Abstract
Females of the SWR/Bm (SWR) inbred mouse strain possess a unique susceptibility to juvenile-onset tumors originating from the granulosa cells (GC) of the ovarian follicles. Tumor susceptibility is an inherited, polygenic trait in SWR females, minimally involving an oncogenic Granulosa cell tumor susceptibility (Gct) locus on chromosome (Chr) 4 (Gct1), and two GC tumor susceptibility modifier genes mapped to distinct regions of Chr X (Gct4 and Gct6). Shifts in the frequency of GC tumor initiation in the SWR female population from low penetrance to moderate penetrance, or phenotype switching between GC tumor-susceptible and GC tumor-resistant, is strongly influenced by the allelic contributions at Gct4 and Gct6. In addition to the allele-specific effects, GC tumor susceptibility is controlled by the mode of X-linked transmission with a dominant, paternal parent-of-origin effect. We took advantage of the robust paternal effect with a recombinant male progeny testing strategy to resolve the Gct4 locus interval to 1.345 million base (Mb) pairs. Based on the mapping resolution and the phenotype sensitivity to endogenous and exogenous androgen exposure, a promising candidate for Gct4 identity is the androgen receptor (Ar) gene. We explored the mechanism of allelic variation for Ar between SWR (low penetrance allele) and SJL/Bm (SJL) (moderate penetrance allele) using an SWR.SJL-X congenic strain resource and a quantitative gene expression method. We report the low GC tumor penetrance allele of the SWR strain correlates with significantly reduced Ar transcript levels in the female ovary at the pubertal transition.
Introduction
In female patients, granulosa cell (GC) tumors of the ovary are classified into two general categories (adult-type or juvenile-type) based on both clinical and pathological criteria, such as tumor histology, nuclear morphology and the potential for disease recurrence.Citation1 As the name suggests, GC tumors of the juvenile-type are most commonly found in children and young women. A comprehensive review of 125 cases of juvenile-type GC tumors found that 78% of the cases occurred in individuals less than 20 y of age, with 44% of the cases presenting in girls 10 y of age or younger, suggesting a strong genetic contribution to susceptibility.Citation1 The relative rarity of cases of juvenile-type GC tumors has precluded human genetic linkage studies to identify candidate susceptibility genes or modes of transmission that confer tumor risk. A somatic mutation in the forkhead box L2 (FOXL2) transcription factor (p.C134W) has been identified in adult-type GC tumors with a high degree of consistency between independent research cohorts, suggesting a strong mechanistic link.Citation2,Citation3 The etiology for juvenile-type GC tumors is not explained by the same FOXL2 mutation, either germline or somatically acquired, suggesting a novel pathway of tumorigenic initiation. Lost or reduced expression of FOXL2 has been associated with 50% of juvenile-type GC tumors of high grade, suggesting a role for FOXL2 as a suppressor of GC tumor progression, but not a requirement for tumor initiation.Citation4 To this end, we are pursuing tumor susceptibility genes in the SWR/Bm (SWR) mouse model of spontaneous, juvenile-onset GC tumor development that parallels the histological, endocrinological and developmental timing characteristics of human juvenile-type GC tumors.Citation5,Citation6
Approximately 1% of SWR inbred female mice develop unilateral or bilateral ovarian GC tumors that are easily identified by 8 weeks of age as large solid or cystic masses ().Citation5 GC tumor initiation in SWR females is restricted to the time of ovarian maturation at puberty, within the window of 3–5 weeks of age.Citation7 Beyond this time frame, females that have not developed GC tumors are no longer at risk, retain their fertility and develop no other visible pathological changes in the ovary through 12 mo of age.Citation8 The penetrance of GC tumor initiation is significantly increased (≈20% incidence) in SWR females with the introduction of androgenic steroids at puberty, while introduction of estrogen suppresses tumor initiation, indicating a strong endocrine contribution to susceptibility.Citation9,Citation10
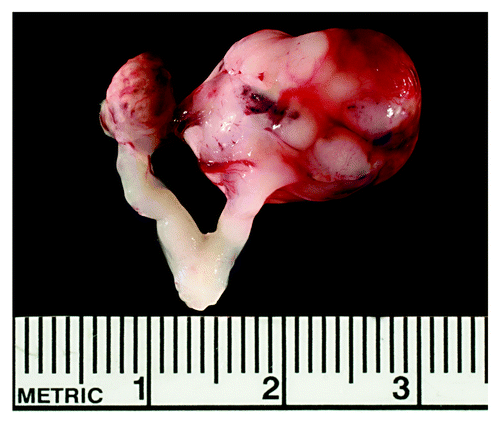
Figure 1. Bilateral GC tumor specimen. Spontaneous GC tumors in SWR-derived mice are easily recognized as large, 5–15 mm cystic or solid masses with blood filled spaces when compared with a normal ovary of 2–3 mm. This image shows a representative bilateral GC tumor specimen with the uterus still attached. The specimen was isolated from an 8 wk old SWR.SJL-X3 congenic female that carries the Gct4J modifier allele. The GC tumor on the left is less vascularized and more necrotic than the larger, highly vascularized tumor on the right.
