Abstract
Histone posttranslational modifications are key components of diverse processes that modulate chromatin structure. These marks function as signals during various chromatin-based events, and act as platforms for recruitment, assembly or retention of chromatin-associated factors. The best-known function of histone phosphorylation takes place during cellular response to DNA damage, when phosphorylated histone H2A(X) demarcates large chromatin domains around the site of DNA breakage. However, multiple studies have also shown that histone phosphorylation plays crucial roles in chromatin remodeling linked to other nuclear processes. In this review, we summarize the current knowledge of histone phosphorylation and describe the many kinases and phosphatases that regulate it. We discuss the key roles played by this histone mark in DNA repair, transcription and chromatin compaction during cell division and apoptosis. Additionally, we describe the intricate crosstalk that occurs between phosphorylation and other histone modifications and allows for sophisticated control over the chromatin remodeling processes.
Introduction
Chromatin is a nucleoprotein structure that compacts and organizes DNA in eukaryotic cells. However, it also establishes a strong barrier for nuclear events that need to access the DNA, such as transcription, DNA repair, replication and meiotic recombination. The fundamental unit of chromatin is the nucleosome, which consists of 146 base pairs of DNA wrapped around an octamer of histones—small basic proteins that are highly evolutionarily conserved. The histone core contains two molecules of each histone H2A, H2B, H3 and H4.Citation1 Unlike the central structured domains of histone proteins that form the globular part of the nucleosome, the N-terminal domain of all four histones and C-terminal domains of H2A and H2B, called “histone tails,” are poorly structured and protrude from the nucleosome.
In order to control DNA accessibility, the chromatin structure must be dynamically modulated. Four mechanisms that modify chromatin compaction are well characterized. They are not mutually exclusive but cooperate to regulate chromatin structure and DNA accessibility. These include (1) ATP-dependent remodeling complexes that use the energy of ATP hydrolysis to slide the nucleosomes and exchange or evict histones from the chromatin fiber; (2) histone variants that possess properties distinct from those of canonical histones and create localized specific domains within the chromatin fiber; (3) histone chaperones that control the supply of free histones and cooperate with chromatin remodelers in histone deposition and eviction and; (4) post-translational modifications (PTMs) of histones, such as acetylation, methylation, phosphorylation and ubiquitination that directly or indirectly influence chromatin structure. These modifications, which occur predominately on the accessible histone tails, can directly modulate histone-DNA interactions and also form docking sites to facilitate recruitment of specific non-histone proteins to chromatin. Importantly, combinations of PTMs occurring on the same histone tail (in cis), or on another tail (in trans), have been proposed to constitute a “histone code” or signature.Citation2-Citation5 This code is deciphered by the so-called readers, proteins that contain binding motifs specific for each modification: for example, chromodomains specifically recognize methylated residues, while bromodomains bind acetylated residues.Citation6,Citation7 Recognition of PTMs results in the local binding of reader-containing complexes known as effectors that interpret the signal, eventually leading to chromatin remodeling.Citation5,Citation8
In this review, we will focus specifically on histone phosphorylation. All four nucleosomal histone tails contain acceptor sites that can be phosphorylated by a number of protein kinases and dephosphorylated by phosphatases. Histone phosphorylation can occur on serine, threonine and tyrosine residues and constitutes an essential part of the “histone code,” or combinatory function of PTMs on chromatin. A number of proteins containing phospho-binding modules such as 14-3-3 and BRCT domains that can recognize phosphorylated histones have been identified and characterized as downstream effectors (for review, see refs. Citation6 and Citation7). While a large number of phosphorylated residues on histones have been described, identification of new phosphorylation sites and characterization of their biological functions remain areas of intensive investigation. In this review, we will summarize current knowledge concerning histone phosphorylation events, their regulation and their known functions in nuclear processes that involve modulation of chromatin structure.
Histone Phosphorylation Involved in DNA Damage Repair
Phosphorylation of H2A(X) is an important histone modification that plays a major role in DNA damage response (for review, see refs. Citation9 and Citation10). In mammalian cells, this modification takes place on serine (S) 139 of the H2AX variant histone, and is commonly referred to as γH2AX.Citation11,Citation12 In yeast, the H2AX variant does not exist, and the corresponding phosphorylation occurs on S129 of H2A.Citation13,Citation14 For simplicity, we will hence refer to both modifications as γH2AX. This phosphorylation occurs in all phases of the cell cycle and is involved in diverse DNA-damage response (DDR) pathways including non-homologous end joining (NHEJ), homologous recombination (HR), and replication-coupled DNA repair.Citation13-Citation15 Protein kinases yTel1 and yMec1 (ATM and ATR in mammals) carry out this phosphorylation.Citation13,Citation16 Mutation of H2AS129 in yeast to non-phosphorylatable alanine leads to hypersensitivity to DNA-damage inducing agents such as phleomycin and methyl methane-sulphonate (MMS), highlighting a crucial role of γH2AX in DNA double strand break (DSB) repair.Citation13 Phosphorylation of H2AS129 in yeast is one of the first events following DNA damage, appearing within 30 min in the immediate vicinity of the DSB.Citation16-Citation19 This modification spreads bidirectionally over several kilobases (~50 kb) on each side of the break in yeast, while mammalian γH2AX spreads over several megabases.Citation16,Citation20-Citation22 This wide distribution of γH2AX around the break is thought to create a specific signaling platform for recruitment and/or retention of DNA damage repair and signaling factors, including the critical mediator protein MDC1 through recognition by its BRCT domainCitation23,Citation24 (for review, see ref. Citation10). Studies performed in yeast suggest that γH2AX also facilitates recruitment of the NuA4 acetyltransferase complex, which is thought to be the first chromatin modifier recruited to the DSB after the γH2AX kinases.Citation17,Citation25 NuA4 relocalization to the break results in H4 hyperacetylation,Citation17,Citation25,Citation26 which is proposed to promote local chromatin relaxation. In addition, it has been suggested that γH2AX allows the recruitment to the DSB of other chromatin-modifying complexes such as the ATP-dependent remodelers INO80 and SWR1. These complexes are thought to act in concert to enhance DNA accessibility in order to facilitate the repair process.Citation9,Citation27,Citation28 NuA4, INO80 and SWR1 all contain a common subunit, Arp4, which was found to bind directly to γH2AX in yeast, suggesting a plausible mechanism for their accumulation near the break.Citation17 Additionally, the 53BP1/Crb2-related adaptor protein Rad9 was demonstrated to interact with γH2AX through its BRCT domain, while its Tudor domain binds methylated K79 of H3.Citation29-Citation31 This specific binding promotes Rad9 recruitment to the DNA break where it is activated by the Mec1 kinase to induce activation of the DNA damage checkpoint. This mechanism plays the crucial role of delaying the cell’s progression through the G1/S phase of the cell cycle, thereby permitting efficient repair of DNA damage.
Once the DNA has been repaired, γH2AX must be removed from chromatin in order to stop the retention of repair proteins at the DSB, and to allow efficient recovery from the DNA damage-induced cell cycle arrest. A study performed in yeast suggested that γH2AX is evicted and replaced with the Htz1 histone variant by the SWR1 remodeler.Citation32 In contrast, Van Attikum and colleagues showed that H2AZ (mammalian Htz1) and γH2AX are both removed from chromatin near the DSB in an INO80-dependent manner.Citation33 Whether it is deposited or removed, the significance of H2AZ dynamics at the site of DNA damage has not been elucidated so far.
In addition to its removal by histone exchange, γH2AX is also dephosphorylated. In yeast, the HTP-C phosphatase complex and its catalytic subunit Pph3 regulate H2AS129 phosphorylation status in vivo.Citation34 HTP-C targets H2AS129ph after its displacement from DNA, and this dephosphorylation is required for DNA damage checkpoint recovery. In parallel, mammalian γH2AX phosphatases such as PP2A, Wip1, PP6 and PP4 have been described.Citation35-Citation39 These enzymes dephosphorylate γH2AX in vivo, and are similarly required for efficient DNA damage repair and recovery from cell cycle arrest.
The Allis group has recently shown that the H2AX variant is also phosphorylated on tyrosine (Y) 142 and that this modification is regulated in a DNA-damage-dependent manner. H2AXY142 is phosphorylated by the WSTF catalytic subunit of the WICH complex and dephosphorylated by the EYA1/3 phosphatase.Citation40 Interestingly, in contrast to γH2AX, H2AXY142 phosphorylation is ubiquitous in the cell and decreases in response to DNA damage. This regulation is critical for γH2AX maintenance as dephosphorylation of H2AXY142ph by EYA is required to maintain γH2AX-containing DNA repair foci.Citation40 A defect of H2AXY142 dephosphorylation inhibits the accumulation of repair factors at damage sites.Citation41 Full understanding of the interplay between the inverse phosphorylation of these two residues during DNA damage response requires further investigation.
Phosphorylation of serine 1 of histone H4 by Casein Kinase II (CKII) is also induced in yeast upon genotoxic stress such as exposure to UV light (UV), methyl methanesulfonate (MMS) and phleomycin.Citation42,Citation43 ChIP experiments demonstrate that H4S1ph is restricted to a much smaller region around the DSB and appears sometime after γH2AX, implicating its involvement in the later stages of the DDR. Interestingly, H4S1ph, which was initially discovered as a mark of newly synthesized histones,Citation44 is inversely correlated with H4 acetylation whose removal takes place as the repair process is nearing completion.Citation42,Citation43 Furthermore, phosphorylation of H4S1 was found to inhibit acetylation of the H4 tail by the NuA4 HAT in vitro, while in vivo the CKII kinase associates with the Rpd3S deacetylase complex.Citation43 Thus, it seems likely that as new nucleosomes are reassembled during restoration of chromatin structure, phosphorylation of H4S1 acts to stabilize them by preventing their acetylation. However, the fact that mutation of H4S1 to a non-phosphorylatable residue does not lead to yeast’s increased sensitivity to genotoxic agentsCitation43 indicates that this phosphorylation event is not essential to the repair process and suggests functional redundancy with other nucleosome-stabilizing mechanisms.
