Abstract
The insulin-like growth factor 2 (IGF2) gene, located within a cluster of imprinted genes on chromosome 11p15, encodes a fetal and placental growth factor affecting birth weight. DNA methylation variability at the IGF2 gene locus has been previously reported but its consequences on fetal growth and development are still mostly unknown in normal pediatric population. We collected one hundred placenta biopsies from 50 women with corresponding maternal and cord blood samples and measured anthropometric indices, blood pressure and metabolic phenotypes using standardized procedures. IGF2/H19 DNA methylation and IGF2 circulating levels were assessed using sodium bisulfite pyrosequencing and ELISA, respectively. Placental IGF2 (DMR0 and DMR2) DNA methylation levels were correlated with newborn’s fetal growth indices, such as weight, and with maternal IGF2 circulating concentration at the third trimester of pregnancy, whereas H19 (DMR) DNA methylation levels were correlated with IGF2 levels in cord blood. The maternal genotype of a known IGF2/H19 polymorphism (rs2107425) was associated with birth weight. Taken together, we showed that IGF2/H19 epigenotype and genotypes independently account for 31% of the newborn’s weight variance. No association was observed with maternal diabetic status, glucose concentrations or prenatal maternal body mass index. This is the first study showing that DNA methylation at the IGF2/H19 genes locus may act as a modulator of IGF2 newborn’s fetal growth and development within normal range. IGF2/H19 DNA methylation could represent a cornerstone in linking birth weight and fetal metabolic programming of late onset obesity.
Introduction
Birth weight at both ends of the spectrum (i.e., intrauterine growth restriction and fetal overgrowth) has been associated with higher risk of obesity, type 2 diabetes (T2D) and cardiovascular disease (CVD) in the adult life.Citation1-Citation3 Intrauterine life is now considered a critical period associated with long-term programming of energy balance regulation.Citation4,Citation5 Events during this critical window are believed to play an important role in the fetal development and growth and in determining children’s susceptibility to energy metabolism-related diseases over the long-term.Citation4 In fact, detrimental fetal and perinatal environments have been repeatedly associated with obesity, T2D and CVD.Citation2,Citation6 However, the underlying molecular mechanisms linking intrauterine growth restriction and fetal overgrowth to children health have been poorly documented. In addition, birth weight heritability is estimated to be around 45%,Citation7,Citation8 but the genetic determinants explaining the variance are still mostly unknown.
Epigenetic profile alterations have been repeatedly proposed as a likely molecular mechanism linking birth weight and later determinant of health in childhood or even later in life.Citation9 Genomic imprinting, an epigenetic phenomenon by which certain genes are expressed in a parent-of-origin-manner, is known to impact genes important in placental and fetal growth and development.Citation10 Insulin-like growth factor 2 (IGF2), also known as the somatomedin A, is one of the key players being involved in fetal growth and development. IGF2 is located upstream of H19 on chromosome 11p15 in a region where gene imprinting was clearly demonstrated to occur. Accordingly, IGF2 is paternally expressed whereas H19 is transcribed into a noncoding RNA of unknown function from the maternal copy of the genome.Citation9 Gene expression regulation of each of the two copies according to their parental origin is under the control of DNA methylation (epigenetic) marks at IGF2/H19 differentially methylated regions (DMRs).Citation11 Briefly, CTCF, a DNA insulator protein, will bind methylated H19 DMR mediating the effect of the H19/IGF2 downstream enhancer.Citation11 Therefore, binding the maternal chromosome, CTCF will suppress IGF2 transcription and activate H19 expression. Absence of CTCF binding on the paternal chromosome has opposite effects. Loss of imprinting (LOI) or deviation from hemimethylation (50% DNA methylation), is expected to unbalance IGF2 expression and induce fetal growth and development defects.Citation9
Gain in methylation at H19 DMR has been associated with syndromes of fetal overgrowth [such as Beckwith-Weidemann syndrome (BWS) and Isolated Hemihyperplasia] while loss of methylation at H19 DMR seems implicated in the Russel-Silver syndrome, characterized by severe prenatal growth restriction.Citation9 This is in line with the hypothesis that higher DMR methylation levels at H19 decrease its expression, allowing its reciprocal IGF2 gene to be more expressed, leading to increase fetal growth (and vice versa). Interestingly, BWS is also associated with neonatal hypoglycemia, hyperinsulinism and cardiomyopathy.
