Abstract
Non-small-cell lung cancer (NSCLC) represents approximately 80% of all types of lung cancer. Here, we report the chemotherapeutic effect of honokiol, a phytochemical from Magnolia grandiflora, on NSCLC cells and the molecular mechanisms underlying these effects using in vitro and in vivo models. Treatment of NSCLC cells (A549, H1299, H460 and H226) with honokiol (20, 40 and 60 µM) inhibited histone deacetylase (HDAC) activity, reduced the levels of class I HDAC proteins and enhanced histone acetyltransferase activity in a dose-dependent manner. These effects of honokiol were associated with a significant reduction in the viability of NSCLC cells. Concomitant treatment of cells with a proteasome inhibitor, MG132, prevented honokiol-induced degradation of class I HDACs, suggesting that honokiol reduced the levels of HDACs in NSCLC cells through proteasomal degradation. Valproic acid, an inhibitor of HDACs, exhibited a similar pattern of reduced viability and induction of death of NSCLC cells. Treatment of A549 and H1299 cells with honokiol resulted in an increase in G1 phase arrest, and a decrease in the levels of cyclin D1, D2 and cyclin dependent kinases. Further, administration of honokiol by oral gavage significantly inhibited the growth of subcutaneous A549 and H1299 tumor xenografts in athymic nude mice, which was associated with the induction of apoptotic cell death and marked inhibition of class I HDACs proteins and HDAC activity in the tumor xenograft tissues. Together, our study provides new insights into the role of class I HDACs in the chemotherapeutic effects of honokiol on lung cancer cells.
Introduction
Histone deacetylases (HDACs) catalyze deacetylation of histones by cleavage of acetyl groups. Typically, this results in a compact chromatin configuration that restricts access of transcription factors to DNA and represses gene expression. In contrast, histone acetyltransferases (HATs) catalyze histone acetylation by neutralizing positive charges and facilitating the binding of transcription factors to the nucleosomal DNA of core histones.Citation1,Citation2 HDACs regulate many biologic processes, including cell cycle progression and cell differentiation.Citation1 Four classes are recognized: class I (HDACs 1–3 and 8), class II (HDACs 4–7, 9 and 10), class III (Sirt1‒Sirt7) and class IV (HDAC 11). Class I HDACs are frequently overexpressed in various human cancers and this overexpression correlates with drug resistance and poor prognosis. Thus, class I HDACs have been considered as potential therapeutic targets for the treatment of cancersCitation2,Citation3 and some promising HDAC inhibitors (HDACi) have been identified. The HDACi can inhibit tumor progression primarily through their ability to regulate gene expression by promoting acetylation of histone and non-histone proteins.Citation4,Citation5 HDACi modulate the expression of several genes that regulate apoptosis, angiogenesis,Citation6,Citation7 cell cycle progression, and cellular differentiation, and have minimal toxicity against normal cells.Citation8-Citation10 Some synthetic HDACi such as vorinostat (SAHA), LBH589, PXD101, MS-275, and FK228, are being examined in clinical trials for their ability to treat various solid and hematological malignancies.Citation11,Citation12 The results of these trials suggest that although HDACi exhibit selective toxicity against tumor cells, their long-term use in patients may result in immune suppression, fatigue and gastrointestinal side effects.Citation11
Some natural plant products have been shown to have anti-carcinogenic effects in multiple animal tumor models and the phytochemicals that have anti-carcinogenic activity and have no significant toxicity in vivo are being investigated as potentially effective chemotherapeutic agents for the prevention and treatment of cancers. The potential of some of these phytochemicals to act as epigenetic regulators, including their effects on histone modifications and HDAC has been investigated.Citation13-Citation15 Honokiol (C18H18O2) is a bioactive constituent of the bark of Magnolia plants that has been used in traditional Japanese medicine for the treatment of various ailments due to its antithrombotic, antidepressant and anti-bacterial properties.Citation16 Previously, we have shown that honokiol exerts a chemopreventive effect on UV radiation-induced skin cancer and that this effect is associated with its targeting of mediators of inflammation and cell cycle regulators.Citation17 The anti-cancer activities of honokiol also have been demonstrated in a variety of cancer cell linesCitation18-Citation22 and animal tumor xenograft models.Citation23 Honokiol has been found to alter various molecular targets that are known to affect tumor cell growth and their survival; however, little is known as to whether honokiol targets alterations in epigenetic regulators in cancer or targets events subsequent to the epigenetic effects. As, it is well known that epigenetic alterations, in particular overexpression of class I HDACs, play a crucial role in carcinogenesis, we sought to determine the chemotherapeutic effect of honokiol on lung cancer cells and whether it is mediated through its effect on HDACs proteins.
To address this issue, we investigated whether honokiol has the ability to suppress the levels of class I HDAC and their activity in human non-small cell lung cancer (NSCLC) cells and whether this effect is associated with its effects on cell growth/viability, cell cycle regulation and apoptosis using in vitro and in vivo models. Lung cancer remains the leading cause of cancer-related deaths in the United States and world-wide.Citation24 One of every three cancer-related deaths is attributable to lung cancer, and the dismal 5-y survival rate of about 14% has shown no improvement over the past three decades.Citation25,Citation26 NSCLC represents approximately 80% of all types of lung cancer and includes adenocarcinomas, large-cell carcinomas and squamous cell carcinomas.Citation27,Citation28 Therefore, the exploration and development of new and effective phytochemicals that are non-toxic in nature and that can target the molecules associated with epigenetic regulators could lead to substantially improved outcomes in patients with this type of cancer.
Here, we report that treatment of NSCLC cells with honokiol suppresses the levels of class I HADC proteins as well as HDAC activity while enhancing HAT activity and that these effects are associated with reduced cell viability, G1 phase arrest and induction of apoptosis of cells in vitro and in vivo in a tumor xenograft model. Thus, our studies provide evidence that honokiol has the ability to inhibit the growth of lung cancer by targeting epigenetic modulators.
Results
Comparative analysis of basal levels of HDAC and HAT activities in NSCLC cell lines
First we assessed the levels of HDAC and HAT activities in various NSCLC cell lines and normal human bronchial epithelial cells (BEAS-2B). Using the HDAC Activity Assay Kit, we found that the levels of HDAC activity were greater in the cultured NSCLC cells as compared with the BEAS-2B cells. The H226 cells had the greatest activity, followed by H460 > H1299 > A549, as shown in (left panel). On analysis of the levels of HAT activity in the cell lines using the EpiQuikTM HAT Activity Assay Kit, we found that the levels of HAT activity were lower in the NSCLC cell lines as compared with BEAS-2B cells. In this case, the A459 and H1299 cells had the greatest activity followed by the H460 and H226 cells as shown in (right panel).
