Abstract
In September 2011, the Second European Workshop on Plant Chromatin took place in Versailles, France. The workshop covered a range of topics related to plant chromatin biology, including regulation of gene expression by Polycomb group proteins, chromatin dynamics, reconfiguration of epigenetic marks in response to various cues and chromatin assembly. Here, we summarize some of the highlights discussed during the meeting.
Introduction
At the second European Workshop on Plant Chromatin in Versailles (France) (colloque.inra.fr/european_workshop_on_plant_chromatin_2011), recent developments in the plant chromatin research field have been presented in the continuity of the first European Workshop on Plant Chromatin held in Zurich in 2009.Citation1 The reports highlighted the fundamental importance of chromatin for major cellular and organismal functions such as genome stability, cell differentiation and development. New players of the histone code have been described, which expand the functional and combinatorial complexity of post-translational histone modifications. Epigenomic approaches were undertaken to decipher the rules of chromatin organization and the dynamics of deposition or erasure of histone marks. Chromatin dynamics in response to environmental cues emerged as a promising topic with major agronomical applications. New plant models, such as the moss Physcomitrella patens and rice (Oryza sativa), a member of the economically important grass family, have joined the plant model Arabidopsis thaliana to address chromatin-related questions. This report summarizes the main topics presented during the meeting and discusses recent insights into this expanding research field. We apologize to those colleagues whose contributions were not mentioned due to space constraints.
What is New About Polycomb Group Proteins in Plants?
Polycomb Repressive Complexes (PRC) are key modulators of the chromatin epigenetic status during cell fate decisions and developmental processes in eukaryotes. Despite the conservation of several core components in plants and animals, the diversity of plant PcG complexes remains to be further investigated to establish their biochemical composition and the specific sets of target genes in different developmental phases. In plants, one of the PRC2 core subunits, the Extra sex combs (Esc) homolog, is encoded by a unique gene, FERTILIZATION INDEPENDENT ENDOSPERM (FIE), whereas the three other PRC2 core subunits are encoded by small gene families with up to five members (e.g., MULTICOPY SUPPRESSOR OF IRA1 to 5 genes (MSI1–5) encoding the PRC2 p55 homolog). Therefore, the combination of the core subunits can possibly give rise to several PRC2 complexes. Until now, three main PRC2-like complexes have been associated with different plant developmental stages [the EMBRYONIC FLOWER (EMF), VERNALIZATION (VRN) and FERTILISATION INDEPENDENT SEED (FIS) complexes].
As part of the PRC2 FIS complex, the Arabidopsis FIE protein is required for the transition between the haploid gametophytic and diploid sporophytic phases. N. Ohad (Tel-Aviv, Israel) reported on the evolution of the FIE function during the 450 million years of plant evolution from bryophytes (early terrestrial plants) to angiosperms (flowering plants). FIE function is conserved despite the dramatic difference in the dominance of the haploid vs. diploid phases, which alternate during the plant life cycle. Indeed, the moss P. patens has a dominant gametophytic haploid phase, while the major phase in flowering plants is sporophytic and diploid.Citation2 Mutant lines carrying a disrupted PpFIE gene are unable to develop leafy gametophores, but develop sporophyte-like structures, similarly to Ppclf mutants affected in the SET-domain histone-methyltransferase PRC2 core subunit.Citation3 This aberrant phenotype may result from failure of the PcG complex to repress proliferation and differentiation of apical stem cells designated to form sporophytes and suggests that PpFIE is associated with the maintenance of pluripotency and cell reprogramming. Transcriptome and epigenome analyses will be future steps to better understand PcG target gene regulation in the moss and their evolution in the plant kingdom.
While biochemical studies had been performed on the FIS and VRN complexes,Citation4,Citation5 the third PRC2-like complex in plants, the EMF complex, was awaiting further characterization. Using protein complex purification coupled with mass spectrometry, M. Derkacheva (Zurich, Switzerland and Uppsala, Sweden) demonstrated that the WD40 repeat protein MSI1 is an essential component of the sporophytic EMF complex. MSI1 binds to EMF2 targets and regulates their expression. Interestingly, crosstalk between PRC complexes was suggested, reinforcing the functional significance of a previous interaction between MSI1 and EMF1, a protein with PRC1-like activity.Citation6 Because MSI1 is not only an essential component of the PRC2-like complexes but also a subunit of Chromatin assembly factor-1, dissecting the various specific functions of MSI1 becomes very challenging.