Taken together, the clinical features of three human syndromes highly suggest that IGF2/H19 DNA methylation is important for fetal growth and development as well as for children’s health. However, the role of DNA methylation at the IGF2/H19 locus in fetal growth and development in normal pediatric population has not yet been established. Accordingly, we tested whether placental IGF2/H19 DNA methylation levels are correlated with newborn’s markers of fetal growth and development and whether fetal environment (dependent on maternal metabolism) is affecting IGF2/H19 epigenetic profile in a cohort of neonates born from women with and without glycemic dysregulation during pregnancy.
Results
Characteristics of the women and their newborn
shows women and newborn characteristics. On average, the mothers were of normal weight with fasting blood glucose within the normal range at first trimester of pregnancy. Twenty-three women developed impaired glucose tolerance (IGT) during pregnancy based on the second trimester oral glucose tolerance test (OGTT).Citation12 Only one of them fulfilled the American Diabetes Association diagnostic value for gestational diabetes mellitus (GDM).Citation13,Citation14 IGT women were on diet (n = 14) alone or on diet and insulin treatment (n = 7). Two mothers with IGT did not receive any treatment. Babies were within the normal birth weight range on average.
Table 1. Women and newborns characteristics
IGF2 circulating concentration
First, associations between maternal IGF2 circulating concentrations and mother’s body composition or newborns’ growth and development markers were assessed. As shown in , maternal IGF2 levels at the third trimester of pregnancy were found negatively correlated with mother’s body weight throughout pregnancy as well as with her percent body mass at 12–14 weeks of gestation (all r ≤ -0.35, p ≤ 0.05). Cord blood IGF2 levels were positively correlated with newborn’s height (r = 0.42, p = 0.005) and placental weight (r = 0.29, p = 0.07) (). Fetal side IGF2 mRNA levels were associated with placenta weight (). No association was observed between neither maternal nor cord blood IGF2 levels and maternal or newborn glucose status.
Table 2. Spearman rank correlation coefficient between circulating IGF2 concentration and mRNA levels and mothers’ and newborns’ body composition-related phenotypes
As epigenetics is involved in gene expression regulation, we verified whether DNA methylation at IGF2/H19 was associated with IGF2 mRNA and circulating levels. As shown in , we demonstrated that higher IGF2-DMR2 DNA methylation levels on the maternal placental side are associated with lower IGF2 concentration at the third trimester of pregnancy. We have also shown that DNA methylation levels of IGF2-DMR0-A1 CpG1 and H19-DMR locus on the maternal side were positively correlated with cord blood IGF2 levels while no significant correlation between cord blood IFG2 levels and fetal side DNA methylation levels at any of the analyzed DMR around IGF2/H19 locus were observed. None of the DNA methylation loci investigated was associated with IGF2 mRNA levels.
Table 3. Spearman rank correlation coefficient between circulating IGF2 concentration and maternal side placenta IGF2/H19 DNA methylation levels
DNA methylation levels at IGF2/H19 locus and markers of fetal growth and development
The main goal of the current study was to verify whether IGF2/H19 DNA methylation levels were associated with markers of fetal growth and development. We found that DNA methylation at each locus analyzed varies widely between individuals (). In addition, we observed that DNA methylation levels between the CpGs within a same locus were generally highly correlated with each other (). Accordingly, DNA methylation measures between correlated CpGs within a same locus were aggregated and only the results for mean DNA methylation levels at the IGF2-DMR2 and H19-DMR are presented. The analyses were conducted in both sides of the placenta separately since corresponding CpGs between placenta sides were only marginally correlated (data not shown).
Figure 1.IGF2/H19 gene locus (chromosome 11p15). Numbers in the diamond shapes report Spearman correlation coefficient between CpG sites (white diamonds) or linkage disequilibrium (LD) values (r2) between H19 SNPs (gray diamonds) as computed with Haploview 4.0. Mean and SD are shown for DNA methylation values.