Figure 1. Treatment of NSCLC cells with honokiol reduces the levels of HDAC activity while increasing HAT activity. (A) Comparative analysis of basal levels of HDAC and HAT activity in four different NSCLC cell lines and non-neoplastic BEAS-2B cells using colorimetric assay kits. (B) A549 and H1299 cells were treated with various concentrations of honokiol (0, 20, 40 and 60 µM) or TSA (100 nm) for 24 or 72 h. Total HDAC activity was determined in nuclear extracts of the cells. Cells treated with TSA, an inhibitor of HDACs, served as a positive control. (C) Treatment of A549 and H1299 cells with honokiol for 72 h enhanced HAT activity in a dose-dependent manner. Data are expressed in terms of percent of control as the mean ± SD of 4 replicates. Significant difference vs. non-honokiol-treated control, ¶p < 0.001, †p < 0.01. (D) Treatment of cells with honokiol for 72 h reduces the expression levels of class l HDACs proteins. After treatment for 72 h, cells were harvested, nuclear extracts were prepared and subjected to western blot analysis. Histone H3 was used as a loading control. Representative blots are shown. The relative intensity (arbitrary) of each band after normalization for histone H3 is shown under each blot as the fold change compared with non-honokiol-treated control, which was assigned an arbitrary unit 1.0 in each case.
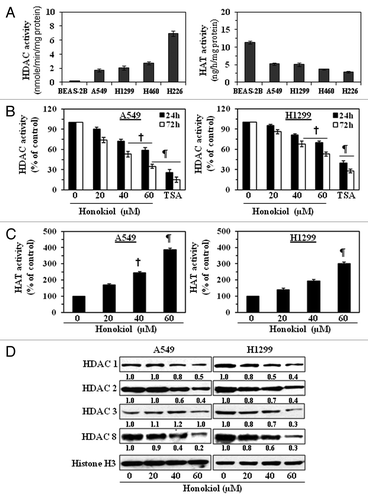
Effect of honokiol and TSA on HDAC and HAT activity in human NSCLC cell lines
To determine the effect of honokiol on HDAC and HAT activities in vitro, we treated A549 and H1299 cells with various concentrations of honokiol (0, 20, 40 and 60 µM) or with TSA (an inhibitor of HDAC) for 24 h and 72 h. As shown in (left and right panels), honokiol treatment of both NSCLC cells resulted in significant inhibition (p < 0.01 and p < 0.001) of HDAC activity as compared with vehicle-treated control cells and that this inhibitory effect occurred in a dose- and time-dependent manner. However, the inhibitory effect of honokiol on HDAC activity was greater in A549 cells than H1299 cells. Treatment of cells with TSA under identical conditions also significantly reduced the levels of HDAC activity in both cell lines. The effects of honokiol on HAT activity in A549 and H1299 cells were determined using the HAT Activity Assay Kit. Treatment with honokiol for 72 h resulted in significantly (p < 0.01, p < 0.001) enhanced levels of HAT activity of both A549 and H1299 cells as compared with the non-honokiol-treated control cells in a dose-dependent manner, as shown in .
Honokiol reduces protein expression of class I HDACs in NSCLC cell lines
As we have found that the NSCLC cells overexpressed HDAC activity and as class I HDACs are overexpressed in human cancers, we investigated whether honokiol affects the protein expression of class I HDACs in NSCLC cells. A549 and H1299 cells were treated with honokiol (0, 20, 40 and 60 µM) for 72 h, then cells were harvested and nuclear extracts prepared. Western blot analysis revealed that treatment of cells with honokiol resulted in a dose-dependent reduction in the levels of the HDAC1, HDAC2, HDAC3 and HDAC8 as compared with the vehicle-treated control cells ().
Reduction of class I HDAC proteins by honokiol in NSCLC cells is mediated through proteasomal degradation of HDACs
To test whether honokiol reduces the levels of HDAC proteins in NSCLC cells through proteasome-mediated degradation, A549 and H1299 cells were treated with honokiol with and without treatment with MG132 (5, 10 and 20 µM), an inhibitor of proteasomal degradation, for 72 h. Nuclear lysates were prepared and were subjected to western blot analysis. This analysis revealed that the levels of class I HDAC proteins were higher in the cells treated with honokiol plus MG132 as compared with levels in the cells treated with honokiol alone (). These results suggest that proteasome-mediated degradation of HDACs may be a possible mechanism through which honokiol reduces the levels of class I HDACs in NSCLC cells.
Figure 2. Effect of honokiol on acetylated histones and cell viability in NSCLC cells. (A) Treatment of NSCLC cells with MG132, a proteasome inhibiter, inhibits the effect of honokiol on HDAC proteins. A549 and H1299 cells were treated with honokiol (60 μM) with and without the treatment of MG132 for 72 h, then cells were harvested and nuclear lysates were subjected to western blot analysis. (B) Treatment of NSCLC cells with honokiol for 72 h enhances the levels of acetylated histone H3 and histone H4, as determined by western blot analysis. Equal loading of samples was verified by re-probing the membrane with anti-histone H3 antibody. The relative intensity of each band after normalization for the levels of histone H3 is shown under each blot. (C) Treatment of NSCLC cells with honokiol inhibits cell viability in a dose-dependent manner, but this effect of honokiol is not seen in BEAS-2B normal human bronchial epithelial cells. NSCLC cells and BEAS-2B cells were treated with various concentrations of honokiol for 48 h and cell viability determined using an MTT assay. (D) Honokiol (60 µM) significantly inhibited cell viability of NSCLC cells in a time-dependent manner, whereas a significant growth inhibitory effect was not observed in BEAS-2B cells. The cell viability data are expressed in terms of percent of control (non-honokiol treatment) cells as the mean ± SD of 5 replicates. Significant difference vs. control, ¶ p < 0.001 †p < 0.05 (left panel). Treatment of A549 and H1299 cells with MG-132 (10 μM), a proteasome inhibitor, reduced the cytotoxicity of honokiol (40 μM) in these lung cancer cells (right panel). Significant reduced cytotoxicity or increased cell viability vs. honokiol alone, †p < 0.05.