Genetic mapping studies for the GC tumor susceptibility phenotype in SWR female mice have been pursued as a means to elucidate novel pathways and genetic mechanisms for juvenile-onset GC tumorigenesis. These studies demonstrated that GC tumor susceptibility is a polygenic, heritable trait involving several Granulosa cell tumor (Gct) susceptibility loci on the autosomes and Chr X.Citation11,Citation12 Both F2 intercross and recombinant inbred strain mapping studies confirmed the SWR allele at the Gct1 locus on Chr 4 is both necessary for GC tumor development and sensitive to dehydroepiandrosterone (DHEA). The Gct1 locus has been fine-mapped to 1.31 Mb on mouse Chr 4 using a congenic strain mapping strategy.Citation6,Citation12,Citation13 When the SWR autosomal contribution to GC tumor susceptibility was genetically fixed, the activities of two independent, tumor incidence modifier alleles were revealed on Chr X: (1) Gct4 was originally identified in the SJL inbred strain that served as a GC tumor resistant mapping partner for the SWR strain and (2) Gct6 was revealed on distal Chr X following transfer of a 72.7 Mb Chr X donor segment from the Castaneus/Ei (CAST) inbred strain as part of an SWR.CAST-X congenic development strategy originally created to fine map Gct4.Citation11,Citation14 The susceptibility loci identified in the mouse model do not overlap with candidate loci identified for human GC tumors based on somatically acquired mutations or altered expression in adult-type (FOXL2) or juvenile-type GC (FOXL2, GNAS) tumors; rather, they may reflect unique, heritable alleles that support juvenile-onset GC tumor initiation events.Citation2,Citation3,Citation15
In the presence of an autosomal SWR strain background, allelic variation at Gct4 modifies GC tumor incidence in the susceptible female population, increasing the phenotypic penetrance from ≤ 1% to ≈20% spontaneous tumor incidence in the absence of hormonal intervention. Gct4SW is a low incidence allele, while Gct4CA and Gct4J support increased penetrance in F1 generation daughters. Based on the allelic difference between Gct4SW and Gct4J, the locus was coarsely mapped with an SWR.SJL-X congenic strain series. The most informative subline—SWR.SJL-X5—localized Gct4 between the markers DXMit45 and DXMit170, an ≈25 Mb interval encompassing the Androgen receptor (Ar) gene and many other candidates.Citation14
The CAST genome was introduced on Chr X in a new SWR.CAST-X congenic strain to increase the number of polymorphisms for high-resolution genetic mapping of the Gct4 locus, but this divergent strain subsequently introduced a unique allele on Chr X that has a dominant influence on the GC tumor susceptibility phenotype. Allelic variation at the Gct6 locus is either permissive or suppressive for spontaneous GC tumor initiation, with tumor suppressor activity contributed by the CAST strain allele (Gct6CA).Citation13 In contrast, both Gct6SW and Gct6J are permissive alleles; thus, Gct6 was not revealed as a functional GC tumor susceptibility locus in the earliest mapping crosses between SWR and SJL strains. We are pursuing the identity of the X-linked Gct candidates given their strong influence over spontaneous and androgen-induced GC tumorigenesis.
In addition to genetic variation, an epigenetic phenomenon in the form of a paternal parent-of-origin effect dominates the activity of the X-linked GC tumor susceptibility alleles in SWR female mice. This unexpected phenomenon was revealed through the differential activity of the Gct4 and Gct6 alleles derived from the SWR, SJL and CAST strains when the X-linked susceptibility alleles were maternally (XM) or paternally (XP) inherited by daughter offspring. In this research report, we have made use of a ten-generation SWR.CAST-X congenic strain resource to confirm the paternal, parent-of-origin effect for the tumor suppressor activity of the Gct6 locus and to fine map the Gct4 locus to a minimal genetic interval and short list of candidate genes with a recombinant male Chr X mapping strategy. Using SWR inbred and SWR.SJL-X5 congenic subline females that retain Gct4J, we report on the mechanism for allelic variation at Gct4 related to the quantitative expression of the androgen receptor (Ar) gene in the ovary, as the primary candidate for Gct4 identity.
Results
Paternal transmission of Gct4CA and Gct6CA allele effects for GC tumor susceptibility
Female mice derived from the homozygous SWR.CAST-X strain were examined for GC tumor susceptibility under the challenge of androgenic steroid supplementation. shows that SWR.CAST-X females homozygous for the CAST donor segment, inclusive of Gct4 and Gct6 loci, are resistant to GC tumor initiation despite the pubertal administration of DHEA or testosterone. Furthermore, tumor resistance is dominantly conferred by Chr XP transmission of the CAST segment, as shown by the resistance of (SWR x SWR.CAST-X) F1 generation female offspring to the development of GC tumors in the presence of DHEA or testosterone. When the CAST-X segment is maternally transmitted, complete tumor suppression is lifted and moderate GC tumor incidence is observed in (SWR.CAST-X x SWR) F1 generation female offspring in the presence of DHEA (7.7%) ().
Table 1. Ovarian GC tumor incidence in female offspring derived from SWR inbred, SWR.CAST-X congenic lines or reciprocal breeding crosses
The SWR.CAST-X1 congenic subline was derived from the SWR.CAST-X founder congenic strain to eliminate GC tumor suppression introduced by the Gct6CA locus while maintaining the Gct4CA locus. As an established homozygous colony, we observed that SWR.CAST-X1 females have enhanced, spontaneous GC tumor incidence in the absence of exogenous androgenic stimulation (20.4%) (). In order to expedite Gct4 mapping with the SWR.CAST-X1 resource, we first confirmed that the paternal parent-of-origin effect was supported by SWR.CAST-X1 males. SWR.CAST-X1 breeders were put through a reciprocal breeding scheme with SWR mates and spontaneous GC tumor incidence was measured in the F1 daughter offspring. We observed that paternal inheritance of the CAST-X1 segment supported GC tumorigenesis incidence at a similar magnitude to the homozygous condition (27.0%), and that maternal inheritance of the same donor segment showed significantly reduced support for GC tumor initiation (4.9%) (). The allelic variants and robust paternal effect for the Gct4CA GC tumor susceptibility locus was subsequently applied to a male progeny testing scheme to refine the Gct4 candidate gene region.
