Abstract
Maintenance of ordered chromatin structure over the body of genes is vital for the regulation of transcription. Increased access to the underlying DNA sequence results in the recruitment of RNA polymerase II to inappropriate, promoter-like sites within genes, resulting in unfettered transcription. Two new papers show how the Set2-mediated methylation of histone H3 on Lys36 (H3K36me) maintains chromatin structure by limiting histone dynamics over gene bodies, either by recruiting chromatin remodelers that preserve ordered nucleosomal distribution or by lowering the binding affinity of histone chaperones for histones, preventing their removal.
Introduction
Under physiological conditions chromatin represents a strong barrier to transcription by RNA polymerase II (RNAP II).Citation1,Citation2 For transcription to take place in a controlled fashion, RNAP II has to move through the nucleosomal template. This process requires extensive modulation of chromatin structure through the remodeling and/or removal of existing nucleosomes and it is achieved through the concerted actions of chromatin remodelers,Citation3 histone modifying enzymesCitation4 and histone chaperones.Citation5
At gene promoters, high turnover of histones is promoted by high levels of histone acetylation and the incorporation of the histone variant H2A.Z, thought to reduce nucleosome affinity for DNA and nucleosome stability, respectively. These measures result in the formation of a nucleosome-depleted region (NDR) that favors binding of transcription factors and formation of RNAP II pre-initiation complexes. In contrast, over gene bodies the original chromatin structure has to be restored once RNAP II has passed. Otherwise, promoter-like sequences within gene bodies become exposed, leading to inappropriate initiation from these sites and the production of so-called cryptic (or internally-initiated) transcripts both in the sense and antisense directions. One of the key pathways involved in suppressing these internally initiated transcripts in a co-transcriptional manner is the Set2/Rpd3S pathway.
Set2/Rpd3S Pathway
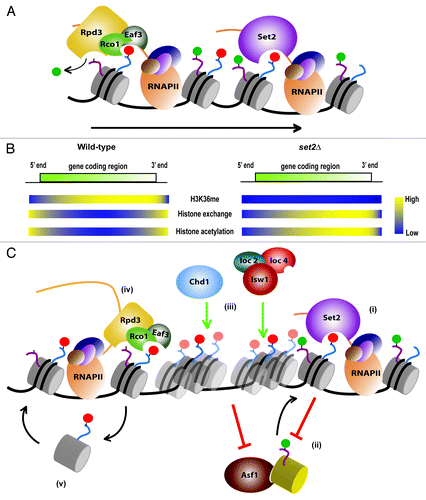
Set2 is a lysine methyltransferase (KMTase) that methylates histone H3 at the K36 residue. Set2 specifically associates with the Ser-2 phosphorylated form of the elongating RNAP II (),Citation6-Citation9 that targets its KMTase activity toward the promoter distal ends of genes ().Citation10,Citation11 While H3K36 methylated nucleosomes are enriched over transcribed genes,Citation9,Citation12 this modification nevertheless fulfills a repressive function. Previous work has shown that the RNAP II-associated Rpd3S histone deacetylase complex requires H3K36 methylation for efficient deacetylation of histones.Citation13-Citation17 Recognition of di- and trimethylated H3K36 through its Eaf3 and Rco1 subunits is required for Rpd3S catalytic activity, ensuring that coding regions remain hypoacetylated (). Thus, Set2 mediated H3K36 methylation acts as a signal from the elongating RNAP II to target the deacetylase activity of Rpd3S toward the 3′ ends of genes. In the absence of Set2, H3K36 methylation or Rpd3S, co-transcriptionally acetylated histones accumulate on coding regions leading to transcription initiation from internal, cryptic promoters.Citation15,Citation18 The hyperacetylation of histones upon loss of Set2 was shown to depend on gene length and its rate of transcription. In particular, 3′ half of long genes and infrequently transcribed genes showed the maximal accumulation of acetylated histones over the coding regions ().Citation18 This observation can be explained by the fact that Ser-2 phosphorylation of RNAP II C-terminal domain (CTD) peaks after the first 500 bases of the coding region are transcribed. Consequently, Set2-mediated H3K36 methylation is targeted to nucleosomes from the mid to the 3′ end of genes, thereby defining the region over which the Set2/Rpd3S pathway acts. This leaves the question as to what pathway targets deacetylases to the 5′ end of genes. Recently, it has been shown that H3K4 di-methylation is necessary to target the deacetylase activity of the Set3 complex at the 5′ end of genes,Citation19 in a manner analogous to the Set2/Rpd3S pathway.
