Abstract
Depressed mood in pregnancy has been linked to low birth weight (LBW, < 2,500 g), a risk factor for adult-onset chronic diseases in offspring. We examined maternal depressed mood in relation to birth weight and evaluated the role of DNA methylation at regulatory sequences of imprinted genes in this association. We measured depressed mood among 922 pregnant women using the CES-D scale and obtained birth weight data from hospital records. Using bisulfite pyrosequencing of cord blood DNA from 508 infants, we measured methylation at differentially methylated regions (DMRs) regulating imprinted genes IGF2/H19, DLK1/MEG3, MEST, PEG3, PEG10/SGCE, NNAT and PLAGL1. Multiple regression models were used to examine the relationship between depressed mood, birth weight and DMR methylation levels. Depressed mood was associated with a more that 3-fold higher risk of LBW, after adjusting for delivery mode, parity, education, cigarette smoking, folic acid use and preterm birth. The association may be more pronounced in offspring of black women and female infants. Compared with infants of women without depressed mood, infants born to women with severe depressed mood had a 2.4% higher methylation at the MEG3 DMR. Whereas LBW infants had 1.6% lower methylation at the IGF2 DMR, high birth weight (> 4,500 g) infants had 5.9% higher methylation at the PLAGL1 DMR compared with normal birth weight infants. Our findings confirm that severe maternal depressed mood in pregnancy is associated with LBW, and that MEG3 and IGF2 plasticity may play important roles.
Introduction
Low birth weight (LBW), defined as birth weight < 2,500 g, is a common measure of overall fetal and maternal health.Citation1 Several lines of evidence suggest that LBW may also function as a marker of adult health,Citation2 as LBW neonates are at increased risk of coronary heart disease, stroke, type 2 diabetes, obesity and some cancers in adulthood.Citation3-Citation8 This epidemiological evidence supports the developmental origin of health and disease (DOHAD) hypothesis, which postulates that the adaptive response to prenatal and early postnatal environment can affect disease susceptibility in adulthood.Citation6,Citation9-Citation11
Prenatal depression has been associated with poor birth outcomes including LBW,Citation12 and a growing emphasis has been placed on screening for psychosocial risk factors during pregnancy as a strategy to reduce poor birth outcomes.Citation13 Depression is one of the most common psychiatric disorders among young adults and is associated with substantial morbidity, particularly during pregnancy.Citation14 In the US, the prevalence of depressive symptoms in the antenatal period ranges from 10–15% depending on gestational age, race/ethnicity and socioeconomic factors.Citation15 As many as 12.7% of pregnant women experience at least one major depressive episode during pregnancy.Citation15 Both maternal depression and poor birth outcomes disproportionately affect Blacks and Hispanics compared with Whites.Citation16-Citation18 Mechanistic insights that can guide public health or therapeutic intervention efforts to alleviate this disparity are required.
Hormonal dysregulation due to abnormalities of the hypothalamus-pituitary-adrenal (HPA) axis driven by epigenetics has been proposed as a potential explanation for associations between maternal depression and LBW.Citation19 Empirical data supportive of epigenetic mechanisms underlying these associations have been limited by a lack of epigenetic targets. Studies largely target promoter regions of genes, as research on tumor suppressor genes has suggested that aberrant methylation at promoter regions may lead to transcriptional silencing.Citation11 However, imprinted genes, or parent of origin dependent monoallelic expression regulated by methylation that is established differentially on the two parental chromosomes, may play a role in many disease processes.Citation20 Epigenetic changes at cis-acting differentially methylated regions (DMRs) that regulate imprinted gene expression are important not only in maintaining the imprinted status of these genes but also in controlling their levels of expression.Citation21,Citation22 Since these methylation marks are established before gastrulation, they are maintained in all somatic tissues.Citation21,Citation22 Because these genes occur in clusters, are enriched for growth regulators and intricately interact to coordinate early growth, methylation alterations at a single DMR may lead to changes in the regulation of multiple genes.Citation21,Citation23,Citation24 The objectives of this analysis were to examine the association between prenatal maternal depressed mood in relation to birth weight and to evaluate the role of DNA methylation at nine imprinted regulatory sequences in this association. Because the effects of depression may be more severe in Blacks compared with WhitesCitation25,Citation26 and epigenetic perturbations may be sex-specific,Citation27,Citation28 we also examine these associations by race/ethnicity of the mother and sex of the offspring.
Results
The distribution of maternal socio-demographic characteristics is summarized in by CES-D and history of depression. The median CES-D scale score was 10 and the mean CES-D scale score was 12.7, SD 9.6. Seven percent (n = 90) of pregnant women were classified as having severe depressed mood, 24% (n = 307) moderate depressed mood, and 872 reported no depressed mood. Compared with women with no depressive symptoms, both groups of women with depressed mood were more likely to be younger (p < 0.01), black (p < 0.01), unmarried (p < 0.01), unemployed (p < 0.01), report poorer health (p < 0.01), be of lower income (p < 0.01) and be enrolled later in gestation (p < 0.001). Women with depressed mood were also more likely to smoke (p < 0.01), have anxiety p < 0.01), use psychotropic medications (p < 0.01), but not take folic acid (p = 0.02). Women at extremes of education, notably those with less than high school or having been to graduate school, were less likely to have depressed mood (p < 0.01). Alcohol use, BMI at last menstrual period (LMP), BMI at enrollment, sex of offspring, parity and mode of delivery were comparable among women with and without depressed mood (p > 0.05).
Table 1. Maternal Demographics by CES-D and History of Depression: NEST
Association between depressed mood and birth weight
Birth weight ranged from 580–5,422 g, with a mean birth weight of 3,285 g (SD 574 g) and median of 3,330 g. Compared with women with no depressed mood, infants born to women with severe (but not moderate) depressed mood were more likely to be LBW (OR 3.6, 95% CI 1.1–11.4), after adjustment for parity, education, smoking, delivery mode, folic acid use and preterm birth (). We also found that this association was limited to infants born to black women (OR 7.2, 95% CI 1.8–28.7) and more pronounced in female infants (7.5, 95% CI 1.5–38.9). However, the cross-product terms for depressed mood and infant sex and for depressed mood and race/ethnicity were not statistically significant (p > 0.05). These associations were not significantly altered by further adjusting for psychotropic medication use and pre-pregnancy or early prenatal maternal BMI, in statistical models. Only 15 infants with completed mood data were born with birth weight > 4,500 g, and 12 of them were born to women without depressed mood. Also, no severely depressed Hispanic woman gave birth to a LBW infant in this study.
