Abstract
Gene polymorphisms associated so far with plasma lipid concentrations explain only a fraction of their heritability, which can reach up to 60%. Recent studies suggest that epigenetic modifications (DNA methylation) could contribute to explain part of this missing heritability. We therefore assessed whether the DNA methylation of key lipoprotein metabolism genes is associated with high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C) and triglyceride levels in patients with familial hypercholesterolemia (FH). Untreated FH patients (61 men and 37 women) were recruited for the measurement of blood DNA methylation levels at the ABCG1, LIPC, PLTP and SCARB1 gene loci using bisulfite pyrosequencing. ABCG1, LIPC and PLTP DNA methylation was significantly associated with HDL-C, LDL-C and triglyceride levels in a sex-specific manner (all P < 0.05). FH subjects with previous history of coronary artery disease (CAD) had higher LIPC DNA methylation levels compared with FH subjects without CAD (P = 0.02). Sex-specific multivariable linear regression models showed that new and previously reported epipolymorphisms (ABCG1-CpGC3, LIPC-CpGA2, mean PLTP-CpGC, LPL-CpGA3, CETP-CpGA2, and CETP-CpGB2) significantly contribute to variations in plasma lipid levels (all P < 0.001 in men and P < 0.02 in women), independently of traditional predictors such as age, waist circumference, blood pressure, fasting plasma lipids and glucose levels. These results suggest that epigenetic perturbations of key lipoprotein metabolism genes are associated with plasma lipid levels, contribute to the interindividual variability and might partially explain the missing heritability of plasma lipid levels, at least in FH.
Introduction
High low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) levels, as well as low high-density lipoprotein cholesterol (HDL-C) concentrations, are strong independent risk factors for cardiovascular disease (CVD).Citation1,Citation2 Several primary (heritable) and secondary (environmental) factors are recognized determinants of plasma lipid concentrations. According to heritability studies, inheritance could account for up to 60% of the interindividual variability in plasma lipid concentration.Citation3,Citation4 However, genetic association studies have so far identified only a few well validated genes that account for a small proportion of the heritability estimates (~20 to 25%).Citation5,Citation6 Therefore, approximately 35 to 40% of plasma lipid level heritability cannot be explained by the current knowledge, a concept called missing heritability.
Previous epigenetic studies have suggested that DNA methylation, the most stable epigenetic mark, could contribute to explain the missing heritability of most complex traits, including plasma lipid levels.Citation7 DNA methylation is a non-traditional heritable factor involved in gene transcription regulation, especially by disrupting the binding of transcription factors.Citation8 It implicates the addition of a methyl group by a DNA methyltransferase at the position 5′ of the cytosine pyrimidine ring, most exclusively when cytosines are located upstream of guanines (sequence called CpG dinucleotides).Citation9 CpG dinucleotides are often clustered in CpG islands (CpGi), which are commonly located in regulatory regions of many genes.Citation10 Some groups have reported that genome-wide and gene specific DNA methylation levels are associated with plasma lipid concentrations and CVD risk.Citation11-Citation15 More recently, our group reported that DNA methylation levels at the ATP-binding cassette transporter A1 (ABCA1), cholesteryl ester transfer protein (CETP) and lipoprotein lipase (LPL) gene promoter loci are especially associated with variations in HDL size and cholesterol contents, as well as previous history of coronary artery diseases (CAD) in familial hypercholesterolemia (FH).Citation16,Citation17 However, the association between DNA methylation levels of other key lipoprotein metabolism genes and plasma lipid concentrations remains to be determined.
In the current study, we analyzed the epigenetic profile at four additional genes implicated in lipoprotein metabolism: ATP-binding cassette transporter G1 (ABCG1; 21q22.3), hepatic lipase (LIPC; 15q21.3), phospholipid transfer protein (PLTP; 20q13.12), and scavenger receptor B1 (SCARB1; 12q24.31). ABCG1 encodes a transmembrane regulator of cholesterol and phospholipid transport, which mediates cholesterol efflux from human macrophages to mature HDL particles. ABCG1 might then be involved in foam cell and atherosclerotic plaque formation.Citation18 Hepatic lipase (HL) is a protein anchored on the endothelial surface specific to the liver where it catalyzes the hydrolysis of lipoproteins’ TG content.Citation19 HL plays an important role in the regulation of plasma lipid concentrations, considering that LIPC polymorphisms were previously associated with dyslipidemia and premature atherosclerosis.Citation19-Citation21 PLTP is secreted in circulation where it mediates the transfer of phospholipids from very low-density lipoprotein (VLDL) and LDL to HDL particles.Citation22 Scavenger receptor B1 (SRBI) is a plasma membrane receptor that catalyzes the bidirectional transfer of cholesterol between most of the peripheral cells and HDL particles. SRBI plays a fundamental role in the reverse cholesterol transport, since it is the major molecular pathway mediating selective cholesterol uptake by the liver. Moreover, common polymorphisms within ABCG1, LIPC, PLTP, and SCARB1 genes have been repeatedly and strongly associated with plasma lipid concentration variability.Citation5,Citation6,Citation23
The aim of the current study was thus to assess whether DNA methylation at key genes involved in lipoprotein metabolism is associated with plasma lipid concentrations and previous history of CAD in FH, a human dyslipidemia model thoroughly studied in CVD research. We focused our analysis primarily on the ABCG1, LIPC, PLTP, and SCARB1 genes. We also examined whether epipolymorphisms within these genes and others that our group has already studied (ABCA1, CETP, and LPL)Citation16,Citation17 might independently contribute to HDL-C, LDL-C, and TG concentration variability in FH.
Results
DNA methylation analyses and CpG selection in the subsample of 28 FH men
DNA methylation variation at the ABCG1, LIPC, PLTP and SCARB1 gene loci () was first assessed in a subsample of finely phenotyped men selected from our FH cohort (n = 28). This allowed us to identify the loci showing DNA methylation variability and that were the most promising to find associations with plasma lipids. According to these initial results, ABCG1-CpGC3, LIPC-CpGA2, and mean PLTP-CpGC were retained to be analyzed in the complete data set (Table S1). None of these CpGs was found significantly associated with leucocyte cellular heterogeneity (Table S2). DNA methylation levels at the SCARB1 gene locus were not associated with plasma lipid profile and either showed very low interindividual variability or were associated with blood leucocyte heterogeneity (Tables S1 and S2). Based on all these subsequent observations, analyses of the association between plasma lipid profile and epipolymorphisms within the ABCG1, LIPC and PLTP genes (respectively ABCG1-CpGC3, LIPC-CpGA2, and mean PLTP-CpGC) were undertaken in the complete FH sample.