Figure 1. Set2-mediated H3K36 methylation and its functional consequences.(A) Set2/Rpd3S pathway. The RNAP II-associated histone methyltransferase Set2 methylates H3K36 (red circle). This mark is recognized by the Rco1 and Eaf3 subunits of the Rpd3S deacetylase complex that maintains genomic regions in a hypoacetylated state by removing acetyl marks (green circle). The arrow indicates the direction of transcription. (B) Distribution of H3K36 methylation (H3K36me), histone exchange and histone acetylation in wild-type or SET2 deleted (set2Δ) yeast strains. (C) Mechanism of Set2-mediated suppression of histone exchange. (i) Co-transcriptional methylation of H3K36 by Set2, results in (ii) preventing Asf1-mediated assembly of newly synthesized pre-acetylated histones (yellow cylinder). (iii) H3K36 methylated nucleosomes are targeted either by the Isw1b complex, or by the RNAP II-associated Chd1 remodeler, resulting in nucleosome remodeling in cis, thus allowing passage of RNAP II and preventing trans-histone exchange by Asf1. (iv) The Rpd3S deacetylase complex is targeted by H3K36 methylation to the coding regions, which is maintained in a hypoacetylated state. (v) The histone chaperone function of Rpd3S may be instrumental in the capture of H3K36 methylated histones and its reassembly following the passage of RNAP II.
Methylation of H3K36 Suppresses Histone Exchange
During chromatin transcription in metazoans, histone exchange occurs over gene bodies whereby histone H3 is replaced by variant H3.3,Citation20,Citation21 in a manner dependent on transcription rates.Citation22,Citation23 In yeast, histone exchange normally occurs over the 5′ and 3′ ends of genes (), while histones over coding regions are replaced less frequently.Citation24,Citation25 In contrast to highly transcribed genes, low transcribed genes do not demonstrate much histone H3 exchange over the coding regions.Citation25,Citation26 However, these genes do exhibit a rapid and continuous exchange of H2A-H2B dimers by histone chaperones over the coding regions, which has been shown to be sufficient for RNAP II elongation.Citation27,Citation28 Interestingly, these infrequently transcribed genes are dependent on the Set2/Rpd3S co-transcriptional deacetylation pathway to regulate histone acetylation over coding regions.Citation18 This observation led to the question whether H3K36 methylation had an additional role in the regulation of histone exchange over coding regions?
Using a specialized yeast strain that allows us to differentiate between “old” and “new” histone H3,Citation26 we can monitor the sites of incorporation for new, soluble histone H3 in response to gene transcription and compare them to genomic loci that preferentially retain existing nucleosomes. In wild-type yeast cells, promoter regions showed maximal accumulation of new histones, while the coding regions retain their existing nucleosomes. Deletion of SET2 in this strain resulted in the increased accumulation of “new” histones over the coding region of genes (). This confirmed our hypothesis that Set2-mediated H3K36 methylation indeed suppresses trans-histone exchange over the coding regions, particularly toward the 3′ ends of long genes.Citation29 The ability of H3K36 methylated nucleosomes to prevent histone exchange could also explain why it suppresses internal initiation of transcription.