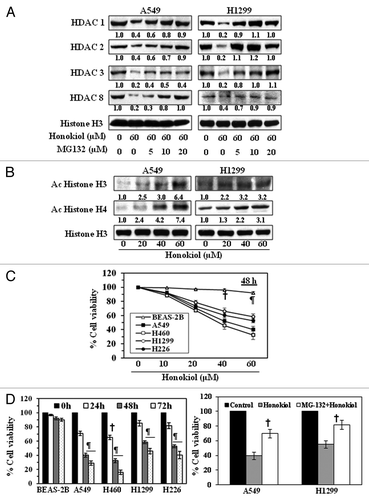
Honokiol increases the levels of acetylated histone H3 and H4 in NSCLC cells
As we have observed that the NSCLC cells had lower levels of HAT activity than normal bronchial epithelial cells, we examined whether honokiol affects the acetylation status of histones in NSCLC cells. A549 and H1299 cells were treated with honokiol (0, 20, 40 and 60 µM) for 72 h, then cells were harvested and nuclear lysates were prepared. Western blot analysis revealed that treatment of cells with honokiol resulted in a dose-dependent increase in the levels of the acetylated histone H3 and histone H4 compared with the vehicle-treated control cells ().
Honokiol inhibits cell growth or viability of human NSCLC cells but not normal human bronchial epithelial cells
To determine whether honokiol-induced inhibition of HDAC activity and induction of HAT activity in NSCLC cells is associated with the growth inhibitory effect of cells, A549, H460, H1299 and H226 NSCLC cell lines were treated with various concentrations of honokiol (0, 10, 20, 40 and 60 µM) for 48 h. BEAS-2B cells also were treated with honokiol under identical conditions. Cell viability was determined using an MTT assay.Citation29 As shown in , treatment of NSCLC cells with honokiol for 48 h resulted in a significant reduction in cell viability in a dose-dependent manner (60 µM dose; 40–70%, p < 0.001). We did not find significant inhibition of cell proliferation of BEAS-2B cells (4–10% inhibition) after honokiol treatment at the concentrations of 10, 20, 40 and 60 µM for 48 h. Moreover, the inhibition of cell viability of BEAS-2B cells by honokiol was significantly lower (p < 0.001) than the effect of equivalent concentrations of honokiol on the NSCLC cells at the same time points. This effect of honokiol on the NSCLC cells was also time dependent, with a 15–33% (p < 0.05) reduction after 24 h, a 40–69% (p < 0.05–0.001) reduction after 48 h, and a 55–84% (p < 0.001) reduction after 72 h (, left panel). The effect of MG-132, a proteasome inhibitor, was also determined on honokiol-induced cancer cell toxicity or cell viability. For this purpose A549 and H1299 cells were treated with honokiol (40 μM) with and without MG-132 (10 μM) for 48 h and then cell viability was determined. As shown in (right panel), treatment of cells with MG-132 plus honokiol significantly reduced (p < 0.05) the cytotoxicity of honokiol when compared with the cells treated with honokiol alone.
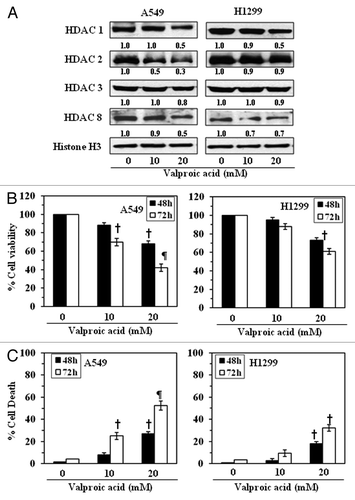
Valproic acid, an inhibitor of HDAC, reduces the levels of class I HDACs and cell viability in NSCLC cells
As the above results suggested that the reduction in the levels of class I HDACs in NSCLC cells by honokiol was associated with the reduction in cell viability of NSCLC cells, we further examined the effects of valproic acid on the levels of class I HDACs in these cell lines. Treatment of A549 and H1299 cells with different concentrations of valproic acid (0, 10 and 20 mM) for 72 h resulted in a reduction in the levels of HDAC1, HDAC2, HDAC3 and HDAC8, as determined by western blot analysis (). Next, we determined the effects of valproic acid on the viability of these cells. Treatment of A549 and H1299 cells with valproic acid (0, 10 and 20 mM) for 48 h or 72 h resulted in a significant reduction (p < 0.05, p < 0.001) in cell viability in a time- and dose-dependent manner, as assessed using an MTT assay (). Treatment of the cells with valproic acid resulted in a significantly (p < 0.05–0.001) higher levels of cell death as compared with the non-valproic acid-treated control cells and this effect was concentration dependent (). The cytotoxic effect of valproic acid on the cells used in this study at a dose of less than 10 mM was not statistically significant. The H1299 cells were less susceptible to the cytotoxic effects of valproic acid than the A549 cells. These observations suggest that the effects of honokiol on the NSCLC cells are similar to those of an inhibitor of class I HDACs.
Figure 3. Effect of valproic acid, an inhibitor of HDACs, on the class I HDAC levels in, and the growth of, NSCLC cells. (A) Treatment of A549 and H1299 cells with valproic acid for 72 h inhibited the expression levels of class I HDACs. Histone H3 was used as a loading control. The relative intensity of each band after normalization for histone H3 is shown under each blot, and it is in terms of fold-change. (B) A549 and H1299 cells were treated with various concentrations of Valproic acid (0, 10 and 20 mM) for 48 and 72 h, and cell viability was determined using an MTT assay. Data on cell viability are presented in terms of percent of control (non-valproic acid-treated) as the mean ± SD of 4–5 replicates. (C) Treatment of NSCLC cells with valproic acid induces cell death. For cell death assay, 5 × 104 cells were plated in six-well culture plates and treated with or without valproic acid for 48 or 72 h. Cell death was determined using a trypan blue exclusion assay. Data are presented as the percent cell death as the mean ± SD from three separate experiments. Significant difference vs. non-valproic acid treated controls, ¶p < 0.001, †p < 0.05.
Honokiol induces G1 phase cell cycle arrest in NSCLC cells
As we had found that treatment of NSCLC cells with honokiol resulted in a reduction in class I HDACs levels and cell viability, we next determined whether this effect of honokiol in NSCLC cells is associated with cell cycle arrest. For this purpose the effect of honokiol on cell cycle progression in H1299 and A549 cells was determined following treatment of cells with honokiol for 72 h. As shown in , cell cycle analysis revealed that treatment of A549 cells with honokiol resulted in arrest of cells in the G1 phase in a dose-dependent manner: 20 µM (86.1%), 40 µM (90.9%) and 60 µM (96.0%) compared with the control cells (68.3%). The effect of honokiol on G1 phase arrest of H1299 cells was also dose-dependent: 20 µM (65.7%), 40 µM (69.3%), and 60 µM (80.4%) compared with control cells (49.0%), as shown in .