V. Gaudin (Versailles, France) addressed the RNA-mediated recruitment of the Arabidopsis PRC1 complex by a RNA-binding protein (LIF2) involved in cell identity and cell fate decision.Citation7 LIF2 could also possibly modulate the function of LIKE HETEROCHROMATIN PROTEIN1 (LHP1), involved in PRC1 H3K27me3 recognition, at specific loci during development or in response to environmental cues. The identification of LIF2 RNA ligands by a RIP-derived approach is expected to give some answers to this important question.
Histone Modifications in the Limelight
One of the major goals in chromatin biology is to establish exhaustive epigenomes and to decipher the underlying rules of genome organization in eukaryotes at the scale of individual cell types. In this perspective, F. Roudier (Paris, France) presented a combinatorial analysis of chromatin marks, thus defining four predominant chromatin states (CSs) forming short domains interspersed with each other along the chromosome and centered on individual transcription units.Citation8 The four CSs display distinct functional properties and relate, respectively, to: (1) active genes; (2) repressed genes under PcG protein control; (3) silent repeat elements (classical heterochromatin) and (4) silent euchromatin, characterized by the absence of any prevalent mark (i.e., intergenic regions and weakly expressed genes). A similar principle of chromatin organization based on five chromatin types or “colors” has been proposed in metazoans.Citation9
M. Lafos (Düsseldorf, Germany) demonstrated dynamic changes of the repressive H3K27me3 mark during differentiation and in a tissue-specific manner by performing ChIP-chip analyses on undifferentiated cells (shoot apical meristems) and differentiated leaf cells.Citation10 Interestingly, besides direct repression of target genes by H3K27 trimethylation, activation of some transcription factors could be mediated by an H3K27me3 repression of their specific regulatory miRNA genes. Thus, PcG protein action allows to fine-tune gene expression of specific gene families during cell differentiation and of genes involved in entire signaling pathways (i.e., auxin regulatory pathway). Interestingly, resetting the H3K27me3 mark is not enough to restore active transcription and additional cues are required.
R. B. Aalen (Oslo, Norway) presented new results on the dynamics of histone lysine methylation. The modulation of histone lysine methyltransferase (HKMT) activity and, importantly, specificity can be achieved by the binding of additional non-enzymatic domains. For instance, the HKMT specificity of the Arabidopsis SU(VAR) 3–9 RELATED protein SUVR4 changes from H3K9 dimethylation to trimethylation when the N-terminal WIYLD domain binds free ubiquitin or ubiquitinylated H2B.Citation11 Aalen also described the targeting of the H3K36 trimethylation activity of ASHH2/SDG8/EFS to H3K4me-marked genes through recognition by the ASHH2 CW zinc finger domain. This new histone recognition module is specific to methylated H3K4Citation12 and reinforces a permissive chromatin state.
As in animals, Arabidopsis H2A monoubiquitylation is mediated by Ring-finger containing proteins belonging to a PRC1-like complex and is associated with H3K27me3 and gene repression, whereas H2B monoubiquitylation (H2Bub1) is deposited before H3K4 and H3K36 trimethylation during transcription activation and should be removed for efficient transcription. W. -H. Shen (Strasbourg, France) addressed the question of the coordination of histone methylation and monoubiquitylation in transcriptional gene regulation and the functions of these modifications. His group previously described the roles of two SET-domain containing proteins, SDG2 and SDG8, with H3K4 and H3K36 methylation activities, in developmental control and in plant defense, respectively.Citation13,Citation14 Extending their study to the Arabidopsis HISTONE MONOUBIQUITINATION1 protein (HUB1) and E2 ubiquitin-conjugating enzymes (UBC) involved in H2Bub1, they propose that these proteins also participate to plant defense.
D.-X. Zhou (Orsay, France) reported on histone-modifying or histone-binding proteins and their effect on rice development. Proceeding through a homology-based study, genes encoding Jumonji or chromo domain-containing proteins were selected and the epigenomes of wild-type and of the corresponding rice mutants were compared. One studied osJmj mutant was affected in H3K9 demethylation and presented floral and seed developmental alterations, whereas another osJmj mutant was affected in H3K4me demethylation and presented modified hormone metabolism and sensitivity.