Figure 1.ABCG1, LIPC, PLTP and SCARB1 gene loci. Three different regions (named from [A‒C]) were epigenotyped at the ABCG1, LIPC and PLTP gene loci and four (named from [A‒D]) at the SCARB1 gene locus. These epigenotyped regions are located at the gene promoter region and near or in a CpG island (represented by thin dashed rectangles). Overall 16 CpG dinucleotides were epigenotyped at the ABCG1 gene locus, 11 at the LIPC gene locus, 9 at the PLTP gene promoter locus and 20 at the SCARB1 gene locus.
![Figure 1.ABCG1, LIPC, PLTP and SCARB1 gene loci. Three different regions (named from [A‒C]) were epigenotyped at the ABCG1, LIPC and PLTP gene loci and four (named from [A‒D]) at the SCARB1 gene locus. These epigenotyped regions are located at the gene promoter region and near or in a CpG island (represented by thin dashed rectangles). Overall 16 CpG dinucleotides were epigenotyped at the ABCG1 gene locus, 11 at the LIPC gene locus, 9 at the PLTP gene promoter locus and 20 at the SCARB1 gene locus.](/cms/asset/76eeb42d-faae-4063-acfd-3b6d76c11ad4/kepi_a_10927981_f0001.gif)
Associations between ABCG1, LIPC and PLTP DNA methylation and plasma lipid levels
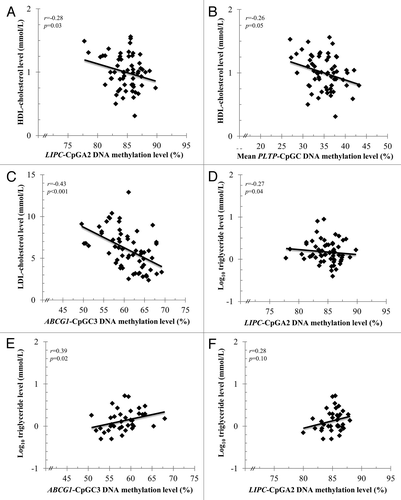
shows the characteristics of subjects from the full sample according to sex. Overall, men and women showed differences in anthropometric parameters and plasma lipid profile. Men (n = 61) were older, had lower HDL-C and LDL-C levels and had a larger waist circumference as compared with women (n = 37). Moreover, we observed significant sex differences in DNA methylation for ABCG1 and PLTP (P < 0.05). Therefore, the analyses between plasma lipid profile and epipolymorphisms were conducted in men and women separately. In men, a higher ABCG1-CpGC3 DNA methylation was associated with age (r = 0.39; P = 0.01), a larger waist circumference (r = 0.34; P = 0.008) and lower total cholesterol (r = -0.46; P < 0.001) and LDL-C concentrations (r = -0.43; P = 0.001). In women, a higher ABCG1-CpGC3 DNA methylation was associated with lower TG concentrations (r = 0.39; P = 0.02). LIPC-CpGA2 DNA methylation was negatively correlated with HDL-C (r = -0.28; P = 0.03) and TG concentrations (r = -0.27; P = 0.04) in men, whereas a trend for a positive association was observed with TG concentrations in women (r = 0.28; P = 0.10). Lower mean PLTP-CpGC DNA methylation levels were associated with higher HDL-C concentrations in men (r = -0.26; P = 0.05) (; Table S3). Results remained unchanged after adjustment for these potential confounding factors: age, waist circumference, HDL-C and TG levels.
Table 1. Characteristics of all subjects according to sex (n = 98)
Figure 2. Partial Pearson’s correlation between DNA methylation levels and plasma lipid profile in FH men and women. (A) LIPC-CpGA2 and HDL-C levels in men (n = 61). (B) Mean PLTP-CpGC and HDL-C levels in men (n = 61). (C) ABCG1-CpGC3 and LDL-C levels in men (n = 61). (D) LIPC-CpGA2 and fasting triglyceridemia in men (n = 61). (E) ABCG1-CpGC3 and fasting triglyceridemia in women (n = 37); and F) LIPC-CpGA2 and fasting triglyceridemia in women (n = 37). All correlation coefficients (r) and P values showed were adjusted for age, waist circumference, and fasting triglyceridemia or HDL-C levels. *Correlation coefficients (r) and P values reported for the associations with fasting triglyceridemia were obtained after log10-transformation.
Next, we investigated which factors among traditional and epigenetic predictors contribute to plasma lipid concentration variability. As expected, traditional risk factors (age, waist circumference, blood pressure, fasting glucose and plasma lipid levels) contributed significantly to the variability in plasma lipid concentrations (). More interestingly, shows that ABCG1, LIPC and PLTP DNA methylation levels contribute independently to plasma lipid level variability in men and women. When previously reported epipolymorphisms found to be associated with plasma lipid levels were added into the statistical models (mean ABCA1-CpGA, CETP-CpGA2, CETP-CpGB2 and LPL-CpGA3 DNA methylation levels), the amount of variance explained was further improved for all lipids tested (R2ajd were then between 16.8% and 49.9%), except for TG levels in women, which remained unchanged at 41.8% (). The independent association between epipolymorphisms and plasma lipid levels was confirmed by the analysis of the residual scores. shows that epipolymorphisms contribute significantly to the variability of residual scores of plasma lipid levels corrected for traditional predictors. Indeed, epipolymorphisms within key genes implicated in lipoprotein metabolism independently explained up to 15.8% of the HDL-C, 24.0% of the LDL-C and 17.4% of the TG concentration variability in FH subjects ().
Table 2. Multivariable linear regression analysis of traditional and epigenetic predictors (ABCG1, LIPC and PLTP DNA methylation) of HDL-C, LDL-C and TG levels in FH men and women
Table 3. Multivariable linear regression analysis of epigenetic and traditional predictors of HDL-C, LDL-C and TG levels in FH men and women
Table 4. Multivariable linear regression analyses of epigenetic predictors of residual scores of HDL-C, LDL-C and triglyceride (TG) levels in FH men and women
Association between LIPC DNA methylation and previous history of CAD
Afterward, we examined whether the ABCG1, LIPC and PLTP epigenetic profile might be associated with a previous history of CAD. shows subjects’ characteristics according to CAD status in the two groups matched for age, gender, BMI and total cholesterol concentrations. FH patients with a prior history of CAD had higher LIPC-CpGA2 DNA methylation levels compared with those without CAD (85.2% vs. 83.8%; P = 0.02). This association remained significant even after consideration for age, sex and plasma lipid profile (P = 0.04). No significant difference was observed between a prior history of CAD, ABCG1-CpGC3 and mean PLTP-CpGC DNA methylation.