In yeast, serine 122 of histone H2A is phosphorylated together with the nearby H2AS129, and their modification is similarly modulated in response to DNA damage.Citation45-Citation48 Systematic site-directed mutagenesis of the C-terminal tail of H2A determined that S122 is important for survival in the presence of DNA damage induced by various agents such as camptothecin, phleomycin, MMS, hydroxyurea (HU) or UV.Citation47,Citation48 Phosphorylation of this residue was suggested to have a direct role in DNA repair, although it is not required for Mec1-dependent signal transduction and occurs independently of S129 phosphorylation. Since inhibition of H2AS122 phosphorylation has no effect on global chromatin structure,Citation47 it can be speculated that this modification has a role in mediating the interaction with the DDR machinery. Phosphorylation of the adjacent S122 and S129 in response to DNA damage suggests a possible interplay or combinatorial action in cis during recruitment, accumulation or retention of repair factors. However, further investigation is required to confirm these hypotheses and to identify any effectors that may interact with these histone modifications.
In mammals, phosphorylation of H2BS14 was also detected upon DNA damage induced by ionizing radiation and shown to colocalize with γH2AX foci.Citation49 The role of this modification in DDR is unknown, while previously linked to cell apoptosis, but it is plausible that this mark plays a role in combination with γH2AX and H2AXY142ph.
Histone Phosphorylation Associated with Transcription Regulation
A substantial number of phosphorylated histone residues are associated with gene expression. Interestingly, these are often related to regulation of proliferative genes. Phosphorylation of serines 10 and 28 of H3 and serine 32 of H2B has been associated with regulation of epidermal growth factor (EGF)-responsive gene transcription. H3S10ph and H2BS32ph have been linked to the expression of proto-oncogenes such as c-fos, c-jun and c-myc.Citation50-Citation52 Furthermore, targeting H3S28 phosphorylation to promoters of genes such as c-fos and α-globin was shown to control their activation.Citation53 Notably, phosphorylation of H3S10 and H3S28 is not exclusive to EGF stimulation, but it also increases upon UVB radiation. Phosphorylation of H3 on T11 and T6 has also been implicated in transcription regulation in response to androgen stimulation,Citation54,Citation55 and to DNA-damage in mouse cells.Citation56
Phosphorylation of H3S10, T11 and S28 has been clearly associated with H3 acetylation, strongly implicating these modifications in transcription activation. Independent studies show that these phosphorylation events are mechanistically linked to Gcn5-dependent H3 acetylation ().Citation57-Citation59 Indeed, in EGF-stimulated cells, phosphorylation of H3S10 is tightly coupled to H3 K9ac and K14ac, both marks of transcriptional activation.Citation51,Citation60,Citation61 It has been shown that phosphorylation of H3S10 promotes acetylation of H3K14 by the Gcn5 acetyltransferase in vitro and allows Gcn5-regulated gene transcription in vivo.Citation57 A similar link has been described in yeast where H3S10 phosphorylated by Snf1 acts in concert with Gcn5-dependent H3K14 acetylation to enhance transcription.Citation62 This functional link between H3S10ph and Gcn5 is likely mediated by the direct interaction of the acetyltransferase with the phosphorylated H3S10 residue, which has been observed in vitro.Citation57,Citation60 However, analysis of the c-jun promoter activation showed that K14ac appears before S10ph and that inhibition of S10 phosphorylation has no effect on K14ac, suggesting that these two events can be uncoupled.Citation61,Citation63 Interestingly, phosphorylation of the H3 tail on T11 in addition to S10 was shown to enhance its interaction with Gcn5 at promoters of Gcn5-dependent genes such as the cell-cycle regulators cyclin B and cdk1, leading to increased H3K9 and K14 acetylation and stimulation of transcription.Citation54,Citation56,Citation58 Likewise, phosphorylation of H3S28 was found to promote K9 acetylation.Citation59 All together, these data suggest a complex crosstalk between phosphorylation of H3S10, T11 and S28 in the control of Gcn5-dependent H3 acetylation, gene expression and cell proliferation ().
Figure 1. Examples of crosstalk between phosphorylation and other histone post-translational modifications. The histone H3 N-terminal tail is presented as example of a platform harboring multiples PTMs showing crosstalks in cis with different sites of phosphorylation. Recent in vitro findings using time-resolved NMR spectroscopy suggest a slightly modified picture in which H3S10ph acts as master switch for subsequent intramolecular modifications, and inhibits T6 and T11 phosphorylation while the reverse does not apply.Citation139 This study also questioned the role of T11ph in the stimulation of K14ac.
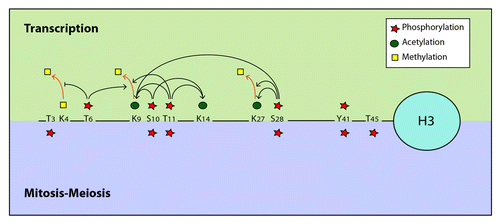
Importantly, H3S28ph also plays a role in combination with H3K27ac in transcription activation.Citation53,Citation64 Mechanistically, H3S28 phosphorylation at gene promoters is thought to displace Polycomb repressive complexes from chromatin and to induce demethylation and acetylation of the adjacent K27 residue at these loci, thereby activating transcription. Additionally, phosphorylation of H3T11 and H3T6 in response to androgen receptor-dependent gene activation has been shown to regulate transcription by controlling H3 methylation. Phosphorylation of both residues promotes removal of the repressive methyl mark on H3K9 by the Jumonji C domain-containing protein JMJD2C.Citation54,Citation55 In addition, H3T6ph was shown to prevent LSD1-mediated removal of mono- and dimethyl-H3K4, marks of actively transcribed chromatin.Citation55 Remarkably, these studies suggest an additional interplay involving phosphorylation and methylation on the N-terminal tail of H3 in transcription regulation. Thus, phosphorylation of the H3 tail plays an important role in controlling in cis its acetylation and methylation in order to regulate gene expression.
H2BS32ph is ubiquitous in normally cycling mammalian cells, but is more abundant in skin cancer cells where RSK2, the kinase responsible for its phosphorylation, is also highly expressed.Citation50,Citation65 In response to EGF treatment, H3 is phosphorylated on S10 by the specific mitogen- or stress-induced kinases RSK2 and MSK1,Citation66,Citation67 and on S28 by MSK1, MSK2 and by the mixed lineage kinase-like mitogen-activated protein triple kinase (MLTK)-α.Citation63,Citation68 Additionally, H3S10 and H3S28 are also phosphorylated in response to UVB radiation by the kinases ERK, p38 and the Src family member Fyn,Citation69,Citation70 while H3S28 is also targeted by MLTK-α, MSK1, ERK1, ERK2, p38 and to a lesser degree JNK1 and JNK2.Citation59,Citation68,Citation71,Citation72 Upon androgen stimulation, phosphorylation of H3 T11 and T6 is catalyzed respectively by the protein kinase-C related kinase 1 (PRK1) and the protein kinase C-β (PKCβ), notably in prostate cancer cells.Citation54,Citation55 Recently, it was demonstrated that H3T11 is also a specific substrate for the tumor-specific pyruvate kinase M2 (PKM2) in a transcriptional process that promotes H3 K9 acetylation and gene transcription upon EGF-signaling, leading to tumor cell proliferation.Citation73 H3T11ph can also be added by the Chk1 kinase in response to DNA damage in mammalian cells.Citation56 Upon DNA damage, this kinase rapidly dissociates from the promoters of cell-cycle regulatory genes, leading to a loss of H3T11ph, a concomitant reduction of permissive acetylation marks from the H3 tail, and a diminution of the transcription of these genes. Thus, this crosstalk between two distinct types of histone modifications appears to be responsible for transcription reduction and cell cycle arrest in response to DNA damage.
Phosphorylation of tyrosine (Y) 41 on H3 by Janus Kinase 2 (JAK2) has also been described to influence transcription.Citation74 Phosphorylation of Y41 disrupts chromatin binding by HP1α (heterochromatin protein 1 α), which directly interacts with this region of H3 through its chromo-shadow domain. Loss of HP1α from chromatin leads to transcriptional activation of JAK2-regulated genes including the oncogene imo2, which encodes a protein with roles in normal hematopoiesis and in leukemia. These observations suggest that, while JAK2 signaling and HP1α chromatin association are tightly regulated in normally growing cells, constitutive activation of JAK2 can lead to oncogenesis by mediating HP1α displacement from chromatin through H3Y41 phosphorylation.
Serine 36 of H2B has been described as a target of AMPK (AMP-activated protein kinase) and its phosphorylation has been linked to gene expression.Citation75 ChIP experiments detect H2B S36ph within promoters and coding regions of AMPK-responsive genes, and loss of this modification leads to their reduced expression and lower rates of cell survival in response to metabolic stress. While both H3Y41ph and H2BS36ph are associated with transcription activation and cell proliferation, neither has been correlated with previously described cis modifications involved in expression of proliferation genes, namely T6, S10, T11 and S28 on H3 and S32 on H2B.
