Abstract
The adaptive immune system is involved in tumor establishment and aggressiveness. Tumors of the ovaries, an immune-privileged organ, spread via transceolomic routes and rarely to distant organs. This is contrary to tumors of non-immune privileged organs, which often disseminate hematogenously to distant organs. Epigenetics-based immune cell quantification allows direct comparison of the immune status in benign and malignant tissues and in blood. Here, we introduce the “cellular ratio of immune tolerance” (immunoCRIT) as defined by the ratio of regulatory T cells to total T lymphocytes. The immunoCRIT was analyzed on 273 benign tissue samples of colorectal, bronchial, renal and ovarian origin as well as in 808 samples from primary colorectal, bronchial, mammary and ovarian cancers. ImmunoCRIT is strongly increased in all cancerous tissues and gradually augmented strictly dependent on tumor aggressiveness. In peripheral blood of ovarian cancer patients, immunoCRIT incrementally increases from primary diagnosis to disease recurrence, at which distant metastases frequently occur. We postulate that non-pathological immunoCRIT values observed in peripheral blood of immune privileged ovarian tumor patients are sufficient to prevent hematogenous spread at primary diagnosis. Contrarily, non-immune privileged tumors establish high immunoCRIT in an immunological environment equivalent to the bloodstream and thus spread hematogenously to distant organs. In summary, our data suggest that the immunoCRIT is a powerful marker for tumor aggressiveness and disease dissemination.
Introduction
The immune system is involved in tumor etiology, the ability of tumors to grow uncontrolled and to invade distinct tissue structures. The hypothesis of immune editing explains the co-evolution of tumors and immune tolerance.Citation1 It postulates that highly antigenic tumor cells are eradicated by the immune system (elimination). Only tumor cells expressing low levels of tumor antigens proliferate until renewed immunological recognition (equilibrium). Eventually, the tumor cells with the lowest antigen levels prevail and are protected from immune recognition and elimination (escape).Citation2 However, even if tumor-associated antigens are expressed, spontaneous immunological counteraction is rare.Citation3 This cannot be explained by the above concept alone. Therefore, as another key parameter in the acceptance and growth of tumors regulatory T cells (Tregs) have been suggested.Citation4 Tregs are part of the tolerogenic immune compartment, balancing necessary aggressiveness against foes with tolerance against self-constituents. Initially, Tregs were characterized by expressing high levels of the IL-2 receptor (CD25),Citation5 which is a rather unspecific activation marker. The identification of the forkhead box transcription factor FOXP3—a master switch for Treg development and function—has provided a more specific tool for Treg characterization.Citation6-Citation9 Functionally, Tregs have been shown to inhibit activation and proliferation of CD4+ and CD8+ effector T lymphocytes either by cell-to-cell contact, cytokine secretion or inducing apoptosis.Citation10 Altogether, non-pathological immune response is thought to be mediated by equilibrium between Tregs and total T lymphocytes (tTL). Here, this equilibrium is determined by the ratio of epigenetically quantified Tregs and tTL and is referred to as “cellular ratio of immune tolerance” (immunoCRIT).
Increased levels of Tregs have been identified in peripheral blood and tumorous tissue from many cancers.Citation11-Citation20 Tumor-induced recruitment of Tregs supposedly leads to active immune modulation toward tolerance, impeding host protective anti-tumor response. Various reports demonstrate that the prognosis is more favorable for patients with higher tTL countsCitation21 or lower Tregs countsCitation19,Citation20,Citation22 in the tumor microenvironment, indicating a complex interdependence between immunological tolerance and tumor biology.
Immunological tolerance is also a hallmark of healthy organs. Most organs are under immunological surveillance and are thus regarded as non-immune privileged. However, in certain tissues or organs of the body, including brain, testis and ovaries, the presence of foreign antigens remains without elicitation of a standard immunological response. These sites are considered immune-privileged. Immune privilege is exhibited by physical tissue-blood barriers hampering tTL infiltration, lack of lymphatic drainage, downregulation of MHC molecules, constitutive expression of Fas ligand and/or local production of immune suppressive cytokines. The role of Tregs, which have been proposed to maintain immune privilege in various organs, remains disputed.Citation23-Citation25
Most tumors of non-immune privileged tissues metastasize either hematogenously or lymphatically into distant organs.Citation26-Citation28 In contrast, cancers from immune privileged sites, such as ovaries and brain, disseminate almost exclusively via cell surfaces and rarely into distant organs.Citation26 In ovarian cancer, involvement of extraperitoneal organs, which require lymphatic and hematogenous dissemination, is rare.Citation29,Citation30 This fact leads to prognostic imbalance between different tumor entities. Prognosis for breast cancer is favorable, if no distant metastases are found. In contrast, ovarian cancer shows an unfavorable prognosis, despite its strict confinement to the abdominal cavity.Citation31
Beside aggressive early and late stage epithelial ovarian cancer (ESOC and LSOC) other tumorous conditions of the ovaries are known. Ovarian cystadenomas (cysts) are composed of simple non-stratified epithelium, with normal cellular proliferation and no nuclear abnormalities. An intermediate degree of cellular proliferation and nuclear atypia with the absence of stromal invasion defines borderline ovarian tumors (BOT). BOT represent a class between benign and malignant tumors with respect to prognosis and histopathology.Citation32,Citation33 Since interaction between tumor and immune system occurs during tumor development, we intended to investigate as to whether shifts on the immunological side of this balance could serve as predictors of tumor behavior.
We have previously shown that constitutive expression of FOXP3 in natural Tregs requires epigenetic DNA methylation-based regulation.Citation34,Citation35 Demethylation at a defined region within the FOXP3 gene (TSDR) was found to be restricted to Tregs when tested in all major blood cell types and a selection of non-hematopoietic cells.Citation36 It was also observed that FOXP3 demethylation occurred only in natural Tregs, but not in activated conventional T lymphocytes transiently expressing FOXP3, thus serving as an excellent Treg marker. In analogy to FOXP3-TSDR, we have determined the epigenetic pattern of the intergenic CD3G/CD3D region as specific marker for identification of total CD3+ T lymphocytes.Citation37 Treg and T lymphocyte-specific epigenetic assays allow quantitative immunophenotyping, i.e., the immunological analysis of defined lymphocyte populations.