Figure 4. Effect of honokiol on cell cycle progression and apoptosis of A549 and H1299 cells. A549 (A) and H1299 (B) cells were treated with either vehicle or honokiol (0, 20, 40 and 60 µM) for 72 h. Cells were harvested, cellular DNA was stained with propidium iodide and flow cytometric analysis performed to analyze the cell cycle distribution. (C) Treatment of cells with honokiol for 72 h inhibits the levels of cyclins and CDKs associated with the G1 phase of the cell cycle in a dose-dependent manner, as analyzed by western blotting. The relative density of each band after normalization for β-actin is shown under each blot.
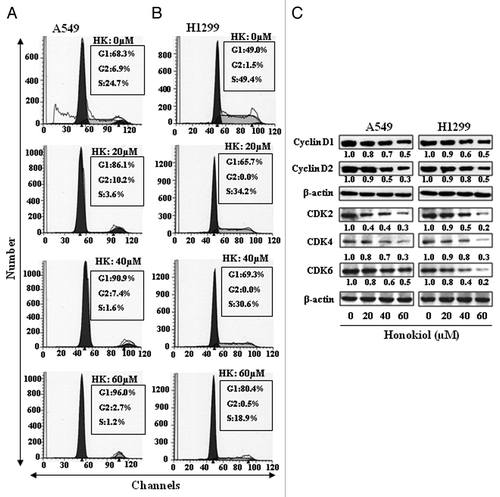
Cyclins and CDKs have been implicated in the regulation of cell cycle;Citation30,Citation31 therefore the effect of honokiol was determined on the levels of cyclins and CDKs. Treatment of H1299 cells with honokiol (0, 20, 40 and 60 µM) for 72 h resulted in a dose-dependent reduction in the expression of cyclin D1 and cyclin D2 (). Similarly, a marked reduction in the expression of CDK2, CDK4 and CDK6 proteins was observed. A549 cells were also treated with honokiol for 72 h and its effect on the G1 phase cell cycle regulatory proteins assessed using western blotting (). Inhibitory effect of honokiol on cyclins and CDKs of G1 phase in A549 cells was identical as found with H1299 cells.
Honokiol suppresses the growth of NSCLC tumor xenografts in athymic nude mice
Next, we determined the effects of honokiol on the growth of A549 and H1299 xenografts in athymic nude mice. Honokiol was administered by oral gavage (100 mg/kg body weight of mice) twice a week. Our recorded data on the average body weight of mice throughout the experiment suggest that there was no significant difference between honokiol-treated and non-honokiol-treated group of mice (). The mice that were treated with honokiol did not exhibit any abnormal behavior or visible sign of toxicity.
Figure 5. Administration of honokiol by gavage inhibits the growth of A549 or H1299 tumor xenografts in athymic nude mice. Mice were inoculated subcutaneously with 2 × 106 cells (A549 or H1299) on the right flank. Twenty-four hours later, mice were treated with either PBS (100 µL) or honokiol (100 mg/kg body weight/100 µL of PBS) by gavage twice per week for a total of eight weeks. (A) The body weight of the mice was monitored weekly. (B) Tumor volumes were recorded on a weekly basis to determine the chemotherapeutic effect of honokiol on the growth of tumor xenografts, and data are presented as tumor volume ± SD/mouse (mm3) in each group. (C) Tumors were harvested at the termination of the experiment, and the wet weight of the tumor/mouse in grams was measured and is reported as a mean ± SD. Statistical significance vs. non-honokiol-fed control group, ¶p < 0.01.
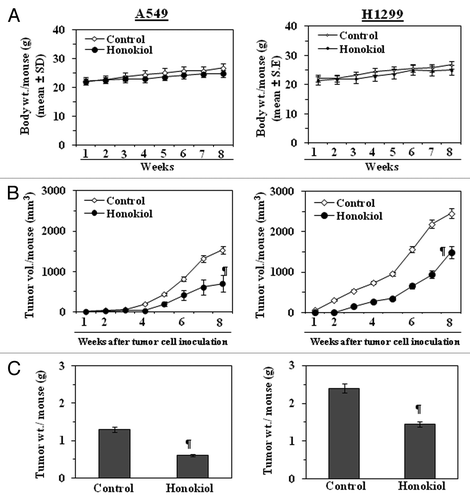
Observations and periodic measurement of the tumor xenograft volume on weekly basis suggested that the treatment with honokiol reduced the growth of lung tumor xenografts throughout the experimental protocol. At the end of the eighth week after tumor cell inoculation, the tumor volumes were again measured. The results indicated that administration of honokiol had a significant inhibitory effect (p < 0.01) on the growth of A549 xenograft tumors (54% reduction in tumor volume) and the growth of H1299 xenograft tumors (40% reduction in tumor volume) (). The weight of the A549 xenograft tumor/mouse was significantly lower (54%, p < 0.01) in the mice administered honokiol than in the mice that did not receive honokiol (, left panel). Similarly, the weight of the H1299 tumor xenograft was also significantly lower (p < 0.01) in the mice administered honokiol than in the mice that did not receive honokiol (, right panel).
Honokiol enhances the number of TUNEL-positive cells in tumor xenografts
To determine the effect of honokiol on the induction of apoptotic cell death of tumor cells, the xenograft tumors were subjected to immunohistochemical detection of TUNEL-positive cells. Immunohistochemical analysis indicated that the percentages of TUNEL-positive cells were significantly higher (p < 0.001) in the H1299 and A549 xenograft tumors of the honokiol-treated mice compared with the xenograft tumors from the control group of mice ().