Gct4 interval mapping
Screening for informative progeny test males useful to map the Gct4 locus progressed until we achieved a minimal genetic resolution and a short list of candidate genes. shows the haplotype map for the resolved Gct4 locus based on the six most informative Chr XP haplotypes identified from hundreds of males genotyped and selected males chosen for progeny testing with SWR mates. The Chr X haplotype for each male in the vicinity of the Gct4 locus is represented as a vertical column, while the overall GC tumor incidence observed in the F1 daughter offspring is indicated below the haplotype for each tested male. The phenotype distinction for mapping Gct4 was the stronger tumorigenic support of the Gct4CA allele vs. the weaker support of the Gct4SW allele. The allelic effects are confirmed by the GC tumor incidence of the F1 daughter population for this subset of males tested, with Gct4CA supporting an average GC tumor incidence of 16% (48/301), vs 0% (0/75) recorded for the single Gct4SW carrier male shown (). Of note, 0% incidence in this case does not imply GC tumor resistance, since the Gct6 locus in all males in this screen was genetically fixed to maintain a Gct6SW tumor susceptibility allele that is permissive for GC tumorigenesis. Informative males 1 through 6 refined the Gct4 locus interval to 1.345 Mb between the markers DXamd3 and DXamd27. lists the annotated genes within this interval that are candidates for Gct4 identity, inclusive of the Ar gene.
Figure 2. Male mice carrying unique combinations of CAST and SWR genome in the region of the Gct4 locus on Chr X were mated to SWR dams and the GC tumor incidence measured in the F1 generation female offspring. White boxes represent SWR alleles present at the DNA marker or gene and black boxes represent CAST alleles. Of the haplotypes shown for six unique animals, males 3, 4 and 6 place the distal interval boundary for the moderate GC tumor susceptibility allele Gct4CA at 99.015 Mb (marker DXamd27), while male 5 places the proximal boundary of the interval at 97.670 Mb (DXamd3). Only male 4 carries the GC tumor susceptibility allele Gct4SW.
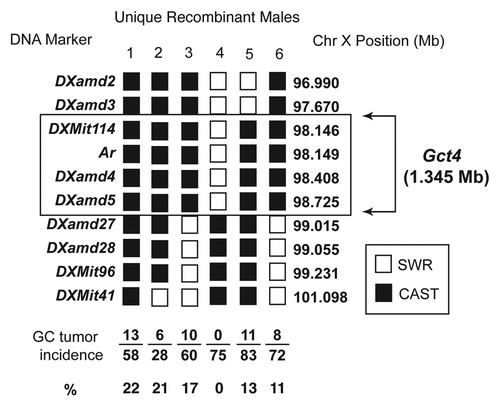
Table 2. Candidate genes within the Gct4 locus interval
Quantitative expression analysis of the Ar gene
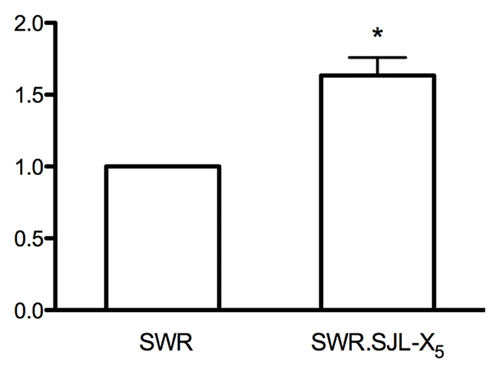
The Ar gene was previously considered a candidate for Gct4 identity based on the activity of androgens to stimulate GC tumor initiation in SWR inbred females, although no coding sequence polymorphisms were identified in the Ar gene between SWR and SJL strains that could explain the allelic differences between Gct4SW and Gct4J.Citation11,Citation14 Given the refined mapping resolution of 1.345 Mb for Gct4 and retention of Ar as a candidate gene, we examined the potential for differential Ar gene expression between SWR and SWR.SJL-X5 pubertal ovaries as an alternate explanation for the allelic difference between strains. shows the gene expression ratios for Ar normalized to the Actin β (Actb) gene in the ovaries of SWR females collected at 4 weeks of age relative to age-matched homozygous SWR.SJL-X5 females that have a higher (≈20 fold) spontaneous incidence of GC tumors.Citation14 Using a rigorous standard curve approach for qPCR analyses, we measured a statistically significant increase in Ar gene expression in the SWR.SJL-X5 ovaries (1.6 ± 0.13-fold) relative to age-matched SWR ovaries.
Figure 3. Quantitative expression of the Ar gene. Ovaries from a minimum of five individual females of the SWR and SWR.SJL-X5 strains were compared for quantitative expression of the Ar gene relative to the Actb reference gene by qPCR. A significantly increased expression was measured in the SWR.SJL-X5 ovaries, equal to 1.6 ± 0.13 fold that of the SWR strain (p = 0.0038).