Co-transcriptional Hstone Acetylation is a Consequence of Histone Exchange
The loss of Set2-mediated H3K36 methylation results in increased histone exchange over coding regions, leading to the enrichment of histone acetylation (). Perturbing the histone exchange pathway (by deleting ASF1)Citation25 in a SET2 deletion mutant reduced enrichment of histone acetylation over the coding regions.Citation29 This result suggests that histone exchange is responsible for co-transcriptional acetylation, by replacing unacetylated histones with pre-acetylated histones from the soluble pool. However, it also raises the question whether histone exchange is the sole means of co-transcriptional acetylation? Interestingly, the deletion of ASF1 in a SET2 mutant decreases acetylation over the coding regions, but does not completely abolish it. This suggests that lysine acetyltransferase (KAT) complexes are also involved in acetylating nucleosomes over coding regionsCitation30 in addition to histone exchange. Therefore, we conclude that exchange is an important mechanism to bring in acetylated histones to a genomic region in addition to the targeted recruitment of the KAT complexes. However, the enrichment of acetylation does not always correlate with histone exchange. Histone exchange levels remain unaffected at the promoters despite loss of Set2, although histone acetylation is reduced compared with the wild type.Citation29 We believe that this could be due to the removal of acetyl marks by a 5′ end specific deacetylase like the Set3 deacetylase complex.Citation19 Interestingly, a recent report has shown that the levels of the Rpd3S specific subunit, Rco1 are greatly increased over the promoter in a SET2 deletion mutant.13The action of these deacetylase complexes could therefore explain the observed decrease in histone acetylation over the promoters upon loss of Set2.
A key question that arises at this point is by what mechanism Set2-mediated H3K36 methylation () prevents histone exchange over coding regions, thereby suppressing both the co-transcriptional acetylation of histones and internal initiation of transcription? In the following sections we discuss two possible mechanisms, both of which may not necessarily be independent of one another.
Methylation of H3K36 Prevents Binding of Histone Chaperones
Histone chaperones play a key role in the assembly and disassembly of nucleosomes. Several histone chaperones including Asf1,Citation31 Spt6Citation32 and the Spt16-containing FACT complexCitation33 have been shown to be involved in the regulation of transcription elongation. It has been suggested that histone chaperones play a role in the capture and reassembly of nucleosomes that are displaced during transcription elongation. Several studies have also pointed to the fact that histone chaperones may facilitate elongation by disassembling nucleosomes ahead of RNAP II. Interestingly, we found that methylation of H3K36 reduces the affinity of the Asf1 histone chaperone for histone H3 as judged from peptide binding studies.Citation29 Asf1 is involved both in the deposition of newly synthesized, pre-acetylated histones onto the DNA during replication, as well as nucleosome disassembly over the promoters during transcription. The presence of the H3K36 methyl mark over coding regions presumably disfavors Asf1 binding, thereby ensuring the retention of existing histones over gene bodies (). While H3K36 tri-methylation reduces the affinity of both Spt6 and Spt16 to bind modified histone H3, H3K36 di-methylation does not seem to affect the binding affinity. This observation is relevant as Spt6 is required to maintain nucleosomal integrity over coding regions,Citation32 presumably over regions enriched for H3K36 di-methylation. Recent studies have indicated that the core subunits of the Rpd3 complex (Rpd3, Ume1 and Sin3) possess histone chaperone activity.Citation34 This gives rise to the interesting possibility that in combination with Rco1 and Eaf3 subunits, the Rpd3 core subunits may act as a H3K36 methyl specific histone chaperone. Therefore, the Rpd3S complex would not only deacetylate nucleosomes methylated at H3K36, but also capture and reassemble the same histones that are displaced by the elongating RNAP II ().
