Abstract
Chromatin structure is regulated by families of proteins that are able to covalently modify the histones and the DNA, as well as to regulate the spacing of nucleosomes along the DNA. Over the years, these chromatin remodeling factors have been proven to be essential to a variety of processes, including gene expression, DNA replication, and chromosome cohesion. The function of these remodeling factors is regulated by a number of chemical and developmental signals and, in turn, changes in the chromatin structure eventually contribute to the response to changes in the cellular environment. Exciting new research findings by the laboratories of Sharon Dent and Steve Jackson indicate, in two different contexts, that changes in the chromatin structure may, in reverse, signal to intracellular signaling pathways to regulate cell fate. The discoveries clearly challenge our traditional view of ‘epigenetics’, and may have important implications in human health.
In eukaryotes, the nucleosome core particle is essential in the packaging of the genomic DNA and the higher-order folding of the chromatin fiber. It also bears post-translational modifications that will be recognized and interpreted by nuclear machineries involved in the regulation of gene expression, DNA replication and DNA repair.Citation1 Over the years, a large number of modifications, a variety of enzymes catalyzing and removing these modifications, as well as a high number of proteins able to recognize these modifications have been described.Citation1-Citation12 A modification may directly modify the interaction between the DNA and histone tails and, subsequently, the conformation of the nucleosome; it may indirectly affect the binding onto the nucleosome of a non-histone protein; it may have different biological consequences depending on the presence and the kind of adjacent modifications; it may have short or long-lasting effects.Citation2,Citation3,Citation6,Citation10,Citation11 Ultimately, this molecular network defines the outcome that a (single) modification will have on the chromatin structure and eventually on cell fate.Citation6,Citation9-Citation11
The role of external cues in the regulation of the chromatin structure has been increasingly appreciated. Signaling pathways, chromatin remodeling factors and epigenetic modifications act in concert to precisely control gene expression, DNA replication and DNA repair during cell differentiation and development. External cues can modify the distribution of histone (and DNA) modifications across the genome and in turn, the chromatin structure can amplify or attenuate signal transduction cascade activity to control gene expression, cell proliferation and cell fate.Citation13-Citation16 As one would expect, dysfunction of chromatin remodeling factors is associated, and often causal, in an array of human genetic disorders, illnesses and cancers.Citation17-Citation24
The laboratories of Steve Jackson and Sharon Dent recently revealed an additional layer of complexity in the role of the chromatin structure.Citation25,Citation26 Their studies nicely show that changes in the chromatin structure can actually act upstream of signaling pathways and signal to the cell.
Signaling to and through Chromatin
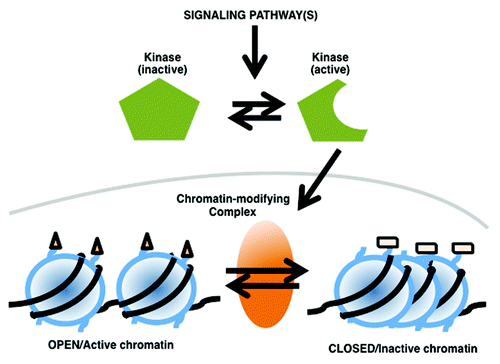
Chromatin-modifying enzymes are sensitive to environmental, developmental and hormonal signals, as well as cell-cell interactions. Hence, the regulation of the stability, the activity and/or the chromatin loading of these enzymes, directly impacts the structure of the chromatin and its function (). Different cues will activate different signal transduction cascades and trigger different, often specific, chromatin remodeling events, locally and globally at the scale of the genome. These events are often highly dynamic leading to transient activation or repression of gene expression and eventually contribute to the cellular response to changes in the environment.Citation9-Citation11
Figure 1. Modulation of chromatin remodeling complexes function by environmental cues and signaling pathways.
