Abstract
During fertilization, two of the most differentiated cells in the mammalian organism, a sperm and oocyte, are combined to form a pluripotent embryo. Dynamic changes in chromatin structure allow the transition of the chromatin on these specialized cells into an embryonic configuration capable of generating every cell type. Initially, this reprogramming activity is supported by oocyte-derived factors accumulated during oogenesis as proteins and mRNAs; however, the underlying molecular mechanisms that govern it remain poorly characterized. Trimethylation of histone H3 at lysine 27 (H3K27me3) is a repressive epigenetic mark that changes dynamically during pre-implantation development in mice, bovine and pig embryos. Here we present data and hypotheses related to the potential mechanisms behind H3K27me3 remodeling during early development. We postulate that the repressive H3K27me3 mark is globally erased from the parental genomes in order to remove the gametic epigenetic program and to establish a pluripotent embryonic epigenome. We discuss information gathered in mice, pigs, and bovine, with the intent of providing a comparative analysis of the reprogramming of this epigenetic mark during early mammalian development.
Introduction
Gametogenesis culminates with the formation of two of the most terminally differentiated cells in the organism: the sperm and the oocyte. When these cells meet during fertilization, they give rise to the zygote, the ultimate totipotent cell. During pre-implantation development, parental chromatin undergoes an extensive and efficient remodeling to generate the embryonic genome. Factors stored in the cytoplasm of the oocyte, in the form of proteins and mRNAs, are responsible for the initial remodeling of the chromatin in the gametes. This chromatin remodeling continues throughout early development as undifferentiated cells are directed to more specialized fates, with factors expressed from the embryonic genome regulating these changes.
Nuclear reprogramming during early development entails epigenetic, rather than genetic, changes. Epigenetic modifications alter chromatin structure in a cell-division heritable way, leaving the DNA sequence unaltered while modifying the cell’s transcriptional landscape. Some epigenetic mechanisms include DNA methylation, post-translational covalent histone-tail modifications, ATP-dependent chromatin remodeling, and changes in histone protein composition. DNA methylation occurs at CpG dinucleotides and is associated with silencing of gene transcription.Citation1 Histone modifications include acetylation, methylation, phosphorylation, ubiquitylation and sumoylation. Individual modifications can be associated with transcriptional activation or repression. In general, histone acetylation is associated with transcriptionally permissive states,Citation2 while histone methylation is associated with gene silencing, with the exception of H3K4, which is associated with increased gene transcription.Citation3,Citation4
Immunolabeling assays using antibodies against different histone modifications and methylated DNA have allowed for the characterization of global epigenetic changes during early pre-implantation development in several species.Citation5-Citation17 These studies showed that dynamic and extensive epigenetic changes take place during early development. However, the nature and extent of such changes vary between species. For example, rapid and active disappearance of paternal DNA methylation is pronounced in mice but undetectable in sheep and rabbits, and is less intense in cattle and humans.Citation8,Citation12,Citation18,Citation19 Moreover, subsequent passive demethylation of the embryonic genome is almost undetectable in rabbits and greatly reduced in pigs.Citation15,Citation16 The developmental consequences of these differences are not fully understood, although it is clear that extrapolating information generated in one species to others can be misleading.
Epigenetic changes during mammalian pre-implantation development are essential, as has been demonstrated by many knockout mice lacking chromatin remodeling enzymes that resulted in early developmental defects or even pre-implantation lethality.Citation20 Among histone modifications, H3K27me3 is involved in silencing of gene expression. During development, H3K27me3 is linked with transcriptional silencingCitation21,Citation22 and has roles in silencing the expression of key developmental genes during embryonic stem cell differentiation.Citation23-Citation25 Therefore, removal of H3K27me3 marks from gametic chromatin would be expected upon fertilization and during early development in order to reactivate silenced genes. In mammals, H3K27me3 is catalyzed by proteins of the polycomb group (PcG), an evolutionally conserved set of long-term transcriptional gene repressors.Citation26 PcG targets are usually genes encoding for transcription factors involved in diverse cellular functions and developmental pathways.Citation24,Citation27-Citation29 PcG proteins form multiprotein complexes including the two transcriptional repressive complexes named polycomb repressive complex 1 and 2 (PRC1 and PRC2).Citation24 In mammals, PCR1 and PCR2 play key roles in establishing and maintaining the H3K27me3 mark.
PRC2, which catalyzes the di- and trimethylation of H3K27,Citation30 contains three essential components: enhancer of zeste homolog 2 (EZH2), embryonic ectoderm development (EED) and suppressor of zeste 12 homolog (SUZ12). The histone lysine methyltransferase activity of PRC2 resides in the SET-domain-containing protein EZH2,Citation31,Citation32 but PRC2 requires the activity of all three core components to function.Citation33-Citation35 Once established, H3K27me3 recruits PRC1, likely aiding in propagation of silencing chromatin states.Citation26 PRC1 is formed by the components polycomb (PC), polyhomeotic (PH), ring finger protein 1 (RING1) and ring finger protein 2 (RNF2), among others.