Figure 6. Administration of honokiol by oral gavage induces apoptosis and lowers the levels of class I HDACs in A549 or H1299 tumor xenografts. At the termination of the experiment described in , tumors were harvested and subjected to immunostaining and western blot analysis. (A) Percent of TUNEL-positive cells in sections of tumor xenograft tissues from honokiol-treated and honokiol-untreated mice. Significant increase vs. non-honokiol-treated controls, *p < 0.005. (B) Honokiol treatment inhibits the expression of anti-apoptotic proteins while increasing the expression of pro-apoptotic protein Bax and enhancing the activation of caspase-3 as determined by western blotting of the A549 and H1299 xenograft tissues. (C) Honokiol treatment inhibits the levels of class I HDACs as determined by western blotting of A549 and H1299 tumor xenograft tissues. Equal loading of samples was verified using the antibodies against histone H3 or β-actin. Relative intensity of each band compared with non-honokiol-treated control group is shown under each blot after normalization for β-actin or histone H3. (D) Total HDAC activity was determined in nuclear extracts of tumor samples using a colorimetric assay kit. Significant inhibition of HDAC activity vs. non-honokiol-treated controls, †p < 0.01.
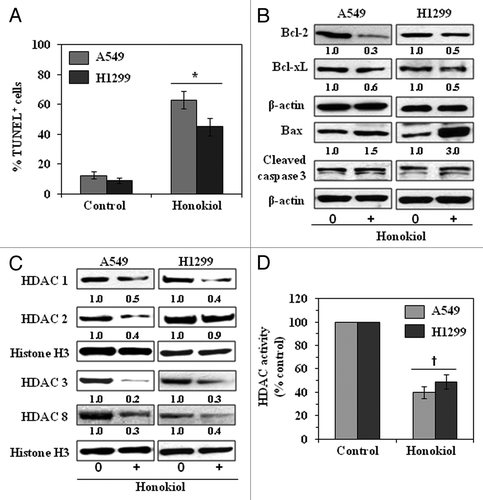
The tumor xenograft samples from each of the treatment groups were subjected to the analysis of the expressions of pro- and anti-apoptotic proteins of the Bcl-2 family. Our western blot analysis revealed that the levels of the anti-apoptotic proteins, Bcl-2 and Bcl-xl, were decreased in the A549 and H1299 xenografts from mice treated with honokiol than in the tumors in non-honokiol-treated control mice. In contrast, the levels of the pro-apoptotic protein, Bax, and cleaved caspase-3 were increased in the tumors from the honokiol-treated mice than the control mice ().
Honokiol suppresses the levels of class I HDAC proteins and HDAC activity in lung tumor xenografts
To further check whether the inhibition of tumor xenografts growth of NSCLC cells by honokiol was associated with the downregulation of class I HDACs in tumors, we determined the levels of class I HDAC proteins in the nuclear extracts of tumor samples using western blot analysis. Our western blot analysis revealed that the levels of class I HDAC1, HDAC2, HDAC3 and HDAC8 proteins were lower in the A549 and H1299 xenografts from honokiol-treated mice than in the tumors from non-honokiol-treated mice, as shown in . Additionally, the HDAC activity was significantly reduced (p < 0.01) in the tumors obtained from honokiol-treated mice as compared with control mice, as shown in .
Discussion
Cancer is a manifestation of epigenetic, as well as genetic, alterations. Post-translational modifications of histone that affect chromatin structure play important roles in epigenetic alterations. Acetylation and deacetylation are the two main histone modifications that have been clinically identified as predictors of cancer progression.Citation32-Citation34 Overexpression of class I HDACs in human cancers has been reported and HDACs are promising targets in oncology and epigenetic therapy.Citation32,Citation35 In the current study, we establish that the levels of HDAC activity are elevated in the human NSCLC cell lines tested, including A549, H1299, H460 and H226, as compared with the normal human bronchial epithelial cell line, BEAS-2B. Additionally, the activity of HAT was lower in these NSCLC cell lines as compared with BEAS-2B cells.
Early clinical trials with synthetic HDAC inhibitors have demonstrated promising therapeutic activity and therefore HDACs have become prime targets in cancer drug development. Preclinical studies suggest that HDACi modulate a wide variety of cellular functions, including cellular differentiation, cell cycle progression, apoptosis and angiogenesis.Citation35 HDAC inhibitors, such as suberoylanilide hydroxamic acid (SAHA) and TSA, have been shown to induce apoptosis in neoplastic cells in vitro and inhibit tumor growth in vivo in animal models.Citation36-Citation39 Honokiol has been shown to have anti-carcinogenic potential in many organ systems.Citation17-Citation23 In the current study, we further demonstrate that treatment of A549 or H1299 cell lines as an in vitro model with honokiol inhibits HDAC activity and suppresses the levels of class I HDAC proteins in a dose- and time-dependent manner, while enhancing HAT activity. Class I HDACs are responsible for deacetylation of the catalytic core for different co-repressor complexes resulting in transcriptional repression. Intriguingly, we found that honokiol had a broad-spectrum effect in that it suppressed the levels of all class I HDACs. Further, our study provides evidence that honokiol suppresses the levels of HDAC proteins in NSCLC cells through their proteasomal degradation.
We found that honokiol significantly reduced the viability/proliferation of A549, H1299, H460 and H226 human NSCLC cell lines but had a non-significant effect on normal human bronchial epithelial cells. The parallel concentration- and time-dependence suggests that the inhibition of cell proliferation by honokiol may be mediated, at least in part, through the downregulation of class I HDAC proteins and inhibition of HDAC activity in these NSCLC cells. Further support for this possibility was suggested by the effects of valproic acid, a known HDAC inhibitor, which also reduced cell viability and induced cell death in NSCLC cell lines. Additionally, treatment of cells with valproic acid decreased the levels of class I HDAC proteins (HDAC1, HDAC2, HDAC3 and HDAC8), which suggests that the action of honokiol against lung cancer cells is similar to that of this synthetic HDAC inhibitor.
As it has been recognized that cell cycle regulators are frequently mutated or deregulated in most of the human malignancies, the control of cell cycle progression in cancer cells may be an effective strategy to inhibit cancer growth.Citation31,Citation40-Citation42 Our study demonstrates that in vitro treatment of NSCLC cells with honokiol induces G1 phase arrest and decreases the expressions of cyclins, and CDKs (CDK2, CDK4 and CDK6), in both A549 and H1299 cell lines suggesting that honokiol induces a marked disruption of the uncontrolled cell cycle progression, and that may be a mechanism by which honokiol inhibits the proliferation or growth of lung cancer cells.