In this report, we investigated the impact of tTL infiltration and immunoCRIT, based on mentioned epigenetic assays, on two characteristics of tumors, i.e., their malignant potential (aggressiveness), and their spreading patterns on altogether 1300 samples from tumor patients. We examined immunological distinctions within tissue samples from immune privileged ovaries and various non-immune privileged organs. The immune privileged ovarian neoplasias and non-immune-privileged colorectal tissues included specimen of different malignant potential, i.e., cysts, BOT, ESOC, LSOC as well as benign colon tissues, non-metastasized and metastasized colorectal cancers (CRC), respectively. In addition, peripheral blood from healthy controls and patients with the ovarian neoplasias were analyzed, both to monitor immunoCRIT values at primary diagnosis and along the course of disease. Furthermore, we correlated T lymphocyte tissue infiltration and immunoCRIT levels with the differing spreading patterns of tumors derived from different organs. For this, we used immune privileged ovarian tumors, metastasizing primarily transceolomically and breast, lung, colorectal and kidney cancers—representing tumors from non-immune privileged tissues.
Results
Differentiation of benign non-immune privileged tissues and immune privileged ovarian tissue by means of total T lymphocyte levels
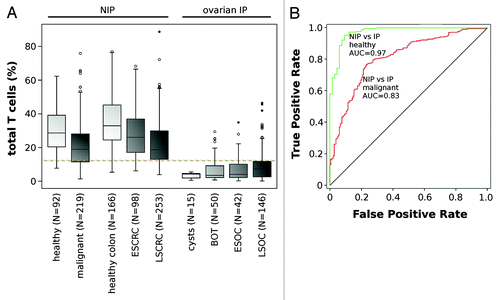
We analyzed the number of tissue infiltrating T lymphocytes in solid healthy tissue samples, applying epigenetic qPCR analyses as previously reported.Citation37 We used this approach on 323 non-malignant samples from colorectal (n = 166), bronchial (n = 75), renal (n = 17) and ovarian origin (n = 65). The median percentage of tTLs was stable throughout all tested non-immune-privileged tissues and ranged from 32.9% in colorectal and 28.7% in bronchial and kidney tissues (). The median tTL proportion in samples derived from immune privileged ovarian cysts was 4.3% and ranged from 0.5% to 5.5%. It remains thus significantly lower (P < 0.001) than in non-immune privileged tissues. To assess differences between benign ovarian—including borderline tumor—and non-immune privileged tissues by means of tTL infiltration levels, we applied receiver operator characteristics (ROC) curve analysis. We observed an area under the curve (AUC) value of 0.97 (confidence interval [CI]: 0.95–0.99, P < 0.001), indicating that low tTL infiltration is an immunological characteristic of benign ovarian tissues and one mechanism of maintaining immune privilege (). The optimal classification accuracy was at 0.94, corresponding to a cut-off value of 12.2% tTL.Citation38
Figure 1. Frequency of total T lymphocytes in benign tissue and solid tumors. (A) Frequency of infiltrating T lymphocytes in different non-immune privileged tissues (i.e., lung, kidney and breast), in benign colon, early stage (ESCRC) and late stage (LSCRC) colorectal carcinoma and in IP ovarian tissues (cysts, borderline ovarian tumor (BOT), early stage (EOSC) and late stage ovarian carcinoma (LSOC)). N indicates the number of samples in each plot. Dotted lines represent the calculated cut-off values for most accurate segregation between healthy (green) and malignant non-immune privileged and immune privileged tissues (red). All measurements were conducted using the epigenetic CD3-specific qPCR assay. (B) ROC curves are plotted for the discrimination between benign non-immune privileged and immune privileged tissue including borderline tumors (green) and malignant non-immune privileged and immune privileged tissue (red) by means of T lymphocyte infiltration. Respective area under the curve values (AUC)—reflecting discriminative power—are indicated.
Differences in tTL infiltration are conserved in both non-immune privileged and privileged tumorous tissue
Next, we analyzed tTL infiltration in 758 cancerous solid tissue samples originating from colorectum (n = 351), lung (n = 87), breast (n = 132) and ovary (n = 188). Upon malignant transformation of ovarian tissues we observed an increase of tTL infiltration along augmenting malignancy, from 3.9% and 3.5% in benign cysts and BOT, respectively, to 4.0% and 7.3% in ESOC and LSOC. The significance of the observed trend was evaluated using the Jonckheere-Terpstra test (P = 0.003).Citation39 Oppositely, non-immune privileged colorectal tissues show an incremental decrease of tTL infiltration parallel with tumor malignancy. While the median level in benign tissue was at 32.9%, this value dropped to 26.1% in early stage (ESCRC) and reached 16.8% in late stage colorectal cancer (LSCRC). Statistical significance of this trend was confirmed (P < 0.001). In a collection of different malignant non-immune privileged (n = 219), including bronchial and mammary tissues, the median tTL proportion was at 21.6%, compared with 28.7% in healthy tissues (). This difference between healthy and malignant non-immune privileged tissues was shown to be statistically significant and confirms the trend seen in colorectal tissue (P < 0.001).
We analyzed whether the differences in immune privilege as determined by tTL infiltration between ovarian and non-immune privileged tissues are conserved in malignancies. Infiltration levels show differences between all non-immune privileged and IP tissues, but were less pronounced than in benign tissues with an AUC of 0.83 (CI: 0.78–0.87, P < 0.001) reaching an optimal classification accuracy of 0.75, at the cut-off of 11.9% ().Citation38 To exclude that these differences were owed to gender effects rather than tissue immune privilege status, we separately analyzed male and female CRC patients and observed no significant differences (data not shown). We conclude that differences in tTL invasion between non-immune privileged and privileged tissues persist, despite a detectable change of tTL infiltration along with malignant transformation.