Methylation of H3K36 Recruits Chromatin Remodelers and Suppresses Histone Exchange
Remodeling factors use the energy generated from ATP hydrolysis to slide or evict nucleosomes, thus affecting chromatin organization. Interestingly, we found chromatin remodelers Isw1 and Chd1 associated with H3K36 methylated mononucleosomes isolated from yeast chromatin.Citation12 Isw1, its homolog Isw2 and Chd1 fulfill partially redundant functions. An isw1Δ isw2Δ chd1Δ strain displays synthetic phenotypesCitation35 as well as widespread disruption of nucleosome positioning throughout the yeast genome.Citation36,Citation37
Two distinct Isw1 complexes exist in yeast. Isw1 associates with Ioc3 or Ioc2 and Ioc4 to form two different remodeling complexes, Isw1a and Isw1b respectively, which are thought to target the remodelers to different genomic locations.Citation38 Indeed, Ioc4 preferentially interacts with H3K36 methylated nucleosomes both in vitro and in vivo.Citation39 It contains an N-terminal PWWP domain that preferentially binds trimethylated H3K36 nucleosomes. Deletion or mutation of the Ioc4 PWWP domain results in reduced nucleosome binding in vitro and in vivo.Citation39,Citation40 Similarly, deletion of SET2 abrogates Ioc4 localization to the bodies of genes genome-wide.Citation39 Chd1 is not recruited directly to coding regions through H3K36 methylation either in vivo or in vitro.Citation41,Citation42 Instead, it interacts with RNAP II-associated factors such as the PAF complex and Spt5 and thus ensures localization to actively transcribed genes ().Citation43-Citation45
Using genome-wide ChIP-chip experiments we showed that both Isw1b and Chd1 play important and complementary roles in the retention of H3K36-methylated nucleosomes over ORFs. Deletion of either ISW1, IOC4 or CHD1 causes increased histone exchange over gene bodies.Citation39,Citation46 Simultaneously, these deletions also result in significant increases in histone acetylation over coding regions, while there is no or little change in H3K36me3 levels. These results suggest that the remodelers are able and required to retain hypoacetylated, H3K36 methylated nucleosomes over gene bodies.Citation39 Catalytic activity is required for this process as an ISW1K227R catalytic mutant also resulted in increased histone exchange over coding regions.
Why are two different remodeling enzymes involved in the regulation of ORF chromatin structure? While Isw1 and Chd1 do have overlapping functions, it is important to note that they are also complimentary. The effect of ISW1 deletion on histone turnover was greatest over infrequently transcribed genes; the same set of genes that are also most reliant on Set2 for efficient regulation. In contrast, deletion of CHD1 increased histone exchange over both frequently and infrequently transcribed genes, in agreement with its purported recruitment through RNAP II-associated factors.
Regulation of Internally-Initiated Transcription
In contrast to other chromatin remodelers, the ISWI and CHD families of remodelers have generally been implicated in the repression rather than activation of gene transcription. However, deletion of either ISW1, ISW2 and/or CHD1 does not cause large-scale changes in gene transcription overall.Citation39,Citation47 Rather, deletion of ISW1 and CHD1 does result in the widespread production of both sense and antisense cryptic transcripts,Citation39,Citation48,Citation49 in agreement with its proposed function in limiting histone turnover and acetylation over coding regions.Citation39 In fact there is good overlap between the genes that exhibit cryptic transcription in an isw1Δ chd1Δ strain and those that display increased cryptic transcription and histone exchange in a set2Δ background.Citation39 As expected, the remodelers function within the Set2 pathway as deletion of the remodelers in a set2Δ background does not result in further increases in either the levels of cryptic transcripts produced or histone acetylation observed over coding sequences. Thus, repression of cryptic transcription in wild-type yeast is achieved through the H3K36me-dependent suppression of histone exchange. Isw1b and Chd1 activities ensure the retention of existing, H3K36 methylated and hypoacetylated nucleosomes and disfavors trans-histone exchange ().
Conclusion
Histone exchange involves the replacement of existing nucleosomes with newly synthesized histones, resulting in the removal or dilution of preexisting histone modification marks. By preventing histone exchange, H3K36 methylation ensures their persistence following transcription elongation. H3K36 methylation behaves as a stable transcription memory mark, indicating the passage of RNAP II, which could be removed by either replication-coupled exchange or the targeted recruitment of specific demethylases. Using histone exchange to deliver histone acetylation ensures rapid delivery of the modification at a genome-wide scale. This feature could help regulate events like transcription and replication without depending on the targeted recruitment of the KAT complexes.