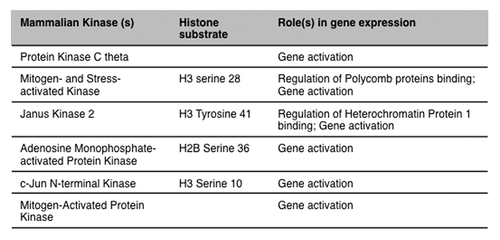
The c-Jun N-terminal kinases (JNKs), a subgroup of mitogen-activated protein kinases, are involved in chromatin remodeling during cell differentiation, tissue morphogenesis, programmed cell death and the stress response.Citation14,Citation27-Citation30 In Drosophila, the histone acetylase (KAT) Chameau and the histone deacetylase HDAC1 act as antagonistic cofactors to modulate JNK-dependent transcription during thorax metamorphosis.Citation14 Still in Drosophila, in the innate immune response, JNK-induces the recruitment of HDAC1 to NF-κB activated promoters, resulting in their repression.Citation31 In Caenorhabditis elegans, JNK activates stress-response genes by removing an HDAC-based repressor complex from the promoter of target genes.Citation32 In human cells, activation of JNK in response to stress blocks acetylase KAT7 binding at origins of replication and induces its recruitment at JNK transcriptional targets, where it facilitates gene expression.Citation33,Citation34 Finally, in Saccharomyces cerevisiae, upon osmotic stress, the JNK-related Hog1 kinase translocates into the nucleus and binds at promoters of target genes where it facilitates gene expression.Citation35 Simultaneously, Hog1 activity blocks DNA replication.Citation36 These few selected studies clearly illustrate the variety of effects that signaling pathways have on the chromatin structure and on the function of chromatin remodeling factors. Intriguingly, these latter studies also suggest that some kinase-dependent chromatin-regulatory pathways, such as the coordination of transcription and replication by a MAPK pathway, might be conserved throughout eukaryotes.
Over the past 5 years, it has also been demonstrated that several kinases actually bind to chromatin at target genes and directly phosphorylate histone tails ().Citation28,Citation37-Citation41 For instance, inhibition of JNK signaling in mouse embryonic stem cells severely impairs their in vitro differentiation into neurons.Citation28 A series of chromatin immunoprecipitation analysis indicates that serine 10 phosphorylation increases at JNK target genes upon differentiation and that the modification is involved in gene expression activation. Additional studies show that JNK directly catalyzes the phosphorylation of histone H3 serine 10, and that this event is critical during in vitro terminal neuronal differentiation.Citation28
These different studies portrayed the chromatin as a structure constantly remodeled by environmental cues, and that integrates and senses the information the cell receive from its environment.Citation1,Citation9,Citation42 These observations led to the hypothesis that the chromatin structure and the location of chromatin modifications, if stably maintained through cell division, could serve as a “foot-print” of the cell type and its past environment. This predominant view lead to the initiation of a number of very large and ambitious research projects over the last few years.Citation4,Citation43,Citation44 The hope being that the characterization of these “foot-prints” will facilitate in the future the detection and diagnosis of human diseases and cancers; the detection of past exposure to drugs and pollutants; and eventually the prediction of disease outcome or cell fate. For instance, yeast cells respond to drugs with a significantly different transcriptional response amplitude and kinetics, if they have been previously exposed to the same drug. This phenomenon was attributed to the presence on the chromatin of modifications deposited during the first exposure and that potentiate the stress response on further exposure (or in response to other stresses).Citation45-Citation47 Identical twins often get different diseases during their lifetime.Citation48 In that case, subtle changes in the distribution of chromatin modifications are detected in the pair of twin, and these changes accumulate with age and might be due to the difference in the twin living environment, including stress, nutrition, pollutants or social interactions.Citation48
Signaling from Chromatin
Most studies pictured the chromatin and chromatin remodeling factors as the ultimate substrates of signaling cascades in the cell. Studies from the Jackson and Dent lab challenge this view and indicate that signals emanating from the chromatin may also control signaling cascades and non-histone regulatory factors.Citation15,Citation16