Table 2. Multivariate Model – Depressed Mood and birth weight, overall and stratified by race/ethnicity and infant sex
DNA methylation in relation to prenatal depressed mood and birth weight
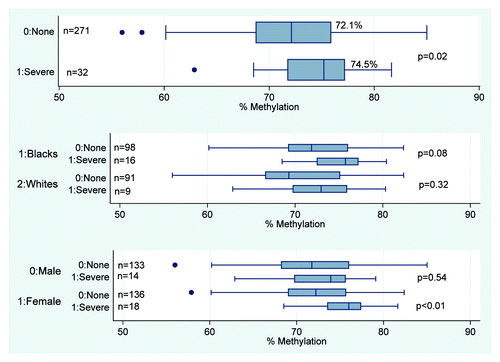
To determine the extent to which DNA methylation marks mediated, at least in part, the association between maternal depressed mood and birth weight, we computed DNA methylation differences among normal birth weight (2,500–4,500 g), LBW (< 2,500 g) and high birth weight (> 4,500 g) infants at nine imprinted gene DMRs (IGF2, H19, MEG3-IG, MEG3, PEG3, MEST, PEG10, NNAT and PLAGL1). We observed that infants born to women with severely depressed mood had 2.4% higher methylation levels at the MEG3 DMR than those born to non-depressed women (p = 0.02; ). This difference persisted after adjusting for maternal DNA methylation levels, despite correlations between maternal and infant methylation profiles at these DMRs (coefficient = 0.18–0.51 depending on DMR). Sex- and race/ethnic-specific analysis revealed that these differences might be larger in female infants (3.6%, p < 0.01), and those born to black women (2.3%, p = 0.08). These methylation differences remained unaltered after excluding all women who reported using psychotropic medications (n = 26). Infants born to women reporting severely depressed mood had similar DNA methylation levels at the other DMRs examined when compared with infants of women with no depressed mood.
Figure 1. Methylation at MEG3 for infants of women with severe and no depressed mood. shows the median and IQR of infant methylation levels at the MEG3 DMR. Overall, MEG3 DMR methylation levels are higher in infants of women with severe compared with no depressed mood, p = 0.02. This difference exists in female infants (75.6% vs. 72.0%, p < 0.01) and Blacks (74.8% vs. 72.5%, p = 0.08).
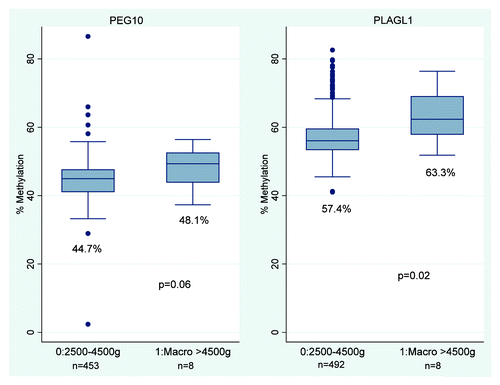
We also observed a 1.6% lower methylation level at the IGF2 DMR among LBW compared with normal birth weight infants () (p = 0.06), a difference that may be larger in female infants (2.3%, p = 0.03) and in those born to black women (2.0%, p = 0.08). Intriguingly, high birth weight infants had a 5.9% (p = 0.02) higher methylation level at the PLAGL1 DMR and a 3.4% (p = 0.06) higher level at the PEG10 DMR () compared with normal birth weight infants. Despite significant methylation differences at the IGF2, PLAGL1, and PEG10 DMRs by birth weight, and significant differences at the MEG3 DMR by maternal mood, inclusion of these DMRs into multivariate models did not alter the strength or direction of the association between maternal mood and birth weight. In addition, maternal DNA methylation at all nine DMRs was comparable between women with severe and no depressed mood as well as between women who gave birth to low or high birth weight infants as compared with normal birth weight infants.
Figure 2. Infant methylation at IGF2 by LBW. shows the median and IQR for infant methylation levels at the IGF2 DMR. Overall, mean methylation at IGF2 DMR is lower for LBW compared with normal weight infants, 49.5% and 51.1%, p = 0.06. This difference persists among female infants (49.2% vs. 51.5%, p = 0.03) and Blacks (47.9% vs. 49.9%, p = 0.08).
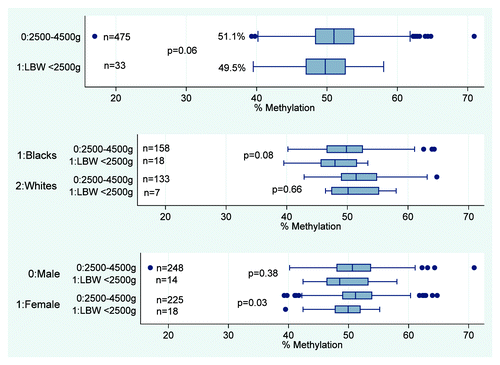
Figure 3. Infant methylation at PLAGL1 and PEG10 for high birth weight infants. shows the median and IQR of infant methylation levels at the PEG10 and PLAGL1 DMRs for high and normal birth weight infants. High birth weight is associated with increased methylation at the PLAGL1 DMR, Wilcoxon rank sum p = 0.02, and at the PEG10 DMR, Wilcoxon rank sum p = 0.06.
Discussion
We examined the association between maternal depressed mood during pregnancy and birth weight and the extent to which this association was altered by DNA methylation differences for DMRs regulating nine imprinted genes. We found that severe maternal depressed mood was associated with a 3-fold increase in the risk of LBW, after adjustment for parity, education, smoking, delivery mode, folic acid use and preterm birth. This association appeared to be stronger in female infants and those born to black women. We also found that while DNA methylation levels at the MEG3 DMR differed significantly by maternal mood, and at the IGF2 and PLAGL1 DMRs by infant birth weight, there was no evidence to suggest that the association between maternal mood and birth weight was mediated by DNA methylation at the nine DMRs examined.
Findings that maternal depressed mood during pregnancy is associated with LBW is consistent with at least six reports in diverse populationsCitation29-Citation35 that found magnitudes of associations in a similar range and direction. However, potential mechanisms that are amenable to prevention are still unknown. Animal studies have implicated epigenetic shifts in the hypothalamus-pituitary-adrenal (HPA) axis;Citation6 however, empirical data are limited to CG-rich promoter regions of a handful of genes. In rats, high levels of maternal licking and grooming have been shown to decrease infant stress responses by dampening HPA axis responses to stress through increased glucocorticoid receptor (GR) expression and negative feedback.Citation36,Citation37 In human infants, prenatal exposure to maternal depression/anxious mood was associated with higher methylation of NR3C1, hippocampal growth receptor (GR) gene, at a predicted binding site (NGFI-A);Citation38 higher DNA methylation levels were also associated with increased salivary cortisol stress response in infants at 3 mo, even after adjustment for maternal SSRI (selective serotonin re-uptake inhibitors) antidepressant use. Depressed mood in the second trimester has also been associated with decreased maternal and infant DNA methylation at the promoter region of the SLC6A4 gene,Citation39 which encodes for the transmembrane serotonin transporter. Studies have shown that depressed mothers have higher cortisol levels and lower dopamine/serotonin levels and that their infants mirrored their biochemical profiles,Citation40,Citation41 leading to the hypothesis that poor birth outcomes, including LBW, are indirectly mediated through hormonal dysregulation of the HPA axis, driven by epigenetic mechanisms. These previous studies, together with our findings of associations between depressed mood and birth weight with several DMRs regulating imprinted genes, support the idea that plasticity in the complex network of epigenetic regulatory elements may play a role. Although epidemiological studies alone cannot establish causation, if perturbations in DNA methylation occur early in gestation, as suggested here, it is possible that these epigenetic regulatory networks can alter metabolic and physiological states that affect growth and development.