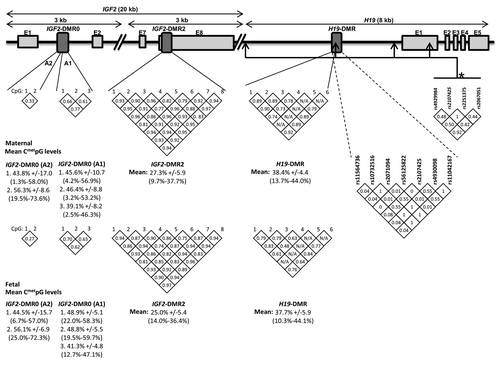
On the fetal side of the placenta, higher mean DNA methylation levels at IGF2-DMR2 locus were associated with higher birth weight, greater height, in addition to larger head and thorax circumferences (). Some neonatal anthropometric measurements were also positively correlated with higher DNA methylation levels at specific CpGs within the DMR0 locus of the IFG2 promoter region (). H19-DMR DNA methylation levels were not found to be associated with any of the anthropometric measurements at birth. Placental weight was not associated with DNA methylation levels on the fetal side of the placenta at any loci investigated.
Table 4. Spearman rank correlation coefficient between placenta IGF2/H19 DNA methylation levels and fetal growth and development markers
On the maternal placental side, we found that higher DNA methylation levels at three CpGs located within the IGF2-DMR0-A1 and DMR0-A2 loci were associated with higher newborn’s thorax circumference and birth and placenta weight (). No significant association between fetal growth and development markers and maternal IGF2-DMR2 or H19-DMR DNA methylation levels was observed ().
IGF2/H19 genotypes and markers of fetal growth and development
H19 DMR has been sequenced for polymorphism inventory at this locus. A total of 7 polymorphisms were found: rs11564736 [minor allele frequency (MAF) = 4%], rs10732516 (MAF = 46%), rs2071094 (MAF = 46%), rs56125822 (MAF = 1%), rs2107425 (MAF = 32%), rs4930098 (MAF = 46%) and rs11042167 (MAF = 46%). rs2107425 is in partial LD with birth weight-associated polymorphisms previously reported by Adkins at al. ().Citation15 We found that mothers carriers of the minor allele at rs10732516 (C/C and C/T) or rs2107425 (AA and AG) gave birth to heavier and longer newborns compared with babies born from T/T or G/G homozygous women (). rs2107425 maternal genotype was also found associated with other fetal growth and development markers (). Newborn’s genotype at rs10732516 was found associated with head and thorax circumferences, whereas rs2107425 newborn’s genotype was not significantly associated with any fetal growth or development marker tested (). Together, placental IGF2/H19 DMR2 (fetal side) epigenotype (18%, p = 0.002) and rs2107425 maternal genotype (13%, p = 0.006) accounted for 31% (p = 0.0002) of the newborn weight variance. rs10732516 did not contribute independently (p > 0.15) to the stepwise regression model. None of the two polymorphisms were associated with DNA methylation at IGF2/H19 DMR2.
Table 5. Association analyses between IGF2/H19 polymorphisms and newborn growth markers
Discussion
In the current study, we showed that placental DNA methylation changes at the IGF2/H19 imprinted locus are associated with fetal development and birth weight in a normal pediatric population. Low and high birth weight have been repeatedly and clearly associated with a long-term increased risk for obesity, diabetes and CVD.Citation1-Citation3 Consequently, understanding the biologic mechanisms determining birth weight brings more insights into early origins of adult chronic diseases.