Histone methylation is regarded as a relatively stable epigenetic mark, with the rate of histone methyl group turnover similar to the rate of histone turnover.Citation36,Citation37 Until recently, histone methylation was thought to be an irreversible mark; however, in 2004, enzymes with lysine-specific histone demethylase activity were reported (LSD1/KDM1)Citation4 and, later, two Jumonji domain-containing enzymes with demethylase activity specific for H3K27 were identified.Citation38-Citation41 These enzymes are the ubiquitously transcribed tetratricopeptide repeat X (UTX, a.k.a. KDM6A) and the jumonji domain-containing protein 3 (JMJD3, a.k.a. KDM6B). UTX, as its name indicates, is expressed in most cells.Citation39 JMJD3 has been involved in temporal gene regulation during wound healing,Citation42 neural cell differentiation,Citation43,Citation44 as well as cellular senescence and tumor suppression in a variety of cancer types.Citation38,Citation45-Citation47 In many of these cases, changes in levels of H3K27me3 were not global, but rather specific changes at certain loci, implying that JMJD3 may be directed to remove the repressive H3K27me3 mark to allow reactivation of specific genes.
Since lysine methyltransferases and demethylases have great substrate specificity with respect to their target lysine residue, and those with specific activity toward H3K27 are well known and characterized, there is a unique opportunity to decipher the mechanisms of H3K27me3 remodeling during early development of mammalian embryos.
Global Levels of H3K27me3 are Dynamically Remodeled During Mammalian Pre-Implantation Development
The availability of anti-H3K27me3 specific antibodies has allowed for the characterization of the global state of this epigenetic mark during pre-implantation development of several species.Citation10,Citation17,Citation48,Citation49 Early embryonic development encompasses the formation of maternal and paternal pronuclei (PNs), cleavage of the zygote, activation of the embryonic genome (at a species-specific state; early 2-cell in mice, 4-cell in pigs, 4- to 8-cell in humans, and 8-cell in cattle), and initial differentiation of embryonic and extra embryonic lineages. At fertilization, the two parental genomes contain distinct chromatin composition as a result of two diverse processes: oogenesis and spermatogenesis. The paternal chromatin is highly compacted by protamine proteins,Citation50 although about 2–15% of mammalian sperm chromatin retains histone-containing nucleosomes. In humans, retained nucleosomes are non-randomly distributedCitation51 and are significantly enriched at loci of developmental importance.Citation52 Furthermore, the epigenetic marks present in sperm histones are transmitted to the developing embryo since they are found in the paternal PN, suggesting that they remain intact during the zygotic stage.Citation53,Citation54 High-resolution analysis of these histones in the sperm nucleus showed that the H3K27me3 mark is significantly enriched at developmental promoters repressed in early embryos.Citation52 These results give biological significance to this small proportion of remaining histones in the paternal genome, highlighting a possible paternal contribution to the epigenetic program of the early embryo. The maternal DNA is present in its meiotic state and differs from somatic chromatin by the presence of an oocyte-specific subtype of the linker histone 1, H1oo,Citation55 which might be involved in creating a more fluid chromatin template permissive of the remodeling observed during cleavage stages.Citation56 Soon after fertilization, maternal and paternal DNA decondense and initiate DNA replication in preparation of the first cleavage division. Paternal chromatin decondensation involves replication-independent replacement of protamines by oocyte-supplied histones stored in the cytoplasm of the egg during growth and maturation.Citation57
Asymmetric staining of H3K27me3 has been observed in the maternal and paternal PNs of mice,Citation14,Citation48,Citation58 pigCitation17,Citation59 and bovine zygotes.Citation10 In all of these species, H3K27me3 is positive in the maternal PN and negative in the paternal PN. This initial asymmetry might be due to the paternal protamines being replaced by maternally-stored histones devoid of H3K27me3, while the maternal chromatin stains intensively for H3K27me3.