The ataxia telangiectasia mutant (ATM) kinase plays a central role in the orchestration of the DNA damage response.Citation49 ATM activity is triggered by its association with the multimeric MRE11-RAD50-NBS1 (MRN) complex at sites of double-strand breaks.Citation50-Citation53 Nonetheless, experimental evidence is piling up to suggest that chromatin structure at sites of double-strand breaks potentiates ATM activation and that ATM accumulation at DNA damage sites is critical for amplification of the DNA damage response.Citation26,Citation51,Citation52,Citation54 For instance, phosphorylation of histone variant H2AX is required for the assembly of DNA repair proteins at the sites containing damaged chromatin as well as for activation of checkpoints proteins which arrest the cell cycle progression.Citation55,Citation56 In a study published in Nature, Kaidi and Jackson recently described the molecular events linking histone H3 lysine 9 methylation at DNA damage sites and ATM activation.Citation26 Acetylase KAT5 (also called Tip60) plays a key role in this process. First, the authors confirmed that KAT5 directly acetylates ATM, and that this reaction enhances ATM auto-phosphorylation and its kinase activity.Citation57 Second, they provide strong evidence that DNA damage triggers KAT5 phosphorylation on tyrosine 44, a highly-conserved residue, in the chromodomain, through which KAT5 interacts with methylated histone H3 lysine 9.Citation26 Using different methods, Kaidi and Jackson demonstrate that ABL kinase phosphorylates KAT5 on tyrosine 44 and that phosphorylated KAT5 tightly binds to methylated histone H3 lysine 9 in vitro and in vivo. Further, in enzymatic reaction assays, they observed that H3 lysine 9 methylation stimulates KAT5-mediated acetylation of ATM while no increase activity is observed on histone tails.Citation26 More importantly, ATM that has been activated by KAT5 in turn phosphorylates bona-fide downstream kinases, including Chk2, and non-histone substrates involved in the DNA damage response.Citation26,Citation58 The authors therefore concluded that exposure of methylated H3 lysine 9 at DNA damage sites directly induces KAT5 activity toward ATM, leading to ATM kinase activation and the induction of the DNA damage response.
Kaidi and Jackson also provide intriguing observations that a related KAT5/ATM-based pathway might be activated upon gross changes of the chromatin structure, independently of detectable level of DNA damage.Citation26,Citation54,Citation59 Indeed, altered chromatin structure, following histone hyper-acetylation (induced by treatment with trichostatin A) or following depletion of heterochromatin protein 1, lead to KAT5 phosphorylation on tyrosine 44, to increased association of KAT5 to chromatin and to KAT5-dependent acetylation and activation of ATM kinase.Citation26,Citation54,Citation59 Experimental evidence therefore suggest that exposure of methylated histone H3 lysine 9 upon gross alteration of the chromatin structure is sufficient to activate KAT5 and ATM signaling, leading the authors to conclude that ATM kinase activity is directly regulated by histone H3 lysine 9 methylation in the cell and that ATM kinase might be a “guardian” of chromatin structure integrity.
The regulation of non-histone factors by changes in the chromatin structure is not restricted to human. A couple of years ago, in a study published in Cell, Sharon Dent lab reported that the function of the yeast kinetochore protein Dam1 was regulated by histone H2B ubiquitination.Citation25 Dam1 is methylated on lysine 233 by the SET1 lysine-methylase.Citation25,Citation60 Using a sophisticated genetic approach and the methylation of Dam1 as a read-out, Latham et al. identified the critical events required for Dam1 function at the kinetochore. They found that ubiquitination of H2B on lysine 123 was required for Set1 interaction and activity toward Dam1. They also identified BRE1, Rad8 and Ubp8 enzymes as regulators of H2B lysine 123 ubiquitination, and hence Dam1 methylation and function.Citation25 All together, results indicate that ubiquitination of H2B lysine 233 at centromere, induces Set1-mediated Dam1 methylation at the kinetochore. This report was the first to describe that the chromatin structure and a specific histone mark may influence, at a distance, the function of a non-histone factor.
Concluding Remarks
Taken together, both reports show that changes in the chromatin structure can regulate the function of non-histone factors. The two studies suggest that a variety of non-histone factors can be differentially regulated directly in response to modifications deposited onto the chromatin. Conversely, a variety of histone (and DNA) modifications may induce these signaling events.
Chromatin remodeling factors have been also shown to post-translationally modify non-histone substrates such as transcription factors, structural components and signaling factors.Citation61 The modification of non-histone factors may affect co-factor recruitment, DNA binding, enzymatic activity, cell localization or protein degradation, among other things. Yet, these studies are not conducted in the context of the chromatin. In light of Jackson and Dent lab reports, it would be worth re-investigating the conclusions drawn from these experiments. Is the activity of kinase and modifying enzymes sensitive to the status of surrounding epigenetic modifications? Is their activity predominantly directed against non-histone substrates rather than histone tails? For instance, in yeast cells and human twins, subtle changes in the distribution of histone (and DNA) modifications detected in specific regions of the genome may modulate the activity of specific intracellular signaling pathways or chromatin-bound enzymes rather than contributing to chromatin structure regulation and gene expression per se.
