Abstract
Epigenetic marks, such as histone methylation, play a central role in chromatin structure and gene expression. During DNA replication, chromatin undergoes a wave of disruption and reassembly. Little is known about how the epigenetic marks are faithfully inherited from one generation to the next. In fission yeast, the hallmark of heterochromatin, a condensed chromatin structure, is H3K9 methylation. This conserved epigenetic mark is mediated by small interference RNAs (siRNAs) in a cell cycle-dependent manner: at S phase, heterochromatin is briefly transcribed by RNAP II and the transcripts are subsequently processed into siRNAs. These small RNAs, together with other key silencing factors, including Dos1/Raf1/Clr8/Cmc1, Dos2/Raf2/Clr7/Cmc2 and Rik1, mediate H3K9 methylation by the histone H3K9 methyltransferase Clr4. Our recent findings indicate that the ε subunit of DNA polymerase, Cdc20, associates with the Dos2-Rik1 complex and is essential for H3K9 methylation and heterochromatin function. Moreover, Cdc20 regulates siRNA generation by promoting RNAP II transcription of heterochromatin. These data suggest that DNA polymerase components may play a key role in the inheritance of histone methylation by coordinating DNA replication, RNAi and histone methylation, and explain previously observed cell cycle-regulated RNAi-dependent heterochromatin silencing. We propose a model in which, at DNA replication forks, DNA polymerase subunits mediate the recruitment of epigenetic factors required for RNAi and histone modification to heterochromatin to promote the faithful transmission of histone methylation.
DNA Replication: Challenge for Epigenetic Inheritance
Epigenetic marks, such as DNA methylation and histone modifications, are essential for the regulation of gene expression and chromatin organization.Citation1 Misregulation of these marks, leads to a variety of diseases, including cancer.Citation1 In eukaryotic cells, chromatin is organized into euchromatin and heterochromatin. While euchromatin is transcriptionally active and loosely packed, heterochromatin is gene poor and highly condensed. However, heterochromatin is not inert, and plays a key role in gene expression, genome stability and sister chromatid cohesion.Citation2 The patterns of histones and their particular modifications, often referred to as the “histone code,” contribute to the organization of chromatin into higher-order structures. The major epigenetic hallmark in heterochromatin is the methylation of histone 3 at lysine 9 (H3K9me), a modification conserved from fission yeast to human. This mark is recognized by the chromo-domain protein Heterochromatin Protein 1 (HP1). HP1, considered a “reader” protein of the histone code, further recruits other proteins to assemble chromatin into heterochromatic structures.Citation2,Citation3 Epigenetic marks in heterochromatin and euchromatin can be faithfully transmitted from generation to generation. However, DNA replication poses a challenge for the inheritance of these marks. During S phase of the cell cycle, chromatin is disassembled ahead of the replication fork causing displacement of existing nucleosomes and dramatic perturbation of epigenetic marks.Citation4 Remarkably, disassembled chromatin quickly returns to its original epigenetic state behind the fork in a manner that remains poorly understood. At replication forks, nascent DNA is catalyzed by DNA polymerase, composed of α, δ and ε (Pol α, δ and ε) subunits. DNA Pol α associates with primase, which synthesizes and extends RNA primers during initiation of replication. DNA Pol α and δ catalyze lagging strand synthesis, while synthesis of the leading strand is achieved by DNA Pol ε.Citation5,Citation6 Once DNA is duplicated, DNA and histones reassemble into nucleosomes with the aid of histone chaperones.Citation7 Importantly, previously existing histone marks are also faithfully restored. The precise mechanism for how these epigenetic marks are reproduced is a subject of intense study.
Histone Methylation and Heterochromatin
Fission yeast has emerged as a model system for studying heterochromatin regulation due to the conserved epigenetic components in this simple, genetic-tractable organism. Heterochromatin in fission yeast is mainly composed of tandem DNA repeats, and can be found in pericentromere, telomere and mating-type loci. Genes inserted within these regions are transcriptionally silenced.Citation2 Unlike budding yeast, which contains relatively small centromeres (~125 bp), S. pombe’s centromeres are large (35–110 kb) and organized into three domains, including a central core (cnt), inner repeat (imr) and outer repeat (otr) region. The cnt region is coated with conserved histone H3 variant CENP-Acnp1, which provides the foundation for assembly of the kinetochore.Citation1 Pericentromeric regions, including imr and otr, are heterochromatic. Similar to mammalian cells, H3K9me is enriched in fission yeast heterochromatin regions.Citation2 This epigenetic mark is stably inherited during each cell generation, thus providing a valuable framework for understanding mechanisms of epigenetic inheritance in eukaryotes.