Our in vivo study provides further evidence that administration of honokiol by gavage inhibits the growth of both A549 and H1299 tumor xenografts without any apparent sign of toxicity in the mice. Furthermore, consistent with the findings in cell culture model, the tumor cells from honokiol-treated mice were undergoing apoptotic cell death as indicated by the TUNEL-positive cells as well as increases in the levels of pro-apoptotic protein Bax and cleaved caspase-3. Our results also indicated that honokiol-induced inhibition of tumor xenograft growth in mice is associated with the reduction in the levels of class I HDAC proteins as well as decrease in HDAC activity in tumor xenograft tissues. In summary, our findings are of importance for understanding the anti-lung cancer effect, mechanisms and clinical applications of honokiol. Further, the new insights into the epigenetic mechanism of action of honokiol may contribute to the chemoprevention or treatment of lung cancer and may have important implications for epigenetic therapy. The use of honokiol in combination with other known HDACi may be more effective for the treatment of lung cancer and needs to be examined further.
Methods
Cell lines and cell culture
NSCLC cell lines, A549, H460, H226 and H1299, and normal human bronchial epithelial cell line (BEAS-2B) were obtained from the American Type Culture Collection. These cell lines were maintained and cultured as detailed previously.Citation43
Chemicals, reagents and antibodies
Purified honokiol was purchased from Quality Phytochemicals, LLC. The primary antibodies were purchased as follows: antibodies for Bcl-2 (Catalog # 2876), Bcl-xl (Catalog # 2762), Bax (Catalog # 2772), cleaved caspase-3 (Catalog # 9664), cyclin D1 (Catalog # 2978), cyclin D2 (Catalog # 2924), CDK6 (Catalog # 3136), class I HDAC, such as anti-HDAC1 (Catalog # 5356), anti-HDAC2 (Catalog # 2540), anti-HDAC3 (Catalog # 2632) and β-actin (Catalog # 4967) were obtained from Cell Signaling Technology, Inc.; antibodies for anti-HDAC8 (Catalog # 07–505), anti-acetyl-histone H3 (Catalog # 06–599), and anti-acetyl-histone H4 (Catalog # 06–866) were obtained from EMD Chemicals. Inc.; antibodies for anti-histone H3 (Catalog # SC-10809), CDK2 (Catalog # SC-6248), and CDK4 (Catalog # SC-601) and the secondary antibodies, horseradish peroxidase-linked anti-mouse immunoglobulin G (Catalog # SC-2005), and anti-rabbit immunoglobulin G (Catalog # SC-2004) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The HDAC Activity Assay Kit was purchased from Active Motif (Catalog # 56210). The western blotting detection reagents were obtained from Amersham Pharmacia Biotech.
HDAC activity assay
HDAC activity was determined using the colorimetric HDAC Activity Assay Kit following the manufacturer’s protocol. This assay kit provides: a positive control (a HeLa nuclear extract), a deacetylated HDAC assay standard, and a control inhibitor (trichostatin A; TSA) as well as the colorimetric HDAC substrate. The absorbance was measured using a microplate reader at 405 nm, and the HDAC activity is reported as nmole/minute/mg protein.
Histone acetyltransferase (HAT) activity assay
HAT activity was determined using the EpiQuikTM HAT Activity/Inhibition Assay Kit (Epigentek Group Inc., Catalog # P-4003) following the manufacturer’s instructions. This assay kit is designed for measurement of total HAT activity/inhibition. The amount of the acetylated histone, which is directly proportional to HAT enzyme activity, can be colorimetrically quantified through an ELISA-like reaction. The color absorbance was read using a microplate reader at 450 nm, and the HAT activity is reported as ng/h/mg protein.
Western blot analysis
Cells were treated with honokiol or valproic acid for desired period of time and cell lysates were prepared as detailed previously.Citation29,Citation43 Proteins were resolved on 8–12% SDS-PAGE and transferred onto the nitrocellulose membrane. Western blot analysis was performed to detect the expression levels of proteins of interest as detailed previously.Citation29,Citation43 Loading of equal protein on the gels was verified by re-probing the membrane with antibodies against β-actin or histone H3. The relative density of protein bands in a blot was measured using an ImageJ program developed at the National Institutes of Health (http://rsb.info.nih.gov/ij) and normalized with the β-actin or histone H3 bands to compare the expression levels of proteins in different treatment groups. In each case non-honokiol-treated control group was assigned an arbitrary unit 1.0.
MTT cell proliferation assay
The effect of honokiol on the viability of cells was determined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, Sigma Chemical Co.) assay as described previously.Citation29 Briefly, 1 × 104 cells per well were treated with various concentrations of honokiol or valproic acid for 24, 48 or 72 h. Thereafter, cells were harvested, washed with PBS buffer and incubated with 50 µL of MTT (5 mg/mL) and the resulting formazan crystals dissolved in 150 µL of DMSO. The absorbance was recorded at 540 nm. The effect of honokiol or valproic acid on cell viability was determined relative to the viability of control-treated cells that were assigned a value of 100%.
Cell cycle analysis
Cells were treated with honokiol (0, 20, 40 and 60 µM) for 72 h. The cells were then harvested and processed for routine analysis of cell cycle progression, as detailed previously.Citation44 The analysis of cell cycle phase distribution was determined using a FACSCalibur instrument (BD Biosciences) equipped with CellQuest 3.3 software.
Tumor xenograft study
Four-to-five weeks old female athymic nude mice were obtained from National Cancer Institute (Frederick, MD) and housed in the Animal Resource Facility at the standard conditions of a 12 h dark/12 h light cycle, relative humidity of 50 ± 10% and a temperature of 24 ± 2°C. The animal protocol was approved by the Institutional Animal Care and Use Committee. Mice were given a sterilized AIN76A diet and water ad libitum.
Exponentially growing A549 or H1299 cells (2 × 106) in 100 µL of PBS were injected subcutaneously in the right flank of each mouse. After 24 h, mice were randomly divided in two groups with 10 mice in each group. One group of mice was treated with 100 mg honokiol/kg body weight in 100 µL of PBS by oral gavage twice per week. Second group of mice received an equal volume of PBS by gavage and served as a control group. The experiment was terminated 8 weeks after tumor cell inoculation. Tumor growth was monitored throughout the protocol. Mice were also monitored for their body weight, diet consumption, and normal behavior including the illness, etc. throughout the experiment period. At the termination of the experiment, mice were sacrificed and the tumor from each mouse was harvested. The wet weight of the tumor was recorded, and tumor lysates were prepared for western blot analysis. A part of the tumor was used to prepare paraffin block for immunohistochemical analysis.
TUNEL assay for apoptotic index analysis
TUNEL-positive cells in tumor sections were detected using DeadEndTM Colorimetric TUNEL System Kit (Promega Corporation) following the manufacturer’s instructions, and as detailed by us previously.Citation45 TUNEL-positive cells were detected using a light microscope and are reported as the percentage of the total cells in the microscopic field.
