Abstract
Maternal cigarette smoking during pregnancy is associated with poor fetal outcome and aberrant miRNA expression is associated with adverse pregnancy outcomes. In 25 human placentas, we analyzed the expression of four candidate miRNA previously implicated in growth and developmental processes: miR-16, miR-21, miR-146a, and miR-182, and used three immortalized placental cell lines to identify if specific components of cigarette smoke were responsible for alterations to miRNA expression. miR-16, miR-21, and miR-146a were significantly downregulated in cigarette smoke-exposed placentas compared to controls. TCL-1 cells exposed to both nicotine and benzo(a)pyrene exhibited significant, dose-dependent downregulation of miR-146a. These results suggest that miR-146a is particularly responsive to exposures, and that smoking may elicit some of its downstream effects through alteration of miRNA expression.
Introduction
Gene-environment interactions occur throughout the lifetime but there are few times when such interactions are more important than during intrauterine development when perturbations can affect fetal growth and development. Further, there can be lasting effects due to altered fetal programming. During in utero development, the placenta plays a critical role supporting normal growth and development, providing the fetus with nutrients, assisting in waste removal, and protecting the fetus from both maternal immune rejection and from other environmental insults. Additionally, the placenta is a metabolic and endocrine center, producing and secreting hormones which support each stage of pregnancy. The placenta also expresses metabolic compounds responsible for the reactions which ultimately protect the fetus from exposure to toxicants. A number of drugs and toxicants, including nicotine,Citation1 alcoholCitation2 and benzo(a)pyrene,Citation3 have been found to accumulate in placenta tissue and affect placental gene expression. Maternal cigarette smoking has been reported to be associated with increased risk for spontaneous abortionCitation4 and preterm delivery.Citation4–Citation6 Previous studies have shown that there are placental complications linked to cigarette smoke exposure during pregnancy, including alterations to the development and function of the placenta.Citation7 There are more than 4,000 chemicals in a cigarette, including nicotine, benzo(a)pyrene and carbon monoxide; more than 43 of these chemicals are known carcinogens.Citation8 Nicotine readily crosses the placenta and can result in fetal concentrations that are 15% higher than maternal concentrations.Citation1 While a number of studies have shown a decrease in overall prevalence of smoking in women in the past 20 years, the prevalence of smoking in young pregnant women has increased.Citation9,Citation10 Additional studies have reported that 12–15% of all women smoke during their pregnancies.Citation11,Citation12 Taken collectively, these observations suggest that maternal smoking during pregnancy remains an important common exposure that can have major ramifications on not only the normal growth and development of the fetus but also on fetal programming.
The mechanisms by which exposures, such as cigarette smoke, affect the complex regulatory mechanisms of the placenta are still being characterized. One such mode of toxicity may be through the altered expression of microRNA (miRNA), small ∼22 nucleotide-long noncoding RNA molecules, which are highly ubiquitous and possess conservation across many species.Citation13 MicroRNA posttranscriptionally regulate gene expression by base-pairing to the 3′-untranslated region of a target mRNA resulting in either translational repression or direct degradation of the mRNA, the exact mechanism of which depends largely on the degree of complementarity of the miRNA to its mRNA target. Because partial complementarity of a miRNA to an mRNA target can still lead to translational repression, a single miRNA has the capability of regulating a large number of genes.Citation14 By negatively regulating their mRNA targets, miRNA have been implicated in regulating cell proliferation, growth and differentiation and apoptosis.Citation15 Highly conserved clusters of primate-specific miRNA are expressed in the placenta and other tissues.Citation16–Citation19 Additionally, multiple groups have further characterized a number of placental miRNA whose aberrant expression is associated with derangements in the intra-uterine environment or maternal condition.Citation20–Citation22 These discoveries have generated much interest in the involvement of miRNA in placental gene regulation and the possible utility of discovering placental miRNA which can serve as clinical biomarkers of exposure or disease.