H3K9 methylation in fission yeast is catalyzed by Clr4, a member of the SUV39 family of histone methyltransferases.Citation8 This modification serves as a binding site for Swi6, a structural and functional homolog of metazoan HP1. Swi6 in turn recruits additional silencing factors including the SHREC complex to drive heterochromatin silencing.Citation9 Swi6 is also required for heterochromatin localization of cohesin, a key factor for sister-chromatid cohesion and chromosome segregation.Citation10 Clr4’s H3K9 methylation activity is mediated by a complex that includes Rik1, Dos1 (Delocalization of Swi6; also known as Raf1, Clr8 and Cmc1), Dos2 (also known as Raf2, Clr7 and Cmc2) and Cul4.Citation8,Citation11-Citation14 Rik1 is a WD repeat protein and shares homology to the DNA damage binding protein, DDB1. Dos1 is also a WD repeat protein while Dos2 contains a zinc finger motif. Loss of Rik1, Dos1 or Dos2 results in complete loss of H3K9 methylation and delocalization of Swi6 from heterochromatin regions.Citation8,Citation11-Citation13 Cul4 is a conserved cullin protein and a component of ubiquitin E3 ligases. In humans, Cul4 serves as a scaffold for DDB1 and other WD repeat proteins to assemble into the ubiquitin ligases E3 complex.Citation15 In fission yeast, Cul4 has been shown to be important for H3K9me and Swi6 heterochromatin distribution.Citation16 Consistent with this, the Cul4-E3 complex was later found to be required for histone modification and transcriptional silencing in other eukaryotic organisms.Citation15,Citation17,Citation18 In fission yeast, the Rik1-Dos1/Dos2 complex physically interacts with Clr4 and is responsible for Clr4’s association with heterochromatin. This complex also associates with the conserved H3K4 histone demethylase Lid2 and is required for the association of Lid2 with heterochromatin.Citation19 Lid2 contains a JmjC domain and promotes removal of H3K4 methylation. Interaction between Clr4 and Lid2, through the Rik1-Dos1/Dos2 complex, is in agreement with the observed H3K9 hypermethylation and H3K4 hypomethylation in heterochromatin.Citation19
RNAi, H3K9 Methylation and the Cell Cycle
Studies in fission yeast revealed that RNA interference (RNAi) plays an essential role in H3K9 methylation and heterochromatin formation. Fission yeast contains one copy of each of the major RNAi components, including Argonaute (Ago1), Dicer (Dcr1), and an RNA-dependent RNA polymerase (Rdp1). In ago1Δ, dcr1Δ and rdp1Δ mutants, silencing is defective, and both H3K9me and Swi6 are lost in pericentromere regions. However, loss of RNAi components has little effect on H3K9me of mating-type and telomeric loci as a result of redundant silencing mechanisms.Citation20,Citation21 Heterochromatin in fission yeast is transcribed by RNA polymerase II (RNAP II). Mutations in RNAP II subunits result in heterochromatin defects.Citation22,Citation23 Heterochromatin transcripts by RNAP II are subsequently processed into siRNA by the RITS (RNA-induced transcriptional silencing), and RDRC (RNA-directed RNA polymerase complex) complexes as well as Dicer.Citation24 RITS contains Ago1, Tas3, and the chromo-domain protein, Chp1. Ago1 has endonucleolytic or slicer activity that slices nascent heterochromatin transcripts at transcription sites. Chp1 binds H3K9 methylation in a manner similar to Swi6, thereby stabilizing the association of RITS with heterochromatin.Citation24,Citation25 Disruption of H3K9me leads to dissociation of RITS from heterochromatin, loss of RNAi-dependent heterochromatin assembly and the accumulation of siRNA precursors, as observed in key silencing mutants such as, Dos1, Dos2 and Rik1.Citation11,Citation24 The RDRC complex consists of the RNA-dependent polymerase, Rdp1, the polyA polymerase, Cid12 (caffeine-induced death protein 12), and the RNA helicase, Hrr1 (Helicase required for RNAi-mediated heterochromatin assembly). This complex, through the action of Rdp1’s polymerase activity presumably converts single stranded heterochromatin transcripts into double stranded RNA (dsRNA) fragments capable of triggering RNAi activity. Newly synthesized dsRNA fragments are then processed by Dicer into siRNAs which are loaded onto Ago1 and facilitate recruitment of H3K9 methylation factors likely via their base pairing with homologous sequences at heterochromatic DNA or newly emerging heterochromatin transcripts.Citation24 In a recent study, the LIM domain protein, Stc1, was shown to have a role in recruiting the Clr4 complex to RITS.Citation26 Small RNA-based chromatin regulatory mechanisms have also been reported in plants, C. elegans, D. melanogaster, and mammals.Citation27-Citation30
RNAi also plays an important role in the establishment of core centromeric histone CENP-Acnp1, which specifies the sites for kinetochore assembly in chromosomes and represents the epigenetic mark responsible for regulating centromere structure and function. In RNAi and clr4 mutants, CENPAcnp1 cannot incorporate properly into a naked centromere DNA. However, RNAi and heterochromatin are dispensable for the maintenance of CENP-Acnp1 at centromeric chromatin as the distribution of CENPAcnp1 remains undisturbed in RNAi and clr4 mutants.Citation31,Citation32 Recently, it was shown that like pericentromeric regions which flank centromeric cores, the latter are also subject to transcription by RNAP II. However, RNAi does not appear to play a role in the processing of core transcripts.Citation33 How RNAi precisely regulates the initial deposition of centromeric histone CENP-Acnp1 remains enigmatic and further analysis is required for the elucidation of this important cellular process.