Discussion
Ovarian tumors diagnosed in girls and young women are most commonly derived from germ cells (teratomas) or mesenchymal tissues (sex cord-stromal tumors), in contrast to the epithelial-derived ovarian carcinomas that usually appear in women of post-menopausal age. GC tumors of the sex cord-stromal class are found in women at both ends of the reproductive spectrum and are placed into two categories—juvenile-type and adult-type—based on differences in the age of onset and other cytological, histological and most recently, molecular genetic features.Citation2 Like adult-type GC tumors, juvenile-type cases have the potential for recurrence and life-threatening metastases, with the additional consequence of fertility loss if the ovaries are removed at an early age.Citation16-Citation18 The SWR mouse model for juvenile-onset, spontaneous GC tumor development shares histological, endocrinological and malignant features with the human tumors, so we have pursued the identity of GC tumor susceptibility genes in the SWR mouse for translation to the human condition as a means to improve prevention, detection and therapeutic strategies. Through this genetic mapping process, we have uncovered a robust epigenetic contribution of the paternal Chr X in mice that has a major influence on GC tumor susceptibility.
All the biological evidence gathered from the SWR mouse model indicates that the site of action for Gct susceptibility genes lies within the GC cells. Alterations of the hormonal signaling profiles within the hypothalamic-pituitary-ovarian axis have been excluded by the fact that grafted, genetically susceptible ovaries can form tumors in fertile female mouse recipients that are not genetically susceptible.Citation7 GC tumors do not form, however, if the transplant recipients are gonadotropin-deficient (hypogonadal, hpg/hpg) mutants that do not undergo sexual maturation. The ovarian transfer experiments indicate that the normal endocrine stimulation during ovarian maturation is necessary and sufficient for GC tumor initiation, and that the maturing SWR hypothalamic-pituitary-ovarian axis is not unique in this regard.Citation7
GC tumor initiation in SWR female mice is restricted to the time of ovarian maturation at puberty, suggesting that the Gct susceptibility genes have a short window of influence over GC fate.Citation7 During puberty in mice, the first wave of ovarian follicles mature, but these growing follicles subsequently undergo atresia rather than maturation and ovulation.Citation19 Follicular atresia also occurs in subsets of follicles in any subsequent reproductive cycle, but this does not correlate with GC tumor initiation potential in this model, suggesting that other GC attributes are unique at puberty. It has been reported that the GC progenitors for the earliest cohorts of ovarian follicles have a different cellular origin than subsequent cohorts of maturing follicles.Citation20 Molecular identification of the Gct candidates will open up complementary and functional approaches to address the hypothesis that a unique ovarian follicle cohort is the GC population of origin for juvenile-type GC tumors.
The SWR.CAST-X congenic strain and SWR.CAST-X1 subline have supported our goal to fine-map the X-linked Gct4 locus that functions as a modifier for GC tumor initiation events. Within the 1.345 Mb interval for Gct4, the Ar gene remains the primary candidate for shared identity with Gct4 based on the testosterone sensitivity of the phenotype in SWR female mice. We have previously examined the Ar gene for coding polymorphisms between SWR, SJL and CAST strains, but this did not provide a clear association with their phenotypic effects, since polymorphisms were identified only in the CAST strain.Citation14 Further support for the Ar gene sharing identity with Gct4 comes from our quantitative gene expression analysis between SWR and SWR.SJL-X5 female ovaries. There is a modest but statistically significant increase in Ar expression in the SWR.SJL-X5 ovary compared with the SWR ovary. We hypothesize a unique regulatory variant of the Ar gene exists in the SWR strain that reduces Ar protein production, such that exogenous androgen administration overcomes this difference and equalizes trait penetrance with SJL or CAST modifier alleles. The Ar protein is a known regulator of ovarian follicle maturation over the reproductive lifespan of the mouse.Citation21 Our model suggests Ar plays a similar role in the unique follicle cohort that has the potential for GC tumor initiation in SWR female mice. We analyzed the Ar expression pattern in a minimum of five females per strain and noted very consistent expression profiles, suggesting that intra-strain variation in Ar expression is not the primary contributor to the stochastic nature of GC tumor initation in this model system.
The Ar gene itself is not essential for life, and there are many variations in the coding sequence evident across human populations; some of these polymorphisms debilitate Ar’s biological functions leading to clinical signs of Androgen Insensitivity Syndrome (AIS) in males.Citation22 We have not observed any signs of AIS, aberrant male development or loss of fertility in SWR inbred strain males, suggesting that genetic polymorphisms leading to a modest reduction in the expression level of Ar does not measurably influence male development or fertility. Conversely, the Ar expression difference may be sex-specific and epigenetically regulated in SWR female ovaries harboring two copies of the Ar gene on Chr X; however, there is no evidence to date that the Ar gene escapes X-inactivation in mice. We are pursuing the genetic explanation as the primary hypothesis for strain-specific allelic differences at the level of transcriptional regulation, with complete sequence analysis of the Ar locus in the SWR strain.
The Gct6 locus was an unexpected discovery that arose from our congenic mapping process and poses a good example of a functional epistatic interaction in vivo. The CAST allele at Gct6 plays a tumor suppressor role in this model of reproductive cancer that is dependent upon endrocrine signaling. The SWR and SJL alleles for Gct6 were essentially invisible in prior mapping strategies, as they had no differential modifying effect on the GC tumor incidence phenotype, nor did they behave as tumor suppressor loci. The Gct6CA allele suppresses the action of both DHEA and testosterone in female mice that carry the autosomal susceptibility alleles for tumor initiation; this suggests the Gct6CA allele interferes with the oncogenic mechanism triggered by the DHEA-responsive Gct1SW locus on Chr 4, or suppresses androgen signaling through Ar. The potential to identify a novel suppressor of androgenic hormone signaling based on Gct6CA identity may have direct benefit for GC tumor prevention or therapeutic targeting of other androgen-sensitive cancers.