As these miRNA play critical roles in development both of the fetus and placenta and because toxicant exposures and stress can alter the expression of miRNA,Citation23,Citation24 we sought to characterize the modulation of placental miRNA by maternal cigarette smoke by analyzing the expression of four candidate miRNA for associations with maternal cigarette smoking during pregnancy.
Results
Twenty-five placentas, eight with a history of maternal cigarette smoking during pregnancy and 17 non-smoking controls, were analyzed for expression of candidate miRNA. illustrates the demographics of the sample population. There were no significant differences in the gender, gestational age, birth weight or maternal age between exposed and unexposed infants. Quantitative RT-PCR analysis revealed downregulation of miR-16 (p < 0.0001), miR-21 (p < 0.0001) and miR-146a (p < 0.01) associated with maternal cigarette smoking during pregnancy (). miR-182 expression showed no differential expression by maternal cigarette smoking during pregnancy. To control for potential confounding effects of clinical variables in these results, we utilized multivariable linear regression to examine the association between miR-16, miR-21 and miR-146a expression with maternal smoking during pregnancy, controlled for gestational age, birth weight, gender and maternal age. The respective regression coefficients and p values are provided in . These models suggest that maternal smoking leads to a statistically significant 3.9% decrease in relative expression of miR-146a, a 5.8% decrease in the relative expression of miR-16 and a 9.4% decrease in the relative expression of miR-21, each independent of infant gender, gestational age, maternal age and infant birth weight.
Three immortalized placental cell lines representing three different stages and aspects of placental development were chosen to examine the effects of nicotine and benzo(a)pyrene specifically on the expression of the three miRNA found to be significantly altered in the primary tissues in cells from these different stages and aspects of placental development. We sought to investigate the effects of nicotine and benzo(a)pyrene exposure on the expression of these three miRNA in the first trimester villous 3A cells, the first trimester extravillous HTR8 cells and the third trimester extravillous TCL-1 cells. As shown in , qRT-PCR analysis revealed that miR-146a was significantly altered across nicotine exposures in TCL-1 (ANOVA p < 0.03), with a specific significant downregulation of approximately 2.5-fold in TCL-1 exposed to 1 µM nicotine compared to mock control (p < 0.02). No differential expression of miR-16 or miR-21 occurred in TCL-1, 3A and HTR8 cells exposed to nicotine (data not shown). Similar to nicotine exposure, qRT-PCR analysis revealed that miR-146a was downregulated in TCL-1 cells across all benzo(a)pyrene exposure levels ( and ANOVA p < 0.006), with 1 µM exposures leading to a greater than 50% downregulation and 10 µM an almost 75% downregulation of the miRNA. Again, no differential expression of miR-16 or miR-21 occurred in TCL-1, 3A and HTR8 cells exposed to benzo(a)pyrene (data not shown).
shows the targets as predicted by using the three-algorithm miRNA target prediction approach described and confirmed targets with respective sourcesCitation6,Citation25–Citation29 for the three candidate miRNA with significant differential expression in primary placenta samples exposed to cigarette smoking in utero. Predicted targets were as follows: for miR-16, BCL2L2 and EDA; for miR-21, PLAG1 and SATB1; and for miR-146a, TRAF6. Minimum free energies (MFE) are listed for each respective miRNA and target mRNA duplex and were determined using RNA-hybrid.
Discussion
Taken as a whole, our work identifies the association of cigarette smoking during pregnancy with the aberrant expression of several miRNA involved in critical cell processes. A number of groups have previously demonstrated that nicotineCitation1 and benzo(a)pyreneCitation3 interact with the placenta and may affect placental growth and development. Previous work demonstrated the effects of toxicants and agents of cell stress on expression of miR-146a,Citation23,Citation24,Citation30,Citation31 as well as on miR-16Citation32 and miR-21.Citation33,Citation34 While our observations are limited by incomplete information regarding the duration of cigarette smoking during pregnancy, cigarette per day usage or more extensive environmental exposure information (such as alcohol usage, environmental pollutant exposure or second-hand cigarette smoke exposure), our data comprise an important first step in determining associations between maternal cigarette smoking during pregnancy and aberrant miRNA expression in the placenta.