These pioneer discoveries also open new paths to the development of drugs and alternative strategies to treat several human diseases, including cancer. Drugs that could mask, convert, or remove, cancer-specific modifications may be relevant therapeutic opportunities. In addition, the use of chromatin-targeting drugs may potentially provide additive or synergistic beneficial effects when combined with more conventional drugs.
Researchers need to address these important issues, and design experiments to identify and characterize these “cross-talks” and “feedback loops” between histone and non-histone chromatin factors, that may fine-tune signaling cascade activity and cell fate. Exploring these pathways will be an important step toward understanding chromatin structure, function and epigenetics.
Acknowledgments
B.M. is supported by a grant from Ligue Nationale Contre le Cancer (Comité de Paris; LNCC OTP 33965) and by a grant from European Commission (Marie Curie Re-integration grant IRG 268448/EPIX).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Badeaux AI, Shi Y. Emerging roles for chromatin as a signal integration and storage platform. Nat Rev Mol Cell Biol 2013; 14:211 - 24; http://dx.doi.org/10.1038/nrm3545
- Bartke T, Vermeulen M, Xhemalce B, Robson SC, Mann M, Kouzarides T. Nucleosome-interacting proteins regulated by DNA and histone methylation. Cell 2010; 143:470 - 84; http://dx.doi.org/10.1016/j.cell.2010.10.012; PMID: 21029866
- Eberl HC, Spruijt CG, Kelstrup CD, Vermeulen M, Mann M. A map of general and specialized chromatin readers in mouse tissues generated by label-free interaction proteomics. Mol Cell 2013; 49:368 - 78; http://dx.doi.org/10.1016/j.molcel.2012.10.026; PMID: 23201125
- Dunham I, Kundaje A, Aldred SF, Collins PJ, Davis CA, Doyle F, et al, ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012; 489:57 - 74; http://dx.doi.org/10.1038/nature11247; PMID: 22955616
- Fischle W, Wang Y, Allis CD. Binary switches and modification cassettes in histone biology and beyond. Nature 2003; 425:475 - 9; http://dx.doi.org/10.1038/nature02017; PMID: 14523437
- Jenuwein T, Allis CD. Translating the histone code. Science 2001; 293:1074 - 80; http://dx.doi.org/10.1126/science.1063127; PMID: 11498575
- Smith E, Shilatifard A. The chromatin signaling pathway: diverse mechanisms of recruitment of histone-modifying enzymes and varied biological outcomes. Mol Cell 2010; 40:689 - 701; http://dx.doi.org/10.1016/j.molcel.2010.11.031; PMID: 21145479
- Spruijt CG, Gnerlich F, Smits AH, Pfaffeneder T, Jansen PW, Bauer C, et al. Dynamic readers for 5-(hydroxy)methylcytosine and its oxidized derivatives. Cell 2013; 152:1146 - 59; http://dx.doi.org/10.1016/j.cell.2013.02.004; PMID: 23434322
- Suganuma T, Workman JL. Chromatin and signaling. Curr Opin Cell Biol 2013; 25:322 - 6; http://dx.doi.org/10.1016/j.ceb.2013.02.016; PMID: 23498660
- Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Annu Rev Biochem 2011; 80:473 - 99; http://dx.doi.org/10.1146/annurev-biochem-061809-175347; PMID: 21529160
- Turner BM. Defining an epigenetic code. Nat Cell Biol 2007; 9:2 - 6; http://dx.doi.org/10.1038/ncb0107-2; PMID: 17199124
- Weiner A, Chen HV, Liu CL, Rahat A, Klien A, Soares L, et al. Systematic dissection of roles for chromatin regulators in a yeast stress response. PLoS Biol 2012; 10:e1001369; http://dx.doi.org/10.1371/journal.pbio.1001369; PMID: 22912562
- Collins RT, Treisman JE. Osa-containing Brahma chromatin remodeling complexes are required for the repression of wingless target genes. Genes Dev 2000; 14:3140 - 52; http://dx.doi.org/10.1101/gad.854300; PMID: 11124806