Analysis of immunoCRIT in benign and malignant non-immune privileged and privileged tissues
We applied the epigenetic FOXP3 assay for determination of Treg numbers in the aforementioned tissue specimen. The obtained Treg values were used to calculate the ratio of Tregs to all T lymphocytes, i.e., the immunoCRIT level.
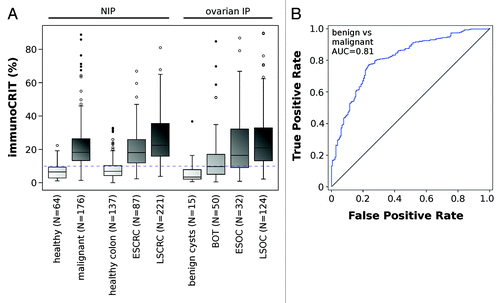
Our data showed minor variations in median immunoCRIT level in benign tissues, ranging from 3.7% in ovarian cysts, 6.8% in colon and 6.5% in other healthy non-immune privileged tissues (). In contrast, cancerous tissues regardless of origin and immune privilege showed median levels of immunoCRIT of approx. 19%, with mild fluctuations between LSCRC (20.7%), other early and late stage malignant non-immune privileged tissues (18.2%) and LSOC (21.7%) cancers. The large difference occurring in immunoCRIT levels between healthy and cancerous tissues suggested that this parameter may be utilized for the segregation of malignant and healthy tissue. The ROC curve provides an AUC of 0.81 (CI: 0.78–0.87, P < 0.001) with an accuracy of 0.78 at an immunoCRIT level of 9.9%, if all malignant tissues were compared with all benign tissues. This difference remains consistent if immunoCRIT is analyzed between individual malignant and benign tissue types (AUC = 0.91 for IP ovarian tissues (CI: 0.80–1.0; P < 0.001), accuracy 0.90 at a cut-off of 9.9%; AUC = 0.85 for non-immune privileged colorectal tissue (CI: 0.81–0.90, P < 0.001), accuracy = 0.81 at a cut-off of 10.6%). In summary, these data show that immunoCRIT is independent of tissue origin, but shows profound differences between benign and malignant tissues.
Figure 2. ImmunoCRIT in benign tissue and solid tumors. (A) ImmunoCRIT in different non-immune privileged tissues (i.e., lung, kidney and breast), in benign colon, early stage (ESCRC) and late stage (LSCRC) colorectal carcinoma and in IP ovarian tissues (cysts, borderline ovarian tumor (BOT), early stage (EOSC) and late stage ovarian carcinoma (LSOC)). Dotted line represents the calculated immunoCRIT cut-off values for most accurate segregation between benign and malignant tissue regardless of immune privilege. All measurements were conducted using the epigenetic CD3 or FOXP3 specific qPCR assay. (B) ROC curve is plotted for the discrimination between any benign and malignant tissue. AUC is indicated.
Correlation of disease aggressiveness with immunoCRIT in ovarian and colorectal tissues
Clear differences in immunoCRIT between healthy and malignant tissue made us interested in the interdependence between immunoCRIT and progressing stages of malignancy. We categorized ovarian and colorectal cancer in early and late stage tumors and analyzed benign ovarian cysts and BOT, the latter as a histologically defined group of ovarian tumors with a low malignant potential. ImmunoCRIT along the histopathologically defined disease stages showed a strong shift. In benign ovarian tissues a median immunoCRIT of 3.4% was observed. This level increased to 9.7% in borderline tumors, further rising to 17.2% in ESOC and reaching 21.7% in LSOC. The same trend was also observed in colorectal tissue, where benign tissue showed median immunoCRIT levels of 6.9%, which increased to 18.1% in ESCRC and reached 22.3% in LSCRC. Jonckheere-Testra test indicated a highly significant group effect as well as a gradually increasing ratio of the immunoCRIT over all malignancy states in both tissues (each with P < 0.001; ). When separately analyzing male and female CRC patients, we observed significantly higher immunoCRIT values in females. However, the observed increasing trend from benign over early to late stage cancer was consistent in either gender (data not shown). Together, these data show a clear and highly significant increase of immunoCRIT parallel to the malignant potential of cancerous diseases.
ImmunoCRIT in peripheral blood in a cohort of patients with ovarian neoplasias
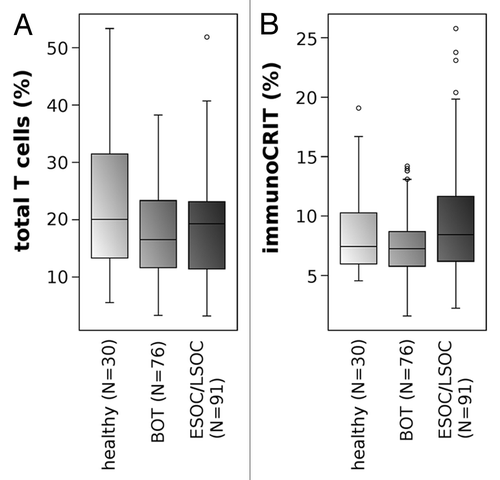
To detect whether this increase in immunoCRIT is also reflected in the peripheral blood of ovarian cancer patients, we analyzed blood samples from healthy donors, borderline ovarian tumor patients and epithelial ovarian cancer patients obtained at primary diagnosis. The difference in tTL levels between ESOC/LSOC patients, BOT patients and healthy donors (median values were: 20.1%, 16.5% and 19.3%, respectively) were minor (). We observed a mild, non-significant (P = 0.22) increase of immunoCRIT when comparing blood from healthy donors (median 7.4%) with BOT and ESOC/LSOC patients (median 7.3% and 8.6%, respectively, ).
ImmunoCRIT in peripheral blood along disease course of ovarian cancer patients
High immunoCRIT in malignant tissue, a tendency toward increased tolerance levels in the periphery at primary diagnosis and increased probability of extraperitoneal metastases upon prolonged treatment, led us to analyze peripheral immunoCRIT in the course of ovarian cancer. We monitored the peripheral immunoCRIT at up to four different time points (t1–t4) of disease progress in 12 patients, by measuring epigenetic cell typing assays for Tregs, tTLs, and the subpopulations of CD4+ and CD8+ T lymphocytes. The latter two assays were utilized as additional confirmation for the tTL count, since CD4+ and CD8+ T lymphocytes should represent the vast majority of tTLs. The patients were diagnosed with confirmed epithelial ovarian cancer of serous (10 patients), undifferentiated (1) or endometroid (1) histopathology and FIGO stage IIb (1 patient), IIIb (1) and IIIc (10) at primary diagnosis.