Our findings that lower DNA methylation at some imprinted loci increase the risk of LBW are consistent with studies of epigenetic dysregulation in growth restriction. Association between severe caloric restriction,Citation5,Citation42 persistent DNA methylation differences of a similar magnitude at multiple epigenetic targets including IGF2 and MEG3,Citation28,Citation42 and poor health outcomes, including LBW, have been previously reported. Some of these epigenetic changes, including at the IGF2 DMR, have been associated with adult-onset colon cancer.Citation43 However, investigators reported no DNA methylation differences at IGF2, GNASAS, INSIGF and LEP DMRs in a subset of 38 adults born small for gestational age and 75 adults born average for gestational age.Citation44 We also have reported lower DNA methylation levels directly associated with elevated levels of IGF2 expression,Citation27 protein level and birth weight in a cohort of primarily term infants.Citation45 However, in this cohort with a large number of preterm and very LBW infants (< 1,500 g), we found lower methylation levels at IGF2 associated with LBW. Guo et al. found no significant DNA methylation differences at the IGF2 DMR between 20 SGA children and non-SGA controls, although IGF2 mRNA levels were decreased in SGA placentae, and one SGA exhibited hypomethylation at the H19 DMR, which may have contributed to growth restriction.Citation46 In addition, Koukora et al. examined IGF2 imprinting and expression in placenta and found significant loss of imprinting in growth restricted placenta compared with controls that correlated with decreased IGF2 mRNA levels; however, this decrease was not significant, and expression levels did not correlate with birth weight.Citation47 Although the source of heterogeneity in these very small studies is unclear, inconsistent findings may reflect the weakness of the use of birth weight as a proxy for a wide range of in utero exposures. It will be important for larger studies to clarify relationships between environmental exposures and epigenetic marks within subgroups of low or high birth weight infants, as these may be heterogeneous. Because some epigenetic mechanisms may be malleable,Citation36,Citation37 they could be important biomarkers for identifying high risk pregnant women and children and potentially provide therapeutic targets and public heath interventions to reduce LBW and its sequelae.
Epigenetic targets including IGF2, MEG3 and PLAGL1 have been identified as key regulators of placental and fetal growth or development.Citation21,Citation48,Citation49 IGF2, in particular, is a well-studied imprinted region that has been implicated in the control of human growth and development, and loss of imprinting may be associated with aberrant expression leading to dysregulated growth.Citation21,Citation50 Moreover, loss of imprinting at the IGF2 DMR has been implicated in Beckwith-Wiedemann syndrome (BWS), a disorder characterized by dysregulation of growth and development resulting in fetal overgrowth and childhood cancers.Citation51 Two potentially important regulatory regions within the MEG3/DLK region have been identified and hypothesized to affect growth and development in both the placenta and the body.Citation52 Recently, the MEG3/DLK region has also been implicated in tumorigenesis,Citation53 neural dysfunction in Rett’s Syndrome,Citation54 and in human clinically non-functioning pituitary adenomas.Citation55 Moreover, PLAGL1 has been shown to interact with, and alter expression of, a network of imprinted genes, including IGF2 and DLK1, which regulate embryonic growth.Citation24 Loss of imprinting at PLAGL1 and bi-allelic expression has been implicated in transient neonatal diabetes mellitus 1 (TNDM), a disorder in which > 95% of patients experience IUGR.Citation56
This study is one of the largest to examine epigenetic markers in relation to maternal depressed mood and birth weight. The study sample encompasses three major ethnic groups in the US and uses well accepted measures of depressed mood. In addition, the exposure of depressed mood was characterized using a well-validated and consistent scale as well as self-reports, which mirror the clinical situation where patients often come to medical attention due to self-identification of depressive symptoms. Moreover, to assess the reliability of our characterization of depressed mood, we repeated multivariate models using various cutoffs of the CES-D scale (≥ 30, 16–29, < 16) to define severe, moderate and not-depressed mood, and results were similar to the results reported here using the CES-D scale in conjunction with a self-reported diagnosis of depression to delineate severe depressed mood. In addition, multiple confounders assessed in our statistical models have plausible association with both depressed mood and LBW in both our study sample and the overall population. Of note, the extremes of education appeared slightly protective of depressed mood, and one possible explanation is that there are differential responses to life events as well as different coping strategies that vary with education level. Although some studies have suggested a possible role of selective serotonin receptor inhibitors (SSRI) antidepressants in poor birth outcomes, we found no significant effect of SSRI or other psychotropic medication use, which is consistent with someCitation38,Citation57-Citation59 but not all studies.Citation60-Citation65 This is most likely due to the inability to determine if the association with birth outcomes is between the degree of depression itself or the psychotropic medications, as severely depressed women are more likely to be taking medications.
Limitations of our study include the small number of LBW infants, which limits the power of our study. Larger studies are needed to replicate these intriguing findings. In addition, our study is limited by the assessment of depression at only one time point during pregnancy; however, depressive symptoms in different trimesters are highly correlated,Citation66 and there may be a threshold effect with susceptibility to poor birth outcomes.Citation67 Moreover, other psychosocial elements such as stress, life events and support networks were not examined. Additionally, the study was limited by the fact that it included a small number of Asians and Native Americans, who also had a low response rate, raising concerns about generalizability of our findings to these population subgroups. Although multiple comparisons may be of concern, it is unlikely that this could explain identification of three of nine DMRs identified here. In addition, the imprint regulatory network (imprintomeCitation23) contains many key regulatory clusters that were not examined in this study and may play critical roles in this association. Genome-wide studies with high resolution in diverse populations will be required to better characterize these epigenetic networks and key regulators in order to determine their interactions and effects.
Conclusion
Despite these limitations, our findings support those of other studies that maternal depressed mood, a common morbidity during pregnancy, can lead to poor birth outcomes, especially in minority populations and female infants. We also identified three regions of epigenetic alterations at IGF2, PLAGL1 and MEG3 that may be associated with birth weight and/or maternal depressed mood. If replicated in larger studies, these findings could provide insights into the mechanisms underlying associations between maternal mood and LBW that could either serve as markers to identify at-risk infants for further surveillance, or as potential therapeutic targets.
