—and there are several hundred such studies including more than 50 human studies—indicates that the current safety guidelines for BPA are not protective of human health.
Based on the available evidence, we are confident of the following:
(1) Consistent, reproducible low dose effects have been demonstrated for BPA in cell lines, primary cells and tissues, laboratory animals, and in some human populations.
(2) Many of these low dose effects should be considered adverse.
(3) Doses that reliably produce statistically significant effects in animals are 1–4 magnitudes of order lower than the current LOAEL of 50 mg/kg/day.
(4) EDCs, including BPA, often pose a greater threat when exposure occurs during early development, organogenesis, or during critical postnatal periods during which tissues are differentiating.
(5) Low dose effects have been demonstrated in rodents following developmental exposures to BPA, including effects on the male and female reproductive tract, brain development and behaviors, metabolism and growth endpoints, and development of preneoplastic and neoplastic lesions of the mammary gland and the mammary gland’s response to carcinogens.
(6) Low dose effects have been demonstrated in rodents following adult exposure to BPA on the brain and behaviors, metabolic parameters, and function of the male reproductive organs.
(7) In wildlife, consistent and reproducible low dose effects have been observed on a number of endpoints, including induction of vitellogenin expression in males of various fish species and feminization of the adult male reproductive tract, and other measures related to reproductive success.
(8) In cultured cells (both primary and immortalized cell lines) and tissue explants, levels in the range of 10−14–10−9 M induce effects on a number of endpoints. These endpoints provide important molecular and mechanistic understanding for some low dose effects observed in vivo.
(9) The epidemiology literature shows consistent relationships between current typical human exposures to BPA and a number of different health outcomes.
Future Directions for Low Dose Research
The major goal of this review was to address whether low dose effects exist for BPA, whether these effects are consistent and reproducible, and whether the endpoints affected represent adverse outcomes. Based on the hundreds of studies published to date, we conclude that there is sufficient evidence for low dose effects of BPA. In this review, we presented evidence for consistent, reproducible, adverse effects on numerous endpoints, including ovarian health (including histopathology and polycystic ovarian syndrome), some behaviors (anxiety, impaired learning), the mammary gland’s response to carcinogens, abnormal glucose/insulin homeostasis, and diabetes. Other adverse effects have been observed in endpoints such as altered immune responses to allergens, decreased fertility, and fecundity, altered brain function, and altered response of the prostate to hormone challenges; we were unable to elaborate on all of these examples. There do remain scientific data gaps and data needs that are worth pursuing. These include:
•The use of a consistent definition of “low dose” and “low dose effects” in the EDC literature.
•An acknowledgment that “low dose” is distinct from NMDRCs.
•Improved data on the sources of BPA exposure.
•Incorporation of low doses and additional sensitive endpoints in guideline studies.
•Improved longitudinal/life-course evaluation of animals and humans developmentally exposed to BPA.
•Better understanding of specific mechanisms of BPA action, including the determination of whether multiple mechanisms contribute to the effects of BPA on animals.
•Evaluation of additive and synergistic effects of BPA with other EDCs including phytoestrogens.
•Study of interactions between developmental BPA exposures and lifestyle or environmental challenges later life.
•To better define critical windows of vulnerability for various endpoints shown to be sensitive to low doses of BPA.
| Abbreviations: | ||
| BPA | = | bisphenol A |
| CNS | = | central nervous system |
| EDC | = | endocrine disrupting chemical |
| EPA | = | US Environmental Protection Agency |
| ER | = | estrogen receptor |
| IVF | = | in vitro fertilization |
| LOAEL | = | lowest observed adverse effect level |
| NIEHS | = | National Institute of Environmental Health Sciences |
| NMDRC | = | non-monotonic dose response curve |
| NTP | = | US National Toxicology Program |
| OECD | = | Organization for Economic Cooperation and Development |
| T4 | = | thyroxine |
| TSH | = | thyroid stimulating hormone |
Additional material
Download Zip (1 MB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This manuscript was prepared as a product of the NIEHS BPA consortium.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/endo_dis/article/26490
References
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV. Human exposure to bisphenol A (BPA). Reprod Toxicol 2007; 24:139 - 77; http://dx.doi.org/10.1016/j.reprotox.2007.07.010; PMID: 17825522
- Watson CS, Jeng YJ, Guptarak J. Endocrine disruption via estrogen receptors that participate in nongenomic signaling pathways. J Steroid Biochem Mol Biol 2011; 127:44 - 50; http://dx.doi.org/10.1016/j.jsbmb.2011.01.015; PMID: 21300151
- Reif DM, Martin MT, Tan SW, Houck KA, Judson RS, Richard AM, Knudsen TB, Dix DJ, Kavlock RJ. Endocrine profiling and prioritization of environmental chemicals using ToxCast data. Environ Health Perspect 2010; 118:1714 - 20; http://dx.doi.org/10.1289/ehp.1002180; PMID: 20826373
- Melnick R, Lucier G, Wolfe M, Hall R, Stancel G, Prins G, Gallo M, Reuhl K, Ho SM, Brown T, et al. Summary of the National Toxicology Program’s report of the endocrine disruptors low-dose peer review. Environ Health Perspect 2002; 110:427 - 31; http://dx.doi.org/10.1289/ehp.02110427; PMID: 11940462
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, et al. Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses. Endocr Rev 2012; 33:378 - 455; http://dx.doi.org/10.1210/er.2011-1050; PMID: 22419778
- Richter CA, Birnbaum LS, Farabollini F, Newbold RR, Rubin BS, Talsness CE, Vandenbergh JG, Walser-Kuntz DR, vom Saal FS. In vivo effects of bisphenol A in laboratory rodent studies. Reprod Toxicol 2007; 24:199 - 224; http://dx.doi.org/10.1016/j.reprotox.2007.06.004; PMID: 17683900
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc GA, Guillette LJ Jr.. An ecological assessment of bisphenol-A: evidence from comparative biology. Reprod Toxicol 2007; 24:225 - 39; http://dx.doi.org/10.1016/j.reprotox.2007.05.008; PMID: 17604601
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM. In vitro molecular mechanisms of bisphenol A action. Reprod Toxicol 2007; 24:178 - 98; http://dx.doi.org/10.1016/j.reprotox.2007.05.010; PMID: 17628395
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ Health Perspect 2005; 113:926 - 33; http://dx.doi.org/10.1289/ehp.7713; PMID: 16079060
- vom Saal FS, Welshons WV. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environ Res 2006; 100:50 - 76; http://dx.doi.org/10.1016/j.envres.2005.09.001; PMID: 16256977
- vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ Jr., Hauser R, Heindel JJ, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol 2007; 24:131 - 8; http://dx.doi.org/10.1016/j.reprotox.2007.07.005; PMID: 17768031
- Keri RA, Ho S-M, Hunt PA, Knudsen KE, Soto AM, Prins GS. An evaluation of evidence for the carcinogenic activity of bisphenol A. Reprod Toxicol 2007; 24:240 - 52; http://dx.doi.org/10.1016/j.reprotox.2007.06.008; PMID: 17706921
- Chapin RE, Adams J, Boekelheide K, Gray LE Jr., Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol 2008; 83:157 - 395; http://dx.doi.org/10.1002/bdrb.20147; PMID: 18613034
- Rochester JR. Bisphenol A and human health: A review of the literature. Reprod Toxicol 2013; 42C:132 - 55; http://dx.doi.org/10.1016/j.reprotox.2013.08.008; PMID: 23994667
- Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJR, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ Health Perspect 2010; 118:1055 - 70; http://dx.doi.org/10.1289/ehp.0901716; PMID: 20338858
- Vandenberg LN, Hunt PA, Myers JP, Vom Saal FS. Human exposures to bisphenol A: mismatches between data and assumptions. Rev Environ Health 2013; 28:37 - 58; http://dx.doi.org/10.1515/reveh-2012-0034; PMID: 23612528
- Taylor JA, Vom Saal FS, Welshons WV, Drury B, Rottinghaus G, Hunt PA, Toutain PL, Laffont CM, VandeVoort CA. Similarity of bisphenol A pharmacokinetics in rhesus monkeys and mice: relevance for human exposure. Environ Health Perspect 2011; 119:422 - 30; http://dx.doi.org/10.1289/ehp.1002514; PMID: 20855240
- Taylor JA, Welshons WV, Vom Saal FS. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24h after administration in neonatal female mice. Reprod Toxicol 2008; 25:169 - 76; http://dx.doi.org/10.1016/j.reprotox.2008.01.001; PMID: 18295446
- Doerge DR, Twaddle NC, Vanlandingham M, Fisher JW. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol Appl Pharmacol 2010; 247:158 - 65; http://dx.doi.org/10.1016/j.taap.2010.06.008; PMID: 20600215
- Prins GS, Ye SH, Birch L, Ho SM, Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod Toxicol 2011; 31:1 - 9; http://dx.doi.org/10.1016/j.reprotox.2010.09.009; PMID: 20887781
- Gayrard V, Lacroix MZ, Collet SH, Viguié C, Bousquet-Melou A, Toutain PL, Picard-Hagen N. High bioavailability of bisphenol A from sublingual exposure. Environ Health Perspect 2013; 121:951 - 6; PMID: 23761051
- Birnbaum LS, Bucher JR, Collman GW, Zeldin DC, Johnson AF, Schug TT, Heindel JJ. Consortium-based science: the NIEHS’s multipronged, collaborative approach to assessing the health effects of bisphenol A. Environ Health Perspect 2012; 120:1640 - 4; PMID: 23052487