H2B has also been recently shown to be phosphorylated on tyrosine 37 in yeast and mammalian cells.Citation76 This mark is added by the WEE1 kinase and is important for suppression of replication-dependent core histone gene transcription. H2B Y37ph directly blocks the binding of a key transcription activator and allows recruitment of the HIRA histone chaperone.Citation76
Studies in yeast have demonstrated that phosphorylation of H4S1 is also regulated during transcription. Indeed, levels of this modification increase upon transcription activation in a CKII-dependent manner.Citation43 Since phosphorylation of H4S1 was shown to negatively regulate H4 acetylation during DNA damage repair, it is surprising to observe the appearance of this mark upon transcription activation, a process usually associated with hyperacetylation. However, H4S1ph is enriched on the coding region compared with the promoter of the inducible hps104 gene, and this is concomitant with a decrease of H4 acetylation at the same locus.Citation43 Thus, H4S1ph appears to play a role in transcription elongation where it regulates acetylation-dependent chromatin relaxation. As phosphorylation of H4 on S1 directly inhibits its acetylation,Citation43 one plausible hypothesis is that phosphorylation of H4 occurs on the coding region in the wake of transcribing RNA polymerase II (RNAP II), where it blocks H4 acetylation. This could be related to stabilization of nucleosomes on the chromatin fiber behind the polymerase, thereby preventing inappropriate initiation of transcription from within the coding regions of active genes, as described for histone chaperones and methylase/deacetylase.Citation77,Citation78
In addition, H4S1 was identified as a substrate of the sporulation specific kinase Sps1, whose deletion alters transcription of mid- and late-sporulation genes.Citation79,Citation80 Unexpectedly, both H4S1ph and Sps1 were found to be enriched on the promoters of these genes, and to be specifically required for optimal timing of transcriptional repression.Citation80,Citation81 While both were also detected at many other genomic loci, no correlation with transcription activity was observed. Recent ChIP-seq studies have specified more precisely H4S1ph localization during meiosis and have found that H4S1ph is enriched at the TSS (transcription start site) of a very large number of genes including sporulation genes.Citation81 Surprisingly, H4S1ph showed a local colocalization with H4ac at the TSS, in contrast to the inverted correlation that was found during DNA repair and transcription elongation. The reason for the colocalization of these two modifications has not been elucidated yet, and Govin and colleaguesCitation81 suggest that it may reflect the existence of “bivalent domains” or “bivalent promoters” presenting positive and negative marks, similar to what has been described in embryonic stem cells.Citation82 Thus, it appears that the cellular context has a determinant influence on the interplay between H4 phosphorylation and acetylation: H4S1ph can block H4 acetylation in order to stabilize nucleosomes during DNA repair or transcription elongation, or it can coexist with H4ac to tightly regulate gene expression during specific cellular events.
H4 is also phosphorylated on S47 in vivo and in vitro in a PAK2-dependent manner.Citation83 PAK2 is able to phosphorylate single H4 or the H3-H4 tetramer but not nucleosomal H4. Although H4S47ph has not been directly associated with transcription regulation, this mark is found to be preferentially enriched in H3.3-containing nucleosomes, generally deposited into chromatin in a transcription-dependent manner.Citation83 Interestingly, it was found that phosphorylation of H4S47 specifically favors H4’s association with the H3.3-specific HIRA chaperone rather than with the canonical H3-specific CAF1, thereby promoting incorporation of H3.3-H4 into nucleosomes. Notably, these results demonstrate a novel mechanism by which a histone PTM can influence incorporation of histone variants into chromatin and thus, specialization of chromatin domains.
An extra-nucleosomal histone, the linker histone H1, is also known to be phosphorylated on multiple residues.Citation84 Since H1 is implicated in the stabilization of the nucleosome (as a chromatosome) and in the formation of the 30nm chromatin fiber, it has long been suggested to play a role in chromatin relaxation to allow transcription (reviewed in ref. Citation85).Citation86 Phosphorylation of H1 is detected at active 45S pre-rRNA promoters and at steroid hormone response elements under hormone treatment; it was also shown to assists RNAP I and RNAP II-dependent transcription.Citation87-Citation89 These observations suggest a role of H1 phosphorylation in ribosome biogenesis and control of cell growth that certainly needs further investigation.
Roles of Histone Phosphorylation in Chromatin Compaction
Histone phosphorylation and chromatin compaction associated with mitosis and meiosis
Histone H3 phosphorylation is highly conserved among eukaryotes from yeast to human, and has been extensively studied for many years. While H3 phosphorylation is involved in chromatin relaxation and regulation of gene expression, this modification was originally identified to be associated with chromosome compaction during mitosis and meiosis. In total, four phosphorylated residues within the N-terminal tail of H3 were discovered to be associated with chromosome condensation and segregation: T3, S10, T11 and S28. However, it is still unclear whether these modifications are functionally related to chromatin condensation and inter-related between each other. Phosphorylation of H3 on S10 is likely the best-documented mark related to chromatin condensation associated with mitosis and meiosis in many eukaryotic organisms, and is commonly used as a reference mark of these events.Citation90-Citation93 Interestingly, the patterns of H3S10 and H3S28 phosphorylation in early mitosis are quite similar, commencing at the onset of chromosome condensation during prophase.Citation94 Phosphorylation of both residues occurs along the same chromosome during mitosis, but it is important to note that there is no evidence that these two marks are present together on the same histone tail. In fact, immunofluorescence and sequential immunoprecipitation analyses performed in quiescent cells have demonstrated that H3S10ph and H3S28ph are found on distinct populations of H3.Citation95,Citation96 Nevertheless, whether their apparent separation applies to mitotic cells has not been described yet. Since both residues present the same consensus sequence, ARKS, it is not entirely surprising that they are phosphorylated by the same kinase Aurora B (yeast Ipl1) and dephosphorylated by Protein Phosphatase 1 (PP1; Glc7 in yeast).Citation93,Citation97,Citation98 The balance between Aurora B/yIpl1 and PP1/yGlc7 was shown to be critical for the control of H3 phosphorylation and for proper chromosome segregation.Citation98 Supporting the notion that these enzymes play an important role in chromosome stability in mammalian cells, Aurora B was reported to be overexpressed in a variety of human cancers.Citation99-Citation101 Interestingly, the H3 K9 residue adjacent to H3S10 was shown to be methylated in silenced regions associated with heterochromatin, and this methylation defines a docking site for the binding of HP1. Moreover, HP1 was shown to dissociate from chromatin during the M phaseCitation102 and, importantly, phosphorylation of H3S10 during mitosis was demonstrated to be sufficient to eject the HP1α, HP1β and HP1γ bound to methylated H3K9.Citation103 This result suggests a role for H3S10ph in the regulation of protein binding to chromatin during mitosis. Full understanding of the functional link between H3S10ph and H3S28ph and of the exact molecular mechanisms by which these PTMs influence chromosome condensation and segregation still needs further examination.
During mitosis, histone H3 is also phosphorylated on threonine 3. Distribution of this PTM is similar to that of H3S10ph in early prophase, but diverges when cells enter prometaphase.Citation104 H3 T3ph becomes highly enriched at inner centromeric regions on prometaphase and metaphase chromosomes, but its levels decline during anaphase and disappear entirely from decondensed chromosomes.Citation105 H3T3 is phosphorylated by Haspin, a mitotic chromatin-associated kinase.Citation105-Citation109 Haspin is known to play a role in sister chromatin cohesion during mitosis, and its function appears to be mediated, at least in part, by phosphorylated H3T3. Recent studies in yeast and human cells have demonstrated that Haspin-dependent phosphorylation of H3T3 is required for recruitment of the Aurora B-containing Chromosomal Passenger Complex (CPC) to the centromere, and this relocalization is mediated by a direct interaction between H3T3ph and Survivin, another subunit of CPC.Citation110-Citation113 Furthermore, Aurora B can also phosphorylate Haspin, promoting further H3T3 phosphorylation at the centromere, and thereby establishing a positive-feedback loop.Citation114 Haspin was also shown to cooperate with the Bub1 kinase in targeting CPC to the centromere.Citation110,Citation114 Bub1 localizes to kinetochores where it phosphorylates H2A on T120 in mammalian cells (and on the corresponding S121 in fission yeast) and regulates H3T3ph distribution.Citation110 H2AT120ph facilitates nucleosome binding by shugoshin, the centromeric CPC adaptor,Citation110,Citation115 and was previously known to be targeted by NHK-1 in Drosophila where it is required for meiotic and mitotic chromosomal architecture.Citation116,Citation117
Depletion of Haspin and reduction of H3T3ph levels by RNA interference affects sister chromatin cohesion, suggesting that H3T3 phosphorylation plays a role in the regulation of chromosome separation rather than in chromosome condensation.Citation106,Citation109 Unexpectedly, it was found that H3T3ph always occurs in cis with tri-methylated K4 and di-methylated R8.Citation118 These marks appear at multiple sites at the periphery of the prophase nucleus, then cluster at the centromeres of metaphase chromosomes, and finally spread to the distal areas of segregating chromatids. The joint role of these histone PTMs has not been fully investigated, and the presence of H3K4me3, a mark of active transcription, together with the mitotic H3T3ph is very surprising.