To further investigate the effects of cigarette smoke, we utilized three placental cell lines from different stages of placental development to investigate mechanisms of aberrant miRNA expression associated with cigarette smoke exposure. Downregulation of miR-146a in TCL-1 cells treated with nicotine and benzo(a) pyrene suggests that miR-146a may be especially sensitive to agents of cellular stress. Moreover, this result suggests that two components of cigarette smoke which affect the expression of miR-146a in term placentas may be nicotine and benzo(a)pyrene. The lack of differential expression of miR-16 and miR-21 in cells treated with nicotine or benzo(a)pyrene does not necessarily rule out that their expression is not modulated by cigarette smoke. Other components of cigarette smoke may modulate the expression of these miRNA. Furthermore, the effect of the mixed exposures encountered with the myriad chemicals in cigarette smoke may be truly responsible for the modulation of expression of these miRNA. Experiments conducted with different components of cigarette smoke and complex mixtures of these components may provide more extensive information on the mechanistic effects of cigarette smoke on miRNA expression.
Previous studies on benzo(a)pyrene-exposed human choriocarcinoma cell lines BeWo and JEG-3 showed that placental cell line proliferation was largely unchanged following exposure to 10 and 50 µM doses of benzo(a)pyrene in serum-containing medium.Citation35 Genbacev and colleagues used nicotine-exposed placental explants to conclude that nicotine is an important molecule that can drastically placental growth and development.Citation36 In our cell culture experiments, we saw no detectable change in cell growth or morphology in any of our cell lines treated with any of the doses of benzo(a)pyrene or nicotine compared to respective mock treated-cells. Doses of benzo(a)pyrene and nicotine higher than the ones we used may have led to increased cell toxicity and alterations in cell growth due to increased cell stress. Future investigations using higher doses may discover miRNA whose expression and function is altered by higher levels of benzo(a) pyrene, nicotine and other agents of environmental stress.
One of the more difficult challenges is miRNA target prediction; complementarity between miRNAs and their target mRNAs is generally far from perfect and is not required for miRNA to functionally silence the expression of a protein product. As a result of this complexity, a simple search for sequence complementarity between the miRNA and its mRNA target, the basis of many target prediction algorithms, can be expected to produce many false-positive hits. Moreover, most miRNAs are thought to have potentially hundreds of targets, some of which will be targeted more strongly than others and in cell-type specific contexts. Thus, it is essential to devise a strategy which predicts targets based not solely on sequence homology, but which also incorporates additional characterizations when choosing which targets should be examined with further testing.
Our target prediction strategy suggested that PLAG1 and SATB1 were targets of miR-21. Both of these genes are transcription factors implicated in tissue-specific control of the cell cycle and cellular proliferation, and PLAG1 has been previously demonstrated to be a target of miR-21.Citation26 Downregulation of miR-21 due to cigarette smoke exposure might lead to overproduction of PLAG1 and SATB1 protein, resulting in the overexpression of these key genes involved in cell cycling and proliferation.
Our target prediction strategy predicted BCL2L2 and EDA as targets for miR-16. BCL2L2 or B-cell CLL/lymphoma 2 like 2 protein, is a pro-survival molecule previously reported to be targeted by miR-133b.Citation37 Like other anti-apoptotic proteins in the BCL-2 family, BCL2L2 has been shown to be regulated both pre-transcriptionally and post-transcriptionally.Citation38 The prediction that BCL2L2 is a target of miR-16 supports hypotheses that by downregulating miR-16, cigarette smoke upregulates BCL2L2 and may enhance signaling through anti-apoptotic pathways. The EDA gene is mutated in anhidrotic ectodermal dysplasia and under normal circumstances, produces the protein ectodysplasin, a member of the TNF superfamily that is involved in activation of the NFκβ signaling pathway.Citation39 Again, miR-16 downregulation by cigarette smoke exposure, could lead to an upregulation of EDA protein thereby enhancing NFκβ signaling and increased tendency toward survival.