We also report that the relationship between DNA methylation and newborns’ growth and developmental markers analyzed is different in both sides (maternal vs. fetal) of the placenta tissues. Briefly, we found that IGF2 DNA methylation levels in the maternal side of the placenta are potentially associated with early unfavorable maternal environment as it was found associated with placenta developmental dysregulation (placenta weight). However, the fetal side DNA methylation levels were associated with overall fetal growth and developmental markers (birth weight and height and head and thorax circumferences) suggesting that late gestation as well as the fetal milieu are likely more important to determine the fetal side epigenetic profile. This is potentially an important finding as exposure to a detrimental fetal environment during early gestation is associated with obesity,Citation16 dyslipidemiaCitation17 and an overall 3-fold increased risk of CVD,Citation18 whereas a late gestation exposure is associated with an increased risk for glucose metabolism dysregulation (impaired glucose tolerance and type 2 diabetes mellitus).Citation19,Citation20 This suggests that DNA methylation signature of both sides of the placenta reflects an exposure to metabolic challenges that occurred at different critical window during fetal development.Citation21 Therefore, studies will be needed to identify the factors predictive of DNA methylation changes at the IGF2/H19 locus. One of these could be maternal obesity as suggested by a well-designed retrospective case-series study of 172 pediatric patients (children) followed between 2 to 18 y old and born from mothers who underwent bariatric surgery.Citation22 In this study, Kral et al. demonstrated a reduction of 50% of obesity in the offspring, but epigenetic profiling has not been investigated.Citation22
Interestingly, the DNA methylation variability at the IGF2/H19 locus was quite large with few individuals with very low levels of DNA methylation (< 10%), although this is a region known to be imprinted. This is in line with results recently published by Coolen et al., also reporting considerable DNA methylation variation both in monozygotic (MZ) and dizygotic (DZ) twins.Citation23 Interestingly, they also reported that the DNA methylation profiles (at the IGF2/H19 locus) of genetically identical twins (MZ) were less discordant than in DZ individuals. This suggests that local genetic sequence affects DNA methylation at IGF2/H19 locus. Nevertheless, genetics only partially accounts for DNA methylation variability (as demonstrated by existing variability in MZ twins) suggesting that other factors (environment) remain to be identified. Explaining the source of this variability could potentially be critical because it would allow providing recommendations for a healthier pregnancy. However, none of the anthropometric and metabolic factors measured in the mothers and the newborns were correlated (or predictive) of DNA methylation levels at the IGF2/H19 locus. Nevertheless, we have shown that the DNA methylation changes observed at the IGF2/H19 locus are likely to be functional, being associated with circulating IGF2 concentrations. This suggests that DNA methylation variability at the IGF2/H19 locus contributes to modulate IGF2 gene expression over that of the effects of gene imprinting. Environmental and biological factors that could modulate DNA methylation at this locus are still to be discovered.
Nevertheless, a number of gene polymorphisms located within the IGF2/H19 gene locus have been studied, as they are potential biological factors modulating IGF2 DNA methylation profile, expression and activity. Recently, Adkins at al. reported a significant association between maternal genotype for three H19 polymorphisms and birth weight.Citation15 These polymorphisms were located surrounding (6 kb) the H19-DMR (). In order to be as close as possible to the epigenotyped region, we have inventoried the polymorphisms located within H19 DMR locus and investigated their association with fetal growth and development markers. This allowed us to confirm the association with birth weight reported by Adkins et al.Citation15 and to show that the H19 maternal polymorphism and IGF2 DNA methylation account for up to 31% of the birth weight variance. Our results suggest that IGF2/H19 DNA methylation and genotypes are independent predictors of birth weight. Although these results have first to be replicated, explaining 31% of the variance of a complex trait such as birth weight with a heritability estimate ranging from 42–45%Citation7,Citation8 with only one genetic variant and one epivariant at a single gene locus is an important finding. Also, it suggests that DNA methylation could explain a fraction of the ‘missing heritability’ of other complex traits (such as adult BMI) that are currently being investigated in many genetic epidemiological studies, looking only at genotypes.Citation24 Moreover, a maternal effect has been observed as already reported by Adkins at al.Citation15 This is also in accordance with the genetic conflict theory,Citation25 which states that the gene allele inherited from the mother will protect her resources exhaustion against the fetus’ needs by downregulating the activity of newborns’ (anabolic) genes such as IGF2. The suspected mechanism would be an interaction between the inherited maternal allele and newborns’ epigenotype. This has still to be carefully studied.