Set2-mediated H3K36 methylation uses multiple mechanisms, such as the targeted recruitment of chromatin remodelers and/or lowering the binding affinity for histone chaperones to prevent histone exchange over coding regions (). Consequently, this process allows for the retention of existing histones over gene bodies, with the ultimate aim of suppressing internally-initiated transcripts. Recent data have shown that cryptic, non-coding transcripts may play a key role in the regulation of delicate biological processes such as sporulation.Citation50 Interestingly, these studies suggest that the process of transcription, rather than the non-coding RNA themselves may be key in regulating gene expression.Citation51
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Kulaeva OI, Hsieh FK, Studitsky VM. RNA polymerase complexes cooperate to relieve the nucleosomal barrier and evict histones. Proc Natl Acad Sci U S A 2010; 107:11325 - 30; http://dx.doi.org/10.1073/pnas.1001148107; PMID: 20534568
- Kulaeva OI, Gaykalova DA, Pestov NA, Golovastov VV, Vassylyev DG, Artsimovitch I, et al. Mechanism of chromatin remodeling and recovery during passage of RNA polymerase II. Nat Struct Mol Biol 2009; 16:1272 - 8; http://dx.doi.org/10.1038/nsmb.1689; PMID: 19935686
- Carey M, Li B, Workman JL. RSC exploits histone acetylation to abrogate the nucleosomal block to RNA polymerase II elongation. Mol Cell 2006; 24:481 - 7; http://dx.doi.org/10.1016/j.molcel.2006.09.012; PMID: 17081996
- Bannister AJ, Kouzarides T. Regulation of chromatin by histone modifications. Cell Res 2011; 21:381 - 95; http://dx.doi.org/10.1038/cr.2011.22; PMID: 21321607
- Workman JL. Nucleosome displacement in transcription. Genes Dev 2006; 20:2009 - 17; http://dx.doi.org/10.1101/gad.1435706; PMID: 16882978
- Li B, Howe L, Anderson S, Yates JR 3rd, Workman JL. The Set2 histone methyltransferase functions through the phosphorylated carboxyl-terminal domain of RNA polymerase II. J Biol Chem 2003; 278:8897 - 903; http://dx.doi.org/10.1074/jbc.M212134200; PMID: 12511561
- Krogan NJ, Kim M, Tong A, Golshani A, Cagney G, Canadien V, et al. Methylation of histone H3 by Set2 in Saccharomyces cerevisiae is linked to transcriptional elongation by RNA polymerase II. Mol Cell Biol 2003; 23:4207 - 18; http://dx.doi.org/10.1128/MCB.23.12.4207-4218.2003; PMID: 12773564
- Schaft D, Roguev A, Kotovic KM, Shevchenko A, Sarov M, Shevchenko A, et al. The histone 3 lysine 36 methyltransferase, SET2, is involved in transcriptional elongation. Nucleic Acids Res 2003; 31:2475 - 82; http://dx.doi.org/10.1093/nar/gkg372; PMID: 12736296
- Li J, Moazed D, Gygi SP. Association of the histone methyltransferase Set2 with RNA polymerase II plays a role in transcription elongation. J Biol Chem 2002; 277:49383 - 8; http://dx.doi.org/10.1074/jbc.M209294200; PMID: 12381723
- Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Lee TI, et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell 2005; 122:517 - 27; http://dx.doi.org/10.1016/j.cell.2005.06.026; PMID: 16122420
- Liu CL, Kaplan T, Kim M, Buratowski S, Schreiber SL, Friedman N, et al. Single-nucleosome mapping of histone modifications in S. cerevisiae. PLoS Biol 2005; 3:e328; http://dx.doi.org/10.1371/journal.pbio.0030328; PMID: 16122352