Our in-silico analysis predicted TRAF6 as a target for miR-146a, suggesting that downregulation of miR-146a could result in an upregulation of TRAF6, a protein also important for signaling through both the NFκβ signaling pathwayCitation40 as well as a mediator of inflammation in the toll-like receptor 4 (TLR4) pathway.Citation41,Citation42 Previous reports using different target prediction strategies have hypothesized TRAF6 as a target of miR-146aCitation28 and have validated TRAF6 as a target in murine macrophages.Citation29 TLR4 mediates the inflammatory response, and aberrant TLR-4 signaling is associated with inflammation-induced preterm delivery.Citation43 Such a consequence could be the result of dysregulated TRAF6 production as a result of downregulation of miR-146a. TRAF6 overexpression due to downregulation of miR-146a could result in overactive TLR signaling which could have a number of downstream consequences for both placenta and fetus. Further investigation into such relationships would prove vital into better understanding both the role of TRAF6 in TLR4 signaling, as well as the association of aberrant expression of miR-146a with adverse pregnancy outcomes.
As with miR-16, downregulation of miR-146a can lead to enhanced signaling through the anti-apoptotic and pro-survival NFκβ pathway, resulting in placental cells that avoid apoptosis and prolong their survival. Components of cigarette smoke have been suggested to function through the NFκβ pathwayCitation44 and, in light of our results with both placental tissues as well as cell culture experiments, both benzo(a)pyrene and nicotine may be acting through the NFκβ signaling pathway through downregulation of miR-146a. Studies by other groups have demonstrated that an NFκβ-modulated pathway upregulates miR-146a when miR-146a expression is induced with IL-1β.Citation45 While this may be true under normal circumstances under which conditions of cellular stress are held to a minimum, our data suggest that agents of cell stress, such as nicotine and benzo(a)pyrene, may downregulate miR-146a expression which, by consequently upregulating TRAF6 expression, enhances signaling through NFκβ and promotes cell survival. The complexities of this potential duality of effect, namely the notion that miR-146a both negatively regulates and can be regulated by the NFκβ pathway, remain to be further elucidated and future experiments involving knock-downs of various components of the pathway, including the miRNA acting as negative regulators of the pathway, may clarify the mechanisms at play. Our results suggest then that TRAF6, and therefore the NFκβ and TLR4 signaling pathways, may be important mediators of the effects of cigarette smoke exposure on the placenta.
Our results indicate a potential cascade of molecular changes that occur in the placenta upon exposure to cigarette smoke. We suggest that cigarette smoke exposure during pregnancy is associated with downregulation of miR-16, miR-21 and miR-146a in the placenta. Collectively, aberrant repression of these miRNA upregulates the targets of these miRNA and may affect cell cycle regulation, growth, immunomodulation and development in the placenta. These changes in target gene expression may have further effects downstream for both placenta and fetus, ultimately resulting in altered fetal programming. Future studies are ongoing to further characterize the effects of environmental exposures on placental miRNA, on understanding the downstream phenotypes of aberrant miRNA expression and in investigating the placenta as a record of the intrauterine environment.
Materials and Methods
Placenta samples.
Placenta samples were collected within two hours of delivery at Women and Infants' Hospital in Providence, RI, USA. An approximately 1 g biopsy of placenta was excised, free of maternal decidua, from the maternal side of the placenta 2 cm from the umbilical cord insertion site, and the sample was placed immediately in RNAlater and stored at 4°C. At least 72 h later, placenta samples were removed from the RNAlater, blotted dry, aliquoted and stored in sample tubes at −80°C until needed for examination. Medical information, including cigarette smoking during pregnancy, available on patient charts was also collected. All samples were collected in under appropriate IRB protocols for Women and Infants' Hospital and Brown University.
RNA extraction.