Significant association between IGF2/H19 and obesity-related complications were also reported. Faienza et al. found an association between an IGF2 polymorphism (6815 A/T) and hypertension in obese children and adolescent, but failed, as other groups,Citation26,Citation27 to find an association with obesity.Citation28 However, significant associations with obesity, hypertension and dyslipidemia in healthy mid-age men were also reported.Citation29 This suggests that the IGF2/H19 epigenotypes we have uncovered could also be associated with obesity and its cardiometabolic risk factors. Whether those associations are linked through the effect on birth weight remain to be tested. This link has still to be established but could have important clinical implication if proven true.
Strengths and limitations
The longitudinal follow-up starting at the first trimester with standardized anthropometric and metabolic measurements is among the strengths of this study as well as having conducted the study in a homogeneous population form European descent (which can be a strength in genetic and epigenetic studies), although the findings might not be applicable to other populations. The bisulfite pyrosequencing technology used to measure DNA methylation levels is well validated and reliable. Our sample size was fairly large according to the strength of the correlations and compared with other studies looking at epigenetic mechanisms. It allows us to take into account possible confounding factors. However, weaker associations could have been missed and prevent finding metabolic factors predictive of DNA methylation modulation for example. Also, our results need to be replicated before strong conclusion can be drawn.
In conclusion, we demonstrated for the first time that both genetic and epigenetic variants at IGF2/H19 locus are potentially major determinants of birth weight in a normal pediatric population. Higher risk of obesity, diabetes and CVD later in life is associated with birth weight at both ends of the spectrum, and one of the mechanisms explaining this association could be DNA methylation variation at key gene loci. Future studies should investigate whether DNA methylation patterns found to be associated with birth weight and other fetal growth and development indices are also predictive of obesity and related metabolic disorders later in life.
Material and Methods
Subjects
Fifty women with a singleton pregnancy were recruited from a founder population of French-Canadian origin. Women over 40 y old, with pre-gestational diabetes or other disorders known to affect glucose metabolism as well as those with a positive history of alcohol and/or drug abuse during the current pregnancy were excluded. The Chicoutimi Hospital Ethics Committee approved the project. All women provided a written informed consent before their inclusion in the study in accordance with the Declaration of Helsinki.
Anthropometric variables [body mass index (BMI), waist and hip girth] and blood pressure were measured using standardized procedures.Citation30 Body composition (lean and fat mass) was measured using bioimpedance (Xitron model 4200, Xitron Technologies). Glucose was evaluated using a Beckman analyzer (model CX7), and insulin measurements were performed using a radioimmunoassay method (Advia Centaur, Simmens). Serum IGF2 levels were measured using ELISA assay as recommended by the manufacturer (ALPCO Immunoassays). IGT was defined as a 2-h glucose ≥ 7.8 mmol/L following a 75-g OGTT performed at 24–28 weeks gestation.Citation12
Placenta tissue sampling
Placenta tissues were sampled minutes following delivery and kept in RNALater (Qiagen) at -80°C until nucleic acid extraction. Tissue biopsies were taken from the fetal and the maternal sides. The first consisted of the intervillous tissues and chorionic villi, and the second consisted mainly of fetal villous tissue but may also contains very little tissue of maternal origin in the deciduas basalis (basal plate). DNA and RNA analyses were performed on both sides separately.
IGF2/H19 molecular analyses
DNA and RNA were purified using the All Prep DNA/RNA/Protein mini Kit (Qiagen). RNA quality was assessed with the Agilent 2100 bioanalyzer RNA Nano Chips (Agilent Technology). On average, RNA was of good to excellent quality (RIN = 7.65 ± 0.06).
Polymorphisms within H19 DMR locus were inventoried and genotyped using direct sequencing. The samples were run on an ABI PRISM 3100 Genetic Analyzer (ABI). Both strands were sequenced using the PCR primers. The primer sequences were: PCR forward: 5'-GGGGAGATGGGAGGAGATAC-3'; PCR reverse: 5'-GTAAAAGGCTGGGGATTTGG-3'. PCR conditions can be provided upon request. Data were analyzed with CodonCode Aligner 3.7 (CodonCode Corporation).