Detailed analysis of the 1-cell embryonic stage in mice has shown that immediately after fertilization, at PN0, H3K27me3 is only localized on the maternal PN, and this asymmetry is maintained until the early PN4 stage when the male PN becomes gradually positive for H3K27me3.Citation14,Citation60,Citation61 The acquisition of this mark in the mouse paternal genome coincides with the onset of DNA replication and maternally-stored EZH2 is necessary for its establishment.Citation62 In mice, the histone variant H3.3 is preferentially incorporated to the male PN, although it is also recruited to the female pronucleus shortly after fertilization.Citation61 This histone variant can be incorporated into chromatin in a replication-independent manner, and is usually associated with a transcriptional permissive chromatin state in somatic cells.Citation63 The acquisition of trimethylation at lysine 27 in the histone variant H3.3 at the paternal late PN in mice is necessary for achieving normal rates of blastocyst formation and is restricted to pericentromeric chromatin. Overexpression of a mutated histone H3.3 that cannot be methylated at K27 leads to de-repression of heterochromatin in the zygote, resulting in an accumulation of major satellite transcripts at the 2-cell stage that hampers further development. These results suggest that incorporation of H3.3 to the paternal PN during the zygotic stage is necessary to first allow the transcription of the paternal heterochromatin repeats during replication (which is essential to propagate the silent stage), and then to silence those regions by acquiring trimethylation at lysine 27 in the later PN stage.Citation61
As pre-implantation development proceeds into cleavage stages, H3K27me3 is further remodeled. In mice, the global signal of H3K27me3 is maintained at the 2-, 4- and 8-cell stages, followed by a decrease at the morula stage and re-establishment in the inner cell mass of the blastocyst, while the TE remains hypomethylated.Citation64 The mechanism and role for the decrease in global levels of H3K27me3 in mouse morulas is unknown. In bovine embryos, the H3K27me3 mark decreases from its level at the 2-cell stage to reach a minimum by the 8-cell stage (a moment that coincides with activation of the embryonic genome in this species) and then increases toward the morula and blastocyst stages.Citation10 Similarly, in pigs, it has been shown that the levels of H3K27me3 decline gradually from the 1-cell embryo to the morula stage, where they reach their lowest level, and then, subsequently, levels of this mark increase in blastocysts.Citation49 Thus, it appears that in species with late embryonic genome activation, such as cattle and pigs, global levels of H3K27me3 decrease from the time of fertilization until EGA.
EGA is a critical step for the correct development of the embryo. At this developmental milestone, the onset of transcription allows the transfer of developmental control from maternal RNA and proteins to the embryonic genome. The precise mechanism of EGA is not well understood; however, it is hypothesized that removal of transcriptionally repressive epigenetic marks is an important step for initiation of embryonic gene expression. The decrease in global levels of H3K27me3 near the time of EGA in pigs and cattle may represent a mechanism to allow for de-repression of pluripotency-related genes silenced during gametogenesis. Correspondingly, DNA methylationCitation8 and H3K9me2 levels,Citation9 both transcriptionally repressive marks, follow a similar pattern, suggesting that removal of repressive epigenetic modifications is an integral part of the EGA mechanism in these species.
Molecular Mechanisms Involved in H3K27me3 Remodeling
Evidence for passive loss of H3K27me3 during cleavage development of mammalian embryos
In species with late embryonic genome activation, like cattle and pigs, the levels of H3K27me3 decrease gradually from the 1-cell state to the time of EGA. PRC2 activity is necessary for maintaining H3K27me3 after DNA replication, and cell division in the absence of H3K27me3 maintenance would lead to a passive decrease in H3K27me3 levels. A comprehensive analysis of the localization and expression of the three core components of PRC2 (EZH2, EED and SUZ12) was performed during bovine pre-implantation development.Citation10 This work shows that only EZH2, the catalytic subunit of the PRC2, is localized to the nucleus of cleavage stage blastomeres. Remarkably, EED was expressed at all stages of pre-implantation development, but localized to the cytoplasm at early stages and then to the nucleus in morulas and blastocysts. Finally, SUZ12 was not detected until the morula stage. The absence of SUZ12 and EED localized to the nucleus of cleavage stage embryos is consistent with a lack of PRC2 activity and, therefore, it would be expected that with each cell division the levels of H3K27me3 would decrease. Interestingly, the absence of a functional PRC2 in this species correlates with the dynamics of H3K27me3. At the morula and blastocyst stages, both with high levels of H3K27me3 staining, all PRC2 components are present and localized to the embryonic nuclei.Citation10
In pigs, EZH2, EED and SUZ12 transcript abundance was positively correlated with global H3K27me3 levels.Citation49 The levels of PRC2 transcripts were low before the 4-cell stage and increased after EGA. These data are consistent with a decreased PRC2 activity before EGA and passive loss of H3K27me3 in the pig; however, further studies are necessary to corroborate the presence and localization of PRC2 proteins in pig pre-implantation embryos.
In contrast to what is observed in cattle and pigs, a gradual decrease in H3K27me3 is not seen during mouse early development, and PRC2 activity is already apparent in late stage zygotes.Citation14,Citation60,Citation61 Nevertheless, differential subcellular localization of PRC2 components is also observed in mice. Initially, EZH2 and EED are preferentially recruited to the maternal PNCitation14 and it is not until late PN stages that this complex is visualized in the paternal PN, coincident with the appearance of H3K27me3 in the male PN.Citation14,Citation65 Therefore, it appears that changes in subcellular localization of PRC2 components represent a potential mechanism by which PRC2 activity is regulated in pre-implantation embryos of different species.
Active removal of H3K27me3 during pre-implantation development
The possibility of an active mechanism involved in the removal of H3K27me3 during cleavage development became compelling when specific demethylases for this mark were discovered (JMJD3 and UTX); however, the involvement of these enzymes in mammalian early development was not reported until recently.