Chromatin Dynamics at the Heart of Developmental Controls
Conserved multimeric SWI/SNF complexes are capable of disrupting DNA-histone contacts and remodeling of chromatin in an ATP-dependent manner and have a wide variety of cellular processes ranging from gene expression to nuclear organization and function. A. Jerzmanowski (Warsaw, Poland) addressed the question of the role of SWI/SNF complexes in hormonal control of plant growth and development and brought very promising results about the crosstalk between chromatin and DELLA repressors involved in plant hormonal homeostasis.
Because the timing of flowering is of great economic interest, the molecular pathways controlling flowering time are intensively studied. Often, such studies revealed the functions of important chromatin-related regulators.Citation15 M. Piñeiro (Madrid, Spain) reported epigenetic repression of genes for integrators of the transition to flowering by two related proteins, EARLY BOLTING IN SHORT DAYS (EBS) and SHORT LIFE (SHL), which both contain BROMOADJACENT HOMOLOGY (BAH) and PLANT HOMEODOMAIN (PHD) domains, and demonstrated their binding to chromatin. J. Jarillo (Madrid, Spain) addressed the characterization of two components of the multiprotein SWR1 remodeling complex, which catalyzes the substitution of histone H2A by the histone variant H2A.Z,Citation16 the Arabidopsis homologs of the SWC4 and YAF9 subunits. SWC4 knockdown lines or double mutants affected in both YAF9-related genes display several modified developmental traits, including leaf shape and size, overall plant architecture or flowering time (Gomez-Zambrano et al., in preparation).
D. Wanke (Tubingen, Germany) discussed the link between transcriptional regulation by transcription factors and chromatin remodeling complexes. His unpublished data on in vivo FLIM-FRET interaction studies proposes a link between different classes of DNA-binding factors and epigenetic regulation.Citation17,Citation18 This link will possibly open interplay between chromatin and mainly all developmental pathways.
How Does Plant Chromatin Assembly Participate to the Maintenance of Genome Stability?
J. Fajkus (Brno, Czech Republic) investigated the relationship between telomere dynamics, genome stability and the DNA replication machinery. Extending earlier findings of his group,Citation19 he showed that dysfunction of Chromatin assembly factor 1, a complex involved in replication-dependent nucleosome assembly including FASCIATA 1 (FAS1), FAS2 and MSI1, leads to telomere erosion and a progressive and specific loss of the 45S rDNA repeats over generations. By studying the reversion of the fas phenotype, new elements to resolve mechanisms leading to the fas pleiotropic phenotype are expected.
Interplay Between PcG Repression and DNA Methylation in Genomic Imprinting
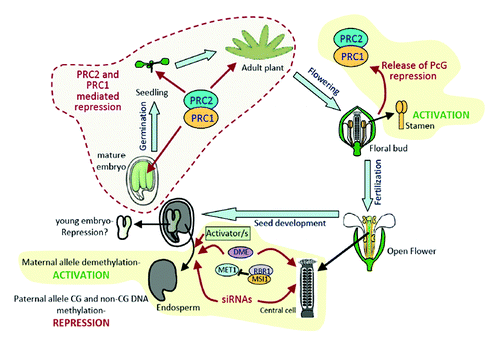
The expression of imprinted genes is dependent on their parent-of-origin, based on an epigenetic asymmetry of maternal and paternal alleles. Both molecular mechanisms of genomic imprinting and the fraction of imprinted genes are still largely unknown. In plants, imprinting predominantly occurs in the endosperm, the triploid nourishing tissue of the seed resulting from the fertilization of the diploid central cell of the female gametophyte by a haploid sperm cell. M. Calonje (Heidelberg, Germany) reported exciting new data on the regulation of AtBMI1C, one of the five BMI1-like genes encoding putative PRC1 H2A monoubiquitin ligases, in relation with imprinting.Citation20 The expression of the maternal allele was detected during seed development in the endosperm, similarly to MEDEA and FIS2, two components of the FIS PRC2 complex. However, the maternal expression depends on DNA demethylation and the silencing of the paternal allele requires high levels of maternal transcripts, CG methylation and siRNA-promoted RdDM suggesting implication of AtBMI1C in its own imprinting. Surprisingly, a biallelic expression during the reproductive phase in stamen resulting from a tissue-specific release of PcG repression was demonstrated. These data open new perspectives on the origin and mechanism of imprinting in plant endosperm, the maintenance and the release of PcG repression.