Concomitant with H3T3ph, mitotic H3T11ph enrichment is restricted from prophase to early anaphase, and is also found to preferentially associate with the centromere.Citation119 Phosphorylation of H3 on T11 was first identified in rat and in the human breast carcinoma cell line MCF7 and shown to be restricted to mitotic cells. Dlk was suggested to be the kinase responsible for T11 phosphorylation since the GFP-Dlk fusion associated with the centromere within the same time frame.Citation119 This modification has also been reported in plants where it temporally correlates with chromatin condensation during mitosis and meiosis.Citation120 In yeast, H3T11 is also phosphorylated during meiosis and this modification is catalyzed by the meiotic Mek1 kinase.Citation120,Citation121 In contrast to the H3S10A substitution in yeast that strikingly had no negative effect on sporulation, the T11A mutant showed a reduction in sporulation efficiency, arguing that phosphorylation of T11 is a key modification during meiosis.Citation121
Phosphorylation of H4S1 and H2BS10 was also demonstrated to occur during meiosis in yeast. Additionally, H4S1ph has been observed during fruit fly and mouse spermatogenesis.Citation80 Examination of H4S1ph timing relative to H3S10 phosphorylation shows that these two marks have a distinct pattern during sporulation.Citation80 Unlike the transient H3S10ph that coincides with early sporulation, H4S1ph appears as a stable mark after meiotic I and II divisions.Citation80,Citation121 In fact, S10 residues of both H2B and H3 are phosphorylated during meiotic chromosome condensation and disappear during meiotic divisions,Citation122 whereas H4S1 phosphorylation appears later in meiosis and increases in post-meiotic cells.Citation80,Citation81 H4S1ph is stable and persists in post-meiotic cells, including mature yeast spores and mature spermatids. This meiotic phosphorylation is dependent on Sps1, a mid-sporulation-specific kinase member of the Ste20 family.Citation80 There is no in vitro evidence that Sps1 phosphorylates H4S1 directly, but deletion of this kinase results in a loss of H4S1ph signal. Supporting the role of H4S1ph in chromatin compaction during later stages of meiosis, deletion of Sps1 as well as mutation of H4S1 to alanine in yeast results in sporulation deficiency and increased DNA and nuclear volumes.Citation80,Citation121
Finally, H2BS10 phosphorylation has been detected during the prophase of meiosis, although the kinase responsible for this modification in meiosis remains unknown.Citation122 While Ipl1 can phosphorylate both H3 and H2B in vitro, the possibility that it phosphorylates H2B on S10 in vivo during meiosis remains to be addressed. Intriguingly, this histone mark was found to colocalize with Zip1, a component of the synaptonemal complex characteristic of meiotic pachytene chromosomes. Overall, current evidence indicates that H2BS10ph plays a major role in chromatin condensation during various cellular processes such as meiosis but the molecular mechanism by which this modification influences chromatin structure still needs to be elucidated.
Phosphorylation of linker histone H1 is also regulated in a cell cycle-dependent manner (reviewed in ref. Citation123). It significantly increases during mitosis and S phase, being more specifically detected through metaphase. Multiple phosphorylated sites have been identifiedCitation84,Citation124 and the state of H1 phosphorylation is thought to be dependent on the balance of protein phosphatase I and CDC2/CDK2 kinases.Citation125 H1 phosphorylation appears to be associated with chromatin decondensation rather that chromatin condensation.Citation123 While H1 was shown to bind HP1 and to stabilize the compacted chromatin structure, its phosphorylation by CDK2 is proposed to disrupt this binding, resulting in chromatin destabilization and efficient cell-cycle progression.Citation126 Whereas N- and C-terminal tails of H1 contain consensus sequences with potential phosphorylatable sites by CDC2/CDK2 kinases, it is suggested that it is the number of phosphorylated sites that is functionally important, not the specific residues per se.Citation127
Histone phosphorylation and chromatin compaction associated with apoptosis
Several intriguing connections exist between histone phosphorylation and apoptosis. Early work demonstrated that phosphorylation of the N-terminal tail of histone H2B was essential for apoptosis-induced chromatin condensation.Citation128,Citation129 Subsequently, serine 14 was identified as the phosphorylated residue in apoptotic mammalian cells.Citation130 The corresponding phosphorylation on S10 of yeast H2B was confirmed to be essential for hydrogen peroxide (H2O2)-induced chromatin condensation and apoptosis.Citation122,Citation131 Phosphorylation of H2B S10 in yeast is mediated by the sterile 20 kinase (Ste20), while the homologous Mst1 modifies H2BS14 in mammals.Citation130,Citation131 Deletion of Ste20 abrogates H2BS10ph, increases cell survival under H2O2 treatment, as does mutation of serine 10 to non-phosphorylatable alanine. In contrast, mutation of S10 to glutamate, which mimics phosphorylation, promotes cell death and induces constitutive chromatin compaction.Citation132 Thus, H2BS10ph appears to play a key role in apoptotic chromatin condensation. While further investigation is necessary to understand the mechanisms by which this mark can induce such drastic chromatin structure changes, a number of studies have addressed the issue of its regulation. It has been demonstrated that the K11, immediately adjacent to H2BS10, can be acetylated in a Gcn5-dependent manner and is enriched in exponentially growing cells.Citation133 More recently, it was shown that H2BS10 phosphorylation is negatively regulated by the acetylation state of H2BK11, pointing to a mutually exclusive existence of these marks in the tail of H2B.Citation132 Deacetylation of H2BK11 by the Hos3 HDAC is a prerequisite for H2BS10 phosphorylation by Ste20 and for subsequent induction of apoptotic chromatin compaction.
In addition to its extensively studied role in DDR, phosphorylation of H2AXS139 in mammals was recently shown to function in apoptosis.Citation93 Notably, H2AX phosphorylation increases upon DNA fragmentation and apoptosis.Citation134,Citation135 Mst1 has been identified as the kinase responsible for apoptotic H2AX phosphorylation.Citation136 Overexpression of Mst1 induces phosphorylation of H2AXS139 in HeLa cells, accompanied by DNA fragmentation. In line with this, a study by the Pommier group found that H2BS14 phosphorylation coincides with γH2AX staining at the nuclear periphery following induction of apoptosis by death receptor agonists such as TRAIL or Fas-Ligand, or by treatment with staurosporine.Citation137 Furthermore, it was demonstrated that a defect of H2AXY142 dephosphorylation resulted in recruitment of the pro-apoptotic factor JNK1, rather than the repair apparatus, to γH2AX.Citation41 Based on such evidence, Solier et al. proposed the existence of an H2AX-H2B phosphorylation code during apoptosis in mammalian cells. They suggested that the interplay between phosphorylation of H2BS14, H2AXS139 and H2AXY142 determines the cell fate decision between repair and apoptosis, with H2BS14ph being a hallmark of the latter.Citation137
H3T45 phosphorylation was also observed to occur during apoptosis. While this site has been identified as the target of the PKCδ kinase in vitro and in vivo in normally cycling human cells,Citation138 its phosphorylation increases in apoptotic cells. This occurs concomitantly with or shortly after DNA nicking, and the kinetics of its appearance closely resembles those of caspase-3 activation. The role of H3T45ph during apoptosis is still unknown and further investigation is needed to understand whether this mark has an influence on nucleosome structure and a function in chromatin condensation during apoptosis.
Concluding Remarks
Identification and characterization of histone phosphorylation events and of the kinases that carry them out have shown that histone phosphorylation is prominently involved in various essential cellular processes associated with chromatin remodeling and gene expression (). It is interesting to observe that the same phosphorylation events can be implicated in multiple cellular processes involving chromatin modulation. For instance, H4S1ph in yeast is involved in chromatin compaction or nucleosome stabilization during such independent processes as DNA repair, transcription and sporulation. Even more remarkable is the fact that the same phosphorylated residue can have significantly distinct effects on chromatin structure depending on the context in which it occurs. Phosphorylation of H3S10 and H3S28 is a good example of this duality: both phosphorylated residues are involved in chromatin condensation associated with mitosis and meiosis, as well as in chromatin relaxation linked to transcription activation (). Such divergent roles played by these phosphorylation events make it clear that, like most other histone modifications, phosphorylation of individual residues cannot be considered in isolation. The cellular context, the crosstalk with other modifications in cis or in trans, and the local histone “code” or signature formed by diverse PTMs determine the identities of the effector proteins that are recruited to particular chromatin domains, thereby dictating the ultimate outcomes of the phosphorylation events ().
Table 1. Sites of histone phosphorylation, their known kinases and functions
Acknowledgments
We apologize to our colleagues for work that could not be cited owing to space limitations. Work in our laboratory is supported by operating grants from the Canadian Institutes of Health Research (CIHR, MOP-14308/64289). N.A. was a CIHR-Institute of Aging Fellow and J. Côté holds a Canada Research Chair.