Evidence for active removal of H3K27me3 during bovine early development came from an experiment showing that the gradual loss of this mark was independent of cell divisions.Citation66 Treatment of 2-cell embryos with Aphidicolin, a reversible inhibitor of DNA synthesis, halted cell division but did not prevent the decrease in global H3K27me3 levels. This suggested that an enzymatic mechanism of H3K27me3 removal is active during bovine cleavage stages. Among the known histone demethylases, only JMJD3 presented high transcript levels in GV and MII bovine oocytes,Citation10,Citation66 which then decreased after fertilization. Conversely, JMJD3 protein was not detected in oocytes but localized to the nucleus in zygotes and cleavage stage embryos. This pattern of expression is consistent with a maternal contribution of JMJD3 mRNA by the oocyte and translation initiation upon oocyte activation by the fertilizing sperm. When oocytes were injected with siRNA targeting JMJD3, the levels of JMJD3 mRNA decreased and the global levels of H3K27me3 at the 4- and 8-cell stages did not decrease as observed for control embryos. This evidence points to JMJD3 as the H3K27me3-specific demethylase responsible for the decrease in H3K27me3 coinciding with EGA in cattle embryos. Moreover, depletion of JMJD3 maternal mRNA decreased the developmental potential of the embryo, manifested by a diminished rate of blastocyst formation.Citation66 Therefore, at least in bovine embryos, the activity of JMJD3 is necessary for the reprogramming of H3K27me3 and for normal embryonic development. It is tempting to speculate that the abnormal reprogramming of H3K27me3 in JMJD3 knockdown embryos prevents correct activation of the embryonic genome resulting in diminished embryonic development; however, H3K27 demethylase-independent roles of JMJD3 cannot be excluded.
In porcine zygotes, incubation in the presence of cycloheximide or aphidicolin diminished the asymmetry of H3K27me3 staining between pronuclei, suggesting the presence of an H3K27me3 demethylase.Citation17 Also in pig embryos, the mRNA levels of histone demethylases inversely correlated with levels of H3K27me3,Citation49 suggesting a role for active histone demethylation during cleavage division progression.
In mice, a detailed analysis of the involvement of histone demethylases in pre-implantation development has not yet been reported. However, it was recently demonstrated that JMJD3 knockout (JMJD3-KO) mice can develop to term but are stillborn or die shortly after birth from respiratory failure.Citation67,Citation68 Given that the JMJD3-KO mice would be expected to have the maternal JMJD3 gene contribution, since they are generated from a heterozygous female, we cannot make any conclusions regarding the necessity of maternal JMJD3 during murine pre-implantation development. However, these results argue that embryonic JMJD3 is not necessary for early mouse development, given the ability of JMJD3-KO embryos to reach advanced developmental stages well beyond pre-implantation development. It is possible that in mice active removal of H3K27me3, as observed at the morula stage, is not required for early development or utilizes mechanisms not involving JMJD3. One possibility is that UTX may substitute for the lack of JMJD3 in these embryos, although as indicated by the later lethality of JMJD3 deletion, this compensation does not seem to be complete. Examination of H3K27me3 global levels in JMJD3-KO pre-implantation embryos will help elucidate embryonic demethylation requirements. Also, generation of an oocyte-specific JMJD3-KO model would be necessary to test the role of maternally-provided JMJD3 and its effect on H3K27me3 reprogramming during mouse pre-implantation development.
Concluding Remarks
Dynamic remodeling of H3K27me3 is observed in mouse, pig, and bovine embryos during early pre-implantation development. In species with delayed embryonic genome activation, the H3K27me3 mark is gradually erased from the chromatin from fertilization to activation of the embryonic genome. It is possible in these species that erasure of H3K27me3 leads to reactivation of developmentally important genes that were silenced during gametogenesis. However, genome-wide analysis of gene-specific states will be necessary to better understand the role of H3K27me3 on gene activation.
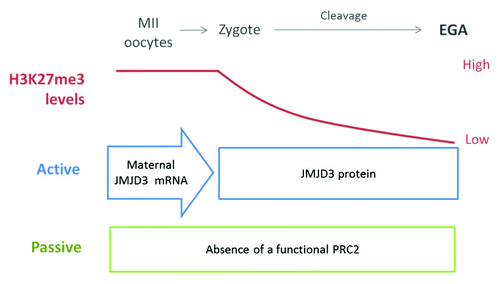
In pig and bovine, which exhibit a late EGA (compared with mice in which EGA occurs at the early 2-cell stage) the erasure is observed by the time of the genome activation. We hypothesize that, at least in late-EGA species, a combination of passive and active mechanisms result in the erasure of H3K27me3, which in turns allows EGA. The presence of a passive mechanism is supported by evidence showing a lack of functional PRC2, which would result in a dilution of H3K27me3 marks due to a lack of maintenance of this mark after each cell division.Citation10,Citation49 Active removal of H3K27me3 was recently reported in bovine embryos and involves maternal contribution of JMJD3 demethylases.Citation66 Both active and passive mechanisms could be involved in the H3K27me3 reprogramming during early development although the effect of the passive mechanism appears to be less pronounced. shows a model of H3K27me3 remodeling before EGA in species with late-EGAs and the possible molecular mechanisms involved. Further studies are needed to analyze specific loci reprogrammed by these mechanisms and their importance for pre-implantation development.