It is well-established that heterochromatin transcription is required for heterochromatin silencing.Citation20,Citation24,Citation25 This seemingly paradoxical phenomenon can be explained by the recent finding that in fission yeast, RNAi-mediated heterochromatin assembly is cell cycle-regulated. In fission yeast, centromere replication takes place during early S phase of the cell cycle and it is also at this time when centromeric transcripts first appear. By an unknown mechanism, RNAP II is recruited to pericentromeric repeats at S phase and presumably drives transcription from these loci as it is at this time that heterochromatin transcripts appear in the cell.Citation34,Citation35 siRNAs reach a peak soon after the pericentromeric transcripts are generated, suggesting rapid processing of the double-stranded transcripts by RNAi machinery. Correspondingly, H3K9me2 levels begin to rise at late S phase and reach their highest level at G2. Similarly to the methyl-phospho switch mechanism described in humans, histone H3 serine 10 (H3S10) phosphorylation prevents the binding of Swi6 with methylated H3K9. As cells enter mitosis, H3S10 phosphorylation levels begin to rise, which results in dissociation of Swi6 from methylated H3K9 as cells enter early S phase. Concordantly, H3K4me2, a hallmark of transcriptionally active chromatin, increases at these sites.Citation34,Citation35 Notably, heterochromatin transcription at S phase coincides with the recruitment of key silencing factors to heterochromatic loci. Rik1, Dos2 and Ago1 associate with heterochromatin as S phase when transcripts from these loci emerge.Citation34 Thus, heterochromatin assembly in fission yeast is a dynamic process for which the S phase of the cycle represents a key window for regulating its activity.
DNA Polymerases and Inheritance of Histone Methylation
The exact role of the zinc finger protein Dos2 remains unknown. To gain further insight into the role of this silencing factor in heterochromatin function we performed Tandem Affinity Purification (TAP) to isolate its interaction partners. We identified two novel factors as binding partners for Dos2: the DNA replication component Cdc20 and a novel protein, Mms19.Citation36 A previous study using a similar approach did not identify Cdc20 and Mms19 to associate with Dos2; this could be due to the use of sub-optimal tag combinations. Cdc20, a DNA polymerase ε subunit, plays a key role in DNA replication and repair, and is involved in the elongation of leading strand synthesis.Citation5,Citation6,Citation37 Our studies further implicate Cdc20 as required for heterochromatin silencing since perturbation of Cdc20 activity resulted in reduced levels of H3K9 methylation and Swi6 in heterochromatin, with a concomitant loss in heterochromatin silencing.Citation36 Cdc20 also appears to be required for siRNA production as loss of siRNAs occurs in strains in which Cdc20 function is compromised.Citation36 Interestingly, despite the significant loss in heterochromatin silencing observed in an N-terminally deleted cdc20 mutant, DNA replication defects in this mutant appear minimal, suggesting that Cdc20 heterochromatin silencing and DNA replication functions can be decoupledCitation36 and that the Cdc20 N-terminal domain may be dispensable for DNA replication.Citation38 Nonetheless, we cannot exclude the possibility that the process of DNA replication itself, rather than any of its individual components, may be required for heterochromatin silencing. Importantly, heterochromatin integrity and Cdc20-Dos2 interactions are concomitantly compromised when Cdc20 is impaired. This suggests that heterochromatin defects may result from inability of these proteins to properly interact. In addition, disruption of Cdc20 results in dissociation of Dos2 and Rik1 from centromeric heterochromatin at S phase, indicating that Cdc20 is required for association of the silencing complex with heterochromatin at this stage.Citation36
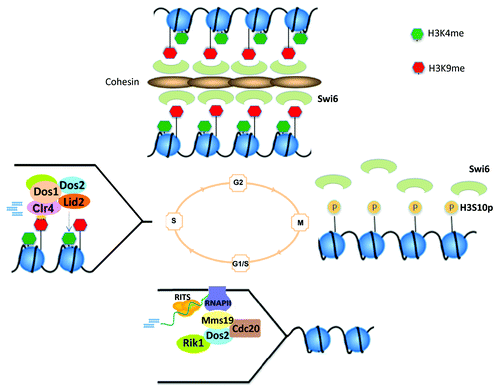
Mms19 is highly conserved and its homolog in budding yeast and human cells has been shown to be important for transcriptional regulation through interaction with the general transcription factor TFIIH.Citation39,Citation40 TFIIH is essential for RNAP II transcription initiation.Citation41 Interestingly, homologs of Mms19 also appear to play a role in DNA repair.Citation39 We show that in fission yeast Mms19 interacts with RNAP II, which we predict would facilitate heterochromatin transcription.Citation36 In favor of this view, we further observed that loss of Mms19 results in a reduction in heterochromatin transcription and siRNA generation. In addition, like Dos2 and Rik1, Mms19 localizes to heterochromatin at S phase in a Cdc20-dependt manner.Citation36 These observations suggest that Mms19 may directly promote the transcription of siRNA precursors at S phase, which