The paternal effect for the X-linked GC tumor susceptibility alleles has not yet been mechanistically explained. When considering phenotypes controlled by Chr X in females, the process of Chr X inactivation must be considered. The X-inactivation center (XIC) at ≈100 Mb on mouse Chr X can exhibit strain-specific bias for which Chr maintains transcriptional activity (XA) vs. the Chr that is functionally silenced (XI). The CAST strain has a strong allele compared with other inbred strain alleles in the context of XA bias.Citation23 The SWR.CAST-X and SWR.CAST-X1 lines include the XIC locus in the CAST congenic segment; however, bias in the X-inactivation ratios does not explain the paternal effect observed for the SWR.SJL-X5 congenic line, which carries a relatively small piece of the SJL-derived Chr X that excludes the XIC.Citation14 Progress toward understanding this epigenetic phenomenon will proceed in parallel with the identification of candidate gene sequence polymorphisms that support allele-specific gene expression assays to address whether or not the ovary, or a specific subset of ovarian follicles, exhibit imprinted or biased expression of candidate GC tumor susceptibility genes. The paternal mode of transmission may or may not be relevant to the human condition, since there is significant variability at the level of epigenetic regulation for Chr X between mice and humans; for example, human female cells are estimated to have 15% of the X-linked genes escape X inactivation compared with 3% of the genes in female mice.Citation24 As the GC tumor susceptibility gene identities are translated to human cases, the significance of the mode of transmission will be further investigated for similar parent-of-origin effects.
In summary, we have identified two modifier genes on Chr X that impact juvenile-type GC tumor development in female mice. In search of a modifier locus for GC tumor susceptibility, the mapping resolution achieved for the Gct4 locus has reduced the genetic interval with particular emphasis on the Ar gene as the primary candidate based on endocrine responsiveness and differential expression of strain-specific Ar alleles. The identification and validation of both Gct4 and Gct6 susceptibility genes in the mouse will provide a foundation for the pursuit of genetic and epigenetic features that contribute to pediatric cases of juvenile-type GC tumors.
Methods
Mouse housing and nutrition
This investigation proceeded across two institutions: The Jackson Laboratory (TJL) and Memorial University of Newfoundland (MUN). For Gct locus mapping and progeny tests, SWR inbred, SWR.SJL-X3, SWR.SJL-X5, SWR.CAST-X and SWR.CAST-X1 congenic mice were maintained in a research colony at TJL, housed under 14:10 h light/dark cycles with pasteurized NIH-31 diet (Purina Mills Intl.) plus HCL-acidified water (pH 2.8–3.2) ad libitum. Following an institutional move (AMD), the strains were transferred to a specific pathogen free barrier facility in the Faculty of Medicine at MUN. Mice were subsequently housed under 12:12 h light/dark cycles and provided Laboratory Autoclavable Rodent Diet 5010 food (Purina Mills Intl.) and autoclaved water ad libitum. Spontaneous and androgen-induced GC tumor susceptibility was measured and found comparable at the new facility before molecular analyses were performed.
Females were weaned at 20 to 23 d of age and housed in groups of 2 to 5 animals per cage in 27.9 cm (L) × 17.8 cm (W) × 12.7 cm (H) rodent cages with high-profile filtered lids, containing sterilized White pine shavings (TJL), or sterilized Bed-O-Cobs® corn-cob bedding material (MUN) (The Andersons). All animal procedures performed at each institution were approved by their respective Animal Care and Use Committees.
Hormonal interventions in SWR.CAST-X female mice
We have previously reported the development of a ten-generation (N10) backcross congenic strain, SWR.CAST-X, that maintains an SWR strain autosomal background but carries a large, homozygous segment of the CAST genome on Chr X.Citation14 The CAST donor segment is 72.7 Mb from DXMit109 to DXMit35, inclusive of the Gct4 locus positioned near 95 Mb and the Gct6 locus that lies ≈38 Mb distal to Gct4. Based on the observation of over 300 homozygous, young adult or aged breeding female mice, SWR.CAST-X females are resistant to spontaneous GC tumor development, despite the presence of fundamental GC tumor susceptibility alleles derived from SWR at Chr 4 (Gct1) and other autosomal loci linked to the phenotype. To test the extent of GC tumor resistance in the presence of androgenic challenge, SWR.CAST-X females homozygous for the CAST-X segment, or N11F1 generation females derived from reciprocal matings between SWR and SWR.CAST-X, were administered DHEA or testosterone (Steraloids Inc.). Androgens were administered to pre-pubertal females (age 20 to 24 d) in the form of a 1.0 cm steroid-powder filled capsule made from Silastic tubing (1.98 mm inner diameter × 3.18 mm outer diameter) (Dow Corning) capped with glass beads. Capsules were implanted subcutaneously on the back at the time of weaning under isoflurane anesthesia (Baxter Corporation), closed with stainless steel wound clips and treated post-operatively with carprofen (5 mg/kg BW) analgesic (Pfizer). For phenotypic examination, female mice were necropsied at 8 weeks of age and ovaries were examined for GC tumors. GC tumors present as unilateral or bilateral, cystic or solid hemorrhagic masses that are macroscopically identifiable (). Females with either unilateral or bilateral GC tumors were counted as one affected animal for statistical purposes.