Figure 1. Model for H3K27me3 dynamics during early development in late-EGA species. Both active and passive mechanisms are involved in the gradual erasure of H3K27me3. The active mechanism involves maternal JMJD3 mRNA stored in the egg cytoplasm that is rapidly translated after fertilization. The passive mechanism involves the lack of a functional PRC2 before EGA.
A greater understanding of the epigenetic mechanisms controlling pre-implantation development and early lineage differentiation is critical to comprehending the basis of early embryonic development and to devise methods and approaches to treat infertility. Defective reprogramming of H3K27me3 has been recently observed in mouse cloned embryos,Citation64 heated-sperm derived mouse embryos,Citation69 and bovine cloned embryos (Ross et al., unpublished). Further analysis of the potential consequence of alterations to the early H3K27me3 reprogramming is therefore warranted.
Acknowledgments
We want to thank James Chitwood and Fernando Garcia Bermudez for comments on the manuscript. Work in the Ross lab related to this manuscript is supported by NIH/NICHD, R01 HD070044. YB was supported by IMSD fellowship from the National Institute of Health.
References
- Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci 2006; 31:89 - 97; http://dx.doi.org/10.1016/j.tibs.2005.12.008; PMID: 16403636
- Fry CJ, Peterson CL. Chromatin remodeling enzymes: who’s on first?. Curr Biol 2001; 11:R185 - 97; http://dx.doi.org/10.1016/S0960-9822(01)00090-2; PMID: 11267889
- Schneider R, Bannister AJ, Myers FA, Thorne AW, Crane-Robinson C, Kouzarides T. Histone H3 lysine 4 methylation patterns in higher eukaryotic genes. Nat Cell Biol 2004; 6:73 - 7; http://dx.doi.org/10.1038/ncb1076; PMID: 14661024
- Shi Y, Lan F, Matson C, Mulligan P, Whetstine JR, Cole PA, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell 2004; 119:941 - 53; http://dx.doi.org/10.1016/j.cell.2004.12.012; PMID: 15620353
- Sega MF, Lee K, Machaty Z, Cabot R. Pronuclear stage porcine embryos do not possess a strict asymmetric distribution of lysine 9 dimethylation of histone H3 based solely on parental origin. Mol Reprod Dev 2007; 74:2 - 7; http://dx.doi.org/10.1002/mrd.20599; PMID: 16941674
- Fulka H, Mrazek M, Tepla O, Fulka J Jr.. DNA methylation pattern in human zygotes and developing embryos. Reproduction 2004; 128:703 - 8; http://dx.doi.org/10.1530/rep.1.00217; PMID: 15579587
- Bourc’his D, Le Bourhis D, Patin D, Niveleau A, Comizzoli P, Renard JP, et al. Delayed and incomplete reprogramming of chromosome methylation patterns in bovine cloned embryos. Curr Biol 2001; 11:1542 - 6; http://dx.doi.org/10.1016/S0960-9822(01)00480-8; PMID: 11591324
- Dean W, Santos F, Stojkovic M, Zakhartchenko V, Walter J, Wolf E, et al. Conservation of methylation reprogramming in mammalian development: aberrant reprogramming in cloned embryos. Proc Natl Acad Sci U S A 2001; 98:13734 - 8; http://dx.doi.org/10.1073/pnas.241522698; PMID: 11717434
- Santos F, Zakhartchenko V, Stojkovic M, Peters A, Jenuwein T, Wolf E, et al. Epigenetic marking correlates with developmental potential in cloned bovine preimplantation embryos. Curr Biol 2003; 13:1116 - 21; http://dx.doi.org/10.1016/S0960-9822(03)00419-6; PMID: 12842010
- Ross PJ, Ragina NP, Rodriguez RM, Iager AE, Siripattarapravat K, Lopez-Corrales N, et al. Polycomb gene expression and histone H3 lysine 27 trimethylation changes during bovine preimplantation development. Reproduction 2008; 136:777 - 85; http://dx.doi.org/10.1530/REP-08-0045; PMID: 18784248
- Maalouf WE, Alberio R, Campbell KH. Differential acetylation of histone H4 lysine during development of in vitro fertilized, cloned and parthenogenetically activated bovine embryos. Epigenetics 2008; 3:199 - 209; http://dx.doi.org/10.4161/epi.3.4.6497; PMID: 18698155
- Mayer W, Niveleau A, Walter J, Fundele R, Haaf T. Demethylation of the zygotic paternal genome. Nature 2000; 403:501 - 2; http://dx.doi.org/10.1038/35000656; PMID: 10676950