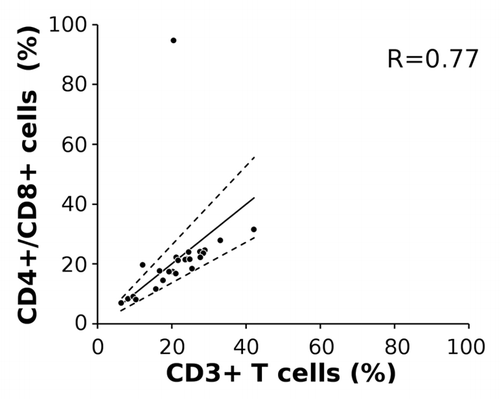
Relative median Treg and tTL numbers at first measured time points, mostly at primary diagnosis, were at 1.6% (range 0.8 to 7.6%) and 21.2% (6.5 to 42.2%), respectively. This corresponds to the data from a larger cohort presented in . tTL values expectedly showed high correlation to combined CD4+ and CD8+ cell numbers (Spearman’s rho = 0.77), with the exception of patient 5, which shows biologically implausible discrepancies at primary diagnosis (). We consider a biological or medical cause, such as mutations or genetic aberrations, unlikely since T lymphocyte numbers of the same patient at later time points of disease showed no abnormalities. Additionally, there were no technical problems associated and therefore we have no explanation for the divergence of this outlier. Nevertheless, this data point was included in all downstream statistical analyses, potentially negatively impacting the clearness of the data, but avoiding an optimistic bias.
Figure 4. Correlation of total CD3+ T lymphocytes with CD4+ and CD8+ T lymphocyte subpopulations. Cell frequencies were measured by epigenetic CD3, CD4 and CD8 qPCR assays in peripheral blood from 10 patients drawn at up to 4 different time points (n = 23). The solid line represents the estimated regression line, broken lines the upper and lower 95% confidence interval. R indicates Spearman rank correlation coefficient for tTLs compared with combined CD4+/CD8+ measurement.
Peripheral immunoCRIT levels at primary diagnosis or the first time point of analysis correspond to the data presented in and ranged from 4.3 to 36.8% with a median of 6.8%. However, we observed a strong increase of peripheral immunoCRIT along with disease progression. In 9 out of 12 patients, the immunoCRIT at later time points was higher than the initially detected values (). In a 10th patient (#8) this was principally confirmed since immunoCRIT was lower at primary diagnosis (t1) compared with both later time points, but was highest at relapse 1 (t2) rather than at relapse 2 (t3). The 11th patient (#5) showed the expected increase starting from aftercare relapse 1 (t2) over relapse 3 through relapse 4 (t3 and t4), but represents the aforementioned outlier at primary diagnosis (t1) with respect to tTL, CD4+ and CD8+ T lymphocyte correlation. Hence, when disregarding this outlier, the increasing trend is confirmed for this patient. Only a single patient (#9) appeared to have a reduction of immunoCRIT when comparing values at primary diagnosis (t1) to relapse values (t2). It is noteworthy that in this latter patient the observed T lymphocyte value was at a pathologically low level at t1, both by tTL and combined CD4+ and CD8+ cell count analysis (data not shown). When taking together all patients and data points, we find the increase of immunoCRIT along with the progress of disease statistically highly significant, when tested with Page's trend test (p < 0.01).Citation40 In contrast, healthy controls showed no statistically significant changes in immunoCRIT over the course of 6 weeks. The stability of the immunoCRIT was also analyzed in 100 healthy donors in an independent study, showing no significant changes in a one-year time frame (data not shown). We conclude that the increase of peripheral immunoCRIT is specific to ovarian cancer progression and does not occur in healthy donors.
Discussion
We applied epigenetic qPCR assays to quantify total T lymphocyte (tTL) and immunoCRIT levels in peripheral blood and tissues of different origin in order to investigate how immune cell levels are associated with malignancy and spreading of tumors. To our knowledge, our data were obtained from a cohort larger than any previous conducted study analyzing these immunological properties in tumor patients. The level of immunoCRIT directly displays the equilibrium between Tregs and tTLs and reflects immune cell-mediated tolerance. This is in contrast to measurement of Tregs alone, which is less objective due to tolerance-independent effects, such as leukocyte fluctuations.
Our data show that tTL infiltration in immune privileged ovarian tissues is significantly lower than in non-immune privileged tissues. For ovarian tumors, we observe that tTL infiltration increases with stage but remains below tissues from non-immune privileged organs. In contrast tumors from non-immune privileged tissues show a reduction of tTL counts compared with their healthy counterparts. Data from different stages of colorectal cancer show that this effect is disease progress dependent. Whereas changes in tTL-infiltration show tissue-dependent characteristics, tumor development generally leads to a distorted tTL balance. Immune privilege specific differences in tTL infiltration are maintained in general, but progressively undermined in later tumor stages.
Since the majority of non-immune privileged samples were FFPE and most immune privileged samples were fresh frozen before analysis, we had to exclude preparation dependent effects. While T cells are somewhat increased (app. 30% overestimation) in FFPE tissues, this cannot account for the observed biological differences, which is an order of magnitude higher than the average technical difference (Table S1).
ImmunoCRIT levels are relatively stable throughout all benign tissues independent of tTL infiltration levels, origin and immune privilege status. A consistent immunoCRIT increase is observed in all analyzed tumor entities. In a detailed analysis of ovarian tissues, we found a gradual increase of immunoCRIT in parallel to histologically classified levels of malignancy: non-invasive cystadenoma show a low immunoCRIT level similar to healthy tissue. Borderline tumors (BOT) cannot invade peritoneal stroma and have a moderately increased immunoCRIT. A further increase in immunoCRIT is observed in malignant ESOC with obvious stromal invasions. LSOC shows the highest immunoCRIT and is associated with highly invasive properties as well as spreading outside the peritoneum. Strict tumor stage-dependent increase of immunoCRIT is also confirmed in colorectal tissue.