Statistical analysis
The statistical significance of differences between control and honokiol-treated groups were calculated by Student’s t-test. Quantitative data are shown as mean ± SD. In the tumor xenograft study, the statistical significance of difference between control and honokiol-treated groups was determined by ANOVA. In each case p < 0.05 was considered statistically significant.
| Abbreviations: | ||
| NSCLC | = | non-small cell lung cancer |
| HDAC | = | histone deacetylases |
| HAT | = | histone acetyl transferase |
| CDK | = | cyclin-dependent kinases |
| BEAS-2B | = | normal human bronchial epithelial cells |
| DMSO | = | dimethylsulfoxide |
| SD | = | standard deviation |
| TSA | = | trichostatin A |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Financial Support
This work was supported by the Veterans Administration Merit Review Award, 1I01BX001059 (S.K.K.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Dr Fiona Hunter for her assistance in editing the manuscript.
References
- Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkühler C. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Res 2007; 17:195 - 211; PMID: 17325692
- Weichert W, Röske A, Niesporek S, Noske A, Buckendahl AC, Dietel M, et al. Class I histone deacetylase expression has independent prognostic impact in human colorectal cancer: specific role of class I histone deacetylases in vitro and in vivo. Clin Cancer Res 2008; 14:1669 - 77; http://dx.doi.org/10.1158/1078-0432.CCR-07-0990; PMID: 18347167
- Weichert W. HDAC expression and clinical prognosis in human malignancies. Cancer Lett 2009; 280:168 - 76; http://dx.doi.org/10.1016/j.canlet.2008.10.047; PMID: 19103471
- Khan O, La Thangue NB. HDAC inhibitors in cancer biology: emerging mechanisms and clinical applications. Immunol Cell Biol 2012; 90:85 - 94; http://dx.doi.org/10.1038/icb.2011.100; PMID: 22124371
- Beumer JH, Tawbi H. Role of histone deacetylases and their inhibitors in cancer biology and treatment. Curr Clin Pharmacol 2010; 5:196 - 208; http://dx.doi.org/10.2174/157488410791498770; PMID: 20406169
- Ellis L, Hammers H, Pili R. Targeting tumor angiogenesis with histone deacetylase inhibitors. Cancer Lett 2009; 280:145 - 53; http://dx.doi.org/10.1016/j.canlet.2008.11.012; PMID: 19111391
- Srivastava RK, Kurzrock R, Shankar S. MS-275 sensitizes TRAIL-resistant breast cancer cells, inhibits angiogenesis and metastasis, and reverses epithelial-mesenchymal transition in vivo. Mol Cancer Ther 2010; 9:3254 - 66; http://dx.doi.org/10.1158/1535-7163.MCT-10-0582; PMID: 21041383
- Martínez-Iglesias O, Ruiz-Llorente L, Sánchez-Martínez R, García L, Zambrano A, Aranda A. Histone deacetylase inhibitors: mechanism of action and therapeutic use in cancer. Clin Transl Oncol 2008; 10:395 - 8; http://dx.doi.org/10.1007/s12094-008-0221-x; PMID: 18628067
- Jazirehi AR. Regulation of apoptosis-associated genes by histone deacetylase inhibitors: implications in cancer therapy. Anticancer Drugs 2010; 21:805 - 13; http://dx.doi.org/10.1097/CAD.0b013e32833dad91; PMID: 20679890
- Nishioka C, Ikezoe T, Yang J, Koeffler HP, Yokoyama A. Blockade of mTOR signaling potentiates the ability of histone deacetylase inhibitor to induce growth arrest and differentiation of acute myelogenous leukemia cells. Leukemia 2008; 22:2159 - 68; http://dx.doi.org/10.1038/leu.2008.243; PMID: 18784743
- Prince HM, Bishton MJ, Harrison SJ. Clinical studies of histone deacetylase inhibitors. Clin Cancer Res 2009; 15:3958 - 69; http://dx.doi.org/10.1158/1078-0432.CCR-08-2785; PMID: 19509172
- Tan J, Cang S, Ma Y, Petrillo RL, Liu D. Novel histone deacetylase inhibitors in clinical trials as anti-cancer agents. J Hematol Oncol 2010; 3:5; http://dx.doi.org/10.1186/1756-8722-3-5; PMID: 20132536
- Thakur VS, Gupta K, Gupta S. Green tea polyphenols causes cell cycle arrest and apoptosis in prostate cancer cells by suppressing class I histone deacetylases. Carcinogenesis 2012; 33:377 - 84; http://dx.doi.org/10.1093/carcin/bgr277; PMID: 22114073
- Vaid M, Prasad R, Singh T, Jones V, Katiyar SK. Grape seed proanthocyanidins reactivate silenced tumor suppressor genes in human skin cancer cells by targeting epigenetic regulators. Toxicol Appl Pharmacol 2012; 263:122 - 30; http://dx.doi.org/10.1016/j.taap.2012.06.013; PMID: 22749965
- Nandakumar V, Vaid M, Katiyar SK. (-)-Epigallocatechin-3-gallate reactivates silenced tumor suppressor genes, Cip1/p21 and p16INK4a, by reducing DNA methylation and increasing histones acetylation in human skin cancer cells. Carcinogenesis 2011; 32:537 - 44; http://dx.doi.org/10.1093/carcin/bgq285; PMID: 21209038
- Li TSC. Chinese and related North American herbs: phytopharmacology and therapeutic values. Boca Raton, FL: CRC Press, 2002.