- Hess-Wilson JK, Webb SL, Daly HK, Leung YK, Boldison J, Comstock CE, Sartor MA, Ho SM, Knudsen KE. Unique bisphenol A transcriptome in prostate cancer: novel effects on ERbeta expression that correspond to androgen receptor mutation status. Environ Health Perspect 2007; 115:1646 - 53; http://dx.doi.org/10.1289/ehp.10283; PMID: 18007998
- Richter CA, Taylor JA, Ruhlen RL, Welshons WV, Vom Saal FS. Estradiol and Bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate mesenchyme cells. Environ Health Perspect 2007; 115:902 - 8; http://dx.doi.org/10.1289/ehp.9804; PMID: 17589598
- Takamiya M, Lambard S, Huhtaniemi IT. Effect of bisphenol A on human chorionic gonadotrophin-stimulated gene expression of cultured mouse Leydig tumour cells. Reprod Toxicol 2007; 24:265 - 75; http://dx.doi.org/10.1016/j.reprotox.2007.07.003; PMID: 17706920
- Bouskine A, Nebout M, Brücker-Davis F, Benahmed M, Fenichel P. Low doses of bisphenol A promote human seminoma cell proliferation by activating PKA and PKG via a membrane G-protein-coupled estrogen receptor. Environ Health Perspect 2009; 117:1053 - 8; PMID: 19654912
- Kim JY, Han EH, Kim HG, Oh KN, Kim SK, Lee KY, Jeong HG. Bisphenol A-induced aromatase activation is mediated by cyclooxygenase-2 up-regulation in rat testicular Leydig cells. Toxicol Lett 2010; 193:200 - 8; http://dx.doi.org/10.1016/j.toxlet.2010.01.011; PMID: 20096755
- Sheng ZG, Zhu BZ. Low concentrations of bisphenol A induce mouse spermatogonial cell proliferation by G protein-coupled receptor 30 and estrogen receptor-α. Environ Health Perspect 2011; 119:1775 - 80; http://dx.doi.org/10.1289/ehp.1103781; PMID: 21813366
- Ye L, Zhao B, Hu G, Chu Y, Ge RS. Inhibition of human and rat testicular steroidogenic enzyme activities by bisphenol A. Toxicol Lett 2011; 207:137 - 42; http://dx.doi.org/10.1016/j.toxlet.2011.09.001; PMID: 21925253
- Chevalier N, Bouskine A, Fenichel P. Bisphenol A promotes testicular seminoma cell proliferation through GPER/GPR30. Int J Cancer 2012; 130:241 - 2; http://dx.doi.org/10.1002/ijc.25972; PMID: 21312194
- Nanjappa MK, Simon L, Akingbemi BT. The industrial chemical bisphenol A (BPA) interferes with proliferative activity and development of steroidogenic capacity in rat Leydig cells. Biol Reprod 2012; 86:135 - , 1-12; http://dx.doi.org/10.1095/biolreprod.111.095349; PMID: 22302688
- N’Tumba-Byn T, Moison D, Lacroix M, Lecureuil C, Lesage L, Prud’homme SM, Pozzi-Gaudin S, Frydman R, Benachi A, Livera G, et al. Differential effects of bisphenol A and diethylstilbestrol on human, rat and mouse fetal leydig cell function. PLoS One 2012; 7:e51579; http://dx.doi.org/10.1371/journal.pone.0051579; PMID: 23284716
- Derouiche S, Warnier M, Mariot P, Gosset P, Mauroy B, Bonnal JL, Slomianny C, Delcourt P, Prevarskaya N, Roudbaraki M. Bisphenol A stimulates human prostate cancer cell migration via remodelling of calcium signalling. Springerplus 2013; 2:54; http://dx.doi.org/10.1186/2193-1801-2-54; PMID: 23450760
- Hulak M, Gazo I, Shaliutina A, Linhartova P. In vitro effects of bisphenol A on the quality parameters, oxidative stress, DNA integrity and adenosine triphosphate content in sterlet (Acipenser ruthenus) spermatozoa. Comp Biochem Physiol C Toxicol Pharmacol 2013; 158:64 - 71; http://dx.doi.org/10.1016/j.cbpc.2013.05.002; PMID: 23680852
- Dominguez MA, Petre MA, Neal MS, Foster WG. Bisphenol A concentration-dependently increases human granulosa-lutein cell matrix metalloproteinase-9 (MMP-9) enzyme output. Reprod Toxicol 2008; 25:420 - 5; http://dx.doi.org/10.1016/j.reprotox.2008.05.059; PMID: 18585891
- Zhou W, Liu J, Liao L, Han S, Liu J. Effect of bisphenol A on steroid hormone production in rat ovarian theca-interstitial and granulosa cells. Mol Cell Endocrinol 2008; 283:12 - 8; http://dx.doi.org/10.1016/j.mce.2007.10.010; PMID: 18191889
- Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res 2008; 651:71 - 81; http://dx.doi.org/10.1016/j.mrgentox.2007.10.017; PMID: 18093867
- Mlynarcíková A, Nagyová E, Ficková M, Scsuková S. Effects of selected endocrine disruptors on meiotic maturation, cumulus expansion, synthesis of hyaluronan and progesterone by porcine oocyte-cumulus complexes. Toxicol In Vitro 2009; 23:371 - 7; http://dx.doi.org/10.1016/j.tiv.2008.12.017; PMID: 19162163
- Grasselli F, Baratta L, Baioni L, Bussolati S, Ramoni R, Grolli S, Basini G. Bisphenol A disrupts granulosa cell function. Domest Anim Endocrinol 2010; 39:34 - 9; http://dx.doi.org/10.1016/j.domaniend.2010.01.004; PMID: 20172683
- Naciff JM, Khambatta ZS, Reichling TD, Carr GJ, Tiesman JP, Singleton DW, Khan SA, Daston GP. The genomic response of Ishikawa cells to bisphenol A exposure is dose- and time-dependent. Toxicology 2010; 270:137 - 49; http://dx.doi.org/10.1016/j.tox.2010.02.008; PMID: 20170705
- Schaefer WR, Fischer L, Deppert WR, Hanjalic-Beck A, Seebacher L, Weimer M, Zahradnik HP. In vitro-Ishikawa cell test for assessing tissue-specific chemical effects on human endometrium. Reprod Toxicol 2010; 30:89 - 93; http://dx.doi.org/10.1016/j.reprotox.2010.02.002; PMID: 20172022
- Li Y, Burns KA, Arao Y, Luh CJ, Korach KS. Differential estrogenic actions of endocrine-disrupting chemicals bisphenol A, bisphenol AF, and zearalenone through estrogen receptor α and β in vitro. Environ Health Perspect 2012; 120:1029 - 35; http://dx.doi.org/10.1289/ehp.1104689; PMID: 22494775
- Ptak A, Gregoraszczuk EL. Bisphenol A induces leptin receptor expression, creating more binding sites for leptin, and activates the JAK/Stat, MAPK/ERK and PI3K/Akt signalling pathways in human ovarian cancer cell. Toxicol Lett 2012; 210:332 - 7; http://dx.doi.org/10.1016/j.toxlet.2012.02.003; PMID: 22343039
- Benachour N, Aris A. Toxic effects of low doses of Bisphenol-A on human placental cells. Toxicol Appl Pharmacol 2009; 241:322 - 8; http://dx.doi.org/10.1016/j.taap.2009.09.005; PMID: 19769995
- Dairkee SH, Seok J, Champion S, Sayeed A, Mindrinos M, Xiao W, Davis RW, Goodson WH. Bisphenol A induces a profile of tumor aggressiveness in high-risk cells from breast cancer patients. Cancer Res 2008; 68:2076 - 80; http://dx.doi.org/10.1158/0008-5472.CAN-07-6526; PMID: 18381411
- Habauzit D, Boudot A, Kerdivel G, Flouriot G, Pakdel F. Development and validation of a test for environmental estrogens: Checking xeno-estrogen activity by CXCL12 secretion in BREAST CANCER CELL LINES (CXCL-test). Environ Toxicol 2010; 25:495 - 503; http://dx.doi.org/10.1002/tox.20594; PMID: 20549624
- Weng YI, Hsu PY, Liyanarachchi S, Liu J, Deatherage DE, Huang YW, Zuo T, Rodriguez B, Lin CH, Cheng AL, et al. Epigenetic influences of low-dose bisphenol A in primary human breast epithelial cells. Toxicol Appl Pharmacol 2010; 248:111 - 21; http://dx.doi.org/10.1016/j.taap.2010.07.014; PMID: 20678512
- Yang CZ, Yaniger SI, Jordan VC, Klein DJ, Bittner GD. Most plastic products release estrogenic chemicals: a potential health problem that can be solved. Environ Health Perspect 2011; 119:989 - 96; http://dx.doi.org/10.1289/ehp.1003220; PMID: 21367689
- Wu S, Wei X, Jiang J, Shang L, Hao W. Effects of bisphenol A on the proliferation and cell cycle of HBL-100 cells. Food Chem Toxicol 2012; 50:3100 - 5; http://dx.doi.org/10.1016/j.fct.2012.06.029; PMID: 22735500
- Dairkee SH, Luciani-Torres MG, Moore DH, Goodson WH 3rd. Bisphenol-A-induced inactivation of the p53 axis underlying deregulation of proliferation kinetics, and cell death in non-malignant human breast epithelial cells. Carcinogenesis 2013; 34:703 - 12; http://dx.doi.org/10.1093/carcin/bgs379; PMID: 23222814
- Lapensee EW, Tuttle TR, Fox SR, Ben-Jonathan N. Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor-alpha-positive and -negative breast cancer cells. Environ Health Perspect 2009; 117:175 - 80; PMID: 19270784
- LaPensee EW, LaPensee CR, Fox S, Schwemberger S, Afton S, Ben-Jonathan N. Bisphenol A and estradiol are equipotent in antagonizing cisplatin-induced cytotoxicity in breast cancer cells. Cancer Lett 2010; 290:167 - 73; http://dx.doi.org/10.1016/j.canlet.2009.09.005; PMID: 19796866
- Goodson WH 3rd, Luciani MG, Sayeed SA, Jaffee IM, Moore DH 2nd, Dairkee SH. Activation of the mTOR pathway by low levels of xenoestrogens in breast epithelial cells from high-risk women. Carcinogenesis 2011; 32:1724 - 33; http://dx.doi.org/10.1093/carcin/bgr196; PMID: 21890461
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One 2008; 3:e2069; http://dx.doi.org/10.1371/journal.pone.0002069; PMID: 18446233
- Soriano S, Alonso-Magdalena P, García-Arévalo M, Novials A, Muhammed SJ, Salehi A, Gustafsson JA, Quesada I, Nadal A. Rapid insulinotropic action of low doses of bisphenol-A on mouse and human islets of Langerhans: role of estrogen receptor β. PLoS One 2012; 7:e31109; http://dx.doi.org/10.1371/journal.pone.0031109; PMID: 22347437
- Sargis RM, Johnson DN, Choudhury RA, Brady MJ. Environmental endocrine disruptors promote adipogenesis in the 3T3-L1 cell line through glucocorticoid receptor activation. Obesity (Silver Spring) 2010; 18:1283 - 8; http://dx.doi.org/10.1038/oby.2009.419; PMID: 19927138
- Ben-Jonathan N, Hugo ER, Brandebourg TD. Effects of bisphenol A on adipokine release from human adipose tissue: Implications for the metabolic syndrome. Mol Cell Endocrinol 2009; 304:49 - 54; http://dx.doi.org/10.1016/j.mce.2009.02.022; PMID: 19433247
- Chamorro-García R, Kirchner S, Li X, Janesick A, Casey SC, Chow C, Blumberg B. Bisphenol A diglycidyl ether induces adipogenic differentiation of multipotent stromal stem cells through a peroxisome proliferator-activated receptor gamma-independent mechanism. Environ Health Perspect 2012; 120:984 - 9; http://dx.doi.org/10.1289/ehp.1205063; PMID: 22763116
- Wang J, Sun B, Hou M, Pan X, Li X. The environmental obesogen bisphenol A promotes adipogenesis by increasing the amount of 11beta-hydroxysteroid dehydrogenase type 1 in the adipose tissue of children. Int J Obes (Lond) 2012.