Exploiting deep sequencing technology and accession polymorphisms, C. Köhler’s group (Uppsala, Sweden) searched genome-wide for new imprinted genes.Citation21 A set of about 70 genes was identified that according to independent confirmation experiments contained more than 90% of truly maternally or paternally expressed genes (MEGs and PEGs). Many MEGs and PEGs encode for transcriptional regulators implicating novel functional roles in seed development. They are often neighbored by transposable elements and are faster evolving compared with all other genes in the genome. MEGS and PEGS are often regulated by the PRC2-like FIS complex, but in the case of MEGs, repression of the paternally inherited alleles largely depends on DNA methylation. In contrast, DNA methylation is required to keep paternally inherited PEG alleles active by preventing FIS-mediated silencing. Whereas the general conclusions of this study are largely in agreement with two related recent studies,Citation22,Citation23 the identified imprinted genes between the studies have only a minor overlap. P. Wolff discussed that genomic imprinting is accession- and developmental-stage dependent, explaining at least in part the discrepancy between different imprinting studies.
How to Reset the Epigenetic Program?
Whereas the germ line in animals is set up during early embryogenesis, spore mother cells in plant are produced by somatic tissues of floral organs in the adult plant, and give rise to the gametes. In previous work, C. Baroux (Zurich, Switzerland) described an epigenetic dimorphism of gametic chromatinCitation24 and is now addressing the question of the epigenetic reprogramming in the plant germ line by studying the role of the H1 linker histone variants and several epigenetic marks. Specific epigenetic landscapes of megaspore mother cell chromatin were presented suggesting a putative link with a meiotic resetting.
P. Crevillen (from the Dean laboratory in Norwich, United Kingdom) investigated the resetting mechanism of the floral transcriptional repressor gene, FLOWERING LOCUS C (FLC). FLC regulation involves several regulatory mechanisms such as PcG proteins, control by long non-coding RNAs, RNA maturation processes and additional plant-specific proteins.Citation25,Citation26 FLC expression needs to be restored at every generation to maximize plant reproductive success. The group of C. Dean performed a forward genetic screen to identify trans-acting factors required for the epigenetic reprogramming of FLC. Crevillen presented data on a promising resetter gene required to upregulate FLC expression during embryogenesis and investigated the impact of mutations in this gene on histone modifications at the FLC locus as well as on FLC sense and antisense expression.
In animals, major histones and replacement histones are mainly distinguished according to the nucleosome incorporation pathway, replication-dependent and replication-independent, respectively. The roles of their plant homologs are not yet well characterized. L. Hennig (Zurich, Switzerland and Uppsala, Sweden) investigated the genome-wide distribution of a H3.3 histone variant in Arabidopsis and showed that H3.3 marks core promoters and transcription start sites and is enriched at transcribed genes, suggesting that the Arabidopsis H3.3 may have a replacement histone function and could play a role in maintenance of transcription memory and regulation of gene expression.
A Chromatin Memory for Stress Responses?
Priming is a phenomenon that enables cells to respond more rapidly and/or sensitively during a second exposure to a stimulus than during the initial exposure.Citation27 Y. Saijo (Cologne, Germany) noted that more than a half of defense-related genes carry H3K27me3 and H3K4me3 in Arabidopsis seedlings, and thus questioned the role of chromatin in the memory of immune response that allows priming to a subsequent elicitation. Following immune response activation triggered upon microbe-derived molecules, he reported that sets of defense-related genes were poised for greater and/or faster activation upon a second weak stimulus that is insufficient for their activation in a mock control. Saijo proposed that priming of transcription defines a common principle that underlies the acquisition of local immune memory, as already shown for systemic memory.Citation28 Moreover, he also demonstrated that mutants for several chromatin modifiers genes exhibited alterations in transcriptional priming of target genes and were compromised in host immunity against pathogen challenges.
Little is known on the effects of stress on chromatin and the recovery of chromatin states after stress release. V. Cavrak (Vienna, Austria) pointed to the role of chromatin destabilization under long heat stress (LHS), which releases transcriptional gene silencing of several repeat targets without DNA demethylation and with only minor changes to histone modifications. LHS activation is accompanied by loss of nucleosomes and heterochromatin decondensation. While decondensation persists, nucleosome loading and transcriptional silencing are restored upon recovery from heat stress but are delayed in mutants with impaired chromatin assembly functions.Citation29 Mapping of significant QTLs linked with LHS-mediated activation is currently in progress, which possibly will allow the identification of trans-acting regulators.