RNA was extracted from placenta samples and cultured cells using the miRvana miRNA Isolation Kit (Ambion) following the manufacturer protocols. For tissue samples, a 200 mg piece was cut and placed in ice cold, sterile PBS and homogenized using a PowerGen 125 Tissue Homogenizer (Fisher Scientific). The homogenized sample was removed from PBS and RNA was extracted using manufacturer protocols. Extracted RNA was quantified using a Nanodrop spectrophotometer and then aliquoted into single-use aliquots and stored at −80°C.
Quantitative RT-PCR (qRT-PCR) for mature miRNA.
Expression of mature miRNAs was measured using commercially available TaqMan MicroRNA Assays or TaqMan Gene Expression Assays (Applied Biosystems, Valencia, CA, USA) on an Applied Biosystems 7500 Real Time PCR system and analyzed with 7500 System Software. All reactions were run in triplicate, with RNU44 serving as the referent for miRNA expression. In addition, a no-RT control was run with each plate.
Cell culture.
3A and TCL-1 placental cell lines were cultured in RPMI 1640 medium (GIBCO) supplemented with 10% FBS and 1% Pen-Strep. HTR8 cells were cultured in RPMI 1640 medium (GIBCO) supplemented with 5% FBS. These three placental cell lines represent three different stages and aspects of placental development and were selected to examine the effects of components of cigarette smoke in models of different time-points during pregnancy. 3A cells are first-trimester villous cells, HTR-8s are first trimester extravillous cells and TCL-1s are third trimester extravillous cells. Placental cell lines were exposed to 0, 1, 5 and 10 µM doses of nicotine (Sigma-Aldrich) in medium for 6 days, with 1 µM being closest to a physiologically relevant dose and higher doses used to investigate the effects of more concentrated doses of nicotine on placental cells.Citation46,Citation47 Placental cell lines were exposed to 0.1, 1 and 10 µM doses of benzo(a)pyrene (Sigma-Aldrich) or DMSO (<0.1%) alone in medium for 6 days based on previously published methods and physiologically-relevant doses.Citation23,Citation48 None of the doses of nicotine or benzo(a)pyrene led to increased cell toxicity compared to controls. Cells were cultured for 6 days, with exposure medium refreshed on days 2 and 4. The 6-day period of exposure was chosen to mimic a long-term chronic exposure, but to avoid sub-culturing of the cells, and has been previously described.Citation23 All experimental and control conditions were performed in triplicate. Following exposure, cells were harvested and RNA was extracted for analysis by qRT-PCR as described above.
miRNA target prediction.
Three target prediction algorithms were used to predict targets for miRNA of interest, and a fourth algorithm was used to evaluate predicted targets for base-pairing and minimum free energy. The three algorithms used for target prediction were miRanda (September 2008 release, available online at www.microrna.org/microrna/home.do), PicTar (as cited in ref. Citation49 and available online at http://pictar.mdc-berlin.de/cgi-bin/new_PicTar_vertebrate.cgi) and TargetScan 5.1 (available online at www.targetscan.org/). In order to be considered a predicted target for further investigation, the target must have appeared in the top 100 targets in all three prediction algorithms. The RNA-hybrid algorithm was used to evaluate predicted targets for information on secondary structure and thermodynamic stability of the miRNA-mRNA duplex.Citation50 RNA-hybrid is available online at http://bibiserv.techfak.uni-bielefeld.de/rnahybrid/submission.html.
Statistical analysis.
Two-tailed t-tests were used to compare miRNA expression levels determined by real-time PCR in human tissue samples. In order to examine the association between exposure to cigarette smoke and miRNA expression, controlling for potential confounders, we employed multivariable linear regression modeling, in SAS 9.1 (SAS Institute, Cary, NC, USA). ANOVA was used to determine differential expression of miRNA across treatments of nicotine and benzo(a)pyrene on cells in cell culture experiments.
Financial Support
This work was funded by NIH grants from the NCRRP20RR018728, the NIEHS P42ES013660 and T32ES007272 (MAM) and the Flight Attendants Medical Research Institute Young Clinical Scientist Award.