are subsequently processed by RNAi machinery into siRNAs to direct the assembly of heterochromatin nucleation sites.Citation36 Human Mms19 has also been implicated in chromatin regulation.Citation42 Combined with observations from others, our data strongly supports a link between DNA replication, siRNA production and heterochromatin inheritance, and suggest that replication components may play an essential role in the restoration of histone methylation marks during genome replication. These analyses further suggest a mechanistic explanation for cell cycle-regulated RNAi and heterochromatin assembly, and the inheritance of H3K9 methylation. Based on these findings, we propose that heterochromatin inheritance encompasses the following steps: 1) at G1/S, concurrent with the replication of heterochromatic DNA repeats at replication forks, DNA pol ε subunit Cdc20 recruits Dos2 and Rik1. It also recruits Mms19, which through its regulatory role on RNAP II transcriptional activity promotes the formation of siRNAs; 2) At S phase, Dos2 and Rik1, in concert with siRNAs and Dos1, mediate H3K9 methylation through their interaction with Cdc20 on the one hand, and the histone methylation enzymes Clr4 and Lid2 on the other, thereby ensuring the faithful transmission of H3K9 methylation patterns onto newly made heterochromatin; 3) Finally, newly deposited H3K9 methylation marks serve as a docking sites for Swi6 binding, which promote the restoration of condensed heterochromatic structures ().Citation36
Figure 1. Co-regulation of DNA replication and RNAi-dependent heterochromatin assembly: Model for inheritance of H3K9me. 1) At G1/S phase, the DNA polymerase ε subunit, Cdc20, in addition to its role in leading strand synthesis at replication forks, recruits Dos2 and Rik1. It also recruits the transcriptional regulator Mms19, which in turn recruits RNAP II to initiate heterochromatin transcription. Nascent heterochromatin transcripts are then processed into siRNAs via RNAi activity; 2) At S phase, newly made siRNAs, in concert with Dos1, Dos2 and Rik1, mediate H3K9 methylation and H3K4 hypomethylation through their interaction with the histone methylation enzymes, Clr4 and Lid2; 3) At G2, newly deposited H3K9 methylation marks serve as a docking sites for Swi6 binding, which promote the restoration of condensed heterochromatic structures; 4) at M phase, H3S10 phosphorylation levels begin to increase steadily into early S phase leading to decreased H3K9 methylation and Swi6 binding required for the initiation of a new cycle of heterochromatin transcription and re-assembly in the next generation.
Previous genetic studies in fission yeast implicate DNA polymerase α subunits, Mcl1 and Swi7, in heterochromatin formation.Citation43,Citation44 However, the precise role, particularly whether these subunits play a role in siRNA regulation, remains unclear. It is conceivable that they may work together with Cdc20 to recruit the Rik1-Dos1/Dos2 complex to heterochromatin. It is also possible that the various polymerase subunits may play distinct roles in other aspects of heterochromatin assembly. For instance, the polymerase subunit Swi7 has been observed to interact with Swi6, and to potentially participate in the spreading of heterochromatin.Citation44 Furthermore, how the E3 ligase subunit, Cul4, functions in heterochromatin regulation and what its target is remain enigmatic. Our findings linking heterochromatin assembly factors with DNA replication components may provide some clues. It is well known that the E3 ligases target replication components to promote the preservation of genome integrity.Citation45,Citation46 It is conceivable that Cul4 may act in a similar fashion to promote co-regulation of heterochromatin assembly and DNA replication processes at replication forks.
One important question is how general replication factors participate in heterochromatin assembly and in the faithful inheritance of epigenetic marks. The answer might lie on the difference in timing between heterochromatic and euchromatic loci replication at S phase. These two distinct chromatin structures replicate at different times: euchromatin is generally replicated prior to heterochromatin (except for centromeric heterochromatin in fission yeast, which is replicated early in S phase before any other part of the genome).Citation35,Citation47,Citation48 Additionally, the spatial organization of heterochromatin in the nucleus may contribute to the specificity of Cdc20 in heterochromatin. It is a widespread phenomenon among eukaryotes that heterochromatin is preferentially localized at the nuclear periphery.Citation49 The difference in replication timing and/or spatial architecture between euchromatin and heterochromatin may create the unique microenvironment needed for Cdc20 specific role. Further studies are needed to resolve this important gap in knowledge.