- Sarmento OF, Digilio LC, Wang Y, Perlin J, Herr JC, Allis CD, et al. Dynamic alterations of specific histone modifications during early murine development. J Cell Sci 2004; 117:4449 - 59; http://dx.doi.org/10.1242/jcs.01328; PMID: 15316069
- Santos F, Peters AH, Otte AP, Reik W, Dean W. Dynamic chromatin modifications characterise the first cell cycle in mouse embryos. Dev Biol 2005; 280:225 - 36; http://dx.doi.org/10.1016/j.ydbio.2005.01.025; PMID: 15766761
- Chen T, Zhang YL, Jiang Y, Liu SZ, Schatten H, Chen DY, et al. The DNA methylation events in normal and cloned rabbit embryos. FEBS Lett 2004; 578:69 - 72; http://dx.doi.org/10.1016/j.febslet.2004.10.073; PMID: 15581618
- Fulka J, Fulka H, Slavik T, Okada K, Fulka J Jr.. DNA methylation pattern in pig in vivo produced embryos. Histochem Cell Biol 2006; 126:213 - 7; http://dx.doi.org/10.1007/s00418-006-0153-x; PMID: 16435122
- Park KE, Magnani L, Cabot RA. Differential remodeling of mono- and trimethylated H3K27 during porcine embryo development. Mol Reprod Dev 2009; 76:1033 - 42; http://dx.doi.org/10.1002/mrd.21061; PMID: 19536841
- Beaujean N, Hartshorne G, Cavilla J, Taylor J, Gardner J, Wilmut I, et al. Non-conservation of mammalian preimplantation methylation dynamics. Curr Biol 2004; 14:R266 - 7; http://dx.doi.org/10.1016/j.cub.2004.03.019; PMID: 15062117
- Wilmut I, Beaujean N, de Sousa PA, Dinnyes A, King TJ, Paterson LA, et al. Somatic cell nuclear transfer. Nature 2002; 419:583 - 6; http://dx.doi.org/10.1038/nature01079; PMID: 12374931
- Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet 2002; 3:662 - 73; http://dx.doi.org/10.1038/nrg887; PMID: 12209141
- Cheung P, Lau P. Epigenetic regulation by histone methylation and histone variants. Mol Endocrinol 2005; 19:563 - 73; http://dx.doi.org/10.1210/me.2004-0496; PMID: 15677708
- Margueron R, Trojer P, Reinberg D. The key to development: interpreting the histone code?. Curr Opin Genet Dev 2005; 15:163 - 76; http://dx.doi.org/10.1016/j.gde.2005.01.005; PMID: 15797199
- Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell 2006; 125:315 - 26; http://dx.doi.org/10.1016/j.cell.2006.02.041; PMID: 16630819
- Lee TI, Jenner RG, Boyer LA, Guenther MG, Levine SS, Kumar RM, et al. Control of developmental regulators by Polycomb in human embryonic stem cells. Cell 2006; 125:301 - 13; http://dx.doi.org/10.1016/j.cell.2006.02.043; PMID: 16630818
- Pan G, Tian S, Nie J, Yang C, Ruotti V, Wei H, et al. Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell 2007; 1:299 - 312; http://dx.doi.org/10.1016/j.stem.2007.08.003; PMID: 18371364
- Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell 2007; 128:735 - 45; http://dx.doi.org/10.1016/j.cell.2007.02.009; PMID: 17320510
- Boyer LA, Plath K, Zeitlinger J, Brambrink T, Medeiros LA, Lee TI, et al. Polycomb complexes repress developmental regulators in murine embryonic stem cells. Nature 2006; 441:349 - 53; http://dx.doi.org/10.1038/nature04733; PMID: 16625203
- Bracken AP, Dietrich N, Pasini D, Hansen KH, Helin K. Genome-wide mapping of Polycomb target genes unravels their roles in cell fate transitions. Genes Dev 2006; 20:1123 - 36; http://dx.doi.org/10.1101/gad.381706; PMID: 16618801
- Squazzo SL, O’Geen H, Komashko VM, Krig SR, Jin VX, Jang SW, et al. Suz12 binds to silenced regions of the genome in a cell-type-specific manner. Genome Res 2006; 16:890 - 900; http://dx.doi.org/10.1101/gr.5306606; PMID: 16751344
- Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002; 298:1039 - 43; http://dx.doi.org/10.1126/science.1076997; PMID: 12351676
- Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell 2002; 111:185 - 96; http://dx.doi.org/10.1016/S0092-8674(02)00975-3; PMID: 12408863
- Müller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, et al. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell 2002; 111:197 - 208; http://dx.doi.org/10.1016/S0092-8674(02)00976-5; PMID: 12408864
- Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell 2004; 15:57 - 67; http://dx.doi.org/10.1016/j.molcel.2004.06.020; PMID: 15225548