For tTL—and immunoCRIT—values in peripheral blood of healthy subjects and patients with BOT or cancer, no statistically relevant differences were found. Considering the low levels of immune cell infiltration in tumor-affected ovarian tissue, such limited tumor effect on the periphery is not surprising. Notably, the absence of Treg-mediated tolerance coincides with a general absence of peripherally disseminated metastases at diagnosis of ovarian cancer, whereas tumors derived from non-immune privileged tissues show hematogenous spread into distant organs. Extra-abdominal metastases in ovarian cancer only pose a frequent problem in the course of disease after diagnosis and upon recurrences. This time span (i.e., the survival time of the patient under treatment) is usually significantly longer than the time span from local immune escape to clinical manifestation and diagnosis. Thus, peripheral immunoCRIT levels may further rise along the course of disease until, eventually, peripheral tolerance is sufficient to foster hematogenous extra-abdominal tumor spread. To test this hypothesis, we analyzed ovarian cancer patients under treatment along their courses of disease. We observed a highly significant correlation of immunoCRIT along disease course in the periphery of 11 out of 12 patients while immunoCRIT was shown to be stable in healthy control donors. These data show that in ovarian cancer patients tolerance gradually develops in the periphery and coincides with a higher likelihood of extraperitoneal metastases.
Our data stress an abnormally high immunoCRIT as characteristic to all solid cancers. A central difference between tumors from different origins is the level of tTL infiltration. In immune-privileged ovarian tissue prolonged overall survival times -which were achieved due to improved treatment- made distant metastases a clinically relevant issue. This is consistent with a decreasing ability of the immune system to fight off tissue invasion,Citation19,Citation20 suggesting co-development of tumor aggressiveness and immune tolerance.
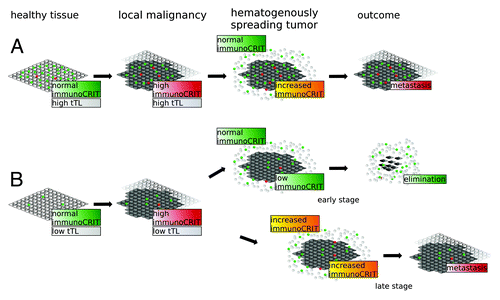
A model that is consistent with our finding is presented in : a shift toward higher immunoCRIT is prerequisite to tumor growth in all tissues regardless of tTL count (). The ability to invade local tissues becomes more pronounced along with elevated immunoCRIT values (). Transceolomic spread proceeds without overcoming immunological barriers. However, for hematogenous spread tumors need to overcome the peripheral immune environment. The immunoCRIT level in healthy blood is at approx. 5% ( and ) and thus significantly lower than in tumorous tissue (approx. 20%, ). The percental share of tTLs in non-immune privileged tissues is nearly equivalent to the bloodstream ( and ). In ovarian tissue tTL infiltration is significantly lower ( and ). The immunoCRIT in the microenvironment of the hematogenously circulating tumor lump is reduced to a non-pathological level by the low immunoCRIT in the surrounding bloodstream, allowing its elimination. Hence, tumor expansion remains confined to the abdominal cavity. If higher peripheral tolerance along the course of disease is established (as shown in ) immunoCRIT in the microenvironment of the circulating tumor structure remains high and metastases evade elimination even outside the abdomen. In contrast, due to higher tTL infiltration level in non-immune privileged tumors the effect of peripheral T lymphocytes on circulating tumor structures is less pronounced. While the immunoCRIT level is mildly decreased, tolerance is sufficient for promoting tumor escape from immune surveillance and metastasizing into distant organs. However, many aspects of this model remain hypothetical, as we did not show a functional link between the observed increasing immune tolerance and tumor aggressiveness.
Figure 5. Model of immunoCRIT-dependent tumor development and metastasis. Development and hematogenous dissemination of a tumor from non-immune privileged organs (A) and immune privileged ovarian tumors (B) are exemplified. On the left hand side, an immunoCRIT of 3–6% as measured in all healthy tissues is depicted. This corresponds for example to 3000 tTLs (green) and 200 Tregs (red) per 10 000 tissue cells (white) in non-immune privileged tissues and, analogously, to 400 tTLs and 13 Tregs per 10 000 in IP ovarian tissues cells. Upon malignant transformation, the immunoCRIT level increases to app. 20% in all tissues. In our example, the immunoCRIT level in non-immune privileged tissues would be reflected by 2000 tTls and 400 Tregs per 10000 cancer cells (dark gray), whereas in malignant IP ovarian tissue by 600 tTLs and 100 Tregs per 10000 tissue cells (2nd panel from the left). For reason of simplicity we assume the tumor is confronted with an equivalent number of leukocytes (white circles), which contain approximately 2000 tTLs and 250 Tregs. In non-immune privileged metastasizing tumor structures the immunoCRIT is little affected by the peripheral immunoCRIT due to the higher infiltration by tTLs in the primary tumor. During hematogenous spreading, the immunoCRIT in the microenvironment of the disseminating tumor lump remains increased to allow immunological evasion and forming of metastases in distant organs (right upper panel). In contrast, tTL infiltration in immune privileged ovarian cancer tissue is significantly lower. ImmunoCRIT in hematogenously circulating metastases in early disease stages can be lowered by non-pathological peripheral immunoCRIT levels in the blood stream, allowing elimination of the circulating cells. Upon disease progression, the peripheral immunoCRIT levels are constantly increased. Thus, immunoCRIT levels in hematogenously spreading tumor lumps in later stages are not decreased enough to enable elimination, leading to distant metastases in ovarian cancer.