Genetic studies in budding yeast have revealed that DNA polymerase ε subunits are required for transcriptional silencing, although DNA replication per se appears to not play a role in the reestablishment of heterochromatin silencing.Citation50-Citation52 DNA Pol ε binds double-stranded DNA and promotes epigenetic silencing at telomeres in budding yeast, but this activity is independent of its polymerase activity.Citation53 The precise mechanism by which DNA Pol ε mediates transcriptional silencing in budding yeast remains unclear. RNAi and H3K9 methylation do not exist in budding yeast, and heterochromatin silencing is mediated by a complex conformed by silent information regulator (SIR) proteins.Citation54 It is possible that in budding yeast DNA Pol ε regulates silencing by interacting with the Sir complex. DNA replication components have been implicated in epigenetic repression in plants. In Arabidopsis, vernalization, a process of acquiring germination through exposure to cold temperature, depends on the FLOWERING LOCUS C (FLC) gene. During wintertime, FLC is epigenetically repressed, which further induces repression of the FLOWERING LOCUS T (FT) gene responsible for promoting flowering. RNAi and histone modifications, such as H3K9me and H3K27me, are indispensible for the transcriptional silencing of FTC and FT.Citation35 In a previous study, DNA replication is suggested to play a role in epigenetic regulation of vernalization.Citation55 Two additional studies report a role for DNA polymerase ε catalytic subunit in histone modification and silencing at FLC and FT loci.Citation56,Citation57 Furthermore, a recent study shows that mutations that disrupt DNA polymerase α (ICU2) catalytic activity result in reactivation of the silenced reporter gene, 35S-NPTII, and lower levels of H3K9me at the 35S promoter.Citation58 In mammals, DNA polymerase ε subunit has been shown to preferentially associate with heterochromatin during S phase.Citation59 In mice in particular, Pol ε B subunits associate with SAP18, which in turn associates with the transcriptional co-repressor Sin3, and together immediate transcriptional silencing.Citation60 The role of DNA replication components in epigenetic inheritance and the underlying mechanisms elucidated by the fission yeast studies will likely apply to multicellular eukaryotes as well.
Concluding Remarks
Our results suggest that DNA polymerase subunits play an essential role in the inheritance of epigenetic states. In the future, it will be interesting to see how other DNA replication components contribute to heterochromatin assembly and H3K9 methylation. It is also of great interest to investigate whether DNA replication per se has a role in RNAi-dependent heterochromatin formation. Another important area that is much less explored is whether DNA polymerases are involved in epigenetic regulation in euchromatin. A better understanding of the mechanisms by which replication components regulate epigenetic inheritance will provide new insight into cell differentiation and development and disease processes.
| Abbreviations: | ||
| RNAi | = | RNA interference |
| siRNA | = | small interference RNA |
| H3K9me | = | methylation of histone H3 at lysine 9 |
| dsRNA | = | double stranded RNA |
| DNA Pol | = | DNA polymerase |
| TAP | = | Tandem Affinity Purification |
Acknowledgments
We thank members of the Li laboratory for their support and discussions.
References
- C. D. Allis TJ. Reinberg D. Epigenetics. New York: Cold Spring Harbor Laboratory Press; 2006.
- Lippman Z, Martienssen R. The role of RNA interference in heterochromatic silencing. Nature 2004; 431:364 - 70; http://dx.doi.org/10.1038/nature02875; PMID: 15372044
- Dillon N. Heterochromatin structure and function. Biol Cell 2004; 96:631 - 7; http://dx.doi.org/10.1016/j.biolcel.2004.06.003; PMID: 15519697
- Probst AV, Dunleavy E, Almouzni G. Epigenetic inheritance during the cell cycle. Nat Rev Mol Cell Biol 2009; 10:192 - 206; http://dx.doi.org/10.1038/nrm2640; PMID: 19234478
- Garg P, Burgers PM. DNA polymerases that propagate the eukaryotic DNA replication fork. Crit Rev Biochem Mol Biol 2005; 40:115 - 28; http://dx.doi.org/10.1080/10409230590935433; PMID: 15814431