- Miotto B, Sagnier T, Berenger H, Bohmann D, Pradel J, Graba Y. Chameau HAT and DRpd3 HDAC function as antagonistic cofactors of JNK/AP-1-dependent transcription during Drosophila metamorphosis. Genes Dev 2006; 20:101 - 12; http://dx.doi.org/10.1101/gad.359506; PMID: 16391236
- von Zelewsky T, Palladino F, Brunschwig K, Tobler H, Hajnal A, Müller F. The C. elegans Mi-2 chromatin-remodelling proteins function in vulval cell fate determination. Development 2000; 127:5277 - 84; PMID: 11076750
- Rottbauer W, Saurin AJ, Lickert H, Shen X, Burns CG, Wo ZG, et al. Reptin and pontin antagonistically regulate heart growth in zebrafish embryos. Cell 2002; 111:661 - 72; http://dx.doi.org/10.1016/S0092-8674(02)01112-1; PMID: 12464178
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 1999; 23:185 - 8; http://dx.doi.org/10.1038/13810; PMID: 10508514
- Gibbons RJ, Picketts DJ, Villard L, Higgs DR. Mutations in a putative global transcriptional regulator cause X-linked mental retardation with alpha-thalassemia (ATR-X syndrome). Cell 1995; 80:837 - 45; http://dx.doi.org/10.1016/0092-8674(95)90287-2; PMID: 7697714
- Hansen RS, Wijmenga C, Luo P, Stanek AM, Canfield TK, Weemaes CM, et al. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc Natl Acad Sci U S A 1999; 96:14412 - 7; http://dx.doi.org/10.1073/pnas.96.25.14412; PMID: 10588719
- Kasprzyk L, Defossez PA, Miotto B. [Epigenetic regulation in neuronal differentiation and brain function]. Biol Aujourdhui 2013; 207:1 - 17; http://dx.doi.org/10.1051/jbio/2013001; PMID: 23694721
- Ko M, Huang Y, Jankowska AM, Pape UJ, Tahiliani M, Bandukwala HS, et al. Impaired hydroxylation of 5-methylcytosine in myeloid cancers with mutant TET2. Nature 2010; 468:839 - 43; http://dx.doi.org/10.1038/nature09586; PMID: 21057493
- Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RC, Masuno M, et al. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 1995; 376:348 - 51; http://dx.doi.org/10.1038/376348a0; PMID: 7630403
- Trivier E, De Cesare D, Jacquot S, Pannetier S, Zackai E, Young I, et al. Mutations in the kinase Rsk-2 associated with Coffin-Lowry syndrome. Nature 1996; 384:567 - 70; http://dx.doi.org/10.1038/384567a0; PMID: 8955270
- Villard L, Gecz J, Mattéi JF, Fontés M, Saugier-Veber P, Munnich A, et al. XNP mutation in a large family with Juberg-Marsidi syndrome. Nat Genet 1996; 12:359 - 60; http://dx.doi.org/10.1038/ng0496-359; PMID: 8630485
- Latham JA, Chosed RJ, Wang S, Dent SY. Chromatin signaling to kinetochores: transregulation of Dam1 methylation by histone H2B ubiquitination. Cell 2011; 146:709 - 19; http://dx.doi.org/10.1016/j.cell.2011.07.025; PMID: 21884933
- Kaidi A, Jackson SP. KAT5 tyrosine phosphorylation couples chromatin sensing to ATM signalling. Nature 2013; 498:70 - 4; http://dx.doi.org/10.1038/nature12201; PMID: 23708966
- Klein AM, Zaganjor E, Cobb MH. Chromatin-tethered MAPKs. Curr Opin Cell Biol 2013; 25:272 - 7; http://dx.doi.org/10.1016/j.ceb.2013.01.002; PMID: 23434067
- Tiwari VK, Stadler MB, Wirbelauer C, Paro R, Schübeler D, Beisel C. A chromatin-modifying function of JNK during stem cell differentiation. Nat Genet 2012; 44:94 - 100; http://dx.doi.org/10.1038/ng.1036; PMID: 22179133
- Suganuma T, Mushegian A, Swanson SK, Abmayr SM, Florens L, Washburn MP, et al. The ATAC acetyltransferase complex coordinates MAP kinases to regulate JNK target genes. Cell 2010; 142:726 - 36; http://dx.doi.org/10.1016/j.cell.2010.07.045; PMID: 20813260