What to Plant for the Next Harvest?
One of the major goals in plant chromatin research will be to pursue the precise identification and listing of the chromatin players (readers, writers and erasers of epigenetic marks) and their specificities, the characterization of the biochemistry of the complexes they belong to and the identification of their genomic targets. The modularity of the players, the redundancy of some chromatin player families and the combinatorial effects of the marks render the task quite challenging but should eventually establish an integrative and dynamic view of the plant chromatin pathways according to cell types and in response to endogenous and environmental cues. Refining the scale of analysis of chromatin and its temporal modifications might reveal a chromatic picture of chromatin. Studies of some of the other genomic functions of chromatin (e.g., replication, DNA repair, recombination) have been initiatedCitation30 and will require further exploration. Finally, many questions remain to be addressed about the biological functions of the various chromatin players and might open interesting applications. This large effort will hopefully be eased by the development of improved techniques such as the INTACT method.Citation31 ChIP-chip and now ChIP-seq approaches have broadened our understanding of plant epigenomesCitation32 and will provide extraordinary large epigenomic resources. In parallel, development of bioinformatics tools and methods will be required to extensively exploit these data and model chromatin behavior.
AUTHOR: Please cite and in the text.
Figure 1. Localization of the 5S (red) and 45S (green) loci in interphase nuclei of wild-type (A) and fas (B) mutant of A. thaliana by Fluorescent in situ hybridization (FISH) showing a 45S rDNA loss in the fas mutant. Courtesy of J. Fajkus (Brno, Czech Republic).
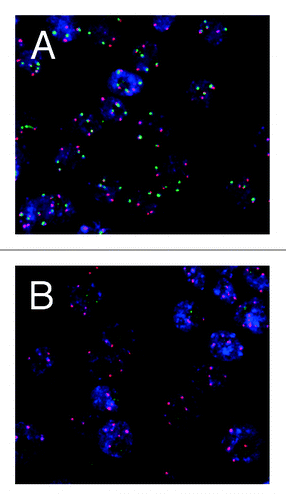
Figure 2. Model of AtBMI1C regulation during plant development. AtBMI1C is an endosperm imprinted gene. The expression of the maternal allele depends on DNA demethylation and a putative transcriptional activator/s, and the silencing of the paternal allele requires high levels of maternal transcripts, CG methylation, and RdDM. AtBMI1C is not expressed during embryo or vegetative development. The silencing in young embryos is achieved through an as-yet unknown mechanism, but in mature embryos and then after germination, the repression of AtBMI1C depends on PcG. During stamen development, the PcG-mediated repression of AtBMI1C is released to allow the biallelic expression of the gene. Courtesy of M. Calonje (Heidelberg, Germany).
Acknowledgments
We thank all participants for their contributions to the second European Workshop on Plant Chromatin 2011 and are grateful to those participants who contributed to this report and allowed us to disclose unpublished results.