Table 5. Characteristics of subjects according to CAD status (n = 44)
Association between DNA methylation and mRNA levels measured for ABCG1 and PLTP genes
Total leucocyte RNA was available for the relative quantification of ABCG1 and PLTP mRNA levels in the subsample of 28 FH men in order to evaluate the potential functional impact of the ABCG1 and PLTP DNA methylation variability. Interestingly, higher ABCG1-CpGC3 DNA methylation levels were associated with lower ABCG1 mRNA levels in leucocytes (r = -0.45; P = 0.02) (Table S1). No significant association was observed between mean PLTP-CpGC DNA methylation and leucocyte PLTP mRNA levels. However, DNA methylation levels at other CpG dinucleotides within the PLTP gene locus (PLTP-CpGA2 and PLTP-CpGB4) were modestly correlated with PLTP mRNA levels (Table S1). Potential binding sites for transcription factors were identified proximal to the epigenotyped regions. Specifically, binding sites for SP2/EKLF (erythroid transcription factor), upstream stimulatory factor (USF) and STAT5 transcription factors were observed respectively close to or within the ABCG1-CpGC3, LIPC-CpGA2, and PLTP-CpGC2 (Fig. S1).
Discussion
To the best of our knowledge, this study is the first to report associations between ABCG1, PLTP, and LIPC DNA methylation levels in leucocytes and the plasma lipid profile. Moreover, we showed that the combination of these and other epipolymorphisms previously identified within key lipoprotein metabolism genes contribute independently of traditional risk factors to interindividual variations in HDL-C, LDL-C, and TG concentrations. Again, the current results support the hypothesis that epigenetic changes within lipid metabolism genes might account for plasma lipid profile variability and could therefore be a molecular mechanism for dyslipidemias.
DNA methylation was previously shown to be partially inherited. Epigenetic modifications might therefore contribute to partly explain the missing heritability observed for many complex traits, including dyslipidemias.Citation7 Indeed, the mean DNA methylation heritability was estimated at 18% in twin studies, whereas it might be as high as 48% at some gene specific loci according to family studies.Citation15,Citation24 The current results, as well as previous studies performed by our groupCitation16,Citation17,Citation25 and others,Citation14,Citation15,Citation24 suggest that epipolymorphisms account for plasma lipid level interindividual variability and could thus be a key molecular mechanism to explain the missing heritability of dyslipidemias and CVD.
The transmembrane transporter ABCG1 is a main player in cholesterol efflux from macrophage to mature HDL. Polymorphisms within the ABCG1 gene have been previously associated with HDL-C levels and CAD risk.Citation23,Citation26,Citation27 Our results suggest that a higher ABCG1 DNA methylation, associated with gene expression repression in leucocytes (potentially through transcriptional activators EKLF and/or SP2 binding disruption), is associated with lower LDL-C in men and higher TG levels in women, whereas no conclusive association was observed with HDL-C levels. Interestingly, prior GWAS and candidate gene studies reported that ABCG1 polymorphisms are associated with TG and total cholesterol levels.Citation6,Citation28 Moreover, a previous study by Olivier et al. suggests that ABCG1 function is required for optimal bioavailability and activity of LPL produced by human macrophages.Citation29 Briefly, they showed that the silencing of ABCG1 expression in macrophages led to the retention of LPL at the cell surface, thus inhibiting its activity in TG-rich lipoprotein metabolism, and consequently decreasing the accumulation of lipids in macrophages. Indeed, an increase in macrophage LPL activity has previously been associated with an acute lipid accumulation in macrophages, which promotes the formation of macrophage foam cells and increases the risk for atherosclerosis.Citation30,Citation31 These observations combined with ours suggest that ABCG1 might not only be involved in reverse cholesterol transport, but also in the metabolism of TG-rich lipoprotein particles by macrophages and the formation of foam cells. Further studies will be needed to confirm the possible impact of epigenetic modifications in the ABCG-mediated lipid accumulation in macrophages.
PLTP is essential for the transfer of excess surface lipids from TG-rich lipoproteins to HDL particles. PLTP-mediated phospholipid transport among HDL particles is also known to be associated with HDL particle size and lipid composition.Citation32 Moreover, PLTP polymorphisms have been previously associated with HDL particle phenotype and TG levels.Citation5,Citation6,Citation23,Citation33 Concordant with this, we observed that a higher PLTP DNA methylation was associated with smaller HDL particles, as well as lower concentrations of HDL-phospholipid and HDL-C in men. Therefore, our observations are also consistent with the known role of DNA methylation in gene expression repression. These results strongly suggest that PLTP-mediated lipid transport is compromised when PLTP DNA methylation increases.
HL hydrolyzes TG and phospholipids in all lipoproteins, but is predominantly implicated in the removal of TG from VLDL, intermediate density lipoprotein and postprandial TG-rich HDL particles.Citation21 Indeed, several genome-wide and gene candidate studies have shown that polymorphisms in the HL gene (LIPC) are associated with fasting TG levels and HDL particle phenotype.Citation5,Citation6,Citation33-Citation35 Accordingly, we report here that a higher LIPC DNA methylation is associated with lower HDL particle size, HDL-phospholipid, HDL-C and fasting TG levels in men, whereas a higher LIPC DNA methylation in women is associated with higher fasting TG levels. Interestingly, we also showed that LIPC DNA methylation was slightly higher in CAD patients compared with patients without a previous history of CAD. Previous studies showed that even a small DNA methylation change might induce substantial differences in gene-expression levels over time and predispose to late-onset disease such as CVD.Citation36,Citation37 The role of HL in CAD and other CVD is still controversial. For instance, a previous study observed a significant association between LIPC polymorphisms and CVD,Citation38-Citation40 while others did not.Citation41-Citation43 Moreover, both increased and decreased HL activity have been associated with CAD.Citation21 However, it was suggested that in patients with isolated hypercholesterolemia, such as FH subjects, an increase in HL activity might be favorable as evidenced by its negative association with CAD risk, possibly because HL increases the reverse cholesterol transport in this specific population.Citation21 Based on our results, a higher LIPC DNA methylation might be associated with an increased CAD risk in FH. This observation is concordant with the expected functional impact of DNA methylation on gene repression. However, this hypothesis will have to be assessed in further studies, as the LIPC gene is not expressed in leucocytes.Citation44 Interestingly, we observed that DNA methylation variability at the LIPC-CpGA2 is located within a potential binging site for the transcription factor USF (previously reported as the -307/-312 E-box). Prior studies made by Van Deursen et al. showed that LIPC gene promoter activity is increased by USF and that this upregulation is mainly mediated through the -307/-312 E-box.Citation45,Citation46 Moreover, a recent study showed that DNA methylation of the USF binding site abolishes the liaison to DNA.Citation47 We therefore suggest that DNA methylation at this specific locus might interfere with the recruitment of USF to the LIPC gene promoter and decrease gene expression.