Methods
Study participants
Study participants were identified among pregnant women during their first prenatal visit and were recruited as part of the Newborn Epigenetics STudy (NEST).Citation68 Between 2009 and 2011, pregnant women were recruited from five prenatal clinics with delivery capabilities at Duke and Durham Regional hospitals, the only two obstetric facilities serving Durham and neighboring counties. Eligibility criteria were age 18 and older and intention to use these obstetric facilities for delivery. Exclusion criteria included plans to relinquish custody of the child, plans to move from the area in the subsequent three years, and infection with HIV due to the limited research on the relationships between HIV, its treatment, and DNA methylation in the offspring.
As of December 2011, 2,548 women had been approached and 1,700 (66.6%) consented to participate. The 848 women who declined were similar to those who consented with respect to age (p = 0.70) but different with respect to race/ethnicity (p < 0.001), with the group that declined more likely to be Asian and Native American but similar with respect to other racial/ethnic groups. Of the 1,700 women, 396 were withdrawn due to miscarriage (n = 109), death of infant after birth (n = 4), illiteracy (n = 1), being underage (n = 1), or other (n = 21); or refused further participation (n = 146); or gave birth at an outside hospital (n = 114), such that 1304 (76.7%) women remained enrolled in the study up to the time of analysis. These analyses are limited to the 922 women in whom both depressed mood and parturition data are available. The study protocol was approved by the Duke University Institutional Review Board (IRB).
Data collection
Assessment of depressed mood
At enrollment (gestational age at visit ranged from 4–32.5 weeks with mean and median of 12 and 11 weeks respectively) all pregnant women completed a questionnaire about socio-demographic characteristics, maternal lifestyle factors, and health conditions. This included an assessment of depressed mood using two measures: a self-reported history of being diagnosed with depression at questionnaire completion and a self-reported assessment of depressive symptoms over the two weeks preceding the interview using the Centers for Epidemiologic Studies Depression Scale (CES-D). The CES-D is a well-validated scale with high internal consistency (Cronbach’s α coefficient of 0.85 in the general population and 0.90 in psychiatric patients), that has also been found to correlate well with other scales, symptoms, and life events.Citation69 The scale consists of 20 items that are scored by summation of responses (0 = rarely, 1 = some of the time, 2 = occasionally or moderately, 3 = most or all the time) with a range of 0–60. Questions 4 (you felt that you were just as good as other people), 8 (you felt hopeful about the future), 12 (you were happy), and 16 (you enjoyed life) were reverse-scored according to guidelines.Citation69
From CES-D scores, women were first dichotomized as either having depressed mood (CES-D scale score ≥ 16) or having no depressed mood (< 16), according to guidelines.Citation69 Because there is no recommended CES-D cutoff for severe depressed mood,Citation69 we further categorized depressed mood using a history of depression. Women who self-reported a history of depression (checked yes when asked if they currently had depression) and also scored 16 or more points on the CES-D were classified as having severe depressed mood. Women who did not self-identify as having a history of depression but scored 16 or more on the CES-D were classified as having moderate depressed mood. All women who did not self-identify as having a history of depression and also scored below 16 on the CES-D depression scale were classified as no depressed mood. Women with missing mood data, defined as one or more missing items (n = 390), and the 68 women who self-identified as having a history of a diagnosis of depression but scored below a 16 on the CES-D were excluded from this analysis. To assess the reliability of our characterization of depressed mood using both the CES-D scale and a history of diagnosis of depression to categorize severe depressed mood, we repeated the multivariate logistic regression analysis of LBW and depressed mood using the CES-D scale only to categorize degree of depressed mood with severe (CES-D scale score of ≥ 30), moderate (CES-D scale score of 16–29) or no (CES-D scale score of < 16) depressed mood corresponding to approximately the 90th and 75th percentiles of the CES-D scale distribution.
Birth outcomes
Birth outcomes data including birth weight, gestational age at birth, infant sex and delivery mode were abstracted by trained personnel from medical records after delivery. Only data from singleton births were included in these analyses. Birth weight was divided into three categories: LBW (< 2,500 g), normal weight (2,500 g-4,500 g) and high birth weight (> 4,500 g), since morbidity increases with birth weight, and > 4,500 g may be a better predictor of neonatal morbidity.Citation70 Gestational age at birth was divided into two categories: preterm < 37 weeks gestation, and term ≥ 37 weeks gestation. Delivery mode was defined as either vaginal delivery or Cesarian section (C-section).
Measurement of co-variables
Maternal age was calculated as the difference between self-reported birthdate year and 2011, year when last delivery occurred. Education was self-reported by highest grade or year of school completed. Annual household income, marital status, employment status, and parity were self-reported. Black women self-identified as Black/African American or as Biracial/ Multiracial and had a Black mother, White women self-identified as Non-Hispanic White or Biracial/Multiracial and had White mothers, Hispanic women self-identified as Hispanic White or Biracial/Multiracial and had Hispanic mothers and Other women self-identified as Asian/Pacific Islander, American Indian/Native American, or Other or if they chose Biracial/Multiracial and had Biracial mothers or if they did not fall into any other category. Smoking status was categorized into three groups: smoking prior to pregnancy only, smoking prior and during pregnancy, and no smoking based on responses to the following questions. Women who answered that they had smoked at least 100 cigarettes in a lifetime and had smoked either in the last six months, during the first trimester, after the LMP (last menstrual period), or at the time of interview were classified as having smoked during pregnancy. Women who smoked at least 100 cigarettes in their lifetime but had not smoked in the past 6 mo, during the 1st trimester, since their LMP or at the time of questionnaire completion, were classified as having smoked prior to pregnancy only. Women who responded that they had not smoked at least 100 cigarettes in their lifetime were classified as non-smokers. Alcohol status was classified as alcohol use during pregnancy if the woman responded “Yes” to drinking > 2 drinks/week in the month after last menstrual period, or “Yes” to drinking any alcoholic beverages at the time of questionnaire completion. All other women without missing data were classified as alcohol non-users during pregnancy. Use of prescribed psychotropic medication was defined as responding “Yes” to using any of the following in the six months prior to the interview: tranquilizers (n = 18), antidepressants (n = 80), sleeping pills (n = 72), or anticonvulsants (n = 6). Folic acid use was defined as responding “Yes” to using a regular dose multivitamin, a multivitamin with additives, prenatal vitamins, or single dose folic acid for at least six of 12 mo prior to interview. Current maternal body mass index (BMI) was calculated from self-reported weight and height at the time of enrollment converted to kilograms and meters using the formula weight in kg/height in m2. Maternal BMI at LMP was calculated using self-reported weight at LMP. Health status was obtained from responses to a single question (how would you describe your current health?) using the responses of excellent, very good, good, fair, and poor. Anxiety was defined as a positive response to a single question (check “yes” for any of the listed ailments you may currently have) to the listed ailment of anxiety/panic attacks.