Figures and Tables
Figure 1 Maternal cigarette smoking during pregnancy is associated with downregulation of miR-16, miR-21 and miR-146a. Quantitative RT-PCR analysis was used to examine the expression of the mature forms of miR-16 (A), miR-21 (B), miR-146a (C) and miR-182 (D), in primary placenta tissue samples from infants whose mothers smoked cigarettes (CS) during pregnancy (n = 8) and infants whose mothers did not smoke during pregnancy (No CS, n = 17). *indicates p < 0.01 and **indicates p < 0.0001 as determined by t-test.
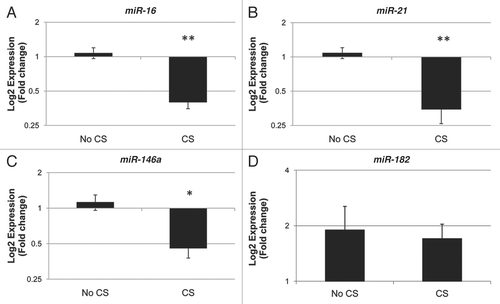
Figure 2 miR-146a is downregulated in TCL-1 cells exposed to nicotine and benzo(a)pyrene. Three placental cell lines (HTR8, 3A and TCL1) were exposed to increasing doses of nicotine (A) and benzo(a)pyrene (B) for 6 days, and the expression of miR-146A was determined through qRT-PCR. Error bars indicated standard error of the mean, *indicates a significant downregulation of miR-146a across doses of nicotine (ANOVA, p < 0.03). **indicates a significant downregulation of miR-146a across doses of benzo(a)pyrene (ANOVA, p < 0.006).
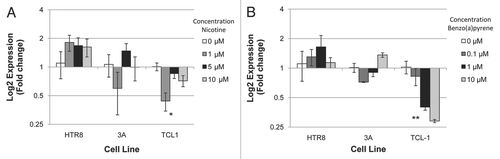
Table 1 Demographics of the study population
Table 2 Multivariable linear regression model of individual miRNA expression
Table 3 mRNA targets predicted and/or experimentally validated for miR-16, miR-21 and miR-146a
Acknowledgements
Many thanks to Devin Koestler, Charlotte Wilhelm, Amanda Filiberto and Luc Gagne for their insightful comments and criticisms during the preparation of this manuscript and to Keila Veiga and Alyse LaLiberte for placenta sample and medical history collection.
References
- Lambers DS, Clark KE. The maternal and fetal physiologic effects of nicotine. Semin Perinatol 1996; 20:115 - 126
- Beattie JO. Transplacental alcohol intoxication. Alcohol Alcohol 1986; 21:163 - 166
- Guyda HJ, Mathieu L, Lai W, Manchester D, Wang SL, Ogilvie S, et al. Benzo(a)pyrene inhibits epidermal growth factor binding and receptor autophosphorylation in human placental cell cultures. Mol Pharmacol 1990; 37:137 - 143
- Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five metaanalyses. Am J Prev Med 1999; 16:208 - 215
- Shah NR, Bracken MB. A systematic review and meta-analysis of prospective studies on the association between maternal cigarette smoking and preterm delivery. Am J Obstet Gynecol 2000; 182:465 - 472
- Kaddar T, Rouault JP, Chien WW, Chebel A, Gadoux M, Salles G, et al. Two new miR-16 targets: caprin-1 and HMGA1, proteins implicated in cell proliferation. Biol Cell 2009; 101:511 - 524
- Einarson A, Riordan S. Smoking in pregnancy and lactation: a review of risks and cessation strategies. Eur J Clin Pharmacol 2009; 65:325 - 330
- Thielen A, Klus H, Muller L. Tobacco smoke: unraveling a controversial subject. Exp Toxicol Pathol 2008; 60:141 - 156
- Mohsin M, Bauman AE. Socio-demographic factors associated with smoking and smoking cessation among 4,26,344 pregnant women in New South Wales, Australia. BMC Public Health 2005; 5:138
- Jaakkola N, Jaakkola MS, Gissler M, Jaakkola JJ. Smoking during pregnancy in Finland: determinants and trends 1987–1997. Am J Public Health 2001; 91:284 - 286
- Cnattingius S. The epidemiology of smoking during pregnancy: smoking prevalence, maternal characteristics and pregnancy outcomes. Nicotine Tob Res 2004; 6:125 - 140