In order to decrease the impact of possible confounding factors such as genetic and environmental heterogeneity, the recruitment of FH subjects was restricted to those carrying the low-density lipoprotein receptor (LDLR) p.W66R mutation, which strengthens our study. Phenotyping of all subjects performed by well-trained research health professionals and experienced lipidologists, analysis of DNA methylation at most key lipoprotein metabolism genes, as well as the use of a robust and reproducible technology for the DNA methylation quantification (i.e., pyrosequencing of bisulfite treated DNA), are among the other strengths of this study. One limitation of this study relates to the impossibility to infer the causal relationship observed between DNA methylation and plasma lipid profile. DNA methylation could both regulate blood lipid concentrations and/or be a marker of plasma lipid profile variability, considering that a patient’s methylome can also be affected by lipoprotein themselves.Citation48,Citation49 Moreover, previous studies advised that the interpretation of whole blood DNA methylation should be performed with great caution since differentially methylated CpG might result from the heterogeneity in leucocyte cell types.Citation50 In order to avoid the potential impact of cellular heterogeneity on data interpretation, we only focused on CpG dinucleotides showing no association with cell blood count. Finally, these results will have to be validated in other FH and non-FH populations before strong conclusions can be drawn.
To conclude, we described for the first time that epipolymorphisms at the ABCG1, LIPC and PLTP gene loci are associated with variations in plasma lipid concentrations, and that hypermethylation of the LIPC gene is associated with previous history of CAD in FH. Moreover, we showed that DNA methylation at five key lipoprotein metabolism gene loci (ABCG1, CETP, LIPC, LPL, and PLTP) contributed independently of well-known traditional predictors to the plasma lipid level variability in a sex-specific manner in FH. Again, the identification of these epipolymorphisms raises very exciting new hypotheses in lipidology and cardiology research. Further larger epigenetic studies integrating genetics and transcriptomics are needed and will contribute to a better understanding of the molecular bases of dyslipidemia and the pathophysiological processes associated with the development of CAD and other cardiovascular phenotypes.
Materials and Methods
Sample and clinical data
This study included 98 FH patients (61 men and 37 women) from the Saguenay-Lac-Saint-Jean (SLSJ) region. All participants were French-Canadians carrying the lowLDLR p.W66R gene founder mutation (rs121908025). These participants were screened and genotyped at the Chicoutimi Hospital Lipid Clinic or the ECOGENE-21 Clinical Research Center between August 1994 and June 2010. All subjects gave their informed consent to participate in studies on genetic determinants of CAD combining genome-wide scans and candidate gene strategies and were assigned a code that systematically de-identifies all clinical data.Citation51 This project received the approval of the Chicoutimi Hospital Ethics Committee in accordance with the Helsinki Declaration.
A registered research nurse and a physician specialized in lipidology performed a complete anthropometric and metabolic profiling of subjects. The acquisition process of all clinical and biochemical characteristics has been described previously.Citation16,Citation17,Citation25 FH subjects were all normoglycemic, naïve of treatment and with no previous history of diabetes, uncontrolled thyroid, inflammatory and pituitary diseases.
Blood samples were obtained after a 12 h overnight fast from the antecubical vein into vacutainer tubes containing EDTA. Plasma cholesterol and TG concentrations were enzymatically measured on a CX7 Analyzer (Beckman). Cholesterol levels were measured in plasma and HDL particles contained in the infranatant after precipitation of VLDL and LDL particles (d < 1.006 g/ml) with heparin (2300 unit/ml) and MnCl2 (1 M). Then, plasma LDL-C levels were estimated using the Friedewald formula.Citation52
Because no blood sample was available for the study’s needs (fine phenotyping, blood cell counts and mRNA measurements), 28 FH men from our initial sample were selected for an additional clinical evaluation, as previously described.Citation17 Briefly, only male subjects were recruited to decrease the confounding effects of sexual hormones. Additional exclusion criteria were also applied: weight instability (± 2 kg) 3 mo prior to the study, severe hypertriglyceridemia (TG > 10 mmol/L), alcohol consumption > 2 drinks/day and the existence of diabetes, uncontrolled thyroid, inflammatory and pituitary diseases. In this subsample of 28 FH men, a fine phenotyping of HDL particles isolated by ultracentrifugation was performed (mean HDL particle size, HDL-TG, HDL-apolipoprotein A1 (apoA1) and HDL-phospholipid levels), as previously described.Citation25 The relative counts (%) of neutrophils, lymphocytes, monocytes, eosinophils and basophils were also determined on an impedance technology hematology counter (COULTER LH 780 hematology system; Beckman Coulter).
Nucleic acid extraction
DNA was purified from whole blood samples with the Gentra Puregen Blood Kit (Qiagen) as previously described.Citation25 Isolation and purification of intracellular RNA from whole blood stabilized in PAXgene Blood RNA Tubes (Qiagen) was completed with the PAXgene Blood RNA kit without DNase treatment (Qiagen). RNA samples were only available for the subsample of 28 FH men. RNA quality was assessed with the Agilent 2100 Bioanalyser RNA Nano Chips (Agilent Technologies). On average, RNA showed good quality (mean RNA integrity number = 8.3 ± 0.6).
DNA methylation measurement
The gold standard pyrosequencing technology is an accurate and quantitative sequencing assay. It was used to determine base-specific cytosine methylation levels at the ABCG1, LIPC, PLTP, and SCARB1genes. Pyrosequencing assays combine sodium bisulfite DNA conversion chemistry (EpiTech Bisulfite Kits; Qiagen), PCR amplification (PyroMark PCR Kit, Qiagen) and sequencing by synthesis assay (PyroMark Gold Q24 Reagents; Qiagen) of the target sequence. Sodium bisulfite preferentially deaminates unmethylated cytosines to thymines (after PCR amplification), whereas methyl-cytosines remain unmodified. Primers were selected using the PyroMark Assay Design software v.2.0.1.15. The PCR and pyrosequencing primers, as well as the primary and bisulfite-treated DNA sequences of both loci, are described in Table S4. DNA methylation levels were measured at genomic regions more likely to be associated with functional impacts on gene expression regulation, i.e., the gene promoter locus as well as regions near (CpGi shores) or within CpGi. Overall, 16 CpG dinucleotides were epigenotyped at the ABCG1 locus, 11 at the LIPC locus, 9 at the PLTP locus and 20 at the SCARB1 locus. The genomic context of the epigenotyped regions is shown in .