- Kizer KO, Phatnani HP, Shibata Y, Hall H, Greenleaf AL, Strahl BD. A novel domain in Set2 mediates RNA polymerase II interaction and couples histone H3 K36 methylation with transcript elongation. Mol Cell Biol 2005; 25:3305 - 16; http://dx.doi.org/10.1128/MCB.25.8.3305-3316.2005; PMID: 15798214
- Drouin S, Laramée L, Jacques PE, Forest A, Bergeron M, Robert F. DSIF and RNA polymerase II CTD phosphorylation coordinate the recruitment of Rpd3S to actively transcribed genes. PLoS Genet 2010; 6:e1001173; http://dx.doi.org/10.1371/journal.pgen.1001173; PMID: 21060864
- Joshi AA, Struhl K. Eaf3 chromodomain interaction with methylated H3-K36 links histone deacetylation to Pol II elongation. Mol Cell 2005; 20:971 - 8; http://dx.doi.org/10.1016/j.molcel.2005.11.021; PMID: 16364921
- Carrozza MJ, Li B, Florens L, Suganuma T, Swanson SK, Lee KK, et al. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell 2005; 123:581 - 92; http://dx.doi.org/10.1016/j.cell.2005.10.023; PMID: 16286007
- Govind CK, Qiu H, Ginsburg DS, Ruan C, Hofmeyer K, Hu C, et al. Phosphorylated Pol II CTD recruits multiple HDACs, including Rpd3C(S), for methylation-dependent deacetylation of ORF nucleosomes. Mol Cell 2010; 39:234 - 46; http://dx.doi.org/10.1016/j.molcel.2010.07.003; PMID: 20670892
- Keogh MC, Kurdistani SK, Morris SA, Ahn SH, Podolny V, Collins SR, et al. Cotranscriptional set2 methylation of histone H3 lysine 36 recruits a repressive Rpd3 complex. Cell 2005; 123:593 - 605; http://dx.doi.org/10.1016/j.cell.2005.10.025; PMID: 16286008
- Li B, Gogol M, Carey M, Pattenden SG, Seidel C, Workman JL. Infrequently transcribed long genes depend on the Set2/Rpd3S pathway for accurate transcription. Genes Dev 2007; 21:1422 - 30; http://dx.doi.org/10.1101/gad.1539307; PMID: 17545470
- Kim T, Buratowski S. Dimethylation of H3K4 by Set1 recruits the Set3 histone deacetylase complex to 5′ transcribed regions. Cell 2009; 137:259 - 72; http://dx.doi.org/10.1016/j.cell.2009.02.045; PMID: 19379692
- Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell 2004; 116:51 - 61; http://dx.doi.org/10.1016/S0092-8674(03)01064-X; PMID: 14718166
- Ahmad K, Henikoff S. Histone H3 variants specify modes of chromatin assembly. Proc Natl Acad Sci U S A 2002; 99:Suppl 4 16477 - 84; http://dx.doi.org/10.1073/pnas.172403699; PMID: 12177448
- Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell 2002; 9:1191 - 200; http://dx.doi.org/10.1016/S1097-2765(02)00542-7; PMID: 12086617
- Deal RB, Henikoff JG, Henikoff S. Genome-wide kinetics of nucleosome turnover determined by metabolic labeling of histones. Science 2010; 328:1161 - 4; http://dx.doi.org/10.1126/science.1186777; PMID: 20508129
- Kaplan T, Liu CL, Erkmann JA, Holik J, Grunstein M, Kaufman PD, et al. Cell cycle- and chaperone-mediated regulation of H3K56ac incorporation in yeast. PLoS Genet 2008; 4:e1000270; http://dx.doi.org/10.1371/journal.pgen.1000270; PMID: 19023413
- Rufiange A, Jacques PE, Bhat W, Robert F, Nourani A. Genome-wide replication-independent histone H3 exchange occurs predominantly at promoters and implicates H3 K56 acetylation and Asf1. Mol Cell 2007; 27:393 - 405; http://dx.doi.org/10.1016/j.molcel.2007.07.011; PMID: 17679090