- Yan S, Chen Y, Dong M, Song W, Belcher SM, Wang HS. Bisphenol A and 17β-estradiol promote arrhythmia in the female heart via alteration of calcium handling. PLoS One 2011; 6:e25455; http://dx.doi.org/10.1371/journal.pone.0025455; PMID: 21980463
- Belcher SM, Chen Y, Yan S, Wang HS. Rapid estrogen receptor-mediated mechanisms determine the sexually dimorphic sensitivity of ventricular myocytes to 17β-estradiol and the environmental endocrine disruptor bisphenol A. Endocrinology 2012; 153:712 - 20; http://dx.doi.org/10.1210/en.2011-1772; PMID: 22166976
- Yan S, Song W, Chen Y, Hong K, Rubinstein J, Wang HS. Low-dose bisphenol A and estrogen increase ventricular arrhythmias following ischemia-reperfusion in female rat hearts. Food Chem Toxicol 2013; 56:75 - 80; http://dx.doi.org/10.1016/j.fct.2013.02.011; PMID: 23429042
- Alonso-Magdalena P, Ropero AB, Carrera MP, Cederroth CR, Baquié M, Gauthier BR, Nef S, Stefani E, Nadal A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS One 2008; 3:e2069; http://dx.doi.org/10.1371/journal.pone.0002069; PMID: 18446233
- Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect 2008; 116:1642 - 7; http://dx.doi.org/10.1289/ehp.11537; PMID: 19079714
- Kochukov MY, Jeng YJ, Watson CS. Alkylphenol xenoestrogens with varying carbon chain lengths differentially and potently activate signaling and functional responses in GH3/B6/F10 somatomammotropes. Environ Health Perspect 2009; 117:723 - 30; PMID: 19479013
- Lapensee EW, Tuttle TR, Fox SR, Ben-Jonathan N. Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor-alpha-positive and -negative breast cancer cells. Environ Health Perspect 2009; 117:175 - 80; PMID: 19270784
- Jeng YJ, Kochukov M, Watson CS. Combinations of physiologic estrogens with xenoestrogens alter calcium and kinase responses, prolactin release, and membrane estrogen receptor trafficking in rat pituitary cells. Environ Health 2010; 9:61; http://dx.doi.org/10.1186/1476-069X-9-61; PMID: 20950447
- Le HH, Belcher SM. Rapid signaling actions of environmental estrogens in developing granule cell neurons are mediated by estrogen receptor ß. Endocrinology 2010; 151:5689 - 99; http://dx.doi.org/10.1210/en.2010-0710; PMID: 20926581
- Thuillier R, Mazer M, Manku G, Boisvert A, Wang Y, Culty M. Interdependence of platelet-derived growth factor and estrogen-signaling pathways in inducing neonatal rat testicular gonocytes proliferation. Biol Reprod 2010; 82:825 - 36; http://dx.doi.org/10.1095/biolreprod.109.081729; PMID: 20089883
- Andersson H, Brittebo E. Proangiogenic effects of environmentally relevant levels of bisphenol A in human primary endothelial cells. Arch Toxicol 2012; 86:465 - 74; http://dx.doi.org/10.1007/s00204-011-0766-2; PMID: 22045264
- Jeng YJ, Watson CS. Combinations of physiologic estrogens with xenoestrogens alter ERK phosphorylation profiles in rat pituitary cells. Environ Health Perspect 2011; 119:104 - 12; http://dx.doi.org/10.1289/ehp.1002512; PMID: 20870566
- Ptak A, Wróbel A, Gregoraszczuk EL. Effect of bisphenol-A on the expression of selected genes involved in cell cycle and apoptosis in the OVCAR-3 cell line. Toxicol Lett 2011; 202:30 - 5; http://dx.doi.org/10.1016/j.toxlet.2011.01.015; PMID: 21277958
- Huc L, Lemarié A, Guéraud F, Héliès-Toussaint C. Low concentrations of bisphenol A induce lipid accumulation mediated by the production of reactive oxygen species in the mitochondria of HepG2 cells. Toxicol In Vitro 2012; 26:709 - 17; http://dx.doi.org/10.1016/j.tiv.2012.03.017; PMID: 22515966
- Sheng ZG, Tang Y, Liu YX, Yuan Y, Zhao BQ, Chao XJ, Zhu BZ. Low concentrations of bisphenol a suppress thyroid hormone receptor transcription through a nongenomic mechanism. Toxicol Appl Pharmacol 2012; 259:133 - 42; http://dx.doi.org/10.1016/j.taap.2011.12.018; PMID: 22227104
- Watson CS, Jeng YJ, Hu G, Wozniak A, Bulayeva N, Guptarak J. Estrogen- and xenoestrogen-induced ERK signaling in pituitary tumor cells involves estrogen receptor-α interactions with G protein-αi and caveolin I. Steroids 2012; 77:424 - 32; http://dx.doi.org/10.1016/j.steroids.2011.12.025; PMID: 22230296
- Sheng ZG, Huang W, Liu YX, Zhu BZ. Bisphenol A at a low concentration boosts mouse spermatogonial cell proliferation by inducing the G protein-coupled receptor 30 expression. Toxicol Appl Pharmacol 2013; 267:88 - 94; http://dx.doi.org/10.1016/j.taap.2012.12.014; PMID: 23274518
- Viñas R, Watson CS. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect 2013; 121:352 - 8; http://dx.doi.org/10.1289/ehp.1205826; PMID: 23458715
- Viñas R, Watson CS. Mixtures of xenoestrogens disrupt estradiol-induced non-genomic signaling and downstream functions in pituitary cells. Environ Health 2013; 12:26; http://dx.doi.org/10.1186/1476-069X-12-26; PMID: 23530988
- Staples CA, Tilghman Hall A, Friederich U, Caspers N, Klecka GM. Early life-stage and multigeneration toxicity study with bisphenol A and fathead minnows (Pimephales promelas). Ecotoxicol Environ Saf 2011; 74:1548 - 57; http://dx.doi.org/10.1016/j.ecoenv.2011.05.010; PMID: 21700340
- Hatef A, Alavi SM, Abdulfatah A, Fontaine P, Rodina M, Linhart O. Adverse effects of bisphenol A on reproductive physiology in male goldfish at environmentally relevant concentrations. Ecotoxicol Environ Saf 2012; 76:56 - 62; http://dx.doi.org/10.1016/j.ecoenv.2011.09.021; PMID: 22036266
- Kamata R, Shiraishi F, Nakajima D, Kageyama S. Estrogenic effects of leachates from industrial waste landfills measured by a recombinant yeast assay and transcriptional analysis in Japanese medaka. Aquat Toxicol 2011; 101:430 - 7; http://dx.doi.org/10.1016/j.aquatox.2010.11.018; PMID: 21216354
- Muncke J, Junghans M, Eggen RI. Testing estrogenicity of known and novel (xeno-)estrogens in the MolDarT using developing zebrafish (Danio rerio). Environ Toxicol 2007; 22:185 - 93; http://dx.doi.org/10.1002/tox.20255; PMID: 17366571
- Zhou J, Zhu XS, Cai ZH. The impacts of bisphenol A (BPA) on abalone (Haliotis diversicolor supertexta) embryonic development. Chemosphere 2011; 82:443 - 50; http://dx.doi.org/10.1016/j.chemosphere.2010.09.056; PMID: 20970156
- Ozlem CA, Hatice P. Effects of bisphenol A on the embryonic development of sea urchin (Paracentrotus lividus). Environ Toxicol 2008; 23:387 - 92; http://dx.doi.org/10.1002/tox.20349; PMID: 18214894
- Mihaich EM, Friederich U, Caspers N, Hall AT, Klecka GM, Dimond SS, Staples CA, Ortego LS, Hentges SG. Acute and chronic toxicity testing of bisphenol A with aquatic invertebrates and plants. Ecotoxicol Environ Saf 2009; 72:1392 - 9; http://dx.doi.org/10.1016/j.ecoenv.2009.02.005; PMID: 19327838
- Ha MH, Choi J. Effects of environmental contaminants on hemoglobin gene expression in Daphnia magna: a potential biomarker for freshwater quality monitoring. Arch Environ Contam Toxicol 2009; 57:330 - 7; http://dx.doi.org/10.1007/s00244-007-9079-0; PMID: 19471991
- Liu HL, Liu XH, Wang XY, Wang XR, Yu HX. [Toxicity of BPA and TBBPA to Daphnia magna and zebrafish Brachydanio rerio]. Huan Jing Ke Xue 2007; 28:1784 - 7; PMID: 17926411
- Park SY, Choi J. Genotoxic effects of nonylphenol and bisphenol A exposure in aquatic biomonitoring species: freshwater crustacean, Daphnia magna, and aquatic midge, Chironomus riparius. Bull Environ Contam Toxicol 2009; 83:463 - 8; http://dx.doi.org/10.1007/s00128-009-9745-1; PMID: 19475328
- Sánchez-Argüello P, Aparicio N, Fernández C. Linking embryo toxicity with genotoxic responses in the freshwater snail Physa acuta: single exposure to benzo(a)pyrene, fluoxetine, bisphenol A, vinclozolin and exposure to binary mixtures with benzo(a)pyrene. Ecotoxicol Environ Saf 2012; 80:152 - 60; http://dx.doi.org/10.1016/j.ecoenv.2012.02.029; PMID: 22417675
- Gibert Y, Sassi-Messai S, Fini JB, Bernard L, Zalko D, Cravedi JP, Balaguer P, Andersson-Lendahl M, Demeneix B, Laudet V. Bisphenol A induces otolith malformations during vertebrate embryogenesis. BMC Dev Biol 2011; 11:4; http://dx.doi.org/10.1186/1471-213X-11-4; PMID: 21269433
- Chow WS, Chan WK, Chan KM. Toxicity assessment and vitellogenin expression in zebrafish (Danio rerio) embryos and larvae acutely exposed to bisphenol A, endosulfan, heptachlor, methoxychlor and tetrabromobisphenol A. J Appl Toxicol 2013; 33:670 - 8; http://dx.doi.org/10.1002/jat.2723; PMID: 22351617
- Duan Z, Zhu L, Zhu L, Kun Y, Zhu X. Individual and joint toxic effects of pentachlorophenol and bisphenol A on the development of zebrafish (Danio rerio) embryo. Ecotoxicol Environ Saf 2008; 71:774 - 80; http://dx.doi.org/10.1016/j.ecoenv.2008.01.021; PMID: 18359083
- McCormick JM, Van Es T, Cooper KR, White LA, Häggblom MM. Microbially mediated O-methylation of bisphenol A results in metabolites with increased toxicity to the developing zebrafish (Danio rerio) embryo. Environ Sci Technol 2011; 45:6567 - 74; http://dx.doi.org/10.1021/es200588w; PMID: 21678910
- Saili KS, Corvi MM, Weber DN, Patel AU, Das SR, Przybyla J, Anderson KA, Tanguay RL. Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology 2012; 291:83 - 92; http://dx.doi.org/10.1016/j.tox.2011.11.001; PMID: 22108044