DNA methylation analysis
Maternal peripheral blood samples were collected at enrollment and infant cord blood specimens were collected at birth. Samples were collected in EDTA-treated tubes and centrifuged using standard protocols to allow for collection of plasma and buffy coat for DNA extraction (Qiagen); samples were stored at -80°C until required. DNA was extracted using Puregene reagents according to the manufacturer’s protocol (Qiagen) and quantity and quality assessed using a Nanodrop 1000 Spectrophotometer (Thermo Scientific). Maternal and infant genomic DNA (800 ng) was modified by treatment with sodium bisulfite using the Zymo EZ DNA Methylation kit (Zymo Research). Bisulfite treatment of denatured DNA converts all unmethylated cytosines to uracils, but leaves methylated cytosines unchanged, allowing quantitative definition of cytosine methylation status. Pyrosequencing was performed using one of two Pyromark Q96 MD pyrosequencers (Qiagen). Nine imprinted DMRs for both mothers and infants were analyzed including: the paternally methylated and expressed IGF2 DMR, the paternally methylated H19DMR, the paternally methylated gametic MEG3-IG DMR (located intergenic to DLK1 and MEG3) and the paternally methylated somatic MEG3 DMR (promoter), the maternally methylated PEG3 DMR, the maternally methylated MEST DMR, the maternally methylated PEG10 DMR, the maternally methylated NNAT DMR and the maternally methylated PLAGL1 DMR. Pyrosequencing assay design, genomic coordinates, assay conditions and assay validation are described in detail in referencesCitation27,Citation71 and Murphy SK et al., submitted. Briefly, assays were designed to query established imprinted gene DMRs using the Pyromark Assay Design Software (Qiagen). PCR conditions were optimized to produce a single, robust amplification product by adjusting annealing temperature and magnesium chloride concentrations. Defined mixtures of fully methylated and unmethylated control DNAs were used to show a linear increase in detection of methylation values as the level of input DNA methylation increased (Pearson r > 0.99 for all DMRs). Once optimal conditions were defined, each DMR was analyzed using the same amount of input DNA from each specimen (40 ng, assuming complete recovery following bisulfite modification), keeping the thermocycler and pyrosequencer constant. Percent methylation for each CpG cytosine was determined using Pyro Q-CpG Software (Qiagen).
Statistical analysis
Pearson’s chi-squared tests were performed to compare the distribution of demographic and obstetric descriptors among women with severe and moderate depressed mood and those with no depressed mood. Variables were selected based on their known or suspected associations with either LBW or depressed mood in the population. Multivariate logistic regression models were fit to examine the relationship between maternal prenatal depressed mood and LBW, adjusting for covariates. Initial models were fit using all variables considered clinically important in this association and included maternal age, race/ethnicity, marital status, employment, parity, household income, education, maternal health status, maternal BMI at enrollment, maternal BMI at last menstrual period, smoking, alcohol use, maternal anxiety, psychotropic use, folic acid use, trimester at enrollment, infant sex, delivery mode, and preterm birth. All non-binary variables were added to multivariate models using indicator variables. A stepwise approach (exclusion p > 0.10 and inclusion p < 0.09) was used to refine the model, and log likelihood tests were used to create the final parsimonious model; with significant covariates parity, education, smoking, delivery mode, folic acid use, and preterm birth. We also repeated multivariate logistic regression analyses using the CES-D scale only stratified at the 75th and 90th percentile (0–15, 16–29, ≥ 30) for none, moderate, and severe depressed mood. In addition, 18 high birth weight (> 4,500 g) infants were excluded in regression models of depressed mood and birth weight because no women with severe depressed mood gave birth to high birth weight infants.
Among the 508 mother-infant pairs where methylation data for at least one of the nine DMRs was available, we examined the extent to which DMR methylation modified the association between maternal depressed mood and LBW. These 508 mother-infant pairs were similar to the original sample with respect to maternal age (p = 0.74), maternal CES-D score (p = 0.85), and proportion of LBW infants (p = 0.81). We first assessed each DMR for normality using the Kolmogorov-Smirnov test. We found that with the exception of PLAGL1 (p < 0.01), PEG3 (p < 0.01), MEG3-IG (p < 0.01) and PEG10 (p = 0.03), all other DMRs were normally distributed (p > 0.05). Confirmatory analysis for individual CpGs revealed Cronbach’s alphas for all DMRs were > 0.89 suggesting mean methylation levels for each DMR could be used in models. T-tests were calculated to compare DMR methylation differences between LBW (< 2,500 grams) and high birth weight (> 4,500 grams) infants, each compared with normal birth weight (2,500–4,500 grams) infants, and among women with severe and no depressed mood. Wilcoxon- rank sum tests were used for DMRs that were not normally distributed. Epidemiologic evidence suggests that although high and low birth weight are both associated with poor outcomes in adulthood, mechanisms may differ;Citation72 thus they were compared separately with normal birth weight infants. In addition, mean values for all nine DMRs were added individually to the model of depressed mood and LBW with covariates described above. DMRs that resulted in attenuation of the odds ratio (ORs) between severe depressed mood and LBW by > 10%, before and after inclusion in the model were considered possible effect modifiers of the association between maternal depressed mood and LBW. To explore possible effects of maternal methylation status,Citation73 all analyses were repeated with adjustments for maternal methylation status. Prior studies have suggested that the effects of depression may be more severe in Blacks and those with low socioeconomic statusCitation66,Citation67 possibly due to increased allostatic load from socioeconomic and cultural stressors.Citation25,Citation74-Citation76 The Dutch Famine studiesCitation28 and our own analysis of the effects of maternal cigarette smokingCitation27 suggest that epigenetic dysregulation may be sex-specific. Hence, refined models adjusting for parity, education, smoking, delivery mode, folic acid use, and preterm birth were used to examine potential effects of a priori defined race/ethnicity and infant sex, and analyses were conducted among three racial/ethnic groups (Blacks, Whites and Hispanics) as well as by sex of the offspring. Race/ethnicity specific models were adjusted for infant sex, and sex-specific models were adjusted for race/ethnicity. All analyses were conducted using STATA 12.0 (StataCorp).
| Abbreviations: | ||
| LBW | = | low birth weight |
| DMR | = | differentially methylated region |
| CES-D | = | Center for Epidemiologic Studies depression scale |
| PTB | = | preterm birth |
| BMI | = | body mass index |
| LMP | = | last menstrual period |
| NEST | = | newborn epigenetics study |
| IQR | = | interquartile range |
| IUGR | = | intrauterine growth restriction |
Acknowledgments
The Newborn Epigenetics Study (NEST) was funded through the US National Institutes of Health (agreement # R01ES016772). We are especially grateful to the women and families involved in the Newborn Epigenetics STudy, and acknowledge the expert contributions of study coordinator Stacy Murray, research nurse Tammy Bishop, and laboratory technicians Carole Grenier, Erin Erginer, Cara Davis and Allison Barratt.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethical Statement
This study was approved by the Duke IRB, and all research was conducted in accordance with guidelines.