- Pursell ZF, Isoz I, Lundstrom EB, Johansson E, Kunkel TA. Yeast DNA polymerase epsilon participates in leading-strand DNA replication. Science 2007; 317:127 - 30; http://dx.doi.org/10.1126/science.1144067; PMID: 17615360
- Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell 2007; 128:721 - 33; http://dx.doi.org/10.1016/j.cell.2007.01.030; PMID: 17320509
- Nakayama J, Rice JC, Strahl BD, Allis CD, Grewal SI. Role of histone H3 lysine 9 methylation in epigenetic control of heterochromatin assembly. Science 2001; 292:110 - 3; http://dx.doi.org/10.1126/science.1060118; PMID: 11283354
- Motamedi MR, Hong EJ, Li X, Gerber S, Denison C, Gygi S, et al. HP1 proteins form distinct complexes and mediate heterochromatic gene silencing by nonoverlapping mechanisms. Mol Cell 2008; 32:778 - 90; http://dx.doi.org/10.1016/j.molcel.2008.10.026; PMID: 19111658
- Bernard P, Maure JF, Partridge JF, Genier S, Javerzat JP, Allshire RC. Requirement of heterochromatin for cohesion at centromeres. Science 2001; 294:2539 - 42; http://dx.doi.org/10.1126/science.1064027; PMID: 11598266
- Li F, Goto DB, Zaratiegui M, Tang X, Martienssen R, Cande WZ. Two novel proteins, Dos1 and Dos2, interact with Rik1 to regulate heterochromatic RNA interference and histone modification. Curr Biol 2005; 15:1448 - 57; http://dx.doi.org/10.1016/j.cub.2005.07.021; PMID: 16040243
- Horn PJ, Bastie JN, Peterson CLA. Rik1-associated, cullin-dependent E3 ubiquitin ligase is essential for heterochromatin formation. Genes Dev 2005; 19:1705 - 14; http://dx.doi.org/10.1101/gad.1328005; PMID: 16024659
- Thon G, Hansen KR, Altes SP, Sidhu D, Singh G, Verhein-Hansen J, et al. The Clr7 and Clr8 directionality factors and the Pcu4 cullin mediate heterochromatin formation in the fission yeast Schizosaccharomyces pombe. Genetics 2005; 171:1583 - 95; http://dx.doi.org/10.1534/genetics.105.048298; PMID: 16157682
- Hong EJ, Villen J, Gerace EL, Gygi SP, Moazed D. A cullin E3 ubiquitin ligase complex associates with Rik1 and the Clr4 histone H3-K9 methyltransferase and is required for RNAi-mediated heterochromatin formation. RNA Biol 2005; 2:106 - 11; http://dx.doi.org/10.4161/rna.2.3.2131; PMID: 17114925
- Higa LA, Wu M, Ye T, Kobayashi R, Sun H, Zhang H. CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat Cell Biol 2006; 8:1277 - 83; http://dx.doi.org/10.1038/ncb1490; PMID: 17041588
- Jia S, Kobayashi R, Grewal SI. Ubiquitin ligase component Cul4 associates with Clr4 histone methyltransferase to assemble heterochromatin. Nat Cell Biol 2005; 7:1007 - 13; http://dx.doi.org/10.1038/ncb1300; PMID: 16127433
- Xu H, Wang J, Hu Q, Quan Y, Chen H, Cao Y, et al. DCAF26, an adaptor protein of Cul4-based E3, is essential for DNA methylation in Neurospora crassa. PLoS Genet 2010; 6:e1001132; http://dx.doi.org/10.1371/journal.pgen.1001132; PMID: 20885793
- Dumbliauskas E, Lechner E, Jaciubek M, Berr A, Pazhouhandeh M, Alioua M, et al. The Arabidopsis CUL4-DDB1 complex interacts with MSI1 and is required to maintain MEDEA parental imprinting. EMBO J 2011; 30:731 - 43; http://dx.doi.org/10.1038/emboj.2010.359; PMID: 21240189
- Li F, Huarte M, Zaratiegui M, Vaughn MW, Shi Y, Martienssen R, et al. Lid2 Is Required for Coordinating H3K4 and H3K9 Methylation of Heterochromatin and Euchromatin. Cell 2008; 135:272 - 83; http://dx.doi.org/10.1016/j.cell.2008.08.036; PMID: 18957202
- Volpe TA, Kidner C, Hall IM, Teng G, Grewal SIS, Martienssen RA. Regulation of heterochromatic silencing and histone H3 lysine-9 methylation by RNAi. Science 2002; 297:1833 - 7; http://dx.doi.org/10.1126/science.1074973; PMID: 12193640
- Jia S, Noma K, Grewal SIS. RNAi-independent heterochromatin nucleation by the stress-activated ATF/CREB family proteins. Science 2004; 304:1971 - 6; http://dx.doi.org/10.1126/science.1099035; PMID: 15218150
- Djupedal I, Portoso M, Spåhr H, Bonilla C, Gustafsson CM, Allshire RC, et al. RNA Pol II subunit Rpb7 promotes centromeric transcription and RNAi-directed chromatin silencing. Genes Dev 2005; 19:2301 - 6; http://dx.doi.org/10.1101/gad.344205; PMID: 16204182
- Kato H, Goto DB, Martienssen RA, Urano T, Furukawa K, Murakami Y. RNA polymerase II is required for RNAi-dependent heterochromatin assembly. Science 2005; 309:467 - 9; http://dx.doi.org/10.1126/science.1114955; PMID: 15947136
- Motamedi MR, Verdel A, Colmenares SU, Gerber SA, Gygi SP, Moazed D. Two RNAi complexes, RITS and RDRC, physically interact and localize to noncoding centromeric RNAs. Cell 2004; 119:789 - 802; http://dx.doi.org/10.1016/j.cell.2004.11.034; PMID: 15607976