- Goodwin RD, Keyes K, Simuro N. Mental disorders and nicotine dependence among pregnant women in the United States. Obstet Gynecol 2007; 109:875 - 883
- Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993; 75:843 - 854
- Du T, Zamore PD. Beginning to understand microRNA function. Cell Res 2007; 17:661 - 663
- Miska EA. How microRNAs control cell division, differentiation and death. Curr Opin Genet Dev 2005; 15:563 - 568
- Bentwich I, Avniel A, Karov Y, Aharonov R, Gilad S, Barad O, et al. Identification of hundreds of conserved and nonconserved human microRNAs. Nat Genet 2005; 37:766 - 770
- Lehnert S, Van Loo P, Thilakarathne PJ, Marynen P, Verbeke G, Schuit FC. Evidence for co-evolution between human microRNAs and Alu-repeats. PLoS One 2009; 4:4456
- Zhang R, Wang YQ, Su B. Molecular evolution of a primate-specific microRNA family. Mol Biol Evol 2008; 25:1493 - 1502
- Tsai KW, Kao HW, Chen HC, Chen SJ, Lin WC. Epigenetic control of the expression of a primate-specific microRNA cluster in human cancer cells. Epigenetics 2009; 4:587 - 592
- Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science 2002; 297:2056 - 2060
- Hu HY, Yan Z, Xu Y, Hu H, Menzel C, Zhou YH, et al. Sequence features associated with microRNA strand selection in humans and flies. BMC Genomics 2009; 10:413
- Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, et al. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 2007; 196:261 - 266
- Marsit CJ, Eddy K, Kelsey KT. MicroRNA responses to cellular stress. Cancer Res 2006; 66:10843 - 10848
- Avissar-Whiting M, Veiga K, Uhl K, Maccani M, Gagne L, Moen E, et al. Bisphenol A exposure leads to specific microRNA alterations in placental cells. Reprod Toxicol 2010; 29:401 - 406
- Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 2007; 133:647 - 658
- Tran N, McLean T, Zhang X, Zhao CJ, Thomson JM, O'Brien C, et al. MicroRNA expression profiles in head and neck cancer cell lines. Biochem Biophys Res Commun 2007; 358:12 - 17
- Rom S, Rom I, Passiatore G, Pacifici M, Radhakrishnan S, Del Valle L, et al. CCL8/MCP-2 is a target for mir-146a in HIV-1-infected human microglial cells. FASEB J 2010; 24:2292 - 2300
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NFkappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA 2006; 103:12481 - 12486
- Hou J, Wang P, Lin L, Liu X, Ma F, An H, et al. MicroRNA-146a feedback inhibits RIG-I-dependent Type I IFN production in macrophages by targeting TRAF6, IRAK1 and IRAK2. J Immunol 2009; 183:2150 - 2158
- Curtale G, Citarella F, Carissimi C, Goldoni M, Carucci N, Fulci V, et al. An emerging player in the adaptive immune response: microRNA-146a is a modulator of IL-2 expression and activation-induced cell death in T lymphocytes. Blood 2010; 115:265 - 273
- Wang JF, Yu ML, Yu G, Bian JJ, Deng XM, Wan XJ, et al. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun 2010; 394:184 - 188
- Pothof J, Verkaik NS, van IW, Wiemer EA, Ta VT, van der Horst GT, et al. MicroRNA-mediated gene silencing modulates the UV-induced DNA-damage response. EMBO J 2009; 28:2090 - 2099
- Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun 2010; 393:643 - 648