References
- Paneth NS. The problem of low birth weight. Future Child 1995; 5:19 - 34; http://dx.doi.org/10.2307/1602505; PMID: 7633862
- Calkins K, Devaskar SU. Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care 2011; 41:158 - 76; http://dx.doi.org/10.1016/j.cppeds.2011.01.001; PMID: 21684471
- Barker DJ. The origins of the developmental origins theory. J Intern Med 2007; 261:412 - 7; http://dx.doi.org/10.1111/j.1365-2796.2007.01809.x; PMID: 17444880
- Huang C, Li Z, Wang M, Martorell R. Early life exposure to the 1959-1961 Chinese famine has long-term health consequences. J Nutr 2010; 140:1874 - 8; http://dx.doi.org/10.3945/jn.110.121293; PMID: 20702751
- Lumey LH, Stein AD, Kahn HS, Romijn JA. Lipid profiles in middle-aged men and women after famine exposure during gestation: the Dutch Hunger Winter Families Study. Am J Clin Nutr 2009; 89:1737 - 43; http://dx.doi.org/10.3945/ajcn.2008.27038; PMID: 19386743
- Meaney MJ, Szyf M, Seckl JR. Epigenetic mechanisms of perinatal programming of hypothalamic-pituitary-adrenal function and health. Trends Mol Med 2007; 13:269 - 77; http://dx.doi.org/10.1016/j.molmed.2007.05.003; PMID: 17544850
- Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976; 295:349 - 53; http://dx.doi.org/10.1056/NEJM197608122950701; PMID: 934222
- St Clair D, Xu M, Wang P, Yu Y, Fang Y, Zhang F, et al. Rates of adult schizophrenia following prenatal exposure to the Chinese famine of 1959-1961. JAMA 2005; 294:557 - 62; http://dx.doi.org/10.1001/jama.294.5.557; PMID: 16077049
- Langley-Evans SC, McMullen S. Developmental origins of adult disease. Med Princ Pract 2010; 19:87 - 98; http://dx.doi.org/10.1159/000273066; PMID: 20134170
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008; 359:61 - 73; http://dx.doi.org/10.1056/NEJMra0708473; PMID: 18596274
- Feinberg AP. Phenotypic plasticity and the epigenetics of human disease. Nature 2007; 447:433 - 40; http://dx.doi.org/10.1038/nature05919; PMID: 17522677
- Alder J, Fink N, Bitzer J, Hösli I, Holzgreve W. Depression and anxiety during pregnancy: a risk factor for obstetric, fetal and neonatal outcome? A critical review of the literature. J Matern Fetal Neonatal Med 2007; 20:189 - 209; http://dx.doi.org/10.1080/14767050701209560; PMID: 17437220
- American College of Obstetricians and Gynecologists Committee on Health Care for Undeserved Women. ACOG Committee Opinion No. 343: psychosocial risk factors: perinatal screening and intervention. Obstet Gynecol 2006; 108:469 - 77; http://dx.doi.org/10.1097/00006250-200608000-00046; PMID: 16880322
- Stewart DE. Clinical practice. Depression during pregnancy. N Engl J Med 2011; 365:1605 - 11; http://dx.doi.org/10.1056/NEJMcp1102730; PMID: 22029982
- Gavin NI, Gaynes BN, Lohr KN, Meltzer-Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol 2005; 106:1071 - 83; http://dx.doi.org/10.1097/01.AOG.0000183597.31630.db; PMID: 16260528
- Hogan VK, Rowley D, Bennett T, Taylor KD. Life Course, Social Determinants, and Health Inequities: Toward a National Plan for Achieving Health Equity for African American Infants-a Concept Paper. Matern Child Health J 2011; In press;; http://dx.doi.org/10.1007/s10995-011-0847-0; PMID: 21748428
- MacDorman M, Mathews T.. Understanding Racial and Ethnic Disparities in U.S. Infant Mortality Rates. NCHS Data Brief 2011; 74:1 - 8
- Zayas LH, Cunningham M, McKee MD, Jankowski KR. Depression and negative life events among pregnant African-American and Hispanic women. Womens Health Issues 2002; 12:16 - 22; http://dx.doi.org/10.1016/S1049-3867(01)00138-4; PMID: 11786288
- Meltzer-Brody S. New insights into perinatal depression: pathogenesis and treatment during pregnancy and postpartum. Dialogues Clin Neurosci 2011; 13:89 - 100; PMID: 21485749
- Das R, Hampton DD, Jirtle RL. Imprinting evolution and human health. Mamm Genome 2009; 20:563 - 72; http://dx.doi.org/10.1007/s00335-009-9229-y; PMID: 19830403
- Skaar DA, Li Y, Bernal AJ, Hoyo C, Murphy SK, Jirtle RL. The Human Imprintome. ILAR 2012; 53:3
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet 2007; 8:253 - 62; http://dx.doi.org/10.1038/nrg2045; PMID: 17363974
- Jirtle RL. Epigenome: the program for human health and disease. Epigenomics 2009; 1:13 - 6; http://dx.doi.org/10.2217/epi.09.16; PMID: 22122631
- Varrault A, Gueydan C, Delalbre A, Bellmann A, Houssami S, Aknin C, et al. Zac1 regulates an imprinted gene network critically involved in the control of embryonic growth. Dev Cell 2006; 11:711 - 22; http://dx.doi.org/10.1016/j.devcel.2006.09.003; PMID: 17084362
- Field T, Diego M, Hernandez-Reif M, Deeds O, Holder V, Schanberg S, et al. Depressed pregnant black women have a greater incidence of prematurity and low birthweight outcomes. Infant Behav Dev 2009; 32:10 - 6; http://dx.doi.org/10.1016/j.infbeh.2008.09.005; PMID: 19004502
- Field T, Diego M, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, et al. Prenatal depression effects on the foetus and neonate in different ethnic and socio-economic status groups. J Reprod Infant Psychol 2002; 20:149 - 57; http://dx.doi.org/10.1080/026468302760270809
- Murphy SK, Adigun A, Huang Z, Overcash F, Wang F, Jirtle RL, et al. Gender-specific methylation differences in relation to prenatal exposure to cigarette smoke. Gene 2012; 494:36 - 43; http://dx.doi.org/10.1016/j.gene.2011.11.062; PMID: 22202639
- Tobi EW, Lumey LH, Talens RP, Kremer D, Putter H, Stein AD, et al. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum Mol Genet 2009; 18:4046 - 53; http://dx.doi.org/10.1093/hmg/ddp353; PMID: 19656776