- Irvine DV, Zaratiegui M, Tolia NH, Goto DB, Chitwood DH, Vaughn MW, et al. Argonaute slicing is required for heterochromatic silencing and spreading. Science 2006; 313:1134 - 7; http://dx.doi.org/10.1126/science.1128813; PMID: 16931764
- Bayne EH, White SA, Kagansky A, Bijos DA, Sanchez-Pulido L, Hoe KL, et al. Stc1: a critical link between RNAi and chromatin modification required for heterochromatin integrity. Cell 2010; 140:666 - 77; http://dx.doi.org/10.1016/j.cell.2010.01.038; PMID: 20211136
- Onodera Y, Haag JR, Ream T, Nunes PC, Pontes O, Pikaard CS. Plant nuclear RNA polymerase IV mediates siRNA and DNA methylation-dependent heterochromatin formation. Cell 2005; 120:613 - 22; http://dx.doi.org/10.1016/j.cell.2005.02.007; PMID: 15766525
- Grishok A, Sinskey JL, Sharp PA. Transcriptional silencing of a transgene by RNAi in the soma of C. elegans. Genes Dev 2005; 19:683 - 96; http://dx.doi.org/10.1101/gad.1247705; PMID: 15741313
- Pal-Bhadra M, Leibovitch BA, Gandhi SG, Rao M, Bhadra U, Birchler JA, et al. Heterochromatic silencing and HP1 localization in Drosophila are dependent on the RNAi machinery. Science 2004; 303:669 - 72; http://dx.doi.org/10.1126/science.1092653; PMID: 14752161
- Girard A, Hannon GJ. Conserved themes in small-RNA-mediated transposon control. Trends Cell Biol 2008; 18:136 - 48; http://dx.doi.org/10.1016/j.tcb.2008.01.004; PMID: 18282709
- Folco HD, Pidoux AL, Urano T, Allshire RC. Heterochromatin and RNAi are required to establish CENP-A chromatin at centromeres. Science 2008; 319:94 - 7; http://dx.doi.org/10.1126/science.1150944; PMID: 18174443
- Kagansky A, Folco HD, Almeida R, Pidoux AL, Boukaba A, Simmer F, et al. Synthetic heterochromatin bypasses RNAi and centromeric repeats to establish functional centromeres. Science 2009; 324:1716 - 9; http://dx.doi.org/10.1126/science.1172026; PMID: 19556509
- Choi ES, Stralfors A, Castillo AG, Durand-Dubief M, Ekwall K, Allshire RC. Identification of noncoding transcripts from within CENP-A chromatin at fission yeast centromeres. J Biol Chem 2011; 286:23600 - 7; http://dx.doi.org/10.1074/jbc.M111.228510; PMID: 21531710
- Chen ES, Zhang K, Nicolas E, Cam HP, Zofall M, Grewal SIS. Cell cycle control of centromeric repeat transcription and heterochromatin assembly. Nature 2008; 451:734 - 7; http://dx.doi.org/10.1038/nature06561; PMID: 18216783
- Kloc A, Zaratiegui M, Nora E, Martienssen R. RNA interference guides histone modification during the S phase of chromosomal replication. Curr Biol 2008; 18:490 - 5; http://dx.doi.org/10.1016/j.cub.2008.03.016; PMID: 18394897
- Li F, Martienssen R, Cande WZ. Coordination of DNA replication and histone modification by the Rik1-Dos2 complex. Nature 2011; 475:244 - 8; http://dx.doi.org/10.1038/nature10161; PMID: 21725325
- D’Urso G, Nurse P. Schizosaccharomyces pombe cdc20(+) encodes DNA polymerase epsilon and is required for chromosomal replication but not for the S phase checkpoint. Proc Natl Acad Sci USA 1997; 94:12491 - 6; http://dx.doi.org/10.1073/pnas.94.23.12491; PMID: 9356477
- Feng W, D'Urso G. Schizosaccharomyces pombe cells lacking the amino-terminal catalytic domains of DNA polymerase epsilon are viable but require the DNA damage checkpoint control. Mol Cell Biol 2001; 21:4495 - 504; http://dx.doi.org/10.1128/MCB.21.14.4495-4504.2001; PMID: 11416129
- Lauder S, Bankmann M, Guzder SN, Sung P, Prakash L, Prakash S. Dual requirement for the yeast MMS19 gene in DNA repair and RNA polymerase II transcription. Mol Cell Biol 1996; 16:6783 - 93; PMID: 8943333
- Hatfield MD, Reis AM, Obeso D, Cook JR, Thompson DM, Rao M, et al. Identification of MMS19 domains with distinct functions in NER and transcription. DNA Repair (Amst) 2006; 5:914 - 24; http://dx.doi.org/10.1016/j.dnarep.2006.05.007; PMID: 16797255
- Drapkin R, Reinberg D. The multifunctional TFIIH complex and transcriptional control. Trends Biochem Sci 1994; 19:504 - 8; http://dx.doi.org/10.1016/0968-0004(94)90139-2; PMID: 7855896
- Ito S, Tan LJ, Andoh D, Narita T, Seki M, Hirano Y, et al. MMXD, a TFIIH-independent XPD-MMS19 protein complex involved in chromosome segregation. Mol Cell 2010; 39:632 - 40; http://dx.doi.org/10.1016/j.molcel.2010.07.029; PMID: 20797633