References
- Luger K, Mäder AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature 1997; 389:251 - 60; http://dx.doi.org/10.1038/38444; PMID: 9305837
- Strahl BD, Allis CD. The language of covalent histone modifications. Nature 2000; 403:41 - 5; http://dx.doi.org/10.1038/47412; PMID: 10638745
- Kouzarides T. Chromatin modifications and their function. Cell 2007; 128:693 - 705; http://dx.doi.org/10.1016/j.cell.2007.02.005; PMID: 17320507
- Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat Struct Mol Biol 2007; 14:1008 - 16; http://dx.doi.org/10.1038/nsmb1337; PMID: 17984963
- Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Annu Rev Biochem 2011; 80:473 - 99; http://dx.doi.org/10.1146/annurev-biochem-061809-175347; PMID: 21529160
- Taverna SD, Li H, Ruthenburg AJ, Allis CD, Patel DJ. How chromatin-binding modules interpret histone modifications: lessons from professional pocket pickers. Nat Struct Mol Biol 2007; 14:1025 - 40; http://dx.doi.org/10.1038/nsmb1338; PMID: 17984965
- Yun M, Wu J, Workman JL, Li B. Readers of histone modifications. Cell Res 2011; 21:564 - 78; http://dx.doi.org/10.1038/cr.2011.42; PMID: 21423274
- Ruthenburg AJ, Li H, Patel DJ, Allis CD. Multivalent engagement of chromatin modifications by linked binding modules. Nat Rev Mol Cell Biol 2007; 8:983 - 94; http://dx.doi.org/10.1038/nrm2298; PMID: 18037899
- van Attikum H, Gasser SM. The histone code at DNA breaks: a guide to repair?. Nat Rev Mol Cell Biol 2005; 6:757 - 65; http://dx.doi.org/10.1038/nrm1737; PMID: 16167054
- Rossetto D, Truman AW, Kron SJ, Côté J. Epigenetic modifications in double-strand break DNA damage signaling and repair. Clin Cancer Res 2010; 16:4543 - 52; http://dx.doi.org/10.1158/1078-0432.CCR-10-0513; PMID: 20823147
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 1998; 273:5858 - 68; http://dx.doi.org/10.1074/jbc.273.10.5858; PMID: 9488723
- Celeste A, Fernandez-Capetillo O, Kruhlak MJ, Pilch DR, Staudt DW, Lee A, et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nat Cell Biol 2003; 5:675 - 9; http://dx.doi.org/10.1038/ncb1004; PMID: 12792649
- Downs JA, Lowndes NF, Jackson SP. A role for Saccharomyces cerevisiae histone H2A in DNA repair. Nature 2000; 408:1001 - 4; http://dx.doi.org/10.1038/35050000; PMID: 11140636
- Redon C, Pilch DR, Rogakou EP, Orr AH, Lowndes NF, Bonner WM. Yeast histone 2A serine 129 is essential for the efficient repair of checkpoint-blind DNA damage. EMBO Rep 2003; 4:678 - 84; http://dx.doi.org/10.1038/sj.embor.embor871; PMID: 12792653
- Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004; 3:959 - 67; http://dx.doi.org/10.1016/j.dnarep.2004.03.024; PMID: 15279782
- Shroff R, Arbel-Eden A, Pilch D, Ira G, Bonner WM, Petrini JH, et al. Distribution and dynamics of chromatin modification induced by a defined DNA double-strand break. Curr Biol 2004; 14:1703 - 11; http://dx.doi.org/10.1016/j.cub.2004.09.047; PMID: 15458641
- Downs JA, Allard S, Jobin-Robitaille O, Javaheri A, Auger A, Bouchard N, et al. Binding of chromatin-modifying activities to phosphorylated histone H2A at DNA damage sites. Mol Cell 2004; 16:979 - 90; http://dx.doi.org/10.1016/j.molcel.2004.12.003; PMID: 15610740
- Morrison AJ, Highland J, Krogan NJ, Arbel-Eden A, Greenblatt JF, Haber JE, et al. INO80 and gamma-H2AX interaction links ATP-dependent chromatin remodeling to DNA damage repair. Cell 2004; 119:767 - 75; http://dx.doi.org/10.1016/j.cell.2004.11.037; PMID: 15607974
- van Attikum H, Fritsch O, Hohn B, Gasser SM. Recruitment of the INO80 complex by H2A phosphorylation links ATP-dependent chromatin remodeling with DNA double-strand break repair. Cell 2004; 119:777 - 88; http://dx.doi.org/10.1016/j.cell.2004.11.033; PMID: 15607975
- Rogakou EP, Boon C, Redon C, Bonner WM. Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 1999; 146:905 - 16; http://dx.doi.org/10.1083/jcb.146.5.905; PMID: 10477747
- Massip L, Caron P, Iacovoni JS, Trouche D, Legube G. Deciphering the chromatin landscape induced around DNA double strand breaks. Cell Cycle 2010; 9:2963 - 72; http://dx.doi.org/10.4161/cc.9.15.12412; PMID: 20714222
- Iacovoni JS, Caron P, Lassadi I, Nicolas E, Massip L, Trouche D, et al. High-resolution profiling of gammaH2AX around DNA double strand breaks in the mammalian genome. EMBO J 2010; 29:1446 - 57; http://dx.doi.org/10.1038/emboj.2010.38; PMID: 20360682
- Stucki M, Clapperton JA, Mohammad D, Yaffe MB, Smerdon SJ, Jackson SP. MDC1 directly binds phosphorylated histone H2AX to regulate cellular responses to DNA double-strand breaks. Cell 2005; 123:1213 - 26; http://dx.doi.org/10.1016/j.cell.2005.09.038; PMID: 16377563
- Jungmichel S, Stucki M. MDC1: The art of keeping things in focus. Chromosoma 2010; 119:337 - 49; http://dx.doi.org/10.1007/s00412-010-0266-9; PMID: 20224865
- Bird AW, Yu DY, Pray-Grant MG, Qiu Q, Harmon KE, Megee PC, et al. Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair. Nature 2002; 419:411 - 5; http://dx.doi.org/10.1038/nature01035; PMID: 12353039
- Tamburini BA, Tyler JK. Localized histone acetylation and deacetylation triggered by the homologous recombination pathway of double-strand DNA repair. Mol Cell Biol 2005; 25:4903 - 13; http://dx.doi.org/10.1128/MCB.25.12.4903-4913.2005; PMID: 15923609
- Vidanes GM, Bonilla CY, Toczyski DP. Complicated tails: histone modifications and the DNA damage response. Cell 2005; 121:973 - 6; http://dx.doi.org/10.1016/j.cell.2005.06.013; PMID: 15989948
- Karagiannis TC, El-Osta A. Chromatin modifications and DNA double-strand breaks: the current state of play. Leukemia 2007; 21:195 - 200; http://dx.doi.org/10.1038/sj.leu.2404478; PMID: 17151702
- Javaheri A, Wysocki R, Jobin-Robitaille O, Altaf M, Côté J, Kron SJ. Yeast G1 DNA damage checkpoint regulation by H2A phosphorylation is independent of chromatin remodeling. Proc Natl Acad Sci U S A 2006; 103:13771 - 6; http://dx.doi.org/10.1073/pnas.0511192103; PMID: 16940359
- Hammet A, Magill C, Heierhorst J, Jackson SP. Rad9 BRCT domain interaction with phosphorylated H2AX regulates the G1 checkpoint in budding yeast. EMBO Rep 2007; 8:851 - 7; http://dx.doi.org/10.1038/sj.embor.7401036; PMID: 17721446
- Wysocki R, Javaheri A, Allard S, Sha F, Côté J, Kron SJ. Role of Dot1-dependent histone H3 methylation in G1 and S phase DNA damage checkpoint functions of Rad9. Mol Cell Biol 2005; 25:8430 - 43; http://dx.doi.org/10.1128/MCB.25.19.8430-8443.2005; PMID: 16166626
- Papamichos-Chronakis M, Krebs JE, Peterson CL. Interplay between Ino80 and Swr1 chromatin remodeling enzymes regulates cell cycle checkpoint adaptation in response to DNA damage. Genes Dev 2006; 20:2437 - 49; http://dx.doi.org/10.1101/gad.1440206; PMID: 16951256
- van Attikum H, Fritsch O, Gasser SM. Distinct roles for SWR1 and INO80 chromatin remodeling complexes at chromosomal double-strand breaks. EMBO J 2007; 26:4113 - 25; http://dx.doi.org/10.1038/sj.emboj.7601835; PMID: 17762868
- Keogh MC, Kim JA, Downey M, Fillingham J, Chowdhury D, Harrison JC, et al. A phosphatase complex that dephosphorylates gammaH2AX regulates DNA damage checkpoint recovery. Nature 2006; 439:497 - 501; http://dx.doi.org/10.1038/nature04384; PMID: 16299494
- Chowdhury D, Xu X, Zhong X, Ahmed F, Zhong J, Liao J, et al. A PP4-phosphatase complex dephosphorylates gamma-H2AX generated during DNA replication. Mol Cell 2008; 31:33 - 46; http://dx.doi.org/10.1016/j.molcel.2008.05.016; PMID: 18614045
- Douglas P, Zhong J, Ye R, Moorhead GB, Xu X, Lees-Miller SP. Protein phosphatase 6 interacts with the DNA-dependent protein kinase catalytic subunit and dephosphorylates gamma-H2AX. Mol Cell Biol 2010; 30:1368 - 81; http://dx.doi.org/10.1128/MCB.00741-09; PMID: 20065038
- Macůrek L, Lindqvist A, Voets O, Kool J, Vos HR, Medema RH. Wip1 phosphatase is associated with chromatin and dephosphorylates gammaH2AX to promote checkpoint inhibition. Oncogene 2010; 29:2281 - 91; http://dx.doi.org/10.1038/onc.2009.501; PMID: 20101220
- Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell 2005; 20:801 - 9; http://dx.doi.org/10.1016/j.molcel.2005.10.003; PMID: 16310392