DNA methylation analyses covered IGF2-DMR0 (2 assays; A1 and A2) located in first intron and IGF2-DMR2 located within exon 8, as well as H19-DMR (). DNA methylation levels at CpG sites were assessed using pyrosequencing (Pyromark Q24, QIAGEN-Biotage). Briefly, methylated cytosines are protected against a C→T transition following sodium bisulfite treatment (EpiTect Bisulfite Kit, QIAGEN). Pyrosequencing is a quantitative sequencing method allowing measuring cytosine methylation levels (%) at each CpG site of a given sequences. PCR primers were selected using PyroMark Assay Design v2.0.1.15 (Qiagen). DNA methylation quality controls were applied. These include NaBis conversion controls and the assessment of pyrosequencing quality (peak height, deviation from reference sequence pattern and unexpected peak height). The PCR and pyrosequencing primers are shown in Table S1.
CDNA (cDNA) was generated from total RNA using a random primer hexamer provided with the High Capacity cDNA Archive kit (Applied Biosystems). Equal amounts of cDNA were run in duplicate and amplified in a 20 µl reaction containing 10 µL of 2X Universal PCR Master Mix (Applied Biosystems). Primers and Taqman probes were designed to cover exon boundaries and were obtained from Applied Biosystems (IGF2: Hs01005963_m1; Applied Biosystems). Each sample was calibrated to the YWHAZ housekeeping gene (endogenous control; YWHAZ: Hs00237047_m1).Citation31,Citation32 Relative quantification estimations were performed using an Applied Biosystems 7500 Real Time PCR System as recommended by the manufacturer (Applied Biosystems).
Statistical analyses
Mean locus DNA methylation levels were computed among the cytosines showing within correlation (r2) levels higher than 0.8 on average (). The computed mean values were used for correlation analyses when applicable. Maternal and the fetal side of the placenta DNA methylation levels were analyzed separately (low pairwise correlation between tissues). IGF2/YWHAZ ct ratio (1/x) values were used for correlation analyses. rs10732516 is in complete LD (r2 = 1) with rs2071094, rs4930098 and rs11042167. Polymorphisms with low MAF were not analyzed (rs11564736 and rs56125822). Accordingly, only rs10732516 and rs2107425 has been statistically analyzed.
Spearman rank correlation coefficients were used to test associations between methylated cytosines and between DNA methylation levels and the main variables of interest: birth and placenta weight, height, head and thorax circumferences, maternal fasting glucose and insulin, glycemic results at OGTT and IGF2 mRNA circulating levels. The correlations with potential confounders (age, BMI, weight change over pregnancy rs11564736 and rs2107425 genotypes) were also tested. Accordingly, confounders were added to the statistical models when they significantly contributed to the models. Student’s t-tests were used to assess statistical differences in mean birth weight and DNA methylation between rs11564736 and rs2107425 genotypic groups. Results were considered statistically significant when p values < 0.05 (two-sided). Statistical analyses were performed using the SAS software, version 9.1.3.
Additional material
Download Zip (108 KB)Acknowledgments
We warmly acknowledge the contribution of Sébastien Claveau (MSc), Nadia Mior, Jeannine Landry (RN) and Chantale Aubut (RN) for their dedicated work in this study. We also express our gratitude to Céline Bélanger, Chicoutimi Hospital, for their thoughtful revision of the manuscript. This project was supported by ECOGENE-21, the Canadian Institutes of Health Research (CIHR team in community genetics (grant #CTP-82941), Fonds de la Recherche en Santé du Québec (FRSQ) and Diabète Québec. S.P.G. was recipient of a doctoral research award (Frederick Banting and Charles Best Canada Graduate Scholarship) from the CIHR. L.B. and M.F.H. are junior research scholars from the FRSQ. P. Poirier is a senior clinician-scientist of the FRSQ. M.F.H. is also supported by a Canadian Diabetes Association clinical scientist award. L.B. and M.F.H. are members of the FRSQ-funded Centre de recherche clinique Étienne-Le Bel.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors Contributions
L.B. and J.S.P. have conceived the study design, performed the data analysis/interpretation and wrote the manuscript. P. Perron participated in the study design conception and revised the manuscript. M.F.H. and P. Poirier participated in the data interpretation and revised the manuscript. S.P.G. performed the data collection and revised the manuscript. D.B. participated in the study design conception, participated in the data analysis/interpretation and revised the manuscript.