- Vaid M, Sharma SD, Katiyar SK. Honokiol, a phytochemical from the Magnolia plant, inhibits photocarcinogenesis by targeting UVB-induced inflammatory mediators and cell cycle regulators: development of topical formulation. Carcinogenesis 2010; 31:2004 - 11; http://dx.doi.org/10.1093/carcin/bgq186; PMID: 20823108
- Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem 2003; 278:35501 - 7; http://dx.doi.org/10.1074/jbc.M302967200; PMID: 12816951
- Battle TE, Arbiser J, Frank DA. The natural product honokiol induces caspase-dependent apoptosis in B-cell chronic lymphocytic leukemia (B-CLL) cells. Blood 2005; 106:690 - 7; http://dx.doi.org/10.1182/blood-2004-11-4273; PMID: 15802533
- Chen F, Wang T, Wu YF, Gu Y, Xu XL, Zheng S, et al. Honokiol: a potent chemotherapy candidate for human colorectal carcinoma. World J Gastroenterol 2004; 10:3459 - 63; PMID: 15526365
- Park EJ, Min HY, Chung HJ, Hong JY, Kang YJ, Hung TM, et al. Down-regulation of c-Src/EGFR-mediated signaling activation is involved in the honokiol-induced cell cycle arrest and apoptosis in MDA-MB-231 human breast cancer cells. Cancer Lett 2009; 277:133 - 40; http://dx.doi.org/10.1016/j.canlet.2008.11.029; PMID: 19135778
- Hahm ER, Arlotti JA, Marynowski SW, Singh SV. Honokiol, a constituent of oriental medicinal herb magnolia officinalis, inhibits growth of PC-3 xenografts in vivo in association with apoptosis induction. Clin Cancer Res 2008; 14:1248 - 57; http://dx.doi.org/10.1158/1078-0432.CCR-07-1926; PMID: 18281560
- Leeman-Neill RJ, Cai Q, Joyce SC, Thomas SM, Bhola NE, Neill DB, et al. Honokiol inhibits epidermal growth factor receptor signaling and enhances the antitumor effects of epidermal growth factor receptor inhibitors. Clin Cancer Res 2010; 16:2571 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-10-0333; PMID: 20388852
- American Cancer Society. Cancer facts and figures. Available: http://www.cancer.org/. Accessed 2011, June 20.
- Proctor RN. Tobacco and the global lung cancer epidemic. Nat Rev Cancer 2001; 1:82 - 6; http://dx.doi.org/10.1038/35094091; PMID: 11900255
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, et al. Cancer statistics, 2005. CA Cancer J Clin 2005; 55:10 - 30; http://dx.doi.org/10.3322/canjclin.55.1.10; PMID: 15661684
- Maziak DE, Markman BR, MacKay JA, Evans WK, Cancer Care Ontario Practice Guidelines Initiative Lung Cancer Disease Site Group. Cancer Care Ontario Practice Guidelines Initiative Lung Cancer Disease Site Group. Photodynamic therapy in non-small cell lung cancer: a systematic review. Ann Thorac Surg 2004; 77:1484 - 91; http://dx.doi.org/10.1016/j.athoracsur.2003.07.017; PMID: 15063303
- Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet 2000; 355:479 - 85; PMID: 10841143
- Sharma SD, Meeran SM, Katiyar SK. Proanthocyanidins inhibit in vitro and in vivo growth of human non-small cell lung cancer cells by inhibiting the prostaglandin E(2) and prostaglandin E(2) receptors. Mol Cancer Ther 2010; 9:569 - 80; http://dx.doi.org/10.1158/1535-7163.MCT-09-0638; PMID: 20145019
- Morgan DO. Principles of CDK regulation. Nature 1995; 374:131 - 4; http://dx.doi.org/10.1038/374131a0; PMID: 7877684
- Pavletich NP. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol 1999; 287:821 - 8; http://dx.doi.org/10.1006/jmbi.1999.2640; PMID: 10222191
- Davis CD, Ross SA. Dietary components impact histone modifications and cancer risk. Nutr Rev 2007; 65:88 - 94; http://dx.doi.org/10.1111/j.1753-4887.2007.tb00285.x; PMID: 17345961
- Fraga MF, Ballestar E, Villar-Garea A, Boix-Chornet M, Espada J, Schotta G, et al. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat Genet 2005; 37:391 - 400; http://dx.doi.org/10.1038/ng1531; PMID: 15765097
- Seligson DB, Horvath S, Shi T, Yu H, Tze S, Grunstein M, et al. Global histone modification patterns predict risk of prostate cancer recurrence. Nature 2005; 435:1262 - 6; http://dx.doi.org/10.1038/nature03672; PMID: 15988529
- Marsoni S, Damia G, Camboni G. A work in progress: the clinical development of histone deacetylase inhibitors. Epigenetics 2008; 3:164 - 71; http://dx.doi.org/10.4161/epi.3.3.6253; PMID: 18487953
- Kelly WK, Marks PA. Drug insight: Histone deacetylase inhibitors--development of the new targeted anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract Oncol 2005; 2:150 - 7; http://dx.doi.org/10.1038/ncponc0106; PMID: 16264908
- Drummond DC, Noble CO, Kirpotin DB, Guo Z, Scott GK, Benz CC. Clinical development of histone deacetylase inhibitors as anticancer agents. Annu Rev Pharmacol Toxicol 2005; 45:495 - 528; http://dx.doi.org/10.1146/annurev.pharmtox.45.120403.095825; PMID: 15822187
- Butler LM, Agus DB, Scher HI, Higgins B, Rose A, Cordon-Cardo C, et al. Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res 2000; 60:5165 - 70; PMID: 11016644
- Yoshida M, Kijima M, Akita M, Beppu T. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem 1990; 265:17174 - 9; PMID: 2211619
- Graña X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995; 11:211 - 9; PMID: 7624138
- Molinari M. Cell cycle checkpoints and their inactivation in human cancer. Cell Prolif 2000; 33:261 - 74; http://dx.doi.org/10.1046/j.1365-2184.2000.00191.x; PMID: 11063129
- Kastan MB, Canman CE, Leonard CJ. P53, cell cycle control and apoptosis: implications for cancer. Cancer Metastasis Rev 1995; 14:3 - 15; http://dx.doi.org/10.1007/BF00690207; PMID: 7606818
- Singh T, Sharma SD, Katiyar SK. Grape proanthocyanidins induce apoptosis by loss of mitochondrial membrane potential of human non-small cell lung cancer cells in vitro and in vivo. PLoS One 2011; 6:e27444; http://dx.doi.org/10.1371/journal.pone.0027444; PMID: 22087318
- Mantena SK, Sharma SD, Katiyar SK. Berberine inhibits growth, induces G1 arrest and apoptosis in human epidermoid carcinoma A431 cells by regulating Cdki-Cdk-cyclin cascade, disruption of mitochondrial membrane potential and cleavage of caspase 3 and PARP. Carcinogenesis 2006; 27:2018 - 27; http://dx.doi.org/10.1093/carcin/bgl043; PMID: 16621886
- Prasad R, Katiyar SK. Bioactive phytochemical proanthocyanidins inhibit growth of head and neck squamous cell carcinoma cells by targeting multiple signaling molecules. PLoS One 2012; 7:e46404; http://dx.doi.org/10.1371/journal.pone.0046404; PMID: 23050025