- Dayan J, Creveuil C, Marks MN, Conroy S, Herlicoviez M, Dreyfus M, et al. Prenatal depression, prenatal anxiety, and spontaneous preterm birth: a prospective cohort study among women with early and regular care. Psychosom Med 2006; 68:938 - 46; http://dx.doi.org/10.1097/01.psy.0000244025.20549.bd; PMID: 17079701
- Field T, Diego M, Hernandez-Reif M, Schanberg S, Kuhn C, Yando R, et al. Pregnancy anxiety and comorbid depression and anger: effects on the fetus and neonate. Depress Anxiety 2003; 17:140 - 51; http://dx.doi.org/10.1002/da.10071; PMID: 12768648
- Field T, Hernandez-Reif M, Diego M, Figueiredo B, Schanberg S, Kuhn C. Prenatal cortisol, prematurity and low birthweight. Infant Behav Dev 2006; 29:268 - 75; http://dx.doi.org/10.1016/j.infbeh.2005.12.010; PMID: 17138282
- Gavin AR, Holzman C, Siefert K, Tian Y. Maternal depressive symptoms, depression, and psychiatric medication use in relation to risk of preterm delivery. Womens Health Issues 2009; 19:325 - 34; http://dx.doi.org/10.1016/j.whi.2009.05.004; PMID: 19733802
- Goedhart G, Snijders AC, Hesselink AE, van Poppel MN, Bonsel GJ, Vrijkotte TGM. Maternal depressive symptoms in relation to perinatal mortality and morbidity: results from a large multiethnic cohort study. Psychosom Med 2010; 72:769 - 76; http://dx.doi.org/10.1097/PSY.0b013e3181ee4a62; PMID: 20668282
- Rahman A, Bunn J, Lovel H, Creed F. Association between antenatal depression and low birthweight in a developing country. Acta Psychiatr Scand 2007; 115:481 - 6; http://dx.doi.org/10.1111/j.1600-0447.2006.00950.x; PMID: 17498160
- Steer RA, Scholl TO, Hediger ML, Fischer RL. Self-reported depression and negative pregnancy outcomes. J Clin Epidemiol 1992; 45:1093 - 9; http://dx.doi.org/10.1016/0895-4356(92)90149-H; PMID: 1474405
- Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. Epigenetic programming by maternal behavior. Nat Neurosci 2004; 7:847 - 54; http://dx.doi.org/10.1038/nn1276; PMID: 15220929
- Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A 2006; 103:3480 - 5; http://dx.doi.org/10.1073/pnas.0507526103; PMID: 16484373
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, Devlin AM. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008; 3:97 - 106; http://dx.doi.org/10.4161/epi.3.2.6034; PMID: 18536531
- Devlin AM, Brain U, Austin J, Oberlander TF. Prenatal exposure to maternal depressed mood and the MTHFR C677T variant affect SLC6A4 methylation in infants at birth. PLoS One 2010; 5:e12201; http://dx.doi.org/10.1371/journal.pone.0012201; PMID: 20808944
- Field T, Diego M, Dieter J, Hernandez-Reif M, Schanberg S, Kuhn C, et al. Prenatal depression effects on the fetus and the newborn. Infant Behav Dev 2004; 27:216 - 29; http://dx.doi.org/10.1016/j.infbeh.2003.09.010; PMID: 17138297
- Field T, Diego M, Hernandez-Reif M, Vera Y, Gil K, Schanberg S, et al. Prenatal maternal biochemistry predicts neonatal biochemistry. Int J Neurosci 2004; 114:933 - 45; http://dx.doi.org/10.1080/00207450490461305; PMID: 15527200
- Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci U S A 2008; 105:17046 - 9; http://dx.doi.org/10.1073/pnas.0806560105; PMID: 18955703
- Cui H, Cruz-Correa M, Giardiello FM, Hutcheon DF, Kafonek DR, Brandenburg S, et al. Loss of IGF2 imprinting: a potential marker of colorectal cancer risk. Science 2003; 299:1753 - 5; http://dx.doi.org/10.1126/science.1080902; PMID: 12637750
- Tobi EW, Heijmans BT, Kremer D, Putter H, Delemarre-van de Waal HA, Finken MJ, et al. DNA methylation of IGF2, GNASAS, INSIGF and LEP and being born small for gestational age. Epigenetics 2011; 6:171 - 6; http://dx.doi.org/10.4161/epi.6.2.13516; PMID: 20930547
- Hoyo C, Fortner K, Murtha AP, Schildkraut JM, Soubry A, Demark-Wahnefried W, et al. Association of cord blood methylation fractions at imprinted insulin-like growth factor 2 (IGF2), plasma IGF2, and birth weight. Cancer Causes Control 2012; 23:635 - 45; http://dx.doi.org/10.1007/s10552-012-9932-y; PMID: 22392079
- Guo L, Choufani S, Ferreira J, Smith A, Chitayat D, Shuman C, et al. Altered gene expression and methylation of the human chromosome 11 imprinted region in small for gestational age (SGA) placentae. Dev Biol 2008; 320:79 - 91; http://dx.doi.org/10.1016/j.ydbio.2008.04.025; PMID: 18550048
- Koukoura O, Sifakis S, Soufla G, Zaravinos A, Apostolidou S, Jones A, et al. Loss of imprinting and aberrant methylation of IGF2 in placentas from pregnancies complicated with fetal growth restriction. Int J Mol Med 2011; 28:481 - 7; PMID: 21805044
- Heijmans BT, Tobi EW, Lumey LH, Slagboom PE. The epigenome: archive of the prenatal environment. Epigenetics 2009; 4:526 - 31; http://dx.doi.org/10.4161/epi.4.8.10265; PMID: 19923908
- Constância M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 2002; 417:945 - 8; http://dx.doi.org/10.1038/nature00819; PMID: 12087403
- Smith FM, Garfield AS, Ward A. Regulation of growth and metabolism by imprinted genes. Cytogenet Genome Res 2006; 113:279 - 91; http://dx.doi.org/10.1159/000090843; PMID: 16575191
- Murrell A, Heeson S, Cooper WN, Douglas E, Apostolidou S, Moore GE, et al. An association between variants in the IGF2 gene and Beckwith-Wiedemann syndrome: interaction between genotype and epigenotype. Hum Mol Genet 2004; 13:247 - 55; http://dx.doi.org/10.1093/hmg/ddh013; PMID: 14645199
- Kagami M, O’Sullivan MJ, Green AJ, Watabe Y, Arisaka O, Masawa N, et al. The IG-DMR and the MEG3-DMR at human chromosome 14q32.2: hierarchical interaction and distinct functional properties as imprinting control centers. PLoS Genet 2010; 6:e1000992; http://dx.doi.org/10.1371/journal.pgen.1000992; PMID: 20585555