Supplemental Material
Supplemental materials may be found here: www.landesbioscience.com/journals/epigenetics/article/21855
References
- Calkins K, Devaskar SU. Fetal origins of adult disease. Curr Probl Pediatr Adolesc Health Care 2011; 41:158 - 76; http://dx.doi.org/10.1016/j.cppeds.2011.01.001; PMID: 21684471
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia 1992; 35:595 - 601; http://dx.doi.org/10.1007/BF00400248; PMID: 1644236
- Ng SK, Olog A, Spinks AB, Cameron CM, Searle J, McClure RJ. Risk factors and obstetric complications of large for gestational age births with adjustments for community effects: results from a new cohort study. BMC Public Health 2010; 10:460; http://dx.doi.org/10.1186/1471-2458-10-460; PMID: 20687966
- Symonds ME, Sebert SP, Hyatt MA, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol 2009; 5:604 - 10; http://dx.doi.org/10.1038/nrendo.2009.195; PMID: 19786987
- St-Pierre J, Bouchard L, Poirier P. The Impact of Obesity on Cardiovascular Structure and Function: From the Very Beginning. Pediatric Health. Medicine and Therapeutics 2012; 3:1 - 8; http://dx.doi.org/10.2147/PHMT.S12931
- Barker DJ, Hales CN, Fall CH, Osmond C, Phipps K, Clark PM. Type 2 (non-insulin-dependent) diabetes mellitus, hypertension and hyperlipidaemia (syndrome X): relation to reduced fetal growth. Diabetologia 1993; 36:62 - 7; http://dx.doi.org/10.1007/BF00399095; PMID: 8436255
- Clausson B, Lichtenstein P, Cnattingius S. Genetic influence on birthweight and gestational length determined by studies in offspring of twins. BJOG 2000; 107:375 - 81; http://dx.doi.org/10.1111/j.1471-0528.2000.tb13234.x; PMID: 10740335
- Svensson AC, Pawitan Y, Cnattingius S, Reilly M, Lichtenstein P. Familial aggregation of small-for-gestational-age births: the importance of fetal genetic effects. Am J Obstet Gynecol 2006; 194:475 - 9; http://dx.doi.org/10.1016/j.ajog.2005.08.019; PMID: 16458649
- Smith AC, Choufani S, Ferreira JC, Weksberg R. Growth regulation, imprinted genes, and chromosome 11p15.5. Pediatr Res 2007; 61:43R - 7R; http://dx.doi.org/10.1203/pdr.0b013e3180457660; PMID: 17413842
- Constância M, Kelsey G, Reik W. Resourceful imprinting. Nature 2004; 432:53 - 7; http://dx.doi.org/10.1038/432053a; PMID: 15525980
- Arney KL. H19 and Igf2--enhancing the confusion?. Trends Genet 2003; 19:17 - 23; http://dx.doi.org/10.1016/S0168-9525(02)00004-5; PMID: 12493244
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva: World Health Organization, 1999.