Breeding strategies for the development of Chr X recombinant males
A GC tumor suppressor allele was first identified during a recombinant male progeny test strategy that was designed to map the robust tumor support conferred by the Gct4J allele.Citation14 The first round of progeny testing revealed that Gct4J and Gct4CA had equivalent tumor modifier activity, and that a unique allele more distal on Chr X had tumor suppressor activity (Gct6CA) when compared with the tumor permissive action of Gct6SW or Gct6J. To improve upon the 25 Mb mapping resolution for Gct4 with the polymorphic potential of the SWR.CAST-X (Gct4CA; Gct6CA) congenic line, we recognized the recombinant male mapping strategy would fail if the Gct6CA resistance locus inhibited GC tumor development, thereby masking the effect of the Gct4 modifier action. We first set out to reduce the CAST-X congenic segment, creating one homozygous subcongenic line that re-instated tumor-permissive SWR alleles on distal Chr X at the Gct6 locus. Following a similar N2F1 cross between SWR.CAST-X females and SWR males, a unique recombinant male was chosen to establish a new subline - SWR.CAST-X1 - that maintains CAST genome from DXMit109 to DXMit149 (124.5 Mb) and re-instates SWR genome on the more distal portion of Chr X. Of note, the founder male for SWR.CAST-X1 was part of the first male progeny testing strategy, and the Gct4CA; Gct6SW combination induced 31% spontaneous GC tumor incidence in F1 daughter generation offspring with SWR dams (n = 77) (reported as Male #10 in ref. Citation14). The robustness of the paternal, parent-of-origin effect for the Gct4CA allele was tested with a measure of the spontaneous GC tumor incidence in a minimum of 50 females in the SWR.CAST-X1 homozygous condition, and following reciprocal matings between SWR and SWR.CAST-X1 breeders. Once the paternal parent-of-origin effect was confirmed with reciprocal crosses, the SWR.CAST-X1 homozygous congenic subline was used to generate males with unique recombinations between SWR and the CAST-X1 segment between DXMit45 to DXMit170, the interval boundaries for Gct4 determined prior with the SWR.SJL-X(n) congenic series.Citation14 Selected males with unique genetic recombinations across the Gct4 interval were mated with SWR dams to determine the GC tumor incidence in F1 generation daughters.
Genotyping protocol for recombinant male selection
All males with potential Chr X recombinations were genotyped prior to selection for progeny testing. Genomic DNA was isolated from 1–2 mm tail tip biopsies using a sodium hydroxide (NaOH) extraction procedure: tail tips were submerged in 500 μl of NaOH (50 mM) and heated at 95°C for 10 min, neutralized with 50 μl of TRIS-HCl (1M, pH 8.0), centrifuged at 13,000 rpm for 5 min and the supernatant transferred to a clean microcentrifuge tube. The DNA was PCR amplified for simple sequence length polymorphic (SSLP) markers around Gct4 using a MasterTaq Kit (5 PRIME Inc.). Each 10 μL PCR reaction contained 5.55 μL of distilled water, 2 μL of 5X TaqMaster PCR enhancer heated to 65°C, 1 μL of 10X reaction buffer, 0.2 μL of 10 mM dNTPs (Life Technologies), 0.2 μL each of 10 μM forward and reverse primers (Integrated DNA Technologies), 0.05 μL of Taq DNA polymerase, and 1 μL of DNA template. PCR conditions were as follows: 97°C for 30 sec; 39 cycles of 94°C for 15 sec, 55°C for 30 sec, and 72°C for 30 sec; 72°C for 10 min; 4°C hold. Informative DNA markers were downloaded from the Mouse Genome Database (DXMit114, DXMit96 and DXMit41).Citation14,Citation25 Custom DNA markers were also designed in-house around novel SSLPs between CAST and SWR alleles in the region of Gct4 (Table S1). SSLP PCR products were separated by horizontal electrophoresis in 4% MetaPhor agarose slab gels (Lonza), 1 x Tris-Boric acid-EDTA buffer at 100 V for 3 h. The bands were visualized by ethidium bromide staining and imaged for allele scoring purposes with a U-Genius imaging system (Syngene). A restriction fragment polymorphism (RFLP) assay for the Ar gene informative for CAST and SWR allele distinction was performed as previously described.Citation14
Male progeny testing and spontaneous GC tumor phenotyping
Males with unique recombinations around the Gct4 locus were selected for progeny testing with multiple SWR dams to collect a minimum cohort of 50 F1 generation daughter offspring for GC tumor phenotyping. At 8 weeks of age, F1 daughter generation females were euthanized with carbon dioxide, necropsied and scored for spontaneous GC tumor development following macroscopic examination of the ovaries.