- Ura S, Nishina H, Gotoh Y, Katada T. Activation of the c-Jun N-terminal kinase pathway by MST1 is essential and sufficient for the induction of chromatin condensation during apoptosis. Mol Cell Biol 2007; 27:5514 - 22; http://dx.doi.org/10.1128/MCB.00199-07; PMID: 17548476
- Kim T, Yoon J, Cho H, Lee WB, Kim J, Song YH, et al. Downregulation of lipopolysaccharide response in Drosophila by negative crosstalk between the AP1 and NF-kappaB signaling modules. Nat Immunol 2005; 6:211 - 8; http://dx.doi.org/10.1038/ni1159; PMID: 15640802
- Hattori A, Mizuno T, Akamatsu M, Hisamoto N, Matsumoto K. The Caenorhabditis elegans JNK signaling pathway activates expression of stress response genes by derepressing the Fos/HDAC repressor complex. PLoS Genet 2013; 9:e1003315; http://dx.doi.org/10.1371/journal.pgen.1003315; PMID: 23437011
- Miotto B, Struhl K. HBO1 histone acetylase is a coactivator of the replication licensing factor Cdt1. Genes Dev 2008; 22:2633 - 8; http://dx.doi.org/10.1101/gad.1674108; PMID: 18832067
- Miotto B, Struhl K. JNK1 phosphorylation of Cdt1 inhibits recruitment of HBO1 histone acetylase and blocks replication licensing in response to stress. Mol Cell 2011; 44:62 - 71; http://dx.doi.org/10.1016/j.molcel.2011.06.021; PMID: 21856198
- Pokholok DK, Zeitlinger J, Hannett NM, Reynolds DB, Young RA. Activated signal transduction kinases frequently occupy target genes. Science 2006; 313:533 - 6; http://dx.doi.org/10.1126/science.1127677; PMID: 16873666
- Duch A, Felipe-Abrio I, Barroso S, Yaakov G, García-Rubio M, Aguilera A, et al. Coordinated control of replication and transcription by a SAPK protects genomic integrity. Nature 2013; 493:116 - 9; http://dx.doi.org/10.1038/nature11675; PMID: 23178807
- Bungard D, Fuerth BJ, Zeng PY, Faubert B, Maas NL, Viollet B, et al. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science 2010; 329:1201 - 5; http://dx.doi.org/10.1126/science.1191241; PMID: 20647423
- Dawson MA, Bannister AJ, Göttgens B, Foster SD, Bartke T, Green AR, et al. JAK2 phosphorylates histone H3Y41 and excludes HP1alpha from chromatin. Nature 2009; 461:819 - 22; http://dx.doi.org/10.1038/nature08448; PMID: 19783980
- Gehani SS, Agrawal-Singh S, Dietrich N, Christophersen NS, Helin K, Hansen K. Polycomb group protein displacement and gene activation through MSK-dependent H3K27me3S28 phosphorylation. Mol Cell 2010; 39:886 - 900; http://dx.doi.org/10.1016/j.molcel.2010.08.020; PMID: 20864036
- Göke J, Chan YS, Yan J, Vingron M, Ng HH. Genome-wide Kinase-Chromatin Interactions Reveal the Regulatory Network of ERK Signaling in Human Embryonic Stem Cells. Mol Cell 2013; 50:844 - 55; http://dx.doi.org/10.1016/j.molcel.2013.04.030; PMID: 23727019
- Sutcliffe EL, Bunting KL, He YQ, Li J, Phetsouphanh C, Seddiki N, et al. Chromatin-associated protein kinase C-θ regulates an inducible gene expression program and microRNAs in human T lymphocytes. Mol Cell 2011; 41:704 - 19; http://dx.doi.org/10.1016/j.molcel.2011.02.030; PMID: 21419345
- Rosenfeld MG, Lunyak VV, Glass CK. Sensors and signals: a coactivator/corepressor/epigenetic code for integrating signal-dependent programs of transcriptional response. Genes Dev 2006; 20:1405 - 28; http://dx.doi.org/10.1101/gad.1424806; PMID: 16751179
- American Association for Cancer Research Human Epigenome Task Force, European Union, Network of Excellence, Scientific Advisory Board. Moving AHEAD with an international human epigenome project. Nature 2008; 454:711 - 5; http://dx.doi.org/10.1038/454711a; PMID: 18685699