Leucocyte DNA methylation at each locus was first analyzed in the subsample of 28 FH men in order to identify loci showing DNA methylation level variability and CpG dinucleotides potentially associated with plasma lipid concentrations. When DNA methylation levels were found to be generally well correlated among the CpG dinucleotides from a same region (r > 0.7), a mean DNA methylation level for these CpGs was computed and used in the subsequent statistical analysis. Otherwise, the CpG dinucleotides were analyzed separately. Overall, four criteria were applied to identify CpG dinucleotides to be further analyzed: (1) a mean DNA methylation level between 10 and 90%; (2) variability in DNA methylation levels (sd > 3%); (3) no significant association between DNA methylation and the relative (%) leucocyte count (|r| < 0.38 and P > 0.05); and (4) at least one significant correlation between DNA methylation and plasma lipid levels (|r| > 0.39 and P < 0.05; after consideration for potential confounders, such as age and waist circumference). ABCG1-CpGC3, LIPC-CPGA2 and mean PLTP-CpGC met all four criteria and were therefore further analyzed in the whole sample of 98 FH subjects (Tables S1 and S2). DNA methylation levels at the ABCA1 (mean ABCA1-CpGA locus), CETP (CETP-CpGA2 and CETP-CpGB2) and LPL (LPL-CpGA3) gene promoter loci were previously determined in the whole sample and already met all four criteria.Citation16,Citation17
Relative leucocyte mRNA level measurement
ABCG1, PLTP and SCARB1 leucocyte mRNA levels were measured in the subsample of 28 FH men. cDNA was generated from total RNA (250 ng) using random primers provided with the High Capacity cDNA Archive Kit (Life Technologies). Equals amounts of cDNA (2 µL) were run in duplicate and amplified in a 20 µL reaction containing 10 µL of 2× Universal PCR Master Mix (Life Technologies). Primers and TaqMan probes were obtained from Life Technologies (ABCG1: Hs00245154_m1; PLTP: Hs00272126_m1 and SCARB1: Hs00969821_m1). Each sample was calibrated to the GAPDH housekeeping gene (reference gene; GAPDH: Hs99999905_m1).Citation53,Citation54 All TaqMan Gene expression assays were performed with probes covering an exon-exon junction thus avoiding the possible detection of genomic DNA. Relative quantification estimations were performed using a 7500Fast Real-Time PCR System as recommended by the manufacturer. The amplification efficiency of all qPCR reactions was determined with a series of serial dilution of a RNA sample pool. The slope computed on the qPCR standard curve was used to calculate the efficiency of the qPCR reactions (efficiency = -1+10(-1/slope)). The amplification efficiency obtained for all qPCR reactions fell between 97% and 103%. ABCG1/GAPDH, PLTP/GAPDH and SCARB1/GAPDH Ct ratio (1/x) values were used in the analyses.
Identification of potential binding motifs of transcription factors
The MatInspector program from the Genomatix software was used to identify potential transcription factor binding motifs within the epigenotyped loci of the ABCG1, LIPC and PLTP genes.Citation55 The primary sequences of the ABCG1-CpGC, LIPC-CpGA and PLTP-CpGC loci were used as the input sequence to determine specific regions where DNA methylation might interfere with transcription factor binding and therefore influence its expression regulation. Only transcription factor binding sites with a matrix similarity score above 0.90 were considered.
Statistical analyses
The normal distribution of all variables was assessed using a Kolmogorov-Smirnov test. Fasting TG and HDL-TG levels did not follow normal distributions and were log10-transformed. The association between DNA methylation, blood leucocyte cellular heterogeneity (relative cell count [%]), relative gene expression and plasma lipid profile was measured using a partial Pearson’s correlation controlling for potential confounding factors (age, waist circumference, and plasma lipid levels). Categorical variables were compared using a Pearson’s χ2-statistic, whereas group differences for continuous variables were compared using a Student t test and general linear models adjusted for potential confounding factors (age, sex, waist circumference, blood pressure and glycemia). Significant differences in DNA methylation according to CAD status were assessed in a subsample of FH subjects matched for age, gender, body mass index (BMI) and total cholesterol concentration (n = 44; 22 subjects with and 22 without previous history of CAD). Results were considered statistically significant when P-values were < 0.05 (two-sided).
Stepwise multivariable linear regression was used to identify predictors of HDL-C, LDL-C and TG levels in men and women among traditional predictors (age, waist circumference, systolic blood pressure, glycemia and plasma lipid profile) and the epipolymorphisms identified (mean ABCA1-CpGA, ABCG1-CpGC3, CETP-CpGA2, CETP-CpGB2, LIPC-CpGA2, LPL-CpGA3, mean PLTP-CpGC). Standardized coefficients (β coefficients) of the significant modulators are reported. Only variables with a P ≤ 0.10 were included in the regression model. The coefficient of determination (adjusted R2) is reported as the estimation of the variance explained by the statistical model. Multivariable regression results were confirmed using residual scores of HDL-C, LDL-C and TG levels corrected for traditional factors by using an unstandardized analysis of residuals computed by linear regression. These regression models included all traditional risk factors that contribute significantly to plasma lipid concentrations (age, waist circumference, systolic blood pressure, glycemia and/or plasma lipid profile). All statistical analyses were performed with the IBM SPSS Statistics 20 software (release 20.0.0, SPSS, Chicago, Il, USA).
| Abbreviations: | ||
| ABCA1 | = | ATP-binding cassette transporter A1 |
| ABCG1 | = | ATP-binding cassette transporter G1 |
| apoA1 | = | apolipoprotein A1 |
| apoB | = | apolipoprotein B |
| AU | = | arbitrary unit |
| BMI | = | body mass index |
| CAD | = | coronary artery disease |
| CETP | = | cholesteryl ester transfer protein |
| CpGi | = | CpG island |
| CVD | = | cardiovascular disease |
| EKLF | = | erythroid transcription factor |
| FH | = | familial hypercholesterolemia |
| HDL-C | = | high-density lipoprotein cholesterol |
| HL | = | hepatic lipase |
| LDL-C | = | low-density lipoprotein cholesterol |
| LDLR | = | low-density lipoprotein receptor |
| LIPC | = | hepatic lipase gene |
| LPL | = | lipoprotein lipase |
| PL | = | phospholipids |
| PLTP | = | phospholipid transfer protein |
| SCARB1 | = | Scavenger receptor B1 gene |
| SLSJ | = | Saguenay-Lac-Saint-Jean |
| SRBI | = | Scavenger receptor B1 |
| TG | = | triglyceride |
| USF | = | upstream stimulatory factor |
| VLDL | = | very low-density lipoprotein cholesterol |
Additional material
Download Zip (321.8 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Authors are thankful to all participants and the staff of the ECOGENE-21 Laboratory and Clinical Research Center. We particularly acknowledge the contribution of Sébastien Claveau (MSc), Nadia Mior, Denise Morin, Jeannine Landry (RN), Chantale Aubut (RN) and Céline Bélanger for their dedicated work. Simon-Pierre Guay was recipient of a doctoral research award from the Canadian Institutes for Health and Research (CIHR). During this study, Dr Daniel Gaudet held the Canada Research Chair in preventive genetics and community genomics. Luigi Bouchard is a junior research scholar from the Fonds de la recherche en santé du Québec (FRQS) and a member of the FRQS-funded Centre de recherche clinique Étienne-Le Bel. This project was supported by ECOGENE-21, the Canada Institutes for Health and Research (CIHR) team in community genetics (grant #CTP-82941), the Fondation des maladies du coeur du Québec, the Fonds de la recherche en santé du Québec (FRQ-S) and the Banting Research Foundation.