- Pasini D, Bracken AP, Jensen MR, Lazzerini Denchi E, Helin K. Suz12 is essential for mouse development and for EZH2 histone methyltransferase activity. EMBO J 2004; 23:4061 - 71; http://dx.doi.org/10.1038/sj.emboj.7600402; PMID: 15385962
- Margueron R, Justin N, Ohno K, Sharpe ML, Son J, Drury WJ 3rd, et al. Role of the polycomb protein EED in the propagation of repressive histone marks. Nature 2009; 461:762 - 7; http://dx.doi.org/10.1038/nature08398; PMID: 19767730
- Bannister AJ, Schneider R, Kouzarides T. Histone methylation: dynamic or static?. Cell 2002; 109:801 - 6; http://dx.doi.org/10.1016/S0092-8674(02)00798-5; PMID: 12110177
- Bannister AJ, Kouzarides T. Reversing histone methylation. Nature 2005; 436:1103 - 6; http://dx.doi.org/10.1038/nature04048; PMID: 16121170
- Xiang Y, Zhu Z, Han G, Lin H, Xu L, Chen CD. JMJD3 is a histone H3K27 demethylase. Cell Res 2007; 17:850 - 7; http://dx.doi.org/10.1038/cr.2007.83; PMID: 17923864
- Lan F, Bayliss PE, Rinn JL, Whetstine JR, Wang JK, Chen S, et al. A histone H3 lysine 27 demethylase regulates animal posterior development. Nature 2007; 449:689 - 94; http://dx.doi.org/10.1038/nature06192; PMID: 17851529
- Agger K, Cloos PA, Christensen J, Pasini D, Rose S, Rappsilber J, et al. UTX and JMJD3 are histone H3K27 demethylases involved in HOX gene regulation and development. Nature 2007; 449:731 - 4; http://dx.doi.org/10.1038/nature06145; PMID: 17713478
- De Santa F, Totaro MG, Prosperini E, Notarbartolo S, Testa G, Natoli G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 2007; 130:1083 - 94; http://dx.doi.org/10.1016/j.cell.2007.08.019; PMID: 17825402
- Shaw T, Martin P. Epigenetic reprogramming during wound healing: loss of polycomb-mediated silencing may enable upregulation of repair genes. EMBO Rep 2009; 10:881 - 6; http://dx.doi.org/10.1038/embor.2009.102; PMID: 19575012
- Burgold T, Spreafico F, De Santa F, Totaro MG, Prosperini E, Natoli G, et al. The histone H3 lysine 27-specific demethylase Jmjd3 is required for neural commitment. PLoS One 2008; 3:e3034; http://dx.doi.org/10.1371/journal.pone.0003034; PMID: 18716661
- Jepsen K, Solum D, Zhou T, McEvilly RJ, Kim HJ, Glass CK, et al. SMRT-mediated repression of an H3K27 demethylase in progression from neural stem cell to neuron. Nature 2007; 450:415 - 9; http://dx.doi.org/10.1038/nature06270; PMID: 17928865
- Agherbi H, Gaussmann-Wenger A, Verthuy C, Chasson L, Serrano M, Djabali M. Polycomb mediated epigenetic silencing and replication timing at the INK4a/ARF locus during senescence. PLoS One 2009; 4:e5622; http://dx.doi.org/10.1371/journal.pone.0005622; PMID: 19462008
- Barradas M, Anderton E, Acosta JC, Li S, Banito A, Rodriguez-Niedenführ M, et al. Histone demethylase JMJD3 contributes to epigenetic control of INK4a/ARF by oncogenic RAS. Genes Dev 2009; 23:1177 - 82; http://dx.doi.org/10.1101/gad.511109; PMID: 19451218
- Jung JW, Lee S, Seo MS, Park SB, Kurtz A, Kang SK, et al. Histone deacetylase controls adult stem cell aging by balancing the expression of polycomb genes and jumonji domain containing 3. Cell Mol Life Sci 2010; 67:1165 - 76; http://dx.doi.org/10.1007/s00018-009-0242-9; PMID: 20049504
- Erhardt S, Su IH, Schneider R, Barton S, Bannister AJ, Perez-Burgos L, et al. Consequences of the depletion of zygotic and embryonic enhancer of zeste 2 during preimplantation mouse development. Development 2003; 130:4235 - 48; http://dx.doi.org/10.1242/dev.00625; PMID: 12900441
- Gao Y, Hyttel P, Hall VJ. Regulation of H3K27me3 and H3K4me3 during early porcine embryonic development. Mol Reprod Dev 2010; 77:540 - 9; http://dx.doi.org/10.1002/mrd.21180; PMID: 20422712
- Miller D, Brinkworth M, Iles D. Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 2010; 139:287 - 301; http://dx.doi.org/10.1530/REP-09-0281; PMID: 19759174
- Arpanahi A, Brinkworth M, Iles D, Krawetz SA, Paradowska A, Platts AE, et al. Endonuclease-sensitive regions of human spermatozoal chromatin are highly enriched in promoter and CTCF binding sequences. Genome Res 2009; 19:1338 - 49; http://dx.doi.org/10.1101/gr.094953.109; PMID: 19584098
- Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature 2009; 460:473 - 8; PMID: 19525931
- van der Heijden GW, Derijck AA, Ramos L, Giele M, van der Vlag J, de Boer P. Transmission of modified nucleosomes from the mouse male germline to the zygote and subsequent remodeling of paternal chromatin. Dev Biol 2006; 298:458 - 69; http://dx.doi.org/10.1016/j.ydbio.2006.06.051; PMID: 16887113
- van der Heijden GW, Ramos L, Baart EB, van den Berg IM, Derijck AA, van der Vlag J, et al. Sperm-derived histones contribute to zygotic chromatin in humans. BMC Dev Biol 2008; 8:34; http://dx.doi.org/10.1186/1471-213X-8-34; PMID: 18377649
- Tanaka M, Kihara M, Meczekalski B, King GJ, Adashi EY. H1oo: a pre-embryonic H1 linker histone in search of a function. Mol Cell Endocrinol 2003; 202:5 - 9; http://dx.doi.org/10.1016/S0303-7207(03)00054-6; PMID: 12770723
- Saeki H, Ohsumi K, Aihara H, Ito T, Hirose S, Ura K, et al. Linker histone variants control chromatin dynamics during early embryogenesis. Proc Natl Acad Sci U S A 2005; 102:5697 - 702; http://dx.doi.org/10.1073/pnas.0409824102; PMID: 15821029
- McLay DW, Clarke HJ. Remodelling the paternal chromatin at fertilization in mammals. Reproduction 2003; 125:625 - 33; http://dx.doi.org/10.1530/rep.0.1250625; PMID: 12713425
- van der Heijden GW, Dieker JW, Derijck AA, Muller S, Berden JH, Braat DD, et al. Asymmetry in histone H3 variants and lysine methylation between paternal and maternal chromatin of the early mouse zygote. Mech Dev 2005; 122:1008 - 22; http://dx.doi.org/10.1016/j.mod.2005.04.009; PMID: 15922569
- Jeong YS, Yeo S, Park JS, Lee KK, Kang YK. Gradual development of a genome-wide H3-K9 trimethylation pattern in paternally derived pig pronucleus. Dev Dyn 2007; 236:1509 - 16; http://dx.doi.org/10.1002/dvdy.21150; PMID: 17474127
- Albert M, Peters AH. Genetic and epigenetic control of early mouse development. Curr Opin Genet Dev 2009; 19:113 - 21; http://dx.doi.org/10.1016/j.gde.2009.03.004; PMID: 19359161
- Santenard A, Ziegler-Birling C, Koch M, Tora L, Bannister AJ, Torres-Padilla ME. Heterochromatin formation in the mouse embryo requires critical residues of the histone variant H3.3. Nat Cell Biol 2010; 12:853 - 62; http://dx.doi.org/10.1038/ncb2089; PMID: 20676102
- Puschendorf M, Terranova R, Boutsma E, Mao X, Isono K, Brykczynska U, et al. PRC1 and Suv39h specify parental asymmetry at constitutive heterochromatin in early mouse embryos. Nat Genet 2008; 40:411 - 20; http://dx.doi.org/10.1038/ng.99; PMID: 18311137
- Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell 2002; 9:1191 - 200; http://dx.doi.org/10.1016/S1097-2765(02)00542-7; PMID: 12086617
- Zhang M, Wang F, Kou Z, Zhang Y, Gao S. Defective chromatin structure in somatic cell cloned mouse embryos. J Biol Chem 2009; 284:24981 - 7; http://dx.doi.org/10.1074/jbc.M109.011973; PMID: 19602512
- Burton A, Torres-Padilla ME. Epigenetic reprogramming and development: a unique heterochromatin organization in the preimplantation mouse embryo. Brief Funct Genomics 2010; 9:444 - 54; http://dx.doi.org/10.1093/bfgp/elq027; PMID: 21186177
- Canovas S, Cibelli JB, Ross PJ. Jumonji domain-containing protein 3 regulates histone 3 lysine 27 methylation during bovine preimplantation development. Proc Natl Acad Sci U S A 2012; 109:2400 - 5; http://dx.doi.org/10.1073/pnas.1119112109; PMID: 22308433
- Satoh T, Takeuchi O, Vandenbon A, Yasuda K, Tanaka Y, Kumagai Y, et al. The Jmjd3-Irf4 axis regulates M2 macrophage polarization and host responses against helminth infection. Nat Immunol 2010; 11:936 - 44; http://dx.doi.org/10.1038/ni.1920; PMID: 20729857
- Tusi B. The role of histone demethylase Jmjd3 in the inflammatory response: generation and analysis of in vivo models. General Pathology. Milan: Università degli studi di Milano, 2011.
- Chao SB, Chen L, Li JC, Ou XH, Huang XJ, Wen S, et al. Defective histone H3K27 trimethylation modification in embryos derived from heated mouse sperm. Microsc Microanal 2012; 18:476 - 82; http://dx.doi.org/10.1017/S1431927612000396; PMID: 22568956