- Natsume T, Tsutsui Y, Sutani T, Dunleavy EM, Pidoux AL, Iwasaki H, et al. A DNA Polymerase alpha Accessory Protein, Mcl1, Is Required for Propagation of Centromere Structures in Fission Yeast. PLoS ONE 2008; 3:e2221; http://dx.doi.org/10.1371/journal.pone.0002221; PMID: 18493607
- Nakayama Ji, Allshire RC, Klar AJS, Grewal SIS. Role for DNA polymerase alpha in epigenetic control of transcriptional silencing in fission yeast. EMBO J 2001; 20:2857 - 66; http://dx.doi.org/10.1093/emboj/20.11.2857; PMID: 11387218
- Abbas T, Sivaprasad U, Terai K, Amador V, Pagano M, Dutta A. PCNA-dependent regulation of p21 ubiquitylation and degradation via the CRL4Cdt2 ubiquitin ligase complex. Genes Dev 2008; 22:2496 - 506; http://dx.doi.org/10.1101/gad.1676108; PMID: 18794347
- Kim Y, Starostina NG, Kipreos ET. The CRL4Cdt2 ubiquitin ligase targets the degradation of p21Cip1 to control replication licensing. Genes Dev 2008; 22:2507 - 19; http://dx.doi.org/10.1101/gad.1703708; PMID: 18794348
- Wallace JA, Orr-Weaver T. Replication of heterochromatin: insights into mechanisms of epigenetic inheritance. Chromosoma 2005; 114:389 - 402; http://dx.doi.org/10.1007/s00412-005-0024-6; PMID: 16220346
- Hiratani I, Gilbert DM. Replication timing as an epigenetic mark. Epigenetics 2009; 4:93 - 7; http://dx.doi.org/10.4161/epi.4.2.7772; PMID: 19242104
- Shaklai S, Amariglio N, Rechavi G, Simon AJ. Gene silencing at the nuclear periphery. FEBS J 2007; 274:1383 - 92; http://dx.doi.org/10.1111/j.1742-4658.2007.05697.x; PMID: 17489096
- Ehrenhofer-Murray AE, Kamakaka RT, Rine J. A role for the replication proteins PCNA, RF-C, polymerase epsilon and Cdc45 in transcriptional silencing in Saccharomyces cerevisiae. Genetics 1999; 153:1171 - 82; PMID: 10545450
- Li YC, Cheng TH, Gartenberg MR. Establishment of transcriptional silencing in the absence of DNA replication. Science 2001; 291:650 - 3; http://dx.doi.org/10.1126/science.291.5504.650; PMID: 11158677
- Kirchmaier AL, Rine J. DNA replication-independent silencing in S-cerevisiae. Science 2001; 291:646 - 50; http://dx.doi.org/10.1126/science.291.5504.646; PMID: 11158676
- Tsubota T, Tajima R, Ode K, Kubota H, Fukuhara N, Kawabata T, et al. Double-stranded DNA binding, an unusual property of DNA polymerase epsilon, promotes epigenetic silencing in Saccharomyces cerevisiae. J Biol Chem 2006; 281:32898 - 908; http://dx.doi.org/10.1074/jbc.M606637200; PMID: 16916794
- Rusche LN, Kirchmaier AL, Rine J. The establishment, inheritance, and function of silenced chromatin in Saccharomyces cerevisiae. Annu Rev Biochem 2003; 72:481 - 516; http://dx.doi.org/10.1146/annurev.biochem.72.121801.161547; PMID: 12676793
- Finnegan EJ, Dennis ES. Vernal ization-induced trimethylation of histone H3 lysine 27 at FLC is not maintainedin mitotically quiescent cells. Curr Biol 2007; 17:1978 - 83; http://dx.doi.org/10.1016/j.cub.2007.10.026; PMID: 17980595
- Yin H, Zhang X, Liu J, Wang Y, He J, Yang T, et al. Epigenetic regulation, somatic homologous recombination, and abscisic acid signaling are influenced by DNA polymerase mutation in Arabidopsis. Plant Cell 2009; 21:386 - 402; http://dx.doi.org/10.1105/tpc.108.061549; PMID: 19244142
- del Olmo I, Lopez-Gonzalez L, Martin-Trillo MM, Martinez-Zapater JM, Pineiro M, Jarillo JA. EARLY IN SHORT DAYS 7 (ESD7) encodes the catalytic subunit of DNA polymerase epsilon and is required for flowering repression through a mechanism involving epigenetic gene silencing. Plant J 2010; 61:623 - 36; http://dx.doi.org/10.1111/j.1365-313X.2009.04093.x; PMID: 19947980
- Liu J, Ren X, Yin H, Wang Y, Xia R, Gong Z. Mutation in the catalytic subunit of DNA polymerase alpha influences transcriptional gene silencing and homologous recombination in Arabidopsis. Plant J 2010; 61:36 - 45; http://dx.doi.org/10.1111/j.1365-313X.2009.04026.x; PMID: 19769574
- Fuss J, Linn S. Human DNA polymerase epsilon colocalizes with proliferating cell nuclear antigen and DNA replication late, but not early, in S phase. J Biol Chem 2002; 277:8658 - 66; http://dx.doi.org/10.1074/jbc.M110615200; PMID: 11741962
- Wada M, Miyazawa H, Wang RS, Mizuno T, Sato A, Asashima M, et al. The second largest subunit of mouse DNA polymerase epsilon, DPE2, interacts with SAP18 and recruits the Sin3 co-repressor protein to DNA. J Biochem 2002; 131:307 - 11; PMID: 11872158