References
- Lamarche B, Després JP, Moorjani S, Cantin B, Dagenais GR, Lupien PJ. Triglycerides and HDL-cholesterol as risk factors for ischemic heart disease. Results from the Québec cardiovascular study. Atherosclerosis 1996; 119:235 - 45; http://dx.doi.org/10.1016/0021-9150(95)05653-X; PMID: 8808500
- Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, et al, INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364:937 - 52; http://dx.doi.org/10.1016/S0140-6736(04)17018-9; PMID: 15364185
- Heller DA, de Faire U, Pedersen NL, Dahlén G, McClearn GE. Genetic and environmental influences on serum lipid levels in twins. N Engl J Med 1993; 328:1150 - 6; http://dx.doi.org/10.1056/NEJM199304223281603; PMID: 8455681
- Almgren P, Lehtovirta M, Isomaa B, Sarelin L, Taskinen MR, Lyssenko V, Tuomi T, Groop L, Botnia Study Group. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia Study. Diabetologia 2011; 54:2811 - 9; http://dx.doi.org/10.1007/s00125-011-2267-5; PMID: 21826484
- Teslovich TM, Musunuru K, Smith AV, Edmondson AC, Stylianou IM, Koseki M, Pirruccello JP, Ripatti S, Chasman DI, Willer CJ, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010; 466:707 - 13; http://dx.doi.org/10.1038/nature09270; PMID: 20686565
- Asselbergs FW, Guo Y, van Iperen EP, Sivapalaratnam S, Tragante V, Lanktree MB, Lange LA, Almoguera B, Appelman YE, Barnard J, et al, LifeLines Cohort Study. Large-scale gene-centric meta-analysis across 32 studies identifies multiple lipid loci. Am J Hum Genet 2012; 91:823 - 38; http://dx.doi.org/10.1016/j.ajhg.2012.08.032; PMID: 23063622
- Eichler EE, Flint J, Gibson G, Kong A, Leal SM, Moore JH, Nadeau JH. Missing heritability and strategies for finding the underlying causes of complex disease. Nat Rev Genet 2010; 11:446 - 50; http://dx.doi.org/10.1038/nrg2809; PMID: 20479774
- Henikoff S, Matzke MA. Exploring and explaining epigenetic effects. Trends Genet 1997; 13:293 - 5; http://dx.doi.org/10.1016/S0168-9525(97)01219-5; PMID: 9260513
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev 2002; 16:6 - 21; http://dx.doi.org/10.1101/gad.947102; PMID: 11782440
- Craig JM, Bickmore WA. The distribution of CpG islands in mammalian chromosomes. Nat Genet 1994; 7:376 - 82; http://dx.doi.org/10.1038/ng0794-376; PMID: 7920655
- Baccarelli A, Wright R, Bollati V, Litonjua A, Zanobetti A, Tarantini L, Sparrow D, Vokonas P, Schwartz J. Ischemic heart disease and stroke in relation to blood DNA methylation. Epidemiology 2010; 21:819 - 28; http://dx.doi.org/10.1097/EDE.0b013e3181f20457; PMID: 20805753
- Cash HL, McGarvey ST, Houseman EA, Marsit CJ, Hawley NL, Lambert-Messerlian GM, Viali S, Tuitele J, Kelsey KT. Cardiovascular disease risk factors and DNA methylation at the LINE-1 repeat region in peripheral blood from Samoan Islanders. Epigenetics 2011; 6:1257 - 64; http://dx.doi.org/10.4161/epi.6.10.17728; PMID: 21937883
- Talens RP, Jukema JW, Trompet S, Kremer D, Westendorp RG, Lumey LH, Sattar N, Putter H, Slagboom PE, Heijmans BT, PROSPER Group. Hypermethylation at loci sensitive to the prenatal environment is associated with increased incidence of myocardial infarction. Int J Epidemiol 2012; 41:106 - 15; http://dx.doi.org/10.1093/ije/dyr153; PMID: 22101166
- Pearce MS, McConnell JC, Potter C, Barrett LM, Parker L, Mathers JC, Relton CL. Global LINE-1 DNA methylation is associated with blood glycaemic and lipid profiles. Int J Epidemiol 2012; 41:210 - 7; http://dx.doi.org/10.1093/ije/dys020; PMID: 22422454
- Zhang Y, Kent JW 2nd, Lee A, Cerjak D, Ali O, Diasio R, Olivier M, Blangero J, Carless MA, Kissebah AH. Fatty acid binding protein 3 (fabp3) is associated with insulin, lipids and cardiovascular phenotypes of the metabolic syndrome through epigenetic modifications in a Northern European family population. BMC Med Genomics 2013; 6:9; http://dx.doi.org/10.1186/1755-8794-6-9; PMID: 23510163
- Guay SP, Brisson D, Munger J, Lamarche B, Gaudet D, Bouchard L. ABCA1 gene promoter DNA methylation is associated with HDL particle profile and coronary artery disease in familial hypercholesterolemia. Epigenetics 2012; 7:464 - 72; http://dx.doi.org/10.4161/epi.19633; PMID: 22419126
- Guay SP, Brisson D, Lamarche B, Marceau P, Vohl MC, Gaudet D, Bouchard L. DNA methylation variations at CETP and LPL gene promoter loci: new molecular biomarkers associated with blood lipid profile variability. Atherosclerosis 2013; 228:413 - 20; http://dx.doi.org/10.1016/j.atherosclerosis.2013.03.033; PMID: 23623643
- Out R, Hoekstra M, Habets K, Meurs I, de Waard V, Hildebrand RB, Wang Y, Chimini G, Kuiper J, Van Berkel TJ, et al. Combined deletion of macrophage ABCA1 and ABCG1 leads to massive lipid accumulation in tissue macrophages and distinct atherosclerosis at relatively low plasma cholesterol levels. Arterioscler Thromb Vasc Biol 2008; 28:258 - 64; http://dx.doi.org/10.1161/ATVBAHA.107.156935; PMID: 18006857
- Connelly PW. The role of hepatic lipase in lipoprotein metabolism. Clin Chim Acta 1999; 286:243 - 55; http://dx.doi.org/10.1016/S0009-8981(99)00105-9; PMID: 10511296
- Hegele RA, Vezina C, Moorjani S, Lupien PJ, Gagne C, Brun LD, Little JA, Connelly PW. A hepatic lipase gene mutation associated with heritable lipolytic deficiency. J Clin Endocrinol Metab 1991; 72:730 - 2; http://dx.doi.org/10.1210/jcem-72-3-730; PMID: 1671786