- Dion MF, Kaplan T, Kim M, Buratowski S, Friedman N, Rando OJ. Dynamics of replication-independent histone turnover in budding yeast. Science 2007; 315:1405 - 8; http://dx.doi.org/10.1126/science.1134053; PMID: 17347438
- Belotserkovskaya R, Oh S, Bondarenko VA, Orphanides G, Studitsky VM, Reinberg D. FACT facilitates transcription-dependent nucleosome alteration. Science 2003; 301:1090 - 3; http://dx.doi.org/10.1126/science.1085703; PMID: 12934006
- Jamai A, Imoberdorf RM, Strubin M. Continuous histone H2B and transcription-dependent histone H3 exchange in yeast cells outside of replication. Mol Cell 2007; 25:345 - 55; http://dx.doi.org/10.1016/j.molcel.2007.01.019; PMID: 17289583
- Venkatesh S, Smolle M, Li H, Gogol MM, Saint M, Kumar S, et al. Set2 methylation of histone H3 lysine 36 suppresses histone exchange on transcribed genes. Nature 2012; 489:452 - 5; http://dx.doi.org/10.1038/nature11326; PMID: 22914091
- Ginsburg DS, Govind CK, Hinnebusch AG. NuA4 lysine acetyltransferase Esa1 is targeted to coding regions and stimulates transcription elongation with Gcn5. Mol Cell Biol 2009; 29:6473 - 87; http://dx.doi.org/10.1128/MCB.01033-09; PMID: 19822662
- Schwabish MA, Struhl K. Asf1 mediates histone eviction and deposition during elongation by RNA polymerase II. Mol Cell 2006; 22:415 - 22; http://dx.doi.org/10.1016/j.molcel.2006.03.014; PMID: 16678113
- Kaplan CD, Laprade L, Winston F. Transcription elongation factors repress transcription initiation from cryptic sites. Science 2003; 301:1096 - 9; http://dx.doi.org/10.1126/science.1087374; PMID: 12934008
- Jamai A, Puglisi A, Strubin M. Histone chaperone spt16 promotes redeposition of the original h3-h4 histones evicted by elongating RNA polymerase. Mol Cell 2009; 35:377 - 83; http://dx.doi.org/10.1016/j.molcel.2009.07.001; PMID: 19683500
- Chen XF, Kuryan B, Kitada T, Tran N, Li JY, Kurdistani S, et al. The Rpd3 core complex is a chromatin stabilization module. Curr Biol 2012; 22:56 - 63; http://dx.doi.org/10.1016/j.cub.2011.11.042; PMID: 22177115
- Tsukiyama T, Palmer J, Landel CC, Shiloach J, Wu C. Characterization of the imitation switch subfamily of ATP-dependent chromatin-remodeling factors in Saccharomyces cerevisiae. Genes Dev 1999; 13:686 - 97; http://dx.doi.org/10.1101/gad.13.6.686; PMID: 10090725
- Gkikopoulos T, Schofield P, Singh V, Pinskaya M, Mellor J, Smolle M, et al. A role for Snf2-related nucleosome-spacing enzymes in genome-wide nucleosome organization. Science 2011; 333:1758 - 60; http://dx.doi.org/10.1126/science.1206097; PMID: 21940898
- Xella B, Goding C, Agricola E, Di Mauro E, Caserta M. The ISWI and CHD1 chromatin remodelling activities influence ADH2 expression and chromatin organization. Mol Microbiol 2006; 59:1531 - 41; http://dx.doi.org/10.1111/j.1365-2958.2005.05031.x; PMID: 16468993
- Vary JC Jr., Gangaraju VK, Qin J, Landel CC, Kooperberg C, Bartholomew B, et al. Yeast Isw1p forms two separable complexes in vivo. Mol Cell Biol 2003; 23:80 - 91; http://dx.doi.org/10.1128/MCB.23.1.80-91.2003; PMID: 12482963
- Smolle M, Venkatesh S, Gogol MM, Li H, Zhang Y, Florens L, et al. Chromatin remodelers Isw1 and Chd1 maintain chromatin structure during transcription by preventing histone exchange. Nat Struct Mol Biol 2012; 19:884 - 92; http://dx.doi.org/10.1038/nsmb.2312; PMID: 22922743