ImmunoCRIT—measured either in tumor and peripheral blood in the course of disease—may provide a powerful diagnostic marker for progression of ovarian cancer. Since this marker is directly associated with the development of the malignant potential and spreading behavior of tumors, immunoCRIT-modifying therapies, such as intraperitoneal autologous peripheral blood T lymphocytes could be considered as therapy approaches. All together, our data from the epigenetic quantification of immune cells allows unmet comparability of the immune status in tissues and blood, providing new insights in the interaction between peripheral and tumor infiltrating immune cells. Such comparability of data obtained in different tissue types is essential for the understanding of the role of the immune system in tumor growth and aggressiveness.
Materials and Methods
Tissue samples and ethical approval
Ovarian cysts, borderline and ovarian cancer tissues were provided by tumor bank ovarian cancer, Charité, Campus Virchow. Sample management and preparation were done as reported.Citation41 Tissue samples of early stage ovarian cancer were collected within the ACTION trial.Citation42 Tissues from late stage ovarian cancer came from the OVCAD consortium and were collected according to their standard procedures.Citation43 Tissue samples for late stage colorectal cancer were obtained from the MRC COIN trial.Citation44 Breast cancer tissue was obtained from the Department of Obstetrics and Gynecology, University Hospital Heidelberg. All other tissue samples were obtained from the Institute of Pathology, Charité, Campus Benjamin Franklin. All institutions and trials had ethical board approval and patient consent. Pre-operative informed consent was obtained from each patient.
Peripheral blood from ovarian cancer patients
All samples were obtained from patients that were treated at the Clinic for Gynecology and Obstatrics Charité, Campus Virchow, Berlin. Each patient included in the longitudinal observation study had at least one recurrence in the followed time span and 7 patients deceased during the follow-up period. First line treatment was based on a platin-taxol chemotherapy regimen, whereas subsequent therapies varied between patients. The age of patients at ID ranged from 23 to 74 (median 57 y). For 9 patients, the first blood draw was at primary diagnosis, for the remaining three 6 mo after initial diagnosis (patient 10), at first recurrence (patient 5, 27 mo after initial diagnosis) and at a later recurrence (patient 12, 57 mo after initial diagnosis). The time points selected to monitor the immunoCRIT in the course of disease were chosen to include blood draws at relapses. In three cases, we also monitored time points at regular aftercare blood draws, i.e., between two disease events.
DNA preparation and bisulfite conversion
DNA from peripheral blood and tissue samples was isolated using DNeasy Blood&Tissue Kit (Qiagen) according to the respective protocol. DNA from formalin-fixed paraffin-embedded tissues was isolated either using QIAamp DNA FFPE Tissue Kit (Qiagen) or VERSANT kPCR Sample Prep (Siemens). As starting material 1–8 sections at 10 µm were used. Purified DNA was quantified using the Qubit fluorometer (Invitrogen) and 2 µg DNA was bisulfite converted using the EpiTect Bisulfite Kit (Qiagen) following the manufacturer's recommendations.
Epigenetic qPCR analyses
qPCR assays for quantification of Tregs,Citation35 tTLs,Citation37 CD4+ and CD8+ T lymphocytes were performed as follows: for each assay, sets of oligonucleotides (i.e., forward/reverse primer and hydrolysis probe) either specific for the TpG (i.e., demethylated) or the CpG (methylated) variant of the particular locus were used to quantify the respective target cells in a given sample. Percental target cells were calculated as previously described.Citation37 Crossing points of template amplification were determined using LightCycler 480 software (Roche Diagnostics) deploying the second derivative maximum method. Copy numbers were calculated by applying serial dilutions of assay-specific plasmid standards (Genscript Inc.). Reactions were performed in triplicates in a total volume of 10 µl using 2× Probe Mastermix (Roche Diagnostics), 15 pmol of each primer, 2.5 pmol probe (both Eurofins MWG Operon), 25 ng λ-DNA (New England Biolabs) and up to 350 ng bisulfite converted template DNA or serial dilutions of plasmid standards. Cycling conditions were 1 × 95 °C for 10 min, and 50 cycles of 95 °C for 15 s and 61 °C (or 58 °C in case of CD8+ T lymphocyte measurements) for 1 min.
Oligonucleotides
Oligonucleotides for qPCR analyses were purchased from Eurofins MWG Operon. Forward (fp), reverse (rp) primers and probes (p) are indicated by chromosomal positions of the human genome assembly NCBI36. CD4 (ENSG00000010610): TpG-specific: fp: 12:6770299–326:1, rp: 12:6770473–99:1, p: 12:6770423–48:1. CpG-specific: fp: 12:6770299–325:1, rp: 12:6770473–500:1, p: 12:6770425–47:1. CD8B (ENSG00000172116): TpG-specific: fp: 2:86902008–34:1, rp: 2:86902097–117:1, p: 2:86902059–91:1. CpG-specific: fp: 2:86902008–34:1, rp: 2:86902110–27:1, p: 2:86902059–86:1. Oligonucleotides for the FOXP3-, CD3- and GAPDH-qPCR assay were described elsewhere.Citation35,Citation37
Plasmid standards
Target regions for the various qPCR assays were designed in silico, synthesized (Genscript Inc.) and inserted into plasmid pUC57. Plasmids were linearized and diluted in 10 ng/µl of λ-phage DNA (New England Biolabs) to obtain qPCR standards with final concentrations of 6250, 1250, 250, 50 and 10 template copies per reaction.
Statistical analysis
Sample groups were tested for normal distribution using Kolmogorov-Smirnov tests. If the normal distribution assumption was retained, mean differences were tested using the Student t-test. Otherwise, Wilcoxon's rank sum test was used to test median differences. For nonparametric trend analyses, Jonckheere-Testra testCitation39 was applied for independent groups and Page's trend test for comparing repeated measurements.Citation40 For the latter comparison all patients but one were observed at only 2 or 3 time points. Thus, we limited the number of observation points to 3 and used the last observation carried forward (LOCF) approach to impute missing values.Citation45 For the assessment of discriminative value between immune privileged and non-immune privileged tissue by tTL infiltration and tissue malignancy by immunoCRIT, area under the receiver operator characteristics (ROC) curve (AUC) was determinded. No discrimination corresponds to an AUC of 0.5, perfect discrimination to an AUC value of 1.0. Cut-off values and accuracies were determined by the data point on the curve with the shortest euclidian distance to the upper left corner.Citation38 All reported P values correspond to two-sided tests. Statistics software SPSS 19.0 (IBM) was employed.