- Brunzell JD, Zambon A, Deeb SS. The effect of hepatic lipase on coronary artery disease in humans is influenced by the underlying lipoprotein phenotype. Biochim Biophys Acta 2012; 1821:365 - 72; http://dx.doi.org/10.1016/j.bbalip.2011.09.008; PMID: 21986251
- Cheung MC, Wolfbauer G, Albers JJ. Plasma phospholipid mass transfer rate: relationship to plasma phospholipid and cholesteryl ester transfer activities and lipid parameters. Biochim Biophys Acta 1996; 1303:103 - 10; http://dx.doi.org/10.1016/0005-2760(96)00082-3; PMID: 8856039
- Edmondson AC, Braund PS, Stylianou IM, Khera AV, Nelson CP, Wolfe ML, Derohannessian SL, Keating BJ, Qu L, He J, et al. Dense genotyping of candidate gene loci identifies variants associated with high-density lipoprotein cholesterol. Circ Cardiovasc Genet 2011; 4:145 - 55; http://dx.doi.org/10.1161/CIRCGENETICS.110.957563; PMID: 21303902
- Bell JT, Tsai PC, Yang TP, Pidsley R, Nisbet J, Glass D, Mangino M, Zhai G, Zhang F, Valdes A, et al, MuTHER Consortium. Epigenome-wide scans identify differentially methylated regions for age and age-related phenotypes in a healthy ageing population. PLoS Genet 2012; 8:e1002629; http://dx.doi.org/10.1371/journal.pgen.1002629; PMID: 22532803
- Guay SP, Voisin G, Brisson D, Munger J, Lamarche B, Gaudet D, Bouchard L. Epigenome-wide analysis in familial hypercholesterolemia identified new loci associated with high-density lipoprotein cholesterol concentration. Epigenomics 2012; 4:623 - 39; http://dx.doi.org/10.2217/epi.12.62; PMID: 23244308
- Xu Y, Wang W, Zhang L, Qi LP, Li LY, Chen LF, Fang Q, Dang AM, Yan XW. A polymorphism in the ABCG1 promoter is functionally associated with coronary artery disease in a Chinese Han population. Atherosclerosis 2011; 219:648 - 54; http://dx.doi.org/10.1016/j.atherosclerosis.2011.05.043; PMID: 21722899
- Furuyama S, Uehara Y, Zhang B, Baba Y, Abe S, Iwamoto T, Miura S, Saku K. Genotypic Effect of ABCG1 gene promoter -257T>G polymorphism on coronary artery disease severity in Japanese men. J Atheroscler Thromb 2009; 16:194 - 200; http://dx.doi.org/10.5551/jat.E380; PMID: 19556716
- Abellán R, Mansego ML, Martínez-Hervás S, Morcillo S, Pineda-Alonso M, Carmena R, Real JT, Redon J, Rojo-Martínez G, Martín-Escudero JC, et al. Dietary polyunsaturated fatty acids may increase plasma LDL-cholesterol and plasma cholesterol concentrations in carriers of an ABCG1 gene single nucleotide polymorphism: study in two Spanish populations. Atherosclerosis 2011; 219:900 - 6; http://dx.doi.org/10.1016/j.atherosclerosis.2011.09.018; PMID: 21978921
- Olivier M, Tanck MW, Out R, Villard EF, Lammers B, Bouchareychas L, Frisdal E, Superville A, Van Berkel T, Kastelein JJ, et al. Human ATP-binding cassette G1 controls macrophage lipoprotein lipase bioavailability and promotes foam cell formation. Arterioscler Thromb Vasc Biol 2012; 32:2223 - 31; http://dx.doi.org/10.1161/ATVBAHA.111.243519; PMID: 22772754
- Van Eck M, Zimmermann R, Groot PH, Zechner R, Van Berkel TJ. Role of macrophage-derived lipoprotein lipase in lipoprotein metabolism and atherosclerosis. Arterioscler Thromb Vasc Biol 2000; 20:E53 - 62; http://dx.doi.org/10.1161/01.ATV.20.9.e53; PMID: 10978269
- Wilson K, Fry GL, Chappell DA, Sigmund CD, Medh JD. Macrophage-specific expression of human lipoprotein lipase accelerates atherosclerosis in transgenic apolipoprotein e knockout mice but not in C57BL/6 mice. Arterioscler Thromb Vasc Biol 2001; 21:1809 - 15; http://dx.doi.org/10.1161/hq1101.097805; PMID: 11701470
- Albers JJ, Vuletic S, Cheung MC. Role of plasma phospholipid transfer protein in lipid and lipoprotein metabolism. Biochim Biophys Acta 2012; 1821:345 - 57; http://dx.doi.org/10.1016/j.bbalip.2011.06.013; PMID: 21736953
- Kaess BM, Tomaszewski M, Braund PS, Stark K, Rafelt S, Fischer M, Hardwick R, Nelson CP, Debiec R, Huber F, et al. Large-scale candidate gene analysis of HDL particle features. PLoS One 2011; 6:e14529; http://dx.doi.org/10.1371/journal.pone.0014529; PMID: 21283740
- Ayyappa KA, Ghosh S, Mohan V, Radha V. Association of hepatic lipase gene polymorphisms with hypertriglyceridemia and low high-density lipoprotein-cholesterol levels among South Indian subjects without diabetes. Diabetes Technol Ther 2013; 15:503 - 12; http://dx.doi.org/10.1089/dia.2012.0302; PMID: 23550552
- Fang DZ, Liu BW. Polymorphism of HL +1075C, but not -480T, is associated with plasma high density lipoprotein cholesterol and apolipoprotein AI in men of a Chinese population. Atherosclerosis 2002; 161:417 - 24; http://dx.doi.org/10.1016/S0021-9150(01)00652-9; PMID: 11888526
- Toperoff G, Aran D, Kark JD, Rosenberg M, Dubnikov T, Nissan B, Wainstein J, Friedlander Y, Levy-Lahad E, Glaser B, et al. Genome-wide survey reveals predisposing diabetes type 2-related DNA methylation variations in human peripheral blood. Hum Mol Genet 2012; 21:371 - 83; http://dx.doi.org/10.1093/hmg/ddr472; PMID: 21994764
- Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, et al. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature 2009; 462:315 - 22; http://dx.doi.org/10.1038/nature08514; PMID: 19829295