- Maltby VE, Martin BJ, Schulze JM, Johnson I, Hentrich T, Sharma A, et al. Histone H3 lysine 36 methylation targets the Isw1b remodeling complex to chromatin. Mol Cell Biol 2012; 32:3479 - 85; http://dx.doi.org/10.1128/MCB.00389-12; PMID: 22751925
- Li B, Jackson J, Simon MD, Fleharty B, Gogol M, Seidel C, et al. Histone H3 lysine 36 dimethylation (H3K36me2) is sufficient to recruit the Rpd3s histone deacetylase complex and to repress spurious transcription. J Biol Chem 2009; 284:7970 - 6; http://dx.doi.org/10.1074/jbc.M808220200; PMID: 19155214
- Quan TK, Hartzog GA. Histone H3K4 and K36 methylation, Chd1 and Rpd3S oppose the functions of Saccharomyces cerevisiae Spt4-Spt5 in transcription. Genetics 2010; 184:321 - 34; http://dx.doi.org/10.1534/genetics.109.111526; PMID: 19948887
- Simic R, Lindstrom DL, Tran HG, Roinick KL, Costa PJ, Johnson AD, et al. Chromatin remodeling protein Chd1 interacts with transcription elongation factors and localizes to transcribed genes. EMBO J 2003; 22:1846 - 56; http://dx.doi.org/10.1093/emboj/cdg179; PMID: 12682017
- Krogan NJ, Kim M, Ahn SH, Zhong G, Kobor MS, Cagney G, et al. RNA polymerase II elongation factors of Saccharomyces cerevisiae: a targeted proteomics approach. Mol Cell Biol 2002; 22:6979 - 92; http://dx.doi.org/10.1128/MCB.22.20.6979-6992.2002; PMID: 12242279
- Warner MH, Roinick KL, Arndt KM. Rtf1 is a multifunctional component of the Paf1 complex that regulates gene expression by directing cotranscriptional histone modification. Mol Cell Biol 2007; 27:6103 - 15; http://dx.doi.org/10.1128/MCB.00772-07; PMID: 17576814
- Radman-Livaja M, Quan TK, Valenzuela L, Armstrong JA, van Welsem T, Kim T, et al. A key role for Chd1 in histone H3 dynamics at the 3′ ends of long genes in yeast. PLoS Genet 2012; 8:e1002811; http://dx.doi.org/10.1371/journal.pgen.1002811; PMID: 22807688
- Pointner J, Persson J, Prasad P, Norman-Axelsson U, Strålfors A, Khorosjutina O, et al. CHD1 remodelers regulate nucleosome spacing in vitro and align nucleosomal arrays over gene coding regions in S. pombe. EMBO J 2012; 31:4388 - 403; http://dx.doi.org/10.1038/emboj.2012.289; PMID: 23103765
- Hennig BP, Bendrin K, Zhou Y, Fischer T. Chd1 chromatin remodelers maintain nucleosome organization and repress cryptic transcription. EMBO Rep 2012; 13:997 - 1003; http://dx.doi.org/10.1038/embor.2012.146; PMID: 23032292
- Shim YS, Choi Y, Kang K, Cho K, Oh S, Lee J, et al. Hrp3 controls nucleosome positioning to suppress non-coding transcription in eu- and heterochromatin. EMBO J 2012; 31:4375 - 87; http://dx.doi.org/10.1038/emboj.2012.267; PMID: 22990236
- van Werven FJ, Neuert G, Hendrick N, Lardenois A, Buratowski S, van Oudenaarden A, et al. Transcription of two long noncoding RNAs mediates mating-type control of gametogenesis in budding yeast. Cell 2012; 150:1170 - 81; http://dx.doi.org/10.1016/j.cell.2012.06.049; PMID: 22959267
- Kim T, Xu Z, Clauder-Münster S, Steinmetz LM, Buratowski S. Set3 HDAC mediates effects of overlapping noncoding transcription on gene induction kinetics. Cell 2012; 150:1158 - 69; http://dx.doi.org/10.1016/j.cell.2012.08.016; PMID: 22959268