References
- Köhler C, Gaudin V, Hennig L. Green chromatin dynamics in Zurich: meeting summary based on the European Workshop on Plant Chromatin 2009 in Zurich, Switzerland. Epigenetics 2010; 5:80 - 3; http://dx.doi.org/10.4161/epi.5.1.10376; PMID: 20009573
- Mosquna A, Katz A, Decker EL, Rensing SA, Reski R, Ohad N. Regulation of stem cell maintenance by the Polycomb protein FIE has been conserved during land plant evolution. Development 2009; 136:2433 - 44; http://dx.doi.org/10.1242/dev.035048; PMID: 19542356
- Okano Y, Aono N, Hiwatashi Y, Murata T, Nishiyama T, Ishikawa T, et al. A polycomb repressive complex 2 gene regulates apogamy and gives evolutionary insights into early land plant evolution. Proc Natl Acad Sci U S A 2009; 106:16321 - 6; http://dx.doi.org/10.1073/pnas.0906997106; PMID: 19805300
- De Lucia F, Crevillen P, Jones AM, Greb T, Dean C. A PHD-polycomb repressive complex 2 triggers the epigenetic silencing of FLC during vernalization. Proc Natl Acad Sci U S A 2008; 105:16831 - 6; http://dx.doi.org/10.1073/pnas.0808687105; PMID: 18854416
- Wood CC, Robertson M, Tanner G, Peacock WJ, Dennis ES, Helliwell CA. The Arabidopsis thaliana vernalization response requires a polycomb-like protein complex that also includes VERNALIZATION INSENSITIVE 3. Proc Natl Acad Sci U S A 2006; 103:14631 - 6; http://dx.doi.org/10.1073/pnas.0606385103; PMID: 16983073
- Calonje M, Sanchez R, Chen L, Sung ZR. EMBRYONIC FLOWER1 participates in polycomb group-mediated AG gene silencing in Arabidopsis. Plant Cell 2008; 20:277 - 91; http://dx.doi.org/10.1105/tpc.106.049957; PMID: 18281509
- Latrasse D, Germann S, Houba-Hérin N, Dubois E, Bui-Prodhomme D, Hourcade D, et al. Control of flowering and cell fate by LIF2, an RNA binding partner of the polycomb complex component LHP1. PLoS One 2011; 6:e16592; http://dx.doi.org/10.1371/journal.pone.0016592; PMID: 21304947
- Roudier F, Ahmed I, Bérard C, Sarazin A, Mary-Huard T, Cortijo S, et al. Integrative epigenomic mapping defines four main chromatin states in Arabidopsis. EMBO J 2011; 30:1928 - 38; http://dx.doi.org/10.1038/emboj.2011.103; PMID: 21487388
- Filion GJ, van Bemmel JG, Braunschweig U, Talhout W, Kind J, Ward LD, et al. Systematic protein location mapping reveals five principal chromatin types in Drosophila cells. Cell 2010; 143:212 - 24; http://dx.doi.org/10.1016/j.cell.2010.09.009; PMID: 20888037
- Lafos M, Kroll P, Hohenstatt ML, Thorpe FL, Clarenz O, Schubert D. Dynamic regulation of H3K27 trimethylation during Arabidopsis differentiation. PLoS Genet 2011; 7:e1002040; http://dx.doi.org/10.1371/journal.pgen.1002040; PMID: 21490956
- Veiseth SV, Rahman MA, Yap KL, Fischer A, Egge-Jacobsen W, Reuter G, et al. The SUVR4 histone lysine methyltransferase binds ubiquitin and converts H3K9me1 to H3K9me3 on transposon chromatin in Arabidopsis. PLoS Genet 2011; 7:e1001325; http://dx.doi.org/10.1371/journal.pgen.1001325; PMID: 21423664
- Hoppmann V, Thorstensen T, Kristiansen PE, Veiseth SV, Rahman MA, Finne K, et al. The CW domain, a new histone recognition module in chromatin proteins. EMBO J 2011; 30:1939 - 52; http://dx.doi.org/10.1038/emboj.2011.108; PMID: 21522130
- Berr A, McCallum EJ, Ménard R, Meyer D, Fuchs J, Dong A, et al. Arabidopsis SET DOMAIN GROUP2 is required for H3K4 trimethylation and is crucial for both sporophyte and gametophyte development. Plant Cell 2010; 22:3232 - 48; http://dx.doi.org/10.1105/tpc.110.079962; PMID: 21037105
- Berr A, McCallum EJ, Alioua A, Heintz D, Heitz T, Shen WH. Arabidopsis histone methyltransferase SET DOMAIN GROUP8 mediates induction of the jasmonate/ethylene pathway genes in plant defense response to necrotrophic fungi. Plant Physiol 2010; 154:1403 - 14; http://dx.doi.org/10.1104/pp.110.161497; PMID: 20810545