- Peloso GM, Demissie S, Collins D, Mirel DB, Gabriel SB, Cupples LA, Robins SJ, Schaefer EJ, Brousseau ME. Common genetic variation in multiple metabolic pathways influences susceptibility to low HDL-cholesterol and coronary heart disease. J Lipid Res 2010; 51:3524 - 32; http://dx.doi.org/10.1194/jlr.P008268; PMID: 20855565
- Willer CJ, Sanna S, Jackson AU, Scuteri A, Bonnycastle LL, Clarke R, Heath SC, Timpson NJ, Najjar SS, Stringham HM, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet 2008; 40:161 - 9; http://dx.doi.org/10.1038/ng.76; PMID: 18193043
- Faggin E, Zambon A, Puato M, Deeb SS, Bertocco S, Sartore S, Crepaldi G, Pessina AC, Pauletto P. Association between the --514 C-->T polymorphism of the hepatic lipase gene promoter and unstable carotid plaque in patients with severe carotid artery stenosis. J Am Coll Cardiol 2002; 40:1059 - 66; http://dx.doi.org/10.1016/S0735-1097(02)02116-2; PMID: 12354428
- Ghatreh Samani K, Noori M, Nobar MR, Chaleshtory MH, Farrokhi E, Amin MD. The -514C/T Polymorphism of Hepatic Lipase Gene among Iranian Patients with Coronary Heart Disease. Iran J Public Health 2012; 41:59 - 65; PMID: 23113123
- Fan YM, Raitakari OT, Kähönen M, Hutri-Kähönen N, Juonala M, Marniemi J, Viikari J, Lehtimäki T. Hepatic lipase promoter C-480T polymorphism is associated with serum lipids levels, but not subclinical atherosclerosis: the Cardiovascular Risk in Young Finns Study. Clin Genet 2009; 76:46 - 53; http://dx.doi.org/10.1111/j.1399-0004.2009.01180.x; PMID: 19558527
- van Acker BA, Botma GJ, Zwinderman AH, Kuivenhoven JA, Dallinga-Thie GM, Sijbrands EJ, Boer JM, Seidell JC, Jukema JW, Kastelein JJ, et al, REGRESS Study Group. High HDL cholesterol does not protect against coronary artery disease when associated with combined cholesteryl ester transfer protein and hepatic lipase gene variants. Atherosclerosis 2008; 200:161 - 7; http://dx.doi.org/10.1016/j.atherosclerosis.2007.11.019; PMID: 18164013
- Rufibach LE, Duncan SA, Battle M, Deeb SS. Transcriptional regulation of the human hepatic lipase (LIPC) gene promoter. J Lipid Res 2006; 47:1463 - 77; http://dx.doi.org/10.1194/jlr.M600082-JLR200; PMID: 16603721
- van Deursen D, Jansen H, Verhoeven AJ. Glucose increases hepatic lipase expression in HepG2 liver cells through upregulation of upstream stimulatory factors 1 and 2. Diabetologia 2008; 51:2078 - 87; http://dx.doi.org/10.1007/s00125-008-1125-6; PMID: 18758746
- van Deursen D, van Leeuwen M, Vaulont S, Jansen H, Verhoeven AJ. Upstream Stimulatory Factors 1 and 2 activate the human hepatic lipase promoter via E-box dependent and independent mechanisms. Biochim Biophys Acta 2009; 1791:229 - 37; http://dx.doi.org/10.1016/j.bbalip.2009.01.017; PMID: 19416648
- Bélanger AS, Tojcic J, Harvey M, Guillemette C. Regulation of UGT1A1 and HNF1 transcription factor gene expression by DNA methylation in colon cancer cells. BMC Mol Biol 2010; 11:9; http://dx.doi.org/10.1186/1471-2199-11-9; PMID: 20096102
- Rangel-Salazar R, Wickström-Lindholm M, Aguilar-Salinas CA, Alvarado-Caudillo Y, Døssing KB, Esteller M, Labourier E, Lund G, Nielsen FC, Rodríguez-Ríos D, et al. Human native lipoprotein-induced de novo DNA methylation is associated with repression of inflammatory genes in THP-1 macrophages. BMC Genomics 2011; 12:582; http://dx.doi.org/10.1186/1471-2164-12-582; PMID: 22118513
- Chen KC, Liao YC, Hsieh IC, Wang YS, Hu CY, Juo SH. OxLDL causes both epigenetic modification and signaling regulation on the microRNA-29b gene: novel mechanisms for cardiovascular diseases. J Mol Cell Cardiol 2012; 52:587 - 95; http://dx.doi.org/10.1016/j.yjmcc.2011.12.005; PMID: 22226905
- Reinius LE, Acevedo N, Joerink M, Pershagen G, Dahlén SE, Greco D, Söderhäll C, Scheynius A, Kere J. Differential DNA methylation in purified human blood cells: implications for cell lineage and studies on disease susceptibility. PLoS One 2012; 7:e41361; http://dx.doi.org/10.1371/journal.pone.0041361; PMID: 22848472
- Gaudet D, Arsenault S, Bélanger C, Hudson T, Perron P, Bernard M, Hamet P. Procedure to protect confidentiality of familial data in community genetics and genomic research. Clin Genet 1999; 55:259 - 64; http://dx.doi.org/10.1034/j.1399-0004.1999.550408.x; PMID: 10361987
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18:499 - 502; PMID: 4337382
- Carrol ED, Salway F, Pepper SD, Saunders E, Mankhambo LA, Ollier WE, Hart CA, Day P. Successful downstream application of the Paxgene Blood RNA system from small blood samples in paediatric patients for quantitative PCR analysis. BMC Immunol 2007; 8:20; http://dx.doi.org/10.1186/1471-2172-8-20; PMID: 17850649
- Spinsanti G, Zannolli R, Panti C, Ceccarelli I, Marsili L, Bachiocco V, Frati F, Aloisi AM. Quantitative Real-Time PCR detection of TRPV1-4 gene expression in human leukocytes from healthy and hyposensitive subjects. Mol Pain 2008; 4:51; http://dx.doi.org/10.1186/1744-8069-4-51; PMID: 18983665
- Cartharius K, Frech K, Grote K, Klocke B, Haltmeier M, Klingenhoff A, Frisch M, Bayerlein M, Werner T. MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 2005; 21:2933 - 42; http://dx.doi.org/10.1093/bioinformatics/bti473; PMID: 15860560