| Abbreviations: | ||
| BOT | = | borderline ovarian tumor |
| CRC | = | colorectal cancer |
| ESC | = | early stage cancer |
| FFPE | = | formalin-fixed paraffin-embedded |
| immunoCRIT | = | cellular ratio of immune tolerance |
| IP | = | immune-privileged |
| LSC | = | late stage cancer |
| OC | = | ovarian cancer |
| Tregs | = | regulatory T cells |
| tTL | = | total T lymphocytes |
Additional material
Download Zip (63.5 KB)Acknowledgments
Ivanka, we dedicate this work to you. To all of us who knew you, you were a great friend, an exemplary scientist and a guide as to how to remain straight and strong, always optimistic and positive, no matter how fate treated you. We will keep fighting your disease. In loving memory. We thank the MRC COIN study group for providing us access to the late stage colorectal samples.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental materials may be found here: www.landesbioscience.com/journals/epigenetics/article/26334
References
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004; 22:329 - 60; http://dx.doi.org/10.1146/annurev.immunol.22.012703.104803; PMID: 15032581
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol 2002; 3:991 - 8; http://dx.doi.org/10.1038/ni1102-991; PMID: 12407406
- Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest 2007; 117:1167 - 74; http://dx.doi.org/10.1172/JCI31202; PMID: 17476346
- Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol 2008; 20:241 - 6; http://dx.doi.org/10.1016/j.coi.2008.04.008; PMID: 18508251
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol 2004; 22:531 - 62; http://dx.doi.org/10.1146/annurev.immunol.21.120601.141122; PMID: 15032588
- Khattri R, Cox T, Yasayko S-A, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003; 4:337 - 42; http://dx.doi.org/10.1038/ni909; PMID: 12612581
- Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol 2003; 4:330 - 6; http://dx.doi.org/10.1038/ni904; PMID: 12612578
- Fontenot JD, Rasmussen JP, Williams LM, Dooley JL, Farr AG, Rudensky AY. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 2005; 22:329 - 41; http://dx.doi.org/10.1016/j.immuni.2005.01.016; PMID: 15780990
- Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science 2003; 299:1057 - 61; http://dx.doi.org/10.1126/science.1079490; PMID: 12522256
- Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med 2007; 13:108 - 16; http://dx.doi.org/10.1016/j.molmed.2007.01.003; PMID: 17257897
- Schaefer C, Kim GG, Albers A, Hoermann K, Myers EN, Whiteside TL. Characteristics of CD4+CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. Br J Cancer 2005; 92:913 - 20; http://dx.doi.org/10.1038/sj.bjc.6602407; PMID: 15714205
- Ormandy LA, Hillemann T, Wedemeyer H, Manns MP, Greten TF, Korangy F. Increased populations of regulatory T cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res 2005; 65:2457 - 64; http://dx.doi.org/10.1158/0008-5472.CAN-04-3232; PMID: 15781662
- Liyanage UK, Moore TT, Joo H-G, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002; 169:2756 - 61; PMID: 12193750
- Gray CP, Arosio P, Hersey P. Association of increased levels of heavy-chain ferritin with increased CD4+ CD25+ regulatory T-cell levels in patients with melanoma. Clin Cancer Res 2003; 9:2551 - 9; PMID: 12855630
- Viguier M, Lemaître F, Verola O, Cho M-S, Gorochov G, Dubertret L, Bachelez H, Kourilsky P, Ferradini L. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 2004; 173:1444 - 53; PMID: 15240741
- Sasada T, Kimura M, Yoshida Y, Kanai M, Takabayashi A. CD4+CD25+ regulatory T cells in patients with gastrointestinal malignancies: possible involvement of regulatory T cells in disease progression. Cancer 2003; 98:1089 - 99; http://dx.doi.org/10.1002/cncr.11618; PMID: 12942579
- Ichihara F, Kono K, Takahashi A, Kawaida H, Sugai H, Fujii H. Increased populations of regulatory T cells in peripheral blood and tumor-infiltrating lymphocytes in patients with gastric and esophageal cancers. Clin Cancer Res 2003; 9:4404 - 8; PMID: 14555512
- Kono K, Kawaida H, Takahashi A, Sugai H, Mimura K, Miyagawa N, Omata H, Fujii H. CD4(+)CD25high regulatory T cells increase with tumor stage in patients with gastric and esophageal cancers. Cancer Immunol Immunother 2006; 55:1064 - 71; http://dx.doi.org/10.1007/s00262-005-0092-8; PMID: 16328385
- Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res 2005; 11:8326 - 31; http://dx.doi.org/10.1158/1078-0432.CCR-05-1244; PMID: 16322292
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942 - 9; http://dx.doi.org/10.1038/nm1093; PMID: 15322536
- Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006; 313:1960 - 4; http://dx.doi.org/10.1126/science.1129139; PMID: 17008531
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006; 24:5373 - 80; http://dx.doi.org/10.1200/JCO.2006.05.9584; PMID: 17135638
- Keino H, Takeuchi M, Kezuka T, Hattori T, Usui M, Taguchi O, et al. Induction of Eye-Derived Tolerance Does Not Depend on Naturally Occurring CD4+CD25+ T Regulatory Cells. Investigative Ophthalmology &. Visual Science 2006; 47:1047 - 55
- McPherson SW, Heuss ND, Gregerson DS. Regulation of CD8(+) T Cell Responses to Retinal Antigen by Local FoxP3(+) Regulatory T Cells. Front Immunol 2012; 3:166; http://dx.doi.org/10.3389/fimmu.2012.00166; PMID: 22737153
- Cervantes-Barragán L, Firner S, Bechmann I, Waisman A, Lahl K, Sparwasser T, Thiel V, Ludewig B. Regulatory T cells selectively preserve immune privilege of self-antigens during viral central nervous system infection. J Immunol 2012; 188:3678 - 85; http://dx.doi.org/10.4049/jimmunol.1102422; PMID: 22407917
- Bacac M, Stamenkovic I. Metastatic cancer cell. Annu Rev Pathol 2008; 3:221 - 47; http://dx.doi.org/10.1146/annurev.pathmechdis.3.121806.151523; PMID: 18233952
- Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer 2002; 94:2698 - 705; http://dx.doi.org/10.1002/cncr.10541; PMID: 12173339
- Roodman GD. Mechanisms of bone metastasis. N Engl J Med 2004; 350:1655 - 64; http://dx.doi.org/10.1056/NEJMra030831; PMID: 15084698
- Sehouli J, Pietzner K, Harter P, Münstedt K, Mahner S, Hasenburg A, Camara O, Wimberger P, Boehmer D, Buehling KJ, et al. Prognostic role of platinum sensitivity in patients with brain metastases from ovarian cancer: results of a German multicenter study. Ann Oncol 2010; 21:2201 - 5; http://dx.doi.org/10.1093/annonc/mdq229; PMID: 20439341
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, et al. SEER Cancer Statistics Review, 1975-2008. National Cancer Institute, 2008.