- Petersen K, Fetter E, Kah O, Brion F, Scholz S, Tollefsen KE. Transgenic (cyp19a1b-GFP) zebrafish embryos as a tool for assessing combined effects of oestrogenic chemicals. Aquat Toxicol 2013; 138-139:88 - 97; http://dx.doi.org/10.1016/j.aquatox.2013.05.001; PMID: 23721851
- Chan WK, Chan KM. Disruption of the hypothalamic-pituitary-thyroid axis in zebrafish embryo-larvae following waterborne exposure to BDE-47, TBBPA and BPA. Aquat Toxicol 2012; 108:106 - 11; http://dx.doi.org/10.1016/j.aquatox.2011.10.013; PMID: 22100034
- Kausch U, Alberti M, Haindl S, Budczies J, Hock B. Biomarkers for exposure to estrogenic compounds: gene expression analysis in zebrafish (Danio rerio). Environ Toxicol 2008; 23:15 - 24; http://dx.doi.org/10.1002/tox.20306; PMID: 18214933
- Ha MH, Choi J. Effects of environmental contaminants on hemoglobin of larvae of aquatic midge, Chironomus riparius (Diptera: Chironomidae): a potential biomarker for ecotoxicity monitoring. Chemosphere 2008; 71:1928 - 36; http://dx.doi.org/10.1016/j.chemosphere.2008.01.018; PMID: 18328532
- Olsvik PA, Lie KK, Sturve J, Hasselberg L, Andersen OK. Transcriptional effects of nonylphenol, bisphenol A and PBDE-47 in liver of juvenile Atlantic cod (Gadus morhua). Chemosphere 2009; 75:360 - 7; http://dx.doi.org/10.1016/j.chemosphere.2008.12.039; PMID: 19167021
- Moens LN, van der Ven K, Van Remortel P, Del-Favero J, De Coen WM. Gene expression analysis of estrogenic compounds in the liver of common carp (Cyprinus carpio) using a custom cDNA microarray. J Biochem Mol Toxicol 2007; 21:299 - 311; http://dx.doi.org/10.1002/jbt.20190; PMID: 17912697
- Lee YM, Rhee JS, Hwang DS, Kim IC, Raisuddin S, Lee JS. Mining of biomarker genes from expressed sequence tags and differential display reverse transcriptase-polymerase chain reaction in the self-fertilizing fish, Kryptolebias marmoratus and their expression patterns in response to exposure to an endocrine-disrupting alkylphenol, bisphenol A. Mol Cells 2007; 23:287 - 303; PMID: 17646703
- Rhee JS, Kim BM, Lee CJ, Yoon YD, Lee YM, Lee JS. Bisphenol A modulates expression of sex differentiation genes in the self-fertilizing fish, Kryptolebias marmoratus. Aquat Toxicol 2011; 104:218 - 29; http://dx.doi.org/10.1016/j.aquatox.2011.04.020; PMID: 21632026
- Rhee JS, Kim RO, Chang HH, Lee J, Lee YM, Lee JS. Endocrine disrupting chemicals modulate expression of O⁶-methylguanine DNA methyltransferase (O⁶-MGMT) gene in the hermaphroditic fish, Kryptolebias marmoratus. Comp Biochem Physiol C Toxicol Pharmacol 2011; 153:141 - 9; http://dx.doi.org/10.1016/j.cbpc.2010.10.005; PMID: 20965277
- Rhee JS, Kim RO, Seo JS, Kang HS, Park CB, Soyano K, Lee J, Lee YM, Lee JS. Bisphenol A modulates expression of gonadotropin subunit genes in the hermaphroditic fish, Kryptolebias marmoratus. Comp Biochem Physiol C Toxicol Pharmacol 2010; 152:456 - 66; http://dx.doi.org/10.1016/j.cbpc.2010.07.003; PMID: 20647052
- Rhee JS, Seo JS, Raisuddin S, Ki JS, Lee KW, Kim IC, Yoon YD, Lee JS. Gonadotropin-releasing hormone receptor (GnRHR) gene expression is differently modulated in gender types of the hermaphroditic fish Kryptolebias marmoratus by endocrine disrupting chemicals. Comp Biochem Physiol C Toxicol Pharmacol 2008; 147:357 - 65; http://dx.doi.org/10.1016/j.cbpc.2008.01.007; PMID: 18280221
- Yu IT, Rhee JS, Raisuddin S, Lee JS. Characterization of the glutathione S-transferase-Mu (GSTM) gene sequence and its expression in the hermaphroditic fish, Kryptolebias marmoratus as a function of development, gender type and chemical exposure. Chem Biol Interact 2008; 174:118 - 25; http://dx.doi.org/10.1016/j.cbi.2008.05.011; PMID: 18571633
- Aluru N, Leatherland JF, Vijayan MM. Bisphenol A in oocytes leads to growth suppression and altered stress performance in juvenile rainbow trout. PLoS One 2010; 5:e10741; http://dx.doi.org/10.1371/journal.pone.0010741; PMID: 20505776
- Liu S, Qin F, Wang H, Wu T, Zhang Y, Zheng Y, Li M, Wang Z. Effects of 17α-ethinylestradiol and bisphenol A on steroidogenic messenger ribonucleic acid levels in the rare minnow gonads. Aquat Toxicol 2012; 122-123:19 - 27; http://dx.doi.org/10.1016/j.aquatox.2012.05.010; PMID: 22710023
- Qin F, Wang L, Wang X, Liu S, Xu P, Wang H, Wu T, Zhang Y, Zheng Y, Li M, et al. Bisphenol A affects gene expression of gonadotropin-releasing hormones and type I GnRH receptors in brains of adult rare minnow Gobiocypris rarus. Comp Biochem Physiol C Toxicol Pharmacol 2013; 157:192 - 202; http://dx.doi.org/10.1016/j.cbpc.2012.11.002; PMID: 23174456
- Baba K, Okada K, Kinoshita T, Imaoka S. Bisphenol A disrupts Notch signaling by inhibiting gamma-secretase activity and causes eye dysplasia of Xenopus laevis. Toxicol Sci 2009; 108:344 - 55; http://dx.doi.org/10.1093/toxsci/kfp025; PMID: 19218331
- Imaoka S, Mori T, Kinoshita T. Bisphenol A causes malformation of the head region in embryos of Xenopus laevis and decreases the expression of the ESR-1 gene mediated by Notch signaling. Biol Pharm Bull 2007; 30:371 - 4; http://dx.doi.org/10.1248/bpb.30.371; PMID: 17268083
- Molina AM, Lora AJ, Blanco A, Monterde JG, Ayala N, Moyano R. Endocrine-active compound evaluation: qualitative and quantitative histomorphological assessment of zebrafish gonads after bisphenol-A exposure. Ecotoxicol Environ Saf 2013; 88:155 - 62; http://dx.doi.org/10.1016/j.ecoenv.2012.11.010; PMID: 23219663
- Tyl RW. Basic exploratory research versus guideline-compliant studies used for hazard evaluation and risk assessment: bisphenol A as a case study. Environ Health Perspect 2009; 117:1644 - 51; PMID: 20049112
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, Vom Saal FS. Endocrine-disrupting chemicals and public health protection: a statement of principles from The Endocrine Society. Endocrinology 2012; 153:4097 - 110; http://dx.doi.org/10.1210/en.2012-1422; PMID: 22733974
- Myers JP, Zoeller RT, vom Saal FS. A clash of old and new scientific concepts in toxicity, with important implications for public health. Environ Health Perspect 2009; 117:1652 - 5; PMID: 20049113
- vom Saal FS, Myers JP. Good laboratory practices are not synonymous with good scientific practices, accurate reporting, or valid data. Environ Health Perspect 2010; 118:A60 - , author reply A61; http://dx.doi.org/10.1289/ehp.0901495; PMID: 20123633
- Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol 2007; 24:253 - 8; http://dx.doi.org/10.1016/j.reprotox.2007.07.006; PMID: 17804194
- Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect 2009; 117:879 - 85; PMID: 19590677
- Signorile PG, Spugnini EP, Mita L, Mellone P, D’Avino A, Bianco M, Diano N, Caputo L, Rea F, Viceconte R, et al. Pre-natal exposure of mice to bisphenol A elicits an endometriosis-like phenotype in female offspring. Gen Comp Endocrinol 2010; 168:318 - 25; http://dx.doi.org/10.1016/j.ygcen.2010.03.030; PMID: 20350546
- Varayoud J, Ramos JG, Bosquiazzo VL, Muñoz-de-Toro M, Luque EH. Developmental exposure to Bisphenol a impairs the uterine response to ovarian steroids in the adult. Endocrinology 2008; 149:5848 - 60; http://dx.doi.org/10.1210/en.2008-0651; PMID: 18653720
- Smith CC, Taylor HS. Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development. FASEB J 2007; 21:239 - 46; http://dx.doi.org/10.1096/fj.06-6635com; PMID: 17093138
- Fernández M, Bourguignon N, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats. Environ Health Perspect 2010; 118:1217 - 22; http://dx.doi.org/10.1289/ehp.0901257; PMID: 20413367
- Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, Gadbois JL, Tharp AP, Whitt GS, Sonnenschein C, et al. Perinatal exposure to environmentally relevant levels of bisphenol A decreases fertility and fecundity in CD-1 mice. Environ Health Perspect 2011; 119:547 - 52; http://dx.doi.org/10.1289/ehp.1002559; PMID: 21126938
- Pacchierotti F, Ranaldi R, Eichenlaub-Ritter U, Attia S, Adler ID. Evaluation of aneugenic effects of bisphenol A in somatic and germ cells of the mouse. Mutat Res 2008; 651:64 - 70; http://dx.doi.org/10.1016/j.mrgentox.2007.10.009; PMID: 18083607
- Muhlhauser A, Susiarjo M, Rubio C, Griswold J, Gorence G, Hassold T, Hunt PA. Bisphenol A effects on the growing mouse oocyte are influenced by diet. Biol Reprod 2009; 80:1066 - 71; http://dx.doi.org/10.1095/biolreprod.108.074815; PMID: 19164168
- Lee SG, Kim JY, Chung JY, Kim YJ, Park JE, Oh S, Yoon YD, Yoo KS, Yoo YH, Kim JM. Bisphenol A exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17β-estradiol synthesis via downregulation of aromatase in rat ovary. Environ Health Perspect 2013; 121:663 - 9; http://dx.doi.org/10.1289/ehp.1205823; PMID: 23512349
- Aldad TS, Rahmani N, Leranth C, Taylor HS. Bisphenol-A exposure alters endometrial progesterone receptor expression in the nonhuman primate. Fertil Steril 2011; 96:175 - 9; http://dx.doi.org/10.1016/j.fertnstert.2011.04.010; PMID: 21536273
- Hewitt SC, Korach KS. Estrogenic activity of bisphenol A and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) demonstrated in mouse uterine gene profiles. Environ Health Perspect 2011; 119:63 - 70; http://dx.doi.org/10.1289/ehp.1002347; PMID: 20826375