Quantitative PCR of the Ar gene
Mouse ovaries were collected from six SWR and five SWR.SJL-X5 females at the age of 4 weeks for quantification of Ar transcript relative to the Actb gene. Two ovaries were pooled to create one RNA sample per female. Ovaries were collected and homogenized in RNase free tubes containing Trizol reagent (Life Technologies) and ceramic beads (Precellys, 1.4 mm) with two 15 sec pulses at 5,000 rpm on a Precellys 24 Dual Tissue Homogenizer (Bertin Technologies). Total RNA was isolated using the Trizol reagent protocol. Extracted RNA was purified using RNeasy MinElute Cleanup kit after DNase I (Qiagen) digestion according to the manufacturer's instructions, followed by storage at -80°C. All RNA samples used for downstream qPCR had 260/280 ratios between 2.02 and 2.07, and RNA integrity was visualized on 1% horizontal agarose gels. cDNA was synthesized from total RNA using the RETROscript kit (MMLV reverse transcriptase) with random decamers (Ambion). qPCR was performed using Power SYBR Green Mastermix (Applied Biosystems) on an Applied Biosystems 7900HT Real-Time PCR System. The Ar oligonucleotide primers (5'-TGTCACTACGGAGCT CTCACTTGT; 5'-AATCGTTTCTGCTGGCACATAGA) and Actb primers (5'-GGCTGTATTCCCCTCCATCG; 5'-CCAGTTGGTAACAATGCCATGT) amplified 97 bp and 154 bp PCR products, respectively, that spanned the Exon 2-Exon 3 juncture of their corresponding cDNA targets. A minimum of six standard curves with correlation coefficients ≥ 0.993 were used to determine the average efficiency of the Ar (94.25%) and Actb (94.88%) PCR reactions. Ar amplification was normalized to Actb using the Pfaffl method and is represented as fold change of the SWR.SJL-X5 ovaries (numerator) relative to the SWR ovaries (denominator).Citation26
Statistical analysis
Spontaneous GC tumor incidence in female mice that possess an SWR autosomal complement and are homozygous for the Gct4 allele from the SJL strain (Gct4J) exhibit ≈20% spontaneous GC tumor incidence. Comparing this incidence to the low penetrance of SWR inbred females (≤ 1%) or a tumor resistant phenotype (0%), we determined a minimum sample size of n = 50 female progeny per recombinant male was required to provide sufficient statistical power. Individual comparisons of GC tumor incidence per paternal haplotype were made to the expected incidence of the Gct4CA/Gct6SW paternal contribution for Gct4 mapping, or the Gct4CA/Gct6J contribution for Gct6 mapping, using a Chi-Square analysis for proportions with a significance level of p < 0.05. A one-way t-test relative to the ratio of 1.0 was used to analyze the qPCR gene expression ratios. All statistical analyses were performed with Prism version 5.00b software (GraphPad).
| Abbreviations: | ||
| Actb | = | actin beta gene |
| AIS | = | androgen insensitivity syndrome |
| Ar | = | androgen receptor gene |
| CA | = | Castaneus/Ei strain allele |
| Chr | = | chromosome |
| dNTP | = | deoxyribonucleotide triphosphate mix |
| FOXL2 | = | forkhead box L2 gene |
| GC | = | granulosa cell |
| Gct | = | Granulosa cell tumor susceptibility locus |
| GNAS | = | guanine nucleotide binding protein, alpha stimulating, complex locus |
| J | = | SJL/Bm strain allele |
| Mb | = | million base pairs |
| MMLV | = | Moloney murine leukemia virus |
| ncRNA | = | non-coding RNA |
| qPCR | = | quantitative real-time PCR |
| SW | = | SWR/Bm strain allele |
| XA | = | Chr X, transcriptionally active |
| XI | = | Chr X, transcriptionally inactive |
| XM | = | Chr X, maternally inherited |
| XP | = | Chr X, paternally inherited |
| XIC | = | X-inactivation center |
Additional material
Download Zip (69.2 KB)Acknowledgments
This work was performed across two institutions, The Jackson Laboratory (TJL) and Memorial University of Newfoundland (MUN). The founder congenic line SWR.CAST-X development, reciprocal matings and male progeny testing at TJL was supported by NIH HD049397 (A.M.D.) and NCI Cancer Core Grant CA34196 (W.G.B.). Other experiments were performed at MUN, supported by Canada Foundation for Innovation, the Canada Research Chairs program and the Faculty of Medicine (A.M.D.). K.N.S. received fellowship support from the Faculty of Medicine and School of Graduate Studies (MUN) and a CIHR Masters award. The authors would like to acknowledge the assistance of the Computational Sciences group at TJL for custom SSLP primer design and the insightful contributions of Dr Eva Eicher throughout this project.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/epigenetics/article/23399
References
- Young RH, Dickersin GR, Scully RE. Juvenile granulosa cell tumor of the ovary. A clinicopathological analysis of 125 cases. Am J Surg Pathol 1984; 8:575 - 96; http://dx.doi.org/10.1097/00000478-198408000-00002; PMID: 6465418
- Shah SP, Köbel M, Senz J, Morin RD, Clarke BA, Wiegand KC, et al. Mutation of FOXL2 in granulosa-cell tumors of the ovary. N Engl J Med 2009; 360:2719 - 29; http://dx.doi.org/10.1056/NEJMoa0902542; PMID: 19516027
- Schrader KA, Gorbatcheva B, Senz J, Heravi-Moussavi A, Melnyk N, Salamanca C, et al. The specificity of the FOXL2 c.402C>G somatic mutation: a survey of solid tumors. PLoS One 2009; 4:e7988; http://dx.doi.org/10.1371/journal.pone.0007988; PMID: 19956657