- Kolles H, Stegmaier C, von Seebach HB, Ziegler H. Deskriptive Epidemiologie und Prognose maligner gynäkologischer Tumoren. Geburtshilfe Frauenheilkd 1989; 49:573 - 8; http://dx.doi.org/10.1055/s-2008-1035842; PMID: 2787260
- Hart WR. Borderline epithelial tumors of the ovary. Mod Pathol 2005; 18:Suppl 2 S33 - 50; http://dx.doi.org/10.1038/modpathol.3800307; PMID: 15761465
- Kaern J, Tropé CG, Kristensen GB, Abeler VM, Pettersen EO. DNA ploidy; the most important prognostic factor in patients with borderline tumors of the ovary. Int J Gynecol Cancer 1993; 3:349 - 58; http://dx.doi.org/10.1046/j.1525-1438.1993.03060349.x; PMID: 11578368
- Floess S, Freyer J, Siewert C, Baron U, Olek S, Polansky J, Schlawe K, Chang HD, Bopp T, Schmitt E, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol 2007; 5:e38 - , e; http://dx.doi.org/10.1371/journal.pbio.0050038; PMID: 17298177
- Wieczorek G, Asemissen A, Model F, Turbachova I, Floess S, Liebenberg V, Baron U, Stauch D, Kotsch K, Pratschke J, et al. Quantitative DNA methylation analysis of FOXP3 as a new method for counting regulatory T cells in peripheral blood and solid tissue. Cancer Res 2009; 69:599 - 608; http://dx.doi.org/10.1158/0008-5472.CAN-08-2361; PMID: 19147574
- Baron U, Floess S, Wieczorek G, Baumann K, Grützkau A, Dong J, Thiel A, Boeld TJ, Hoffmann P, Edinger M, et al. DNA demethylation in the human FOXP3 locus discriminates regulatory T cells from activated FOXP3(+) conventional T cells. Eur J Immunol 2007; 37:2378 - 89; http://dx.doi.org/10.1002/eji.200737594; PMID: 17694575
- Sehouli J, Loddenkemper C, Cornu T, Schwachula T, Hoffmüller U, Grützkau A, Lohneis P, Dickhaus T, Gröne J, Kruschewski M, et al. Epigenetic quantification of tumor-infiltrating T-lymphocytes. Epigenetics 2011; 6:236 - 46; http://dx.doi.org/10.4161/epi.6.2.13755; PMID: 20962591
- Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem 1993; 39:561 - 77; PMID: 8472349
- Jonckheere AR. A test of significance for the relation between m rankings and k ranked categories. Br J Stat Psychol 1954; 7:93 - 100; http://dx.doi.org/10.1111/j.2044-8317.1954.tb00148.x
- Page EB. Ordered hypotheses for multiple treatments: a significance test for linear ranks. J Am Stat Assoc 1963; 58:216 - 30; http://dx.doi.org/10.1080/01621459.1963.10500843
- Chekerov R, Koensgen D, Klaman I, Rosenthal A, Oskay-Oezcelik G, Mustea A, et al. Tumor- and stromal cell-specific expression of topoisomerase IIa and HER-2/neu in primary and recurrent ovarian cancer: Results of a prospective study. Mol Med Report 2009; 2:1011 - 6; http://dx.doi.org/10.3892/mmr_00000207
- Trimbos JB, Parmar M, Vergote I, Guthrie D, Bolis G, Colombo N, Vermorken JB, Torri V, Mangioni C, Pecorelli S, et al, International Collaborative Ovarian Neoplasm 1, European Organisation for Research and Treatment of Cancer Collaborators-Adjuvant ChemoTherapy un Ovarian Neoplasm. International Collaborative Ovarian Neoplasm trial 1 and Adjuvant ChemoTherapy In Ovarian Neoplasm trial: two parallel randomized phase III trials of adjuvant chemotherapy in patients with early-stage ovarian carcinoma. J Natl Cancer Inst 2003; 95:105 - 12; http://dx.doi.org/10.1093/jnci/95.2.113; PMID: 12529343
- Aust S, Bachmayr-Heyda A, Pateisky P, Tong D, Darb-Esfahani S, Denkert C, Chekerov R, Sehouli J, Mahner S, Van Gorp T, et al. Role of TRAP1 and estrogen receptor alpha in patients with ovarian cancer -a study of the OVCAD consortium. Mol Cancer 2012; 11:69; http://dx.doi.org/10.1186/1476-4598-11-69; PMID: 22978347
- Adams RA, Meade AM, Seymour MT, Wilson RH, Madi A, Fisher D, Kenny SL, Kay E, Hodgkinson E, Pope M, et al, MRC COIN Trial Investigators. Intermittent versus continuous oxaliplatin and fluoropyrimidine combination chemotherapy for first-line treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet Oncol 2011; 12:642 - 53; http://dx.doi.org/10.1016/S1470-2045(11)70102-4; PMID: 21641867
- Streiner DL. The case of the missing data: methods of dealing with dropouts and other research vagaries. Can J Psychiatry 2002; 47:68 - 75; PMID: 11873711