- Tharp AP, Maffini MV, Hunt PA, VandeVoort CA, Sonnenschein C, Soto AM. Bisphenol A alters the development of the rhesus monkey mammary gland. Proc Natl Acad Sci U S A 2012; 109:8190 - 5; http://dx.doi.org/10.1073/pnas.1120488109; PMID: 22566636
- Wadia PR, Vandenberg LN, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM. Perinatal bisphenol A exposure increases estrogen sensitivity of the mammary gland in diverse mouse strains. Environ Health Perspect 2007; 115:592 - 8; http://dx.doi.org/10.1289/ehp.9640; PMID: 17450229
- Moral R, Wang R, Russo IH, Lamartiniere CA, Pereira J, Russo J. Effect of prenatal exposure to the endocrine disruptor bisphenol A on mammary gland morphology and gene expression signature. J Endocrinol 2008; 196:101 - 12; http://dx.doi.org/10.1677/JOE-07-0056; PMID: 18180321
- Vandenberg LN, Maffini MV, Schaeberle CM, Ucci AA, Sonnenschein C, Rubin BS, Soto AM. Perinatal exposure to the xenoestrogen bisphenol-A induces mammary intraductal hyperplasias in adult CD-1 mice. Reprod Toxicol 2008; 26:210 - 9; http://dx.doi.org/10.1016/j.reprotox.2008.09.015; PMID: 18938238
- Ayyanan A, Laribi O, Schuepbach-Mallepell S, Schrick C, Gutierrez M, Tanos T, Lefebvre G, Rougemont J, Yalcin-Ozuysal O, Brisken C. Perinatal exposure to bisphenol a increases adult mammary gland progesterone response and cell number. Mol Endocrinol 2011; 25:1915 - 23; http://dx.doi.org/10.1210/me.2011-1129; PMID: 21903720
- Durando M, Kass L, Perdomo V, Bosquiazzo VL, Luque EH, Muñoz-de-Toro M. Prenatal exposure to bisphenol A promotes angiogenesis and alters steroid-mediated responses in the mammary glands of cycling rats. J Steroid Biochem Mol Biol 2011; 127:35 - 43; http://dx.doi.org/10.1016/j.jsbmb.2011.04.001; PMID: 21513798
- Kass L, Altamirano GA, Bosquiazzo VL, Luque EH, Muñoz-de-Toro M. Perinatal exposure to xenoestrogens impairs mammary gland differentiation and modifies milk composition in Wistar rats. Reprod Toxicol 2012; 33:390 - 400; http://dx.doi.org/10.1016/j.reprotox.2012.02.002; PMID: 22349186
- Vandenberg LN, Schaeberle CM, Rubin BS, Sonnenschein C, Soto AM. The male mammary gland: a target for the xenoestrogen bisphenol A. Reprod Toxicol 2013; 37:15 - 23; http://dx.doi.org/10.1016/j.reprotox.2013.01.002; PMID: 23348055
- Jenkins S, Raghuraman N, Eltoum I, Carpenter M, Russo J, Lamartiniere CA. Oral exposure to bisphenol a increases dimethylbenzanthracene-induced mammary cancer in rats. Environ Health Perspect 2009; 117:910 - 5; PMID: 19590682
- Betancourt AM, Eltoum IA, Desmond RA, Russo J, Lamartiniere CA. In utero exposure to bisphenol A shifts the window of susceptibility for mammary carcinogenesis in the rat. Environ Health Perspect 2010; 118:1614 - 9; http://dx.doi.org/10.1289/ehp.1002148; PMID: 20675265
- Weber Lozada K, Keri RA. Bisphenol A increases mammary cancer risk in two distinct mouse models of breast cancer. Biol Reprod 2011; 85:490 - 7; http://dx.doi.org/10.1095/biolreprod.110.090431; PMID: 21636739
- Acevedo N, Davis B, Schaeberle CM, Sonnenschein C, Soto AM. Perinatally administered bisphenol a as a potential mammary gland carcinogen in rats. Environ Health Perspect 2013; 121:1040 - 6; PMID: 23876597
- Yan H, Takamoto M, Sugane K. Exposure to Bisphenol A prenatally or in adulthood promotes T(H)2 cytokine production associated with reduction of CD4CD25 regulatory T cells. Environ Health Perspect 2008; 116:514 - 9; PMID: 18414636
- Midoro-Horiuti T, Tiwari R, Watson CS, Goldblum RM. Maternal bisphenol a exposure promotes the development of experimental asthma in mouse pups. Environ Health Perspect 2010; 118:273 - 7; http://dx.doi.org/10.1289/ehp.0901259; PMID: 20123615
- Bauer SM, Roy A, Emo J, Chapman TJ, Georas SN, Lawrence BP. The effects of maternal exposure to bisphenol A on allergic lung inflammation into adulthood. Toxicol Sci 2012; 130:82 - 93; http://dx.doi.org/10.1093/toxsci/kfs227; PMID: 22821851
- Nakajima Y, Goldblum RM, Midoro-Horiuti T. Fetal exposure to bisphenol A as a risk factor for the development of childhood asthma: an animal model study. Environ Health 2012; 11:8; http://dx.doi.org/10.1186/1476-069X-11-8; PMID: 22353195
- Roy A, Bauer SM, Lawrence BP. Developmental exposure to bisphenol A modulates innate but not adaptive immune responses to influenza A virus infection. PLoS One 2012; 7:e38448; http://dx.doi.org/10.1371/journal.pone.0038448; PMID: 22675563
- Leranth C, Hajszan T, Szigeti-Buck K, Bober J, MacLusky NJ. Bisphenol A prevents the synaptogenic response to estradiol in hippocampus and prefrontal cortex of ovariectomized nonhuman primates. Proc Natl Acad Sci U S A 2008; 105:14187 - 91; http://dx.doi.org/10.1073/pnas.0806139105; PMID: 18768812
- Leranth C, Szigeti-Buck K, Maclusky NJ, Hajszan T. Bisphenol A prevents the synaptogenic response to testosterone in the brain of adult male rats. Endocrinology 2008; 149:988 - 94; http://dx.doi.org/10.1210/en.2007-1053; PMID: 18048497
- Hajszan T, Leranth C. Bisphenol A interferes with synaptic remodeling. Front Neuroendocrinol 2010; 31:519 - 30; http://dx.doi.org/10.1016/j.yfrne.2010.06.004; PMID: 20609373
- Eilam-Stock T, Serrano P, Frankfurt M, Luine V. Bisphenol-A impairs memory and reduces dendritic spine density in adult male rats. Behav Neurosci 2012; 126:175 - 85; http://dx.doi.org/10.1037/a0025959; PMID: 22004261
- Inagaki T, Frankfurt M, Luine V. Estrogen-induced memory enhancements are blocked by acute bisphenol A in adult female rats: role of dendritic spines. Endocrinology 2012; 153:3357 - 67; http://dx.doi.org/10.1210/en.2012-1121; PMID: 22569790
- Batista TM, Alonso-Magdalena P, Vieira E, Amaral ME, Cederroth CR, Nef S, Quesada I, Carneiro EM, Nadal A. Short-term treatment with bisphenol-A leads to metabolic abnormalities in adult male mice. PLoS One 2012; 7:e33814; http://dx.doi.org/10.1371/journal.pone.0033814; PMID: 22470480
- Marmugi A, Ducheix S, Lasserre F, Polizzi A, Paris A, Priymenko N, Bertrand-Michel J, Pineau T, Guillou H, Martin PG, et al. Low doses of bisphenol A induce gene expression related to lipid synthesis and trigger triglyceride accumulation in adult mouse liver. Hepatology 2012; 55:395 - 407; http://dx.doi.org/10.1002/hep.24685; PMID: 21932408
- Rönn M, Kullberg J, Karlsson H, Berglund J, Malmberg F, Orberg J, Lind L, Ahlström H, Lind PM. Bisphenol A exposure increases liver fat in juvenile fructose-fed Fischer 344 rats. Toxicology 2013; 303:125 - 32; http://dx.doi.org/10.1016/j.tox.2012.09.013; PMID: 23142792
- Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, Maghuin-Rogister G, Pironnet AM, Pussemier L, Scippo ML, et al. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol 2012; 50:3725 - 40; http://dx.doi.org/10.1016/j.fct.2012.07.059; PMID: 22889897
- Teeguarden JG, Hanson-Drury S. A systematic review of Bisphenol A “Low Dose” studies in the context of human exposure: A case for establishing standards for reporting “low-dose” effects of chemicals. Food Chem Toxicol 2013; Forthcoming PMID: 23867546
- Salian S, Doshi T, Vanage G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A. Life Sci 2009; 85:11 - 8; http://dx.doi.org/10.1016/j.lfs.2009.04.005; PMID: 19379760
- Jones LP, Sampson A, Kang HJ, Kim HJ, Yi YW, Kwon SY, Babus JK, Wang A, Bae I. Loss of BRCA1 leads to an increased sensitivity to Bisphenol A. Toxicol Lett 2010; 199:261 - 8; http://dx.doi.org/10.1016/j.toxlet.2010.09.008; PMID: 20868731
- Matsuda S, Saika S, Amano K, Shimizu E, Sajiki J. Changes in brain monoamine levels in neonatal rats exposed to bisphenol A at low doses. Chemosphere 2010; 78:894 - 906; http://dx.doi.org/10.1016/j.chemosphere.2009.11.010; PMID: 20006895
- Ryan KK, Haller AM, Sorrell JE, Woods SC, Jandacek RJ, Seeley RJ. Perinatal exposure to bisphenol-a and the development of metabolic syndrome in CD-1 mice. Endocrinology 2010; 151:2603 - 12; http://dx.doi.org/10.1210/en.2009-1218; PMID: 20351315
- Bai Y, Chang F, Zhou R, Jin PP, Matsumoto H, Sokabe M, Chen L. Increase of anteroventral periventricular kisspeptin neurons and generation of E2-induced LH-surge system in male rats exposed perinatally to environmental dose of bisphenol-A. Endocrinology 2011; 152:1562 - 71; http://dx.doi.org/10.1210/en.2010-1042; PMID: 21303948
- Ishido M, Masuo Y, Terasaki M, Morita M. Rat hyperactivity by bisphenol A, but not by its derivatives, 3-hydroxybisphenol A or bisphenol A 3,4-quinone. Toxicol Lett 2011; 206:300 - 5; http://dx.doi.org/10.1016/j.toxlet.2011.08.011; PMID: 21884766
- Jain S, Kumar CH, Suranagi UD, Mediratta PK. Protective effect of N-acetylcysteine on bisphenol A-induced cognitive dysfunction and oxidative stress in rats. Food Chem Toxicol 2011; 49:1404 - 9; http://dx.doi.org/10.1016/j.fct.2011.03.032; PMID: 21440025
- Jenkins S, Wang J, Eltoum I, Desmond R, Lamartiniere CA. Chronic oral exposure to bisphenol A results in a nonmonotonic dose response in mammary carcinogenesis and metastasis in MMTV-erbB2 mice. Environ Health Perspect 2011; 119:1604 - 9; http://dx.doi.org/10.1289/ehp.1103850; PMID: 21988766