- Kalfa N, Philibert P, Patte C, Ecochard A, Duvillard P, Baldet P, et al. Extinction of FOXL2 expression in aggressive ovarian granulosa cell tumors in children. Fertil Steril 2007; 87:896 - 901; http://dx.doi.org/10.1016/j.fertnstert.2006.11.016; PMID: 17430735
- Beamer WG, Hoppe PC, Whitten WK. Spontaneous malignant granulosa cell tumors in ovaries of young SWR mice. Cancer Res 1985; 45:5575 - 81; PMID: 4053032
- Beamer WG, Shultz KL, Tennent BJ, Azumi N, Sundberg JP. Mouse model for malignant juvenile ovarian granulosa cell tumors. Toxicol Pathol 1998; 26:704 - 10; PMID: 9789961
- Beamer WG, Shultz KL, Tennent BJ, Shultz LD. Granulosa cell tumorigenesis in genetically hypogonadal-immunodeficient mice grafted with ovaries from tumor-susceptible donors. Cancer Res 1993; 53:3741 - 6; PMID: 8339285
- Tennent BJ, Shultz KL, Sundberg JP, Beamer WG. Ovarian granulosa cell tumorigenesis in SWR-derived F1 hybrid mice: preneoplastic follicular abnormality and malignant disease progression. Am J Obstet Gynecol 1990; 163:625 - 34; PMID: 2386155
- Tennent BJ, Shultz KL, Beamer WG. Genetic susceptibility for C19 androgen induction of ovarian granulosa cell tumorigenesis in SWXJ strains of mice. Cancer Res 1993; 53:1059 - 63; PMID: 8439952
- Dorward AM, Shultz KL, Beamer WG. LH analog and dietary isoflavones support ovarian granulosa cell tumor development in a spontaneous mouse model. Endocr Relat Cancer 2007; 14:369 - 79; http://dx.doi.org/10.1677/erc.1.01232; PMID: 17639051
- Beamer WG, Shultz KL, Tennent BJ, Nadeau JH, Churchill GA, Eicher EM. Multigenic and imprinting control of ovarian granulosa cell tumorigenesis in mice. Cancer Res 1998; 58:3694 - 9; PMID: 9721880
- Dorward AM, Shultz KL, Horton LG, Li R, Churchill GA, Beamer WG. Distal Chr 4 harbors a genetic locus (Gct1) fundamental for spontaneous ovarian granulosa cell tumorigenesis in a mouse model. Cancer Res 2005; 65:1259 - 64; http://dx.doi.org/10.1158/0008-5472.CAN-04-2992; PMID: 15735010
- Smith KN, Halfyard SJ, Yaskowiak ES, Shultz KL, Beamer WG, Dorward AM. Fine map of the Gct1 spontaneous ovarian granulosa cell tumor locus. Mamm Genome 2012; http://dx.doi.org/10.1007/s00335-012-9439-6; PMID: 23179634
- Dorward AM, Shultz KL, Ackert-Bicknell CL, Eicher EM, Beamer WG. High-resolution genetic map of X-linked juvenile-type granulosa cell tumor susceptibility genes in mouse. Cancer Res 2003; 63:8197 - 202; PMID: 14678975
- Kalfa N, Ecochard A, Patte C, Duvillard P, Audran F, Pienkowski C, et al. Activating mutations of the stimulatory g protein in juvenile ovarian granulosa cell tumors: a new prognostic factor?. J Clin Endocrinol Metab 2006; 91:1842 - 7; http://dx.doi.org/10.1210/jc.2005-2710; PMID: 16507630
- Ali SZ. Metastatic granulosa-cell tumor in the liver: cytopathologic findings and staining with inhibin. Diagn Cytopathol 1998; 19:293 - 7; http://dx.doi.org/10.1002/(SICI)1097-0339(199810)19:4<293::AID-DC14>3.0.CO;2-I; PMID: 9784996
- Williams RJ, Kamel HM, Jilaihawi AN, Prakash D. Metastatic granulosa cell tumour of the diaphragm 15 years after the primary neoplasm. Eur J Cardiothorac Surg 2001; 19:516 - 8; http://dx.doi.org/10.1016/S1010-7940(01)00605-4; PMID: 11306325
- Jozwicki W, Brożyna AA, Walentowicz M, Grabiec M. Bilateral aggressive malignant granulosa cell tumour with essentially different immunophenotypes in primary and metastatic lesions comprising predominantly sarcomatoid and fibrothecomatous patterns - looking for prognostic markers: a case report. Arch Med Sci 2011; 7:918 - 22; http://dx.doi.org/10.5114/aoms.2011.25573; PMID: 22291843
- Hirshfield AN. Development of follicles in the mammalian ovary. Int Rev Cytol 1991; 124:43 - 101; http://dx.doi.org/10.1016/S0074-7696(08)61524-7; PMID: 2001918
- Mork L, Maatouk DM, McMahon JA, Guo JJ, Zhang P, McMahon AP, et al. Temporal differences in granulosa cell specification in the ovary reflect distinct follicle fates in mice. Biol Reprod 2012; 86:37; http://dx.doi.org/10.1095/biolreprod.111.095208; PMID: 21976597
- Sen A, Hammes SR. Granulosa cell-specific androgen receptors are critical regulators of ovarian development and function. Mol Endocrinol 2010; 24:1393 - 403; http://dx.doi.org/10.1210/me.2010-0006; PMID: 20501640
- Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Hum Mutat 2012; 33:887 - 94; http://dx.doi.org/10.1002/humu.22046; PMID: 22334387
- Simmler MC, Cattanach BM, Rasberry C, Rougeulle C, Avner P. Mapping the murine Xce locus with (CA)n repeats. Mamm Genome 1993; 4:523 - 30; http://dx.doi.org/10.1007/BF00364788; PMID: 8118102
- Berletch JB, Yang F, Xu J, Carrel L, Disteche CM. Genes that escape from X inactivation. Hum Genet 2011; 130:237 - 45; http://dx.doi.org/10.1007/s00439-011-1011-z; PMID: 21614513
- Eppig JT, Blake JA, Bult CJ, Kadin JA, Richardson JE, Mouse Genome Database Group. The Mouse Genome Database (MGD): comprehensive resource for genetics and genomics of the laboratory mouse. Nucleic Acids Res 2012; 40:Database issue D881 - 6; http://dx.doi.org/10.1093/nar/gkr974; PMID: 22075990
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001; 29:e45; http://dx.doi.org/10.1093/nar/29.9.e45; PMID: 11328886