- Wang P, Zou F, Zhang X, Li H, Dulak A, Tomko RJ Jr, et al. microRNA-21 negatively regulates Cdc25A and cell cycle progression in colon cancer cells. Cancer Res 2009; 69:8157 - 8165
- Zhang L, Connor EE, Chegini N, Shiverick KT. Modulation by benzo[a]pyrene of epidermal growth factor receptors, cell proliferation and secretion of human chorionic gonadotropin in human placental cell lines. Biochem Pharmacol 1995; 50:1171 - 1180
- Genbacev O, McMaster MT, Lazic J, Nedeljkovic S, Cvetkovic M, Joslin R, et al. Concordant in situ and in vitro data show that maternal cigarette smoking negatively regulates placental cytotrophoblast passage through the cell cycle. Reprod Toxicol 2000; 14:495 - 506
- Crawford M, Batte K, Yu L, Wu X, Nuovo GJ, Marsh CB, et al. MicroRNA 133B targets pro-survival molecules MCL-1 and BCL2L2 in lung cancer. Biochem Biophys Res Commun 2009; 388:483 - 489
- Fabian D, Cikos S, Koppel J. Gene expression in mouse preimplantation embryos affected by apoptotic inductor actinomycin D. J Reprod Dev 2009; 55:576 - 582
- Esibizione D, Cui CY, Schlessinger D. Candidate EDA targets revealed by expression profiling of primary keratinocytes from Tabby mutant mice. Gene 2008; 427:42 - 46
- Qian Y, Commane M, Ninomiya-Tsuji J, Matsumoto K, Li X. IRAK-mediated translocation of TRAF6 and TAB2 in the interleukin-1-induced activation of NFkappaB. J Biol Chem 2001; 276:41661 - 41667
- Wang Q, Dziarski R, Kirschning CJ, Muzio M, Gupta D. Micrococci and peptidoglycan activate TLR2→MyD88→IRAK→TRAF→NIK→IKK→NFkappaB signal transduction pathway that induces transcription of interleukin-8. Infect Immun 2001; 69:2270 - 2276
- Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, Hacker H, et al. Endocytosed HSP60s use toll-like receptor 2 (TLR2) and TLR4 to activate the toll/interleukin-1 receptor signaling pathway in innate immune cells. J Biol Chem 2001; 276:31332 - 31339
- Li L, Kang J, Lei W. Role of Toll-like receptor 4 in inflammation-induced preterm delivery. Mol Hum Reprod 2010; 16:267 - 272
- Nakao S, Ogata Y, Sugiya H. Nicotine stimulates the expression of cyclooxygenase-2 mRNA via NFkappaB activation in human gingival fibroblasts. Arch Oral Biol 2009; 54:251 - 257
- Perry MM, Williams AE, Tsitsiou E, Larner-Svensson HM, Lindsay MA. Divergent intracellular pathways regulate interleukin-1beta-induced miR-146a and miR-146b expression and chemokine release in human alveolar epithelial cells. FEBS Lett 2009; 583:3349 - 3355
- Russell MA, Jarvis M, Iyer R, Feyerabend C. Relation of nicotine yield of cigarettes to blood nicotine concentrations in smokers. Br Med J 1980; 280:972 - 976
- Waggoner SE, Wang X. Effect of nicotine on proliferation of normal, malignant and human papillomavirus-transformed human cervical cells. Gynecol Oncol 1994; 55:91 - 95
- Drukteinis JS, Medrano T, Ablordeppey EA, Kitzman JM, Shiverick KT. Benzo[a]pyrene, but not 2, 3, 7, 8-TCDD, induces G2/M cell cycle arrest, p21CIP1 and p53 phosphorylation in human choriocarcinoma JEG-3 cells: a distinct signaling pathway. Placenta 2005; 26:87 - 95
- Lall S, Grun D, Krek A, Chen K, Wang YL, Dewey CN, et al. A genome-wide map of conserved microRNA targets in C. elegans. Curr Biol 2006; 16:460 - 471
- Kruger J, Rehmsmeier M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res 2006; 34:451 - 454