- Nakada S, Chen GI, Gingras AC, Durocher D. PP4 is a gamma H2AX phosphatase required for recovery from the DNA damage checkpoint. EMBO Rep 2008; 9:1019 - 26; http://dx.doi.org/10.1038/embor.2008.162; PMID: 18758438
- Xiao A, Li H, Shechter D, Ahn SH, Fabrizio LA, Erdjument-Bromage H, et al. WSTF regulates the H2A.X DNA damage response via a novel tyrosine kinase activity. Nature 2009; 457:57 - 62; http://dx.doi.org/10.1038/nature07668; PMID: 19092802
- Cook PJ, Ju BG, Telese F, Wang X, Glass CK, Rosenfeld MG. Tyrosine dephosphorylation of H2AX modulates apoptosis and survival decisions. Nature 2009; 458:591 - 6; http://dx.doi.org/10.1038/nature07849; PMID: 19234442
- Cheung WL, Turner FB, Krishnamoorthy T, Wolner B, Ahn SH, Foley M, et al. Phosphorylation of histone H4 serine 1 during DNA damage requires casein kinase II in S. cerevisiae. Curr Biol 2005; 15:656 - 60; http://dx.doi.org/10.1016/j.cub.2005.02.049; PMID: 15823538
- Utley RT, Lacoste N, Jobin-Robitaille O, Allard S, Côté J. Regulation of NuA4 histone acetyltransferase activity in transcription and DNA repair by phosphorylation of histone H4. Mol Cell Biol 2005; 25:8179 - 90; http://dx.doi.org/10.1128/MCB.25.18.8179-8190.2005; PMID: 16135807
- Ruiz-Carrillo A, Wangh LJ, Allfrey VG. Processing of newly synthesized histone molecules. Science 1975; 190:117 - 28; http://dx.doi.org/10.1126/science.1166303; PMID: 1166303
- Wyatt HR, Liaw H, Green GR, Lustig AJ. Multiple roles for Saccharomyces cerevisiae histone H2A in telomere position effect, Spt phenotypes and double-strand-break repair. Genetics 2003; 164:47 - 64; PMID: 12750320
- Chambers AL, Downs JA. The contribution of the budding yeast histone H2A C-terminal tail to DNA-damage responses. Biochem Soc Trans 2007; 35:1519 - 24; http://dx.doi.org/10.1042/BST0351519; PMID: 18031258
- Harvey AC, Jackson SP, Downs JA. Saccharomyces cerevisiae histone H2A Ser122 facilitates DNA repair. Genetics 2005; 170:543 - 53; http://dx.doi.org/10.1534/genetics.104.038570; PMID: 15781691
- Moore JD, Yazgan O, Ataian Y, Krebs JE. Diverse roles for histone H2A modifications in DNA damage response pathways in yeast. Genetics 2007; 176:15 - 25; http://dx.doi.org/10.1534/genetics.106.063792; PMID: 17028320
- Fernandez-Capetillo O, Allis CD, Nussenzweig A. Phosphorylation of histone H2B at DNA double-strand breaks. J Exp Med 2004; 199:1671 - 7; http://dx.doi.org/10.1084/jem.20032247; PMID: 15197225
- Lau AT, Lee SY, Xu YM, Zheng D, Cho YY, Zhu F, et al. Phosphorylation of histone H2B serine 32 is linked to cell transformation. J Biol Chem 2011; 286:26628 - 37; http://dx.doi.org/10.1074/jbc.M110.215590; PMID: 21646345
- Chadee DN, Hendzel MJ, Tylipski CP, Allis CD, Bazett-Jones DP, Wright JA, et al. Increased Ser-10 phosphorylation of histone H3 in mitogen-stimulated and oncogene-transformed mouse fibroblasts. J Biol Chem 1999; 274:24914 - 20; http://dx.doi.org/10.1074/jbc.274.35.24914; PMID: 10455166
- Choi HS, Choi BY, Cho YY, Mizuno H, Kang BS, Bode AM, et al. Phosphorylation of histone H3 at serine 10 is indispensable for neoplastic cell transformation. Cancer Res 2005; 65:5818 - 27; http://dx.doi.org/10.1158/0008-5472.CAN-05-0197; PMID: 15994958
- Lau PN, Cheung P. Histone code pathway involving H3 S28 phosphorylation and K27 acetylation activates transcription and antagonizes polycomb silencing. Proc Natl Acad Sci U S A 2011; 108:2801 - 6; http://dx.doi.org/10.1073/pnas.1012798108; PMID: 21282660
- Metzger E, Yin N, Wissmann M, Kunowska N, Fischer K, Friedrichs N, et al. Phosphorylation of histone H3 at threonine 11 establishes a novel chromatin mark for transcriptional regulation. Nat Cell Biol 2008; 10:53 - 60; http://dx.doi.org/10.1038/ncb1668; PMID: 18066052
- Metzger E, Imhof A, Patel D, Kahl P, Hoffmeyer K, Friedrichs N, et al. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature 2010; 464:792 - 6; http://dx.doi.org/10.1038/nature08839; PMID: 20228790
- Shimada M, Niida H, Zineldeen DH, Tagami H, Tanaka M, Saito H, et al. Chk1 is a histone H3 threonine 11 kinase that regulates DNA damage-induced transcriptional repression. Cell 2008; 132:221 - 32; http://dx.doi.org/10.1016/j.cell.2007.12.013; PMID: 18243098
- Lo WS, Trievel RC, Rojas JR, Duggan L, Hsu JY, Allis CD, et al. Phosphorylation of serine 10 in histone H3 is functionally linked in vitro and in vivo to Gcn5-mediated acetylation at lysine 14. Mol Cell 2000; 5:917 - 26; http://dx.doi.org/10.1016/S1097-2765(00)80257-9; PMID: 10911986
- Clements A, Poux AN, Lo WS, Pillus L, Berger SL, Marmorstein R. Structural basis for histone and phosphohistone binding by the GCN5 histone acetyltransferase. Mol Cell 2003; 12:461 - 73; http://dx.doi.org/10.1016/S1097-2765(03)00288-0; PMID: 14536085
- Zhong S, Goto H, Inagaki M, Dong Z. Phosphorylation at serine 28 and acetylation at lysine 9 of histone H3 induced by trichostatin A. Oncogene 2003; 22:5291 - 7; http://dx.doi.org/10.1038/sj.onc.1206507; PMID: 12917630
- Cheung P, Tanner KG, Cheung WL, Sassone-Corsi P, Denu JM, Allis CD. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol Cell 2000; 5:905 - 15; http://dx.doi.org/10.1016/S1097-2765(00)80256-7; PMID: 10911985
- Clayton AL, Rose S, Barratt MJ, Mahadevan LC. Phosphoacetylation of histone H3 on c-fos- and c-jun-associated nucleosomes upon gene activation. EMBO J 2000; 19:3714 - 26; http://dx.doi.org/10.1093/emboj/19.14.3714; PMID: 10899125
- Lo WS, Duggan L, Emre NC, Belotserkovskya R, Lane WS, Shiekhattar R, et al. Snf1--a histone kinase that works in concert with the histone acetyltransferase Gcn5 to regulate transcription. Science 2001; 293:1142 - 6; http://dx.doi.org/10.1126/science.1062322; PMID: 11498592
- Soloaga A, Thomson S, Wiggin GR, Rampersaud N, Dyson MH, Hazzalin CA, et al. MSK2 and MSK1 mediate the mitogen- and stress-induced phosphorylation of histone H3 and HMG-14. EMBO J 2003; 22:2788 - 97; http://dx.doi.org/10.1093/emboj/cdg273; PMID: 12773393
- Gehani SS, Agrawal-Singh S, Dietrich N, Christophersen NS, Helin K, Hansen K. Polycomb group protein displacement and gene activation through MSK-dependent H3K27me3S28 phosphorylation. Mol Cell 2010; 39:886 - 900; http://dx.doi.org/10.1016/j.molcel.2010.08.020; PMID: 20864036
- Cho YY, Yao K, Pugliese A, Malakhova ML, Bode AM, Dong Z. A regulatory mechanism for RSK2 NH(2)-terminal kinase activity. Cancer Res 2009; 69:4398 - 406; http://dx.doi.org/10.1158/0008-5472.CAN-08-4959; PMID: 19435896
- Sassone-Corsi P, Mizzen CA, Cheung P, Crosio C, Monaco L, Jacquot S, et al. Requirement of Rsk-2 for epidermal growth factor-activated phosphorylation of histone H3. Science 1999; 285:886 - 91; http://dx.doi.org/10.1126/science.285.5429.886; PMID: 10436156
- Thomson S, Clayton AL, Hazzalin CA, Rose S, Barratt MJ, Mahadevan LC. The nucleosomal response associated with immediate-early gene induction is mediated via alternative MAP kinase cascades: MSK1 as a potential histone H3/HMG-14 kinase. EMBO J 1999; 18:4779 - 93; http://dx.doi.org/10.1093/emboj/18.17.4779; PMID: 10469656
- Choi HS, Choi BY, Cho YY, Zhu F, Bode AM, Dong Z. Phosphorylation of Ser28 in histone H3 mediated by mixed lineage kinase-like mitogen-activated protein triple kinase alpha. J Biol Chem 2005; 280:13545 - 53; http://dx.doi.org/10.1074/jbc.M410521200; PMID: 15684425
- Zhong SP, Ma WY, Dong Z. ERKs and p38 kinases mediate ultraviolet B-induced phosphorylation of histone H3 at serine 10. J Biol Chem 2000; 275:20980 - 4; http://dx.doi.org/10.1074/jbc.M909934199; PMID: 10806218
- He Z, Cho YY, Ma WY, Choi HS, Bode AM, Dong Z. Regulation of ultraviolet B-induced phosphorylation of histone H3 at serine 10 by Fyn kinase. J Biol Chem 2005; 280:2446 - 54; http://dx.doi.org/10.1074/jbc.M402053200; PMID: 15537652
- Zhong S, Zhang Y, Jansen C, Goto H, Inagaki M, Dong Z. MAP kinases mediate UVB-induced phosphorylation of histone H3 at serine 28. J Biol Chem 2001; 276:12932 - 7; http://dx.doi.org/10.1074/jbc.M010931200; PMID: 11278789