- Benetatos L, Vartholomatos G, Hatzimichael E. MEG3 imprinted gene contribution in tumorigenesis. Int J Cancer 2011; 129:773 - 9; http://dx.doi.org/10.1002/ijc.26052; PMID: 21400503
- Jordan C, Li HH, Kwan HC, Francke U. Cerebellar gene expression profiles of mouse models for Rett syndrome reveal novel MeCP2 targets. BMC Med Genet 2007; 8:36; http://dx.doi.org/10.1186/1471-2350-8-36; PMID: 17584923
- Gejman R, Batista DL, Zhong Y, Zhou Y, Zhang X, Swearingen B, et al. Selective loss of MEG3 expression and intergenic differentially methylated region hypermethylation in the MEG3/DLK1 locus in human clinically nonfunctioning pituitary adenomas. J Clin Endocrinol Metab 2008; 93:4119 - 25; http://dx.doi.org/10.1210/jc.2007-2633; PMID: 18628527
- Mackay DJ, Temple IK. Transient neonatal diabetes mellitus type 1. Am J Med Genet C Semin Med Genet 2010; 154C:335 - 42; http://dx.doi.org/10.1002/ajmg.c.30272; PMID: 20803656
- Suri R, Altshuler L, Hellemann G, Burt VK, Aquino A, Mintz J. Effects of antenatal depression and antidepressant treatment on gestational age at birth and risk of preterm birth. Am J Psychiatry 2007; 164:1206 - 13; http://dx.doi.org/10.1176/appi.ajp.2007.06071172; PMID: 17671283
- Suri R, Altshuler L, Hendrick V, Rasgon N, Lee E, Mintz J. The impact of depression and fluoxetine treatment on obstetrical outcome. Arch Womens Ment Health 2004; 7:193 - 200; http://dx.doi.org/10.1007/s00737-004-0057-5; PMID: 15241665
- Soubry A, Murphy S, Huang Z, Murtha A, Schildkraut J, Jirtle R, et al. The effects of depression and use of antidepressive medicines during pregnancy on the methylation status of the IGF2 imprinted control regions in the offspring. Clin Epigenetics 2011; 3:2; http://dx.doi.org/10.1186/1868-7083-3-2; PMID: 22414206
- Källén B. Neonate characteristics after maternal use of antidepressants in late pregnancy. Arch Pediatr Adolesc Med 2004; 158:312 - 6; http://dx.doi.org/10.1001/archpedi.158.4.312; PMID: 15066868
- Lund N, Pedersen LH, Henriksen TB. Selective serotonin reuptake inhibitor exposure in utero and pregnancy outcomes. Arch Pediatr Adolesc Med 2009; 163:949 - 54; http://dx.doi.org/10.1001/archpediatrics.2009.164; PMID: 19805715
- Maschi S, Clavenna A, Campi R, Schiavetti B, Bernat M, Bonati M. Neonatal outcome following pregnancy exposure to antidepressants: a prospective controlled cohort study. BJOG 2008; 115:283 - 9; http://dx.doi.org/10.1111/j.1471-0528.2007.01518.x; PMID: 17903222
- Oberlander TF, Warburton W, Misri S, Aghajanian J, Hertzman C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen Psychiatry 2006; 63:898 - 906; http://dx.doi.org/10.1001/archpsyc.63.8.898; PMID: 16894066
- Simon GE, Cunningham ML, Davis RL. Outcomes of prenatal antidepressant exposure. Am J Psychiatry 2002; 159:2055 - 61; http://dx.doi.org/10.1176/appi.ajp.159.12.2055; PMID: 12450956
- Wisner KL, Sit DK, Hanusa BH, Moses-Kolko EL, Bogen DL, Hunker DF, et al. Major depression and antidepressant treatment: impact on pregnancy and neonatal outcomes. Am J Psychiatry 2009; 166:557 - 66; http://dx.doi.org/10.1176/appi.ajp.2008.08081170; PMID: 19289451
- Hoffman S, Hatch MC. Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol 2000; 19:535 - 43; http://dx.doi.org/10.1037/0278-6133.19.6.535; PMID: 11129356
- Orr ST, James SA, Blackmore Prince C. Maternal prenatal depressive symptoms and spontaneous preterm births among African-American women in Baltimore, Maryland. Am J Epidemiol 2002; 156:797 - 802; http://dx.doi.org/10.1093/aje/kwf131; PMID: 12396996
- Hoyo C, Murtha AP, Schildkraut JM, Forman MR, Calingaert B, Demark-Wahnefried W, et al. Folic acid supplementation before and during pregnancy in the Newborn Epigenetics STudy (NEST). BMC Public Health 2011; 11:46; http://dx.doi.org/10.1186/1471-2458-11-46; PMID: 21255390
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas 1977; 1:385 - 401; http://dx.doi.org/10.1177/014662167700100306
- Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the united states: determinants, outcomes, and proposed grades of risk. Am J Obstet Gynecol 2003; 188:1372 - 8; http://dx.doi.org/10.1067/mob.2003.302; PMID: 12748514
- Hoyo C, Murtha AP, Schildkraut JM, Jirtle RL, Demark-Wahnefried W, Forman MR, et al. Methylation variation at IGF2 differentially methylated regions and maternal folic acid use before and during pregnancy. Epigenetics 2011; 6:928 - 36; http://dx.doi.org/10.4161/epi.6.7.16263; PMID: 21636975
- Ornoy A. Prenatal origin of obesity and their complications: Gestational diabetes, maternal overweight and the paradoxical effects of fetal growth restriction and macrosomia. Reprod Toxicol 2011; 32:205 - 12; http://dx.doi.org/10.1016/j.reprotox.2011.05.002; PMID: 21620955
- Kile ML, Baccarelli A, Tarantini L, Hoffman E, Wright RO, Christiani DC. Correlation of global and gene-specific DNA methylation in maternal-infant pairs. PLoS One 2010; 5:e13730; http://dx.doi.org/10.1371/journal.pone.0013730; PMID: 21060777
- Geronimus AT. Black/white differences in the relationship of maternal age to birthweight: a population-based test of the weathering hypothesis. Soc Sci Med 1996; 42:589 - 97; http://dx.doi.org/10.1016/0277-9536(95)00159-X; PMID: 8643983
- Geronimus AT. Understanding and eliminating racial inequalities in women’s health in the United States: the role of the weathering conceptual framework. J Am Med Womens Assoc 2001; 56:133 - 6, 149-50; PMID: 11759779
- Rosenthal L, Lobel M. Explaining racial disparities in adverse birth outcomes: unique sources of stress for Black American women. Soc Sci Med 2011; 72:977 - 83; http://dx.doi.org/10.1016/j.socscimed.2011.01.013; PMID: 21345565