- Bernstein BE, Stamatoyannopoulos JA, Costello JF, Ren B, Milosavljevic A, Meissner A, et al, The NIH Roadmap Epigenomics Mapping Consortium. The NIH Roadmap Epigenomics Mapping Consortium. Nat Biotechnol 2010; 28:1045 - 8; http://dx.doi.org/10.1038/nbt1010-1045; PMID: 20944595
- Weiner A, Chen HV, Liu CL, Rahat A, Klien A, Soares L, et al. Systematic dissection of roles for chromatin regulators in a yeast stress response. PLoS Biol 2012; 10:e1001369; http://dx.doi.org/10.1371/journal.pbio.1001369; PMID: 22912562
- Zanton SJ, Pugh BF. Full and partial genome-wide assembly and disassembly of the yeast transcription machinery in response to heat shock. Genes Dev 2006; 20:2250 - 65; http://dx.doi.org/10.1101/gad.1437506; PMID: 16912275
- Proft M, Struhl K. Hog1 kinase converts the Sko1-Cyc8-Tup1 repressor complex into an activator that recruits SAGA and SWI/SNF in response to osmotic stress. Mol Cell 2002; 9:1307 - 17; http://dx.doi.org/10.1016/S1097-2765(02)00557-9; PMID: 12086627
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A 2005; 102:10604 - 9; http://dx.doi.org/10.1073/pnas.0500398102; PMID: 16009939
- Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol 2013; 14:197 - 210; http://dx.doi.org/10.1038/nrm3546
- Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J 2003; 22:5612 - 21; http://dx.doi.org/10.1093/emboj/cdg541; PMID: 14532133
- Lee JH, Paull TT. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 2004; 304:93 - 6; http://dx.doi.org/10.1126/science.1091496; PMID: 15064416
- Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science 2005; 308:551 - 4; http://dx.doi.org/10.1126/science.1108297; PMID: 15790808
- Falck J, Coates J, Jackson SP. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 2005; 434:605 - 11; http://dx.doi.org/10.1038/nature03442; PMID: 15758953
- Kim YC, Gerlitz G, Furusawa T, Catez F, Nussenzweig A, Oh KS, et al. Activation of ATM depends on chromatin interactions occurring before induction of DNA damage. Nat Cell Biol 2009; 11:92 - 6; http://dx.doi.org/10.1038/ncb1817; PMID: 19079244
- Lukas J, Lukas C, Bartek J. More than just a focus: The chromatin response to DNA damage and its role in genome integrity maintenance. Nat Cell Biol 2011; 13:1161 - 9; http://dx.doi.org/10.1038/ncb2344; PMID: 21968989
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr Biol 2000; 10:886 - 95; http://dx.doi.org/10.1016/S0960-9822(00)00610-2; PMID: 10959836
- Sun Y, Jiang X, Chen S, Fernandes N, Price BD. A role for the Tip60 histone acetyltransferase in the acetylation and activation of ATM. Proc Natl Acad Sci U S A 2005; 102:13182 - 7; http://dx.doi.org/10.1073/pnas.0504211102; PMID: 16141325
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER 3rd, Hurov KE, Luo J, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science 2007; 316:1160 - 6; http://dx.doi.org/10.1126/science.1140321; PMID: 17525332
- Ayoub N, Jeyasekharan AD, Bernal JA, Venkitaraman AR. HP1-beta mobilization promotes chromatin changes that initiate the DNA damage response. Nature 2008; 453:682 - 6; http://dx.doi.org/10.1038/nature06875; PMID: 18438399
- Zhang K, Lin W, Latham JA, Riefler GM, Schumacher JM, Chan C, et al. The Set1 methyltransferase opposes Ipl1 aurora kinase functions in chromosome segregation. Cell 2005; 122:723 - 34; http://dx.doi.org/10.1016/j.cell.2005.06.021; PMID: 16143104
- Kouzarides T. Acetylation: a regulatory modification to rival phosphorylation?. EMBO J 2000; 19:1176 - 9; http://dx.doi.org/10.1093/emboj/19.6.1176; PMID: 10716917