- Zhong S, Jansen C, She QB, Goto H, Inagaki M, Bode AM, et al. Ultraviolet B-induced phosphorylation of histone H3 at serine 28 is mediated by MSK1. J Biol Chem 2001; 276:33213 - 9; http://dx.doi.org/10.1074/jbc.M103973200; PMID: 11441012
- Yang W, Xia Y, Hawke D, Li X, Liang J, Xing D, et al. PKM2 Phosphorylates Histone H3 and Promotes Gene Transcription and Tumorigenesis. Cell 2012; 150:685 - 96; http://dx.doi.org/10.1016/j.cell.2012.07.018; PMID: 22901803
- Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature 2009; 461:819 - 22; http://dx.doi.org/10.1038/nature08448; PMID: 19783980
- Bungard D, Fuerth BJ, Zeng PY, Faubert B, Maas NL, Viollet B, et al. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science 2010; 329:1201 - 5; http://dx.doi.org/10.1126/science.1191241; PMID: 20647423
- Mahajan K, Fang B, Koomen JM, Mahajan NP. H2B Tyr37 phosphorylation suppresses expression of replication-dependent core histone genes. Nat Struct Mol Biol 2012; In press http://dx.doi.org/10.1038/nsmb.2356; PMID: 22885324
- Cheung V, Chua G, Batada NN, Landry CR, Michnick SW, Hughes TR, et al. Chromatin- and transcription-related factors repress transcription from within coding regions throughout the Saccharomyces cerevisiae genome. PLoS Biol 2008; 6:e277; http://dx.doi.org/10.1371/journal.pbio.0060277; PMID: 18998772
- Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell 2007; 128:707 - 19; http://dx.doi.org/10.1016/j.cell.2007.01.015; PMID: 17320508
- Friesen H, Lunz R, Doyle S, Segall J. Mutation of the SPS1-encoded protein kinase of Saccharomyces cerevisiae leads to defects in transcription and morphology during spore formation. Genes Dev 1994; 8:2162 - 75; http://dx.doi.org/10.1101/gad.8.18.2162; PMID: 7958886
- Krishnamoorthy T, Chen X, Govin J, Cheung WL, Dorsey J, Schindler K, et al. Phosphorylation of histone H4 Ser1 regulates sporulation in yeast and is conserved in fly and mouse spermatogenesis. Genes Dev 2006; 20:2580 - 92; http://dx.doi.org/10.1101/gad.1457006; PMID: 16980586
- Govin J, Schug J, Krishnamoorthy T, Dorsey J, Khochbin S, Berger SL. Genome-wide mapping of histone H4 serine-1 phosphorylation during sporulation in Saccharomyces cerevisiae. Nucleic Acids Res 2010; 38:4599 - 606; http://dx.doi.org/10.1093/nar/gkq218; PMID: 20375100
- Pietersen AM, van Lohuizen M. Stem cell regulation by polycomb repressors: postponing commitment. Curr Opin Cell Biol 2008; 20:201 - 7; http://dx.doi.org/10.1016/j.ceb.2008.01.004; PMID: 18291635
- Kang B, Pu M, Hu G, Wen W, Dong Z, Zhao K, et al. Phosphorylation of H4 Ser 47 promotes HIRA-mediated nucleosome assembly. Genes Dev 2011; 25:1359 - 64; http://dx.doi.org/10.1101/gad.2055511; PMID: 21724829
- Deterding LJ, Bunger MK, Banks GC, Tomer KB, Archer TK. Global changes in and characterization of specific sites of phosphorylation in mouse and human histone H1 Isoforms upon CDK inhibitor treatment using mass spectrometry. J Proteome Res 2008; 7:2368 - 79; http://dx.doi.org/10.1021/pr700790a; PMID: 18416567
- Caterino TL, Hayes JJ. Structure of the H1 C-terminal domain and function in chromatin condensation. Biochem Cell Biol 2011; 89:35 - 44; http://dx.doi.org/10.1139/O10-024; PMID: 21326361
- Dou Y, Mizzen CA, Abrams M, Allis CD, Gorovsky MA. Phosphorylation of linker histone H1 regulates gene expression in vivo by mimicking H1 removal. Mol Cell 1999; 4:641 - 7; http://dx.doi.org/10.1016/S1097-2765(00)80215-4; PMID: 10549296
- Zheng Y, John S, Pesavento JJ, Schultz-Norton JR, Schiltz RL, Baek S, et al. Histone H1 phosphorylation is associated with transcription by RNA polymerases I and II. J Cell Biol 2010; 189:407 - 15; http://dx.doi.org/10.1083/jcb.201001148; PMID: 20439994
- Lee HL, Archer TK. Prolonged glucocorticoid exposure dephosphorylates histone H1 and inactivates the MMTV promoter. EMBO J 1998; 17:1454 - 66; http://dx.doi.org/10.1093/emboj/17.5.1454; PMID: 9482742
- Koop R, Di Croce L, Beato M. Histone H1 enhances synergistic activation of the MMTV promoter in chromatin. EMBO J 2003; 22:588 - 99; http://dx.doi.org/10.1093/emboj/cdg052; PMID: 12554659
- Wei Y, Mizzen CA, Cook RG, Gorovsky MA, Allis CD. Phosphorylation of histone H3 at serine 10 is correlated with chromosome condensation during mitosis and meiosis in Tetrahymena. Proc Natl Acad Sci U S A 1998; 95:7480 - 4; http://dx.doi.org/10.1073/pnas.95.13.7480; PMID: 9636175
- Sauvé DM, Anderson HJ, Ray JM, James WM, Roberge M. Phosphorylation-induced rearrangement of the histone H3 NH2-terminal domain during mitotic chromosome condensation. J Cell Biol 1999; 145:225 - 35; http://dx.doi.org/10.1083/jcb.145.2.225; PMID: 10209020
- de la Barre AE, Gerson V, Gout S, Creaven M, Allis CD, Dimitrov S. Core histone N-termini play an essential role in mitotic chromosome condensation. EMBO J 2000; 19:379 - 91; http://dx.doi.org/10.1093/emboj/19.3.379; PMID: 10654937
- Wei Y, Yu L, Bowen J, Gorovsky MA, Allis CD. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell 1999; 97:99 - 109; http://dx.doi.org/10.1016/S0092-8674(00)80718-7; PMID: 10199406
- Goto H, Tomono Y, Ajiro K, Kosako H, Fujita M, Sakurai M, et al. Identification of a novel phosphorylation site on histone H3 coupled with mitotic chromosome condensation. J Biol Chem 1999; 274:25543 - 9; http://dx.doi.org/10.1074/jbc.274.36.25543; PMID: 10464286
- Dunn KL, Davie JR. Stimulation of the Ras-MAPK pathway leads to independent phosphorylation of histone H3 on serine 10 and 28. Oncogene 2005; 24:3492 - 502; http://dx.doi.org/10.1038/sj.onc.1208521; PMID: 15735677
- Dyson MH, Thomson S, Inagaki M, Goto H, Arthur SJ, Nightingale K, et al. MAP kinase-mediated phosphorylation of distinct pools of histone H3 at S10 or S28 via mitogen- and stress-activated kinase 1/2. J Cell Sci 2005; 118:2247 - 59; http://dx.doi.org/10.1242/jcs.02373; PMID: 15870105
- Goto H, Yasui Y, Nigg EA, Inagaki M. Aurora-B phosphorylates Histone H3 at serine28 with regard to the mitotic chromosome condensation. Genes Cells 2002; 7:11 - 7; http://dx.doi.org/10.1046/j.1356-9597.2001.00498.x; PMID: 11856369
- Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell 2000; 102:279 - 91; http://dx.doi.org/10.1016/S0092-8674(00)00034-9; PMID: 10975519
- Gopalan G, Chan CS, Donovan PJ. A novel mammalian, mitotic spindle-associated kinase is related to yeast and fly chromosome segregation regulators. J Cell Biol 1997; 138:643 - 56; http://dx.doi.org/10.1083/jcb.138.3.643; PMID: 9245792
- Bischoff JR, Anderson L, Zhu Y, Mossie K, Ng L, Souza B, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. EMBO J 1998; 17:3052 - 65; http://dx.doi.org/10.1093/emboj/17.11.3052; PMID: 9606188
- Tatsuka M, Katayama H, Ota T, Tanaka T, Odashima S, Suzuki F, et al. Multinuclearity and increased ploidy caused by overexpression of the aurora- and Ipl1-like midbody-associated protein mitotic kinase in human cancer cells. Cancer Res 1998; 58:4811 - 6; PMID: 9809983
- Minc E, Allory Y, Worman HJ, Courvalin JC, Buendia B. Localization and phosphorylation of HP1 proteins during the cell cycle in mammalian cells. Chromosoma 1999; 108:220 - 34; http://dx.doi.org/10.1007/s004120050372; PMID: 10460410
- Fischle W, Tseng BS, Dormann HL, Ueberheide BM, Garcia BA, Shabanowitz J, et al. Regulation of HP1-chromatin binding by histone H3 methylation and phosphorylation. Nature 2005; 438:1116 - 22; http://dx.doi.org/10.1038/nature04219; PMID: 16222246
- Polioudaki H, Markaki Y, Kourmouli N, Dialynas G, Theodoropoulos PA, Singh PB, et al. Mitotic phosphorylation of histone H3 at threonine 3. FEBS Lett 2004; 560:39 - 44; http://dx.doi.org/10.1016/S0014-5793(04)00060-2; PMID: 14987995
- Dai J, Higgins JM. Haspin: a mitotic histone kinase required for metaphase chromosome alignment. Cell Cycle 2005; 4:665 - 8; http://dx.doi.org/10.4161/cc.4.5.1683; PMID: 15846065
- Dai J, Sultan S, Taylor SS, Higgins JM. The kinase haspin is required for mitotic histone H3 Thr 3 phosphorylation and normal metaphase chromosome alignment. Genes Dev 2005; 19:472 - 88; http://dx.doi.org/10.1101/gad.1267105; PMID: 15681610