- Zhou R, Bai Y, Yang R, Zhu Y, Chi X, Li L, Chen L, Sokabe M, Chen L. Abnormal synaptic plasticity in basolateral amygdala may account for hyperactivity and attention-deficit in male rat exposed perinatally to low-dose bisphenol-A. Neuropharmacology 2011; 60:789 - 98; http://dx.doi.org/10.1016/j.neuropharm.2011.01.031; PMID: 21277317
- Anderson OS, Nahar MS, Faulk C, Jones TR, Liao C, Kannan K, Weinhouse C, Rozek LS, Dolinoy DC. Epigenetic responses following maternal dietary exposure to physiologically relevant levels of bisphenol A. Environ Mol Mutagen 2012; 53:334 - 42; http://dx.doi.org/10.1002/em.21692; PMID: 22467340
- Brannick KE, Craig ZR, Himes AD, Peretz JR, Wang W, Flaws JA, Raetzman LT. Prenatal exposure to low doses of bisphenol A increases pituitary proliferation and gonadotroph number in female mice offspring at birth. Biol Reprod 2012; 87:82; http://dx.doi.org/10.1095/biolreprod.112.100636; PMID: 22875908
- D’Cruz SC, Jubendradass R, Jayakanthan M, Rani SJ, Mathur PP. Bisphenol A impairs insulin signaling and glucose homeostasis and decreases steroidogenesis in rat testis: an in vivo and in silico study. Food Chem Toxicol 2012; 50:1124 - 33; http://dx.doi.org/10.1016/j.fct.2011.11.041; PMID: 22142692
- D’Cruz SC, Jubendradass R, Mathur PP. Bisphenol A induces oxidative stress and decreases levels of insulin receptor substrate 2 and glucose transporter 8 in rat testis. Reprod Sci 2012; 19:163 - 72; http://dx.doi.org/10.1177/1933719111415547; PMID: 22101236
- Ferguson SA, Law CD, Abshire JS. Developmental treatment with bisphenol A causes few alterations on measures of postweaning activity and learning. Neurotoxicol Teratol 2012; 34:598 - 606; http://dx.doi.org/10.1016/j.ntt.2012.09.006; PMID: 23041373
- He Z, Paule MG, Ferguson SA. Low oral doses of bisphenol A increase volume of the sexually dimorphic nucleus of the preoptic area in male, but not female, rats at postnatal day 21. Neurotoxicol Teratol 2012; 34:331 - 7; http://dx.doi.org/10.1016/j.ntt.2012.03.004; PMID: 22507915
- Jones BA, Watson NV. Perinatal BPA exposure demasculinizes males in measures of affect but has no effect on water maze learning in adulthood. Horm Behav 2012; 61:605 - 10; http://dx.doi.org/10.1016/j.yhbeh.2012.02.011; PMID: 22370244
- Kendig EL, Buesing DR, Christie SM, Cookman CJ, Gear RB, Hugo ER, Kasper SN, Kendziorski JA, Ungi KR, Williams K, et al. Estrogen-like disruptive effects of dietary exposure to bisphenol A or 17α-ethinyl estradiol in CD1 mice. Int J Toxicol 2012; 31:537 - 50; http://dx.doi.org/10.1177/1091581812463254; PMID: 23160314
- Kendziorski JA, Kendig EL, Gear RB, Belcher SM. Strain specific induction of pyometra and differences in immune responsiveness in mice exposed to 17α-ethinyl estradiol or the endocrine disrupting chemical bisphenol A. Reprod Toxicol 2012; 34:22 - 30; http://dx.doi.org/10.1016/j.reprotox.2012.03.001; PMID: 22429997
- Matsuda S, Matsuzawa D, Ishii D, Tomizawa H, Sutoh C, Nakazawa K, Amano K, Sajiki J, Shimizu E. Effects of perinatal exposure to low dose of bisphenol A on anxiety like behavior and dopamine metabolites in brain. Prog Neuropsychopharmacol Biol Psychiatry 2012; 39:273 - 9; http://dx.doi.org/10.1016/j.pnpbp.2012.06.016; PMID: 22760093
- Pant J, Pant MK, Deshpande SB. Bisphenol A attenuates phenylbiguanide-induced cardio-respiratory reflexes in anaesthetized rats. Neurosci Lett 2012; 530:69 - 74; http://dx.doi.org/10.1016/j.neulet.2012.09.046; PMID: 23041044
- Anderson OS, Peterson KE, Sanchez BN, Zhang Z, Mancuso P, Dolinoy DC. Perinatal bisphenol A exposure promotes hyperactivity, lean body composition, and hormonal responses across the murine life course. FASEB J 2013; 27:1784 - 92; http://dx.doi.org/10.1096/fj.12-223545; PMID: 23345456
- Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, Welshons WV, Besch-Williford CL, Palanza P, Parmigiani S, et al. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol 2013; In press http://dx.doi.org/10.1016/j.reprotox.2013.07.017; PMID: 23892310
- Cabaton NJ, Canlet C, Wadia PR, Tremblay-Franco M, Gautier R, Molina J, Sonnenschein C, Cravedi JP, Rubin BS, Soto AM, et al. Effects of low doses of bisphenol A on the metabolome of perinatally exposed CD-1 mice. Environ Health Perspect 2013; 121:586 - 93; PMID: 23425943
- Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, Patisaul HB. Prenatal bisphenol a exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala. Toxicol Sci 2013; 133:157 - 73; http://dx.doi.org/10.1093/toxsci/kft035; PMID: 23457122
- Folia M, Boudalia S, Ménétrier F, Decocq L, Pasquis B, Schneider C, Bergès R, Artur Y, Canivenc-Lavier MC. Oral homeostasis disruption by medical plasticizer component bisphenol A in adult male rats. Laryngoscope 2013; 123:1405 - 10; http://dx.doi.org/10.1002/lary.23791; PMID: 23686345
- Jin P, Wang X, Chang F, Bai Y, Li Y, Zhou R, Chen L. Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. J Biomed Res 2013; 27:135 - 44; http://dx.doi.org/10.7555/JBR.27.20120076; PMID: 23554804
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne FA. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A 2013; 110:9956 - 61; http://dx.doi.org/10.1073/pnas.1214056110; PMID: 23716699
- Matsuda S, Matsuzawa D, Ishii D, Tomizawa H, Sajiki J, Shimizu E. Perinatal exposure to bisphenol A enhances contextual fear memory and affects the serotoninergic system in juvenile female mice. Horm Behav 2013; 63:709 - 16; http://dx.doi.org/10.1016/j.yhbeh.2013.03.016; PMID: 23567477
- Patel BB, Raad M, Sebag IA, Chalifour LE. Lifelong exposure to bisphenol a alters cardiac structure/function, protein expression, and DNA methylation in adult mice. Toxicol Sci 2013; 133:174 - 85; http://dx.doi.org/10.1093/toxsci/kft026; PMID: 23418087
- Wadia PR, Cabaton NJ, Borrero MD, Rubin BS, Sonnenschein C, Shioda T, Soto AM. Low-dose BPA exposure alters the mesenchymal and epithelial transcriptomes of the mouse fetal mammary gland. PLoS One 2013; 8:e63902; http://dx.doi.org/10.1371/journal.pone.0063902; PMID: 23704952
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 2011; 128:873 - 82; http://dx.doi.org/10.1542/peds.2011-1335; PMID: 22025598
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP. Prenatal bisphenol A exposure and early childhood behavior. Environ Health Perspect 2009; 117:1945 - 52; PMID: 20049216
- Sathyanarayana S, Braun JM, Yolton K, Liddy S, Lanphear BP. Case report: high prenatal bisphenol a exposure and infant neonatal neurobehavior. Environ Health Perspect 2011; 119:1170 - 5; http://dx.doi.org/10.1289/ehp.1003064; PMID: 21524981
- Perera F, Vishnevetsky J, Herbstman JB, Calafat AM, Xiong W, Rauh V, Wang S. Prenatal bisphenol a exposure and child behavior in an inner-city cohort. Environ Health Perspect 2012; 120:1190 - 4; http://dx.doi.org/10.1289/ehp.1104492; PMID: 22543054
- Bellinger DC, Trachtenberg F, Zhang A, Tavares M, Daniel D, McKinlay S. Dental amalgam and psychosocial status: the New England Children’s Amalgam Trial. J Dent Res 2008; 87:470 - 4; http://dx.doi.org/10.1177/154405910808700504; PMID: 18434579
- Maserejian NN, Trachtenberg FL, Hauser R, McKinlay S, Shrader P, Bellinger DC. Dental composite restorations and neuropsychological development in children: treatment level analysis from a randomized clinical trial. Neurotoxicology 2012; 33:1291 - 7; http://dx.doi.org/10.1016/j.neuro.2012.08.001; PMID: 22906860
- Maserejian NN, Trachtenberg FL, Hauser R, McKinlay S, Shrader P, Tavares M, Bellinger DC. Dental composite restorations and psychosocial function in children. Pediatrics 2012; 130:e328 - 38; http://dx.doi.org/10.1542/peds.2011-3374; PMID: 22802599
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, Calafat AM, Wolff MS. Endocrine disruptors and childhood social impairment. Neurotoxicology 2011; 32:261 - 7; http://dx.doi.org/10.1016/j.neuro.2010.12.009; PMID: 21182865
- Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol 2011; 33:558 - 66; http://dx.doi.org/10.1016/j.ntt.2011.08.003; PMID: 21854843
- Lang IA, Galloway TS, Scarlett A, Henley WE, Depledge M, Wallace RB, Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008; 300:1303 - 10; http://dx.doi.org/10.1001/jama.300.11.1303; PMID: 18799442
- Mahalingaiah S, Meeker JD, Pearson KR, Calafat AM, Ye X, Petrozza J, Hauser R. Temporal variability and predictors of urinary bisphenol A concentrations in men and women. Environ Health Perspect 2008; 116:173 - 8; http://dx.doi.org/10.1289/ehp.10605; PMID: 18288314
- Hong YC, Park EY, Park MS, Ko JA, Oh SY, Kim H, Lee KH, Leem JH, Ha EH. Community level exposure to chemicals and oxidative stress in adult population. Toxicol Lett 2009; 184:139 - 44; http://dx.doi.org/10.1016/j.toxlet.2008.11.001; PMID: 19049859
- Galloway T, Cipelli R, Guralnik J, Ferrucci L, Bandinelli S, Corsi AM, Money C, McCormack P, Melzer D. Daily bisphenol A excretion and associations with sex hormone concentrations: results from the InCHIANTI adult population study. Environ Health Perspect 2010; 118:1603 - 8; http://dx.doi.org/10.1289/ehp.1002367; PMID: 20797929