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009; 32:Suppl 1 S62 - 7; http://dx.doi.org/10.2337/dc09-S062; PMID: 19118289
- American Diabetes Association. Standards of medical care in diabetes--2011. Diabetes Care 2011; 34:Suppl 1 S11 - 61; http://dx.doi.org/10.2337/dc11-S011; PMID: 21193625
- Adkins RM, Somes G, Morrison JC, Hill JB, Watson EM, Magann EF, et al. Association of birth weight with polymorphisms in the IGF2, H19, and IGF2R genes. Pediatr Res 2010; 68:429 - 34; PMID: 20639793
- Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr 1999; 70:811 - 6; PMID: 10539740
- Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr 2000; 72:1101 - 6; PMID: 11063435
- Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Schroeder-Tanka JM, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944-45. Heart 2000; 84:595 - 8; http://dx.doi.org/10.1136/heart.84.6.595; PMID: 11083734
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet 1998; 351:173 - 7; http://dx.doi.org/10.1016/S0140-6736(97)07244-9; PMID: 9449872
- Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Twin Res 2001; 4:293 - 8; PMID: 11869479
- McCance RA, Widdowson EM. The determinants of growth and form. Proc R Soc Lond B Biol Sci 1974; 185:1 - 17; http://dx.doi.org/10.1098/rspb.1974.0001; PMID: 4149051
- Kral JG, Biron S, Simard S, Hould FS, Lebel S, Marceau S, et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years. Pediatrics 2006; 118:e1644 - 9; http://dx.doi.org/10.1542/peds.2006-1379; PMID: 17142494
- Coolen MW, Statham AL, Qu W, Campbell MJ, Henders AK, Montgomery GW, et al. Impact of the genome on the epigenome is manifested in DNA methylation patterns of imprinted regions in monozygotic and dizygotic twins. PLoS One 2011; 6:e25590; http://dx.doi.org/10.1371/journal.pone.0025590; PMID: 21991322
- Meaburn EL, Schalkwyk LC, Mill J. Allele-specific methylation in the human genome: implications for genetic studies of complex disease. Epigenetics 2010; 5:578 - 82; http://dx.doi.org/10.4161/epi.5.7.12960; PMID: 20716955
- Wilkins JF, Haig D. What good is genomic imprinting: the function of parent-specific gene expression. Nat Rev Genet 2003; 4:359 - 68; http://dx.doi.org/10.1038/nrg1062; PMID: 12728278
- Gomes MV, Soares MR, Pasqualim-Neto A, Marcondes CR, Lôbo RB, Ramos ES. Association between birth weight, body mass index and IGF2/ApaI polymorphism. Growth Horm IGF Res 2005; 15:360 - 2; http://dx.doi.org/10.1016/j.ghir.2005.06.016; PMID: 16102992
- Heude B, Ong KK, Luben R, Wareham NJ, Sandhu MS. Study of association between common variation in the insulin-like growth factor 2 gene and indices of obesity and body size in middle-aged men and women. J Clin Endocrinol Metab 2007; 92:2734 - 8; http://dx.doi.org/10.1210/jc.2006-1948; PMID: 17488802
- Faienza MF, Santoro N, Lauciello R, Calabrò R, Giordani L, Di Salvo G, et al. IGF2 gene variants and risk of hypertension in obese children and adolescents. Pediatr Res 2010; 67:340 - 4; http://dx.doi.org/10.1203/PDR.0b013e3181d22757; PMID: 20057340
- Rodríguez S, Gaunt TR, O’Dell SD, Chen XH, Gu D, Hawe E, et al. Haplotypic analyses of the IGF2-INS-TH gene cluster in relation to cardiovascular risk traits. Hum Mol Genet 2004; 13:715 - 25; http://dx.doi.org/10.1093/hmg/ddh070; PMID: 14749349
- Lohman TG, Roche AF, Martorell R. R RAF, Martorell. Anthropometric standardization reference manual. Human Kinetics Books. Champaign, IL, 1988:55-80.
- Meller M, Vadachkoria S, Luthy DA, Williams MA. Evaluation of housekeeping genes in placental comparative expression studies. Placenta 2005; 26:601 - 7; http://dx.doi.org/10.1016/j.placenta.2004.09.009; PMID: 16085039
- Murthi P, Fitzpatrick E, Borg AJ, Donath S, Brennecke SP, Kalionis B. GAPDH, 18S rRNA and YWHAZ are suitable endogenous reference genes for relative gene expression studies in placental tissues from human idiopathic fetal growth restriction. Placenta 2008; 29:798 - 801; http://dx.doi.org/10.1016/j.placenta.2008.06.007; PMID: 18684503
- American Diabetes Association. Standards of medical care in diabetes--2006. Diabetes Care 2006; 29:Suppl 1 S4 - 42; PMID: 16373931