- Ashtiyani RK, Moghaddam AM, Schubert V, Rutten T, Fuchs J, Demidov D, et al. AtHaspin phosphorylates histone H3 at threonine 3 during mitosis and contributes to embryonic patterning in Arabidopsis. Plant J 2011; 68:443 - 54; http://dx.doi.org/10.1111/j.1365-313X.2011.04699.x; PMID: 21749502
- Kurihara D, Matsunaga S, Omura T, Higashiyama T, Fukui K. Identification and characterization of plant Haspin kinase as a histone H3 threonine kinase. BMC Plant Biol 2011; 11:73; http://dx.doi.org/10.1186/1471-2229-11-73; PMID: 21527018
- Dai J, Sullivan BA, Higgins JM. Regulation of mitotic chromosome cohesion by Haspin and Aurora B. Dev Cell 2006; 11:741 - 50; http://dx.doi.org/10.1016/j.devcel.2006.09.018; PMID: 17084365
- Yamagishi Y, Honda T, Tanno Y, Watanabe Y. Two histone marks establish the inner centromere and chromosome bi-orientation. Science 2010; 330:239 - 43; http://dx.doi.org/10.1126/science.1194498; PMID: 20929775
- Kelly AE, Ghenoiu C, Xue JZ, Zierhut C, Kimura H, Funabiki H. Survivin reads phosphorylated histone H3 threonine 3 to activate the mitotic kinase Aurora B. Science 2010; 330:235 - 9; http://dx.doi.org/10.1126/science.1189505; PMID: 20705815
- Wang F, Dai J, Daum JR, Niedzialkowska E, Banerjee B, Stukenberg PT, et al. Histone H3 Thr-3 phosphorylation by Haspin positions Aurora B at centromeres in mitosis. Science 2010; 330:231 - 5; http://dx.doi.org/10.1126/science.1189435; PMID: 20705812
- Jeyaprakash AA, Basquin C, Jayachandran U, Conti E. Structural basis for the recognition of phosphorylated histone h3 by the survivin subunit of the chromosomal passenger complex. Structure 2011; 19:1625 - 34; http://dx.doi.org/10.1016/j.str.2011.09.002; PMID: 22032967
- Wang F, Ulyanova NP, van der Waal MS, Patnaik D, Lens SM, Higgins JM. A positive feedback loop involving Haspin and Aurora B promotes CPC accumulation at centromeres in mitosis. Curr Biol 2011; 21:1061 - 9; http://dx.doi.org/10.1016/j.cub.2011.05.016; PMID: 21658950
- Kawashima SA, Yamagishi Y, Honda T, Ishiguro K, Watanabe Y. Phosphorylation of H2A by Bub1 prevents chromosomal instability through localizing shugoshin. Science 2010; 327:172 - 7; http://dx.doi.org/10.1126/science.1180189; PMID: 19965387
- Ivanovska I, Khandan T, Ito T, Orr-Weaver TL. A histone code in meiosis: the histone kinase, NHK-1, is required for proper chromosomal architecture in Drosophila oocytes. Genes Dev 2005; 19:2571 - 82; http://dx.doi.org/10.1101/gad.1348905; PMID: 16230526
- Aihara H, Nakagawa T, Yasui K, Ohta T, Hirose S, Dhomae N, et al. Nucleosomal histone kinase-1 phosphorylates H2A Thr 119 during mitosis in the early Drosophila embryo. Genes Dev 2004; 18:877 - 88; http://dx.doi.org/10.1101/gad.1184604; PMID: 15078818
- Markaki Y, Christogianni A, Politou AS, Georgatos SD. Phosphorylation of histone H3 at Thr3 is part of a combinatorial pattern that marks and configures mitotic chromatin. J Cell Sci 2009; 122:2809 - 19; http://dx.doi.org/10.1242/jcs.043810; PMID: 19622635
- Preuss U, Landsberg G, Scheidtmann KH. Novel mitosis-specific phosphorylation of histone H3 at Thr11 mediated by Dlk/ZIP kinase. Nucleic Acids Res 2003; 31:878 - 85; http://dx.doi.org/10.1093/nar/gkg176; PMID: 12560483
- Houben A, Demidov D, Rutten T, Scheidtmann KH. Novel phosphorylation of histone H3 at threonine 11 that temporally correlates with condensation of mitotic and meiotic chromosomes in plant cells. Cytogenet Genome Res 2005; 109:148 - 55; http://dx.doi.org/10.1159/000082394; PMID: 15753571
- Govin J, Dorsey J, Gaucher J, Rousseaux S, Khochbin S, Berger SL. Systematic screen reveals new functional dynamics of histones H3 and H4 during gametogenesis. Genes Dev 2010; 24:1772 - 86; http://dx.doi.org/10.1101/gad.1954910; PMID: 20713519
- Ahn SH, Henderson KA, Keeney S, Allis CD. H2B (Ser10) phosphorylation is induced during apoptosis and meiosis in S. cerevisiae. Cell Cycle 2005; 4:780 - 3; http://dx.doi.org/10.4161/cc.4.6.1745; PMID: 15970663
- Roth SY, Allis CD. Chromatin condensation: does histone H1 dephosphorylation play a role?. Trends Biochem Sci 1992; 17:93 - 8; http://dx.doi.org/10.1016/0968-0004(92)90243-3; PMID: 1412698
- Sarg B, Helliger W, Talasz H, Förg B, Lindner HH. Histone H1 phosphorylation occurs site-specifically during interphase and mitosis: identification of a novel phosphorylation site on histone H1. J Biol Chem 2006; 281:6573 - 80; http://dx.doi.org/10.1074/jbc.M508957200; PMID: 16377619
- Paulson JR, Patzlaff JS, Vallis AJ. Evidence that the endogenous histone H1 phosphatase in HeLa mitotic chromosomes is protein phosphatase 1, not protein phosphatase 2A. J Cell Sci 1996; 109:1437 - 47; PMID: 8799831
- Hale TK, Contreras A, Morrison AJ, Herrera RE. Phosphorylation of the linker histone H1 by CDK regulates its binding to HP1alpha. Mol Cell 2006; 22:693 - 9; http://dx.doi.org/10.1016/j.molcel.2006.04.016; PMID: 16762841
- Dou Y, Gorovsky MA. Phosphorylation of linker histone H1 regulates gene expression in vivo by creating a charge patch. Mol Cell 2000; 6:225 - 31; http://dx.doi.org/10.1016/S1097-2765(00)00024-1; PMID: 10983971
- Ajiro K. Histone H2B phosphorylation in mammalian apoptotic cells. An association with DNA fragmentation. J Biol Chem 2000; 275:439 - 43; http://dx.doi.org/10.1074/jbc.275.1.439; PMID: 10617636
- de la Barre AE, Angelov D, Molla A, Dimitrov S. The N-terminus of histone H2B, but not that of histone H3 or its phosphorylation, is essential for chromosome condensation. EMBO J 2001; 20:6383 - 93; http://dx.doi.org/10.1093/emboj/20.22.6383; PMID: 11707409
- Cheung WL, Ajiro K, Samejima K, Kloc M, Cheung P, Mizzen CA, et al. Apoptotic phosphorylation of histone H2B is mediated by mammalian sterile twenty kinase. Cell 2003; 113:507 - 17; http://dx.doi.org/10.1016/S0092-8674(03)00355-6; PMID: 12757711
- Ahn SH, Cheung WL, Hsu JY, Diaz RL, Smith MM, Allis CD. Sterile 20 kinase phosphorylates histone H2B at serine 10 during hydrogen peroxide-induced apoptosis in S. cerevisiae. Cell 2005; 120:25 - 36; http://dx.doi.org/10.1016/j.cell.2004.11.016; PMID: 15652479
- Ahn SH, Diaz RL, Grunstein M, Allis CD. Histone H2B deacetylation at lysine 11 is required for yeast apoptosis induced by phosphorylation of H2B at serine 10. Mol Cell 2006; 24:211 - 20; http://dx.doi.org/10.1016/j.molcel.2006.09.008; PMID: 17052455
- Suka N, Suka Y, Carmen AA, Wu J, Grunstein M. Highly specific antibodies determine histone acetylation site usage in yeast heterochromatin and euchromatin. Mol Cell 2001; 8:473 - 9; http://dx.doi.org/10.1016/S1097-2765(01)00301-X; PMID: 11545749
- Rogakou EP, Nieves-Neira W, Boon C, Pommier Y, Bonner WM. Initiation of DNA fragmentation during apoptosis induces phosphorylation of H2AX histone at serine 139. J Biol Chem 2000; 275:9390 - 5; http://dx.doi.org/10.1074/jbc.275.13.9390; PMID: 10734083
- Mukherjee B, Kessinger C, Kobayashi J, Chen BP, Chen DJ, Chatterjee A, et al. DNA-PK phosphorylates histone H2AX during apoptotic DNA fragmentation in mammalian cells. DNA Repair (Amst) 2006; 5:575 - 90; http://dx.doi.org/10.1016/j.dnarep.2006.01.011; PMID: 16567133
- Wen W, Zhu F, Zhang J, Keum YS, Zykova T, Yao K, et al. MST1 promotes apoptosis through phosphorylation of histone H2AX. J Biol Chem 2010; 285:39108 - 16; http://dx.doi.org/10.1074/jbc.M110.151753; PMID: 20921231
- Solier S, Sordet O, Kohn KW, Pommier Y. Death receptor-induced activation of the Chk2- and histone H2AX-associated DNA damage response pathways. Mol Cell Biol 2009; 29:68 - 82; http://dx.doi.org/10.1128/MCB.00581-08; PMID: 18955500
- Hurd PJ, Bannister AJ, Halls K, Dawson MA, Vermeulen M, Olsen JV, et al. Phosphorylation of histone H3 Thr-45 is linked to apoptosis. J Biol Chem 2009; 284:16575 - 83; http://dx.doi.org/10.1074/jbc.M109.005421; PMID: 19363025
- Liokatis S, Stützer A, Elsässer SJ, Theillet FX, Klingberg R, van Rossum B, et al. Phosphorylation of histone H3 Ser10 establishes a hierarchy for subsequent intramolecular modification events. Nat Struct Mol Biol 2012; 19:819 - 23; http://dx.doi.org/10.1038/nsmb.2310; PMID: 22796964