- Melzer D, Rice NE, Lewis C, Henley WE, Galloway TS. Association of urinary bisphenol a concentration with heart disease: evidence from NHANES 2003/06. PLoS One 2010; 5:e8673; http://dx.doi.org/10.1371/journal.pone.0008673; PMID: 20084273
- Carwile JL, Michels KB. Urinary bisphenol A and obesity: NHANES 2003-2006. Environ Res 2011; 111:825 - 30; http://dx.doi.org/10.1016/j.envres.2011.05.014; PMID: 21676388
- Ning G, Bi Y, Wang T, Xu M, Xu Y, Huang Y, Li M, Li X, Wang W, Chen Y, et al. Relationship of urinary bisphenol A concentration to risk for prevalent type 2 diabetes in Chinese adults: a cross-sectional analysis. Ann Intern Med 2011; 155:368 - 74; http://dx.doi.org/10.7326/0003-4819-155-6-201109200-00005; PMID: 21930854
- Shankar A, Teppala S. Relationship between urinary bisphenol A levels and diabetes mellitus. J Clin Endocrinol Metab 2011; 96:3822 - 6; http://dx.doi.org/10.1210/jc.2011-1682; PMID: 21956417
- Silver MK, O’Neill MS, Sowers MR, Park SK. Urinary bisphenol A and type-2 diabetes in U.S. adults: data from NHANES 2003-2008. PLoS One 2011; 6:e26868; http://dx.doi.org/10.1371/journal.pone.0026868; PMID: 22046388
- Kim K, Park H. Association between urinary concentrations of bisphenol A and type 2 diabetes in Korean adults: a population-based cross-sectional study. Int J Hyg Environ Health 2013; 216:467 - 71; http://dx.doi.org/10.1016/j.ijheh.2012.07.007; PMID: 22921714
- LaKind JS, Goodman M, Naiman DQ. Use of NHANES data to link chemical exposures to chronic diseases: a cautionary tale. PLoS One 2012; 7:e51086; http://dx.doi.org/10.1371/journal.pone.0051086; PMID: 23227235
- Shankar A, Teppala S, Sabanayagam C. Urinary bisphenol a levels and measures of obesity: results from the national health and nutrition examination survey 2003-2008. ISRN Endocrinol 2012; 2012:965243; http://dx.doi.org/10.5402/2012/965243; PMID: 22852093
- Teppala S, Madhavan S, Shankar A. Bisphenol A and Metabolic Syndrome: Results from NHANES. Int J Endocrino. 2012
- Trasande L, Attina TM, Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA 2012; 308:1113 - 21; http://dx.doi.org/10.1001/2012.jama.11461; PMID: 22990270
- Wang T, Li M, Chen B, Xu M, Xu Y, Huang Y, Lu J, Chen Y, Wang W, Li X, et al. Urinary bisphenol A (BPA) concentration associates with obesity and insulin resistance. J Clin Endocrinol Metab 2012; 97:E223 - 7; http://dx.doi.org/10.1210/jc.2011-1989; PMID: 22090277
- Wang HX, Zhou Y, Tang CX, Wu JG, Chen Y, Jiang QW. Association between bisphenol A exposure and body mass index in Chinese school children: a cross-sectional study. Environ Health 2012; 11:79; http://dx.doi.org/10.1186/1476-069X-11-79; PMID: 23083070
- Shankar A, Teppala S, Sabanayagam C. Bisphenol A and peripheral arterial disease: results from the NHANES. Environ Health Perspect 2012; 120:1297 - 300; http://dx.doi.org/10.1289/ehp.1104114; PMID: 22645278
- Melzer D, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Luben R, Khaw KT, Wareham NJ, et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 2012; 125:1482 - 90; http://dx.doi.org/10.1161/CIRCULATIONAHA.111.069153; PMID: 22354940
- Melzer D, Gates P, Osborne NJ, Henley WE, Cipelli R, Young A, Money C, McCormack P, Schofield P, Mosedale D, et al. Urinary bisphenol a concentration and angiography-defined coronary artery stenosis. PLoS One 2012; 7:e43378; http://dx.doi.org/10.1371/journal.pone.0043378; PMID: 22916252
- Chevrier J, Gunier RB, Bradman A, Holland NT, Calafat AM, Eskenazi B, Harley KG. Maternal urinary bisphenol a during pregnancy and maternal and neonatal thyroid function in the CHAMACOS study. Environ Health Perspect 2013; 121:138 - 44; PMID: 23052180
- Brucker-Davis F, Ferrari P, Boda-Buccino M, Wagner-Mahler K, Pacini P, Gal J, Azuar P, Fenichel P. Cord blood thyroid tests in boys born with and without cryptorchidism: correlations with birth parameters and in utero xenobiotics exposure. Thyroid 2011; 21:1133 - 41; http://dx.doi.org/10.1089/thy.2010.0459; PMID: 21875366
- Meeker JD, Calafat AM, Hauser R. Urinary bisphenol A concentrations in relation to serum thyroid and reproductive hormone levels in men from an infertility clinic. Environ Sci Technol 2010; 44:1458 - 63; http://dx.doi.org/10.1021/es9028292; PMID: 20030380
- Meeker JD, Ferguson KK. Relationship between urinary phthalate and bisphenol A concentrations and serum thyroid measures in U.S. adults and adolescents from the National Health and Nutrition Examination Survey (NHANES) 2007-2008. Environ Health Perspect 2011; 119:1396 - 402; http://dx.doi.org/10.1289/ehp.1103582; PMID: 21749963
- Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl 2010; 33:385 - 93; http://dx.doi.org/10.1111/j.1365-2605.2009.01014.x; PMID: 20002217
- Bloom MS, Kim D, Vom Saal FS, Taylor JA, Cheng G, Lamb JD, Fujimoto VY. Bisphenol A exposure reduces the estradiol response to gonadotropin stimulation during in vitro fertilization. Fertil Steril 2011; 96:672 - , e2; http://dx.doi.org/10.1016/j.fertnstert.2011.06.063; PMID: 21813122
- Fujimoto VY, Kim D, vom Saal FS, Lamb JD, Taylor JA, Bloom MS. Serum unconjugated bisphenol A concentrations in women may adversely influence oocyte quality during in vitro fertilization. Fertil Steril 2011; 95:1816 - 9; http://dx.doi.org/10.1016/j.fertnstert.2010.11.008; PMID: 21122836
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod 2012; 27:3583 - 92; http://dx.doi.org/10.1093/humrep/des328; PMID: 23014629
- Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, Ye X, Petrozza JC, Wright D, Hauser R. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect 2012; 120:978 - 83; http://dx.doi.org/10.1289/ehp.1104307; PMID: 22484414
- Vandenberg LN. Non-monotonic dose responses in studies of endocrine disrupting chemicals: bisphenol A as a case study. Dose Response 2013; Forthcoming
- Birnbaum LS. Environmental chemicals: evaluating low-dose effects. Environ Health Perspect 2012; 120:A143 - 4; http://dx.doi.org/10.1289/ehp.1205179; PMID: 22470049
- Jiao B, Cheng CH. Disrupting actions of bisphenol A and malachite green on growth hormone receptor gene expression and signal transduction in seabream. Fish Physiol Biochem 2010; 36:251 - 61; http://dx.doi.org/10.1007/s10695-008-9227-0; PMID: 20467862
- Asahi J, Kamo H, Baba R, Doi Y, Yamashita A, Murakami D, Hanada A, Hirano T. Bisphenol A induces endoplasmic reticulum stress-associated apoptosis in mouse non-parenchymal hepatocytes. Life Sci 2010; 87:431 - 8; http://dx.doi.org/10.1016/j.lfs.2010.08.007; PMID: 20807545
- Jeong HG, Kimand JY, Choi CY. Down-regulation of murine Cyp1a-1 in mouse hepatoma Hepa-1c1c7 cells by bisphenol A. Biochem Biophys Res Commun 2000; 277:594 - 8; http://dx.doi.org/10.1006/bbrc.2000.3717; PMID: 11061999
- Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, Aubert ML, Hüppi PS. Perinatal exposure to bisphenol a alters early adipogenesis in the rat. Environ Health Perspect 2009; 117:1549 - 55; PMID: 20019905
- Hassan ZK, Elobeid MA, Virk P, Omer SA, ElAmin M, Daghestani MH, AlOlayan EM. Bisphenol A induces hepatotoxicity through oxidative stress in rat model. Oxid Med Cell Longev 2012; 2012:194829; http://dx.doi.org/10.1155/2012/194829; PMID: 22888396
- Moon MK, Kim MJ, Jung IK, Koo YD, Ann HY, Lee KJ, Kim SH, Yoon YC, Cho BJ, Park KS, et al. Bisphenol A impairs mitochondrial function in the liver at doses below the no observed adverse effect level. J Korean Med Sci 2012; 27:644 - 52; http://dx.doi.org/10.3346/jkms.2012.27.6.644; PMID: 22690096
- Nahar MS, Liao C, Kannan K, Dolinoy DC. Fetal liver bisphenol A concentrations and biotransformation gene expression reveal variable exposure and altered capacity for metabolism in humans. J Biochem Mol Toxicol 2013; 27:116 - 23; http://dx.doi.org/10.1002/jbt.21459; PMID: 23208979
- Adachi T, Yasuda K, Mori C, Yoshinaga M, Aoki N, Tsujimoto G, Tsuda K. Promoting insulin secretion in pancreatic islets by means of bisphenol A and nonylphenol via intracellular estrogen receptors. Food Chem Toxicol 2005; 43:713 - 9; http://dx.doi.org/10.1016/j.fct.2005.01.009; PMID: 15778011
- Alonso-Magdalena P, Vieira E, Soriano S, Menes L, Burks D, Quesada I, Nadal A. Bisphenol A exposure during pregnancy disrupts glucose homeostasis in mothers and adult male offspring. Environ Health Perspect 2010; 118:1243 - 50; http://dx.doi.org/10.1289/ehp.1001993; PMID: 20488778
- Wei J, Lin Y, Li Y, Ying C, Chen J, Song L, Zhou Z, Lv Z, Xia W, Chen X, et al. Perinatal exposure to bisphenol A at reference dose predisposes offspring to metabolic syndrome in adult rats on a high-fat diet. Endocrinology 2011; 152:3049 - 61; http://dx.doi.org/10.1210/en.2011-0045; PMID: 21586551
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environ Health Perspect 2006; 114:106 - 12; http://dx.doi.org/10.1289/ehp.8451; PMID: 16393666
- Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, Palimeri S, Panidis D, Diamanti-Kandarakis E. Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. J Clin Endocrinol Metab 2011; 96:E480 - 4; http://dx.doi.org/10.1210/jc.2010-1658; PMID: 21193545
- Tarantino G, Valentino R, Di Somma C, D’Esposito V, Passaretti F, Pizza G, Brancato V, Orio F, Formisano P, Colao A, et al. Bisphenol A in polycystic ovary syndrome and its association with liver-spleen axis. Clin Endocrinol (Oxf) 2013; 78:447 - 53; http://dx.doi.org/10.1111/j.1365-2265.2012.04500.x; PMID: 22805002
- Hong Y-C, Park E-Y, Park M-S, Ko JA, Oh S-Y, Kim H, Lee K-H, Leem J-H, Ha E-H. Community level exposure to chemicals and oxidative stress in adult population. Toxicol Lett 2009; 184:139 - 44; http://dx.doi.org/10.1016/j.toxlet.2008.11.001; PMID: 19049859
- Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav 2007; 52:307 - 16; http://dx.doi.org/10.1016/j.yhbeh.2007.05.006; PMID: 17568585
- Gonçalves CR, Cunha RW, Barros DM, Martínez PE. Effects of prenatal and postnatal exposure to a low dose of bisphenol A on behavior and memory in rats. Environ Toxicol Pharmacol 2010; 30:195 - 201; http://dx.doi.org/10.1016/j.etap.2010.06.003; PMID: 21787652
- Tian YH, Baek JH, Lee SY, Jang CG. Prenatal and postnatal exposure to bisphenol a induces anxiolytic behaviors and cognitive deficits in mice. Synapse 2010; 64:432 - 9; http://dx.doi.org/10.1002/syn.20746; PMID: 20169576
- Nakamura K, Itoh K, Dai H, Han L, Wang X, Kato S, Sugimoto T, Fushiki S. Prenatal and lactational exposure to low-doses of bisphenol A alters adult mice behavior. Brain Dev 2012; 34:57 63; http://dx.doi.org/10.1016/j.braindev.2010.12.011
- Yu C, Tai F, Song Z, Wu R, Zhang X, He F. Pubertal exposure to bisphenol A disrupts behavior in adult C57BL/6J mice. Environ Toxicol Pharmacol 2011; 31:88 - 99; http://dx.doi.org/10.1016/j.etap.2010.09.009; PMID: 21787673
- Xu X, Tian D, Hong X, Chen L, Xie L. Sex-specific influence of exposure to bisphenol-A between adolescence and young adulthood on mouse behaviors. Neuropharmacology 2011; 61:565 - 73; http://dx.doi.org/10.1016/j.neuropharm.2011.04.027; PMID: 21570416
- Jang YJ, Park HR, Kim TH, Yang WJ, Lee JJ, Choi SY, Oh SB, Lee E, Park JH, Kim HP, et al. High dose bisphenol A impairs hippocampal neurogenesis in female mice across generations. Toxicology 2012; 296:73 - 82; http://dx.doi.org/10.1016/j.tox.2012.03.007; PMID: 22484357
- Xu XH, Zhang J, Wang YM, Ye YP, Luo QQ. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-D-aspartate receptors of hippocampus in male offspring mice. Horm Behav 2010; 58:326 - 33; http://dx.doi.org/10.1016/j.yhbeh.2010.02.012; PMID: 20206181
- Viberg H, Fredriksson A, Buratovic S, Eriksson P. Dose-dependent behavioral disturbances after a single neonatal Bisphenol A dose. Toxicology 2011; 290:187 - 94; http://dx.doi.org/10.1016/j.tox.2011.09.006; PMID: 21971502
- Chapin RE, Adams J, Boekelheide K, Gray LEJ Jr., Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res B Dev Reprod Toxicol 2008; 83:157 - 395; http://dx.doi.org/10.1002/bdrb.20147; PMID: 18613034
- Murray TJ, Maffini MV, Ucci AA, Sonnenschein C, Soto AM. Induction of mammary gland ductal hyperplasias and carcinoma in situ following fetal bisphenol A exposure. Reprod Toxicol 2007; 23:383 - 90; http://dx.doi.org/10.1016/j.reprotox.2006.10.002; PMID: 17123778
- Durando M, Kass L, Piva J, Sonnenschein C, Soto AM, Luque EH, Muñoz-de-Toro M. Prenatal bisphenol A exposure induces preneoplastic lesions in the mammary gland in Wistar rats. Environ Health Perspect 2007; 115:80 - 6; http://dx.doi.org/10.1289/ehp.9282; PMID: 17366824
- Hunt PA, Lawson C, Gieske M, Murdoch B, Smith H, Marre A, Hassold T, VandeVoort CA. Bisphenol A alters early oogenesis and follicle formation in the fetal ovary of the rhesus monkey. Proc Natl Acad Sci U S A 2012; 109:17525 - 30; http://dx.doi.org/10.1073/pnas.1207854109; PMID: 23012422
- Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet 2007; 3:e5; http://dx.doi.org/10.1371/journal.pgen.0030005; PMID: 17222059
- Rodríguez HA, Santambrosio N, Santamaría CG, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary. Reprod Toxicol 2010; 30:550 - 7; http://dx.doi.org/10.1016/j.reprotox.2010.07.008; PMID: 20692330
- Rivera OE, Varayoud J, Rodríguez HA, Muñoz-de-Toro M, Luque EH. Neonatal exposure to bisphenol A or diethylstilbestrol alters the ovarian follicular dynamics in the lamb. Reprod Toxicol 2011; 32:304 - 12; http://dx.doi.org/10.1016/j.reprotox.2011.06.118; PMID: 21722727
- Karavan JR, Pepling ME. Effects of estrogenic compounds on neonatal oocyte development. Reprod Toxicol 2012; 34:51 - 6; http://dx.doi.org/10.1016/j.reprotox.2012.02.005; PMID: 22406039
- Zhang HQ, Zhang XF, Zhang LJ, Chao HH, Pan B, Feng YM, Li L, Sun XF, Shen W. Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes. Mol Biol Rep 2012; 39:5651 - 7; http://dx.doi.org/10.1007/s11033-011-1372-3; PMID: 22187349
- Markey CM, Coombs MA, Sonnenschein C, Soto AM. Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs. Evol Dev 2003; 5:67 - 75; http://dx.doi.org/10.1046/j.1525-142X.2003.03011.x; PMID: 12492412
- Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod 2009; 81:690 - 9; http://dx.doi.org/10.1095/biolreprod.109.078261; PMID: 19535786
- Allard P, Colaiácovo MP. Bisphenol A impairs the double-strand break repair machinery in the germline and causes chromosome abnormalities. Proc Natl Acad Sci U S A 2010; 107:20405 - 10; http://dx.doi.org/10.1073/pnas.1010386107; PMID: 21059909
- Brieño-Enríquez MA, Robles P, Camats-Tarruella N, García-Cruz R, Roig I, Cabero L, Martínez F, Caldés MG. Human meiotic progression and recombination are affected by Bisphenol A exposure during in vitro human oocyte development. Hum Reprod 2011; 26:2807 - 18; http://dx.doi.org/10.1093/humrep/der249; PMID: 21795248
- Mlynarcíková A, Kolena J, Ficková M, Scsuková S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol Cell Endocrinol 2005; 244:57 - 62; http://dx.doi.org/10.1016/j.mce.2005.02.009; PMID: 16225985
- Peretz J, Gupta RK, Singh J, Hernández-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci 2011; 119:209 - 17; http://dx.doi.org/10.1093/toxsci/kfq319; PMID: 20956811
- Eichenlaub-Ritter U, Vogt E, Cukurcam S, Sun F, Pacchierotti F, Parry J. Exposure of mouse oocytes to bisphenol A causes meiotic arrest but not aneuploidy. Mutat Res 2008; 651:82 - 92; http://dx.doi.org/10.1016/j.mrgentox.2007.10.014; PMID: 18096426
- Peretz J, Craig ZR, Flaws JA. Bisphenol A inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol Reprod 2012; 87:63; http://dx.doi.org/10.1095/biolreprod.112.101899; PMID: 22743301
- Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol 2003; 13:546 - 53; http://dx.doi.org/10.1016/S0960-9822(03)00189-1; PMID: 12676084
- Hunt PA, Susiarjo M, Rubio C, Hassold TJ. The bisphenol A experience: a primer for the analysis of environmental effects on mammalian reproduction. Biol Reprod 2009; 81:807 - 13; http://dx.doi.org/10.1095/biolreprod.109.077008; PMID: 19458313
- Bloom MS, Vom Saal FS, Kim D, Taylor JA, Lamb JD, Fujimoto VY. Serum unconjugated bisphenol A concentrations in men may influence embryo quality indicators during in vitro fertilization. Environ Toxicol Pharmacol 2011; 32:319 - 23; http://dx.doi.org/10.1016/j.etap.2011.06.003; PMID: 21843814
- WHO. State of the science of endocrine disrupting chemicals - 2012. An assessment of the state of the science of endocrine disruptors prepared by a group of experts for the United Nations Environment Programme (UNEP) and WHO. In: WHO/UNEP, ed., 2013.
- Silva E, Rajapakse N, Kortenkamp A. Something from “nothing”--eight weak estrogenic chemicals combined at concentrations below NOECs produce significant mixture effects. Environ Sci Technol 2002; 36:1751 - 6; http://dx.doi.org/10.1021/es0101227; PMID: 11993873
- Crofton KM. Thyroid disrupting chemicals: mechanisms and mixtures. Int J Androl 2008; 31:209 - 23; http://dx.doi.org/10.1111/j.1365-2605.2007.00857.x; PMID: 18217984
- McCarthy MM, Wright CL, Schwarz JM. New tricks by an old dogma: mechanisms of the Organizational/Activational Hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm Behav 2009; 55:655 - 65; http://dx.doi.org/10.1016/j.yhbeh.2009.02.012; PMID: 19682425
- Champagne FA, Rissman EF. Behavioral epigenetics: a new frontier in the study of hormones and behavior. Horm Behav 2011; 59:277 - 8; http://dx.doi.org/10.1016/j.yhbeh.2011.02.011; PMID: 21419246
- Prins GS, Tang WY, Belmonte J, Ho SM. Perinatal exposure to oestradiol and bisphenol A alters the prostate epigenome and increases susceptibility to carcinogenesis. Basic Clin Pharmacol Toxicol 2008; 102:134 - 8; http://dx.doi.org/10.1111/j.1742-7843.2007.00166.x; PMID: 18226066
- Kundakovic M, Champagne FA. Epigenetic perspective on the developmental effects of bisphenol A. Brain Behav Immun 2011; 25:1084 - 93; http://dx.doi.org/10.1016/j.bbi.2011.02.005; PMID: 21333735
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, Myers JP, Shioda T, Soto AM, vom Saal FS, et al. Regulatory decisions on endocrine disrupting chemicals should be based on the principles of endocrinology. Reprod Toxicol 2013; 38:1 - 15; http://dx.doi.org/10.1016/j.reprotox.2013.02.002; PMID: 23411111
- Integrated Risk EPAU. Information Systems (IRIS) Glossary. US EPA, 2012.
- IPCS. IPCS risk assessment terminology. Geneva, Switzerland: WHO Document Production Services, 2004.