- del Olmo I, López-González L, Martín-Trillo MM, Martínez-Zapater JM, Piñeiro M, Jarillo JA. EARLY IN SHORT DAYS 7 (ESD7) encodes the catalytic subunit of DNA polymerase epsilon and is required for flowering repression through a mechanism involving epigenetic gene silencing. Plant J 2010; 61:623 - 36; http://dx.doi.org/10.1111/j.1365-313X.2009.04093.x; PMID: 19947980
- March-Díaz R, Reyes JC. The beauty of being a variant: H2A.Z and the SWR1 complex in plants. Mol Plant 2009; 2:565 - 77; http://dx.doi.org/10.1093/mp/ssp019; PMID: 19825639
- Wanke D, Hohenstatt ML, Dynowski M, Bloss U, Hecker A, Elgass K, et al. Alanine zipper-like coiled-coil domains are necessary for homotypic dimerization of plant GAGA-factors in the nucleus and nucleolus. PLoS One 2011; 6:e16070; http://dx.doi.org/10.1371/journal.pone.0016070; PMID: 21347358
- Brand LH, Kirchler T, Hummel S, Chaban C, Wanke D. DPI-ELISA: a fast and versatile method to specify the binding of plant transcription factors to DNA in vitro. Plant Methods 2010; 6:25; http://dx.doi.org/10.1186/1746-4811-6-25; PMID: 21108821
- Mozgová I, Mokros P, Fajkus J. Dysfunction of chromatin assembly factor 1 induces shortening of telomeres and loss of 45S rDNA in Arabidopsis thaliana.. Plant Cell 2010; 22:2768 - 80; http://dx.doi.org/10.1105/tpc.110.076182; PMID: 20699390
- Bratzel F, Yang C, Angelova A, Lopez-Torrejon G, Koch M, Del Pozo JC, et al. Regulation of the New Arabidopsis Imprinted Gene AtBMI1C Requires the Interplay of Different Epigenetic Mechanisms. Mol Plant 2012; 5:260 - 9; http://dx.doi.org/10.1093/mp/ssr078; PMID: 21914649
- Wolff P, Weinhofer I, Seguin J, Roszak P, Beisel C, Donoghue MT, et al. High-resolution analysis of parent-of-origin allelic expression in the Arabidopsis Endosperm. PLoS Genet 2011; 7:e1002126; http://dx.doi.org/10.1371/journal.pgen.1002126; PMID: 21698132
- Gehring M, Missirian V, Henikoff S. Genomic analysis of parent-of-origin allelic expression in Arabidopsis thaliana seeds. PLoS One 2011; 6:e23687; http://dx.doi.org/10.1371/journal.pone.0023687; PMID: 21858209
- Hsieh TF, Shin J, Uzawa R, Silva P, Cohen S, Bauer MJ, et al. Regulation of imprinted gene expression in Arabidopsis endosperm. Proc Natl Acad Sci U S A 2011; 108:1755 - 62; http://dx.doi.org/10.1073/pnas.1019273108; PMID: 21257907
- Baroux C, Raissig MT, Grossniklaus U. Epigenetic regulation and reprogramming during gamete formation in plants. Curr Opin Genet Dev 2011; 21:124 - 33; http://dx.doi.org/10.1016/j.gde.2011.01.017; PMID: 21324672
- Crevillén P, Dean C. Regulation of the floral repressor gene FLC: the complexity of transcription in a chromatin context. Curr Opin Plant Biol 2011; 14:38 - 44; http://dx.doi.org/10.1016/j.pbi.2010.08.015; PMID: 20884277
- De Lucia F, Dean C. Long non-coding RNAs and chromatin regulation. Curr Opin Plant Biol 2011; 14:168 - 73; http://dx.doi.org/10.1016/j.pbi.2010.11.006; PMID: 21168359
- Conrath U. Molecular aspects of defence priming. Trends Plant Sci 2011; 16:524 - 31; http://dx.doi.org/10.1016/j.tplants.2011.06.004; PMID: 21782492
- Jaskiewicz M, Conrath U, Peterhänsel C. Chromatin modification acts as a memory for systemic acquired resistance in the plant stress response. EMBO Rep 2011; 12:50 - 5; http://dx.doi.org/10.1038/embor.2010.186; PMID: 21132017
- Pecinka A, Dinh HQ, Baubec T, Rosa M, Lettner N, Mittelsten Scheid O. Epigenetic regulation of repetitive elements is attenuated by prolonged heat stress in Arabidopsis. Plant Cell 2010; 22:3118 - 29; http://dx.doi.org/10.1105/tpc.110.078493; PMID: 20876829
- Costas C, de la Paz Sanchez M, Stroud H, Yu Y, Oliveros JC, Feng S, et al. Genome-wide mapping of Arabidopsis thaliana origins of DNA replication and their associated epigenetic marks. Nat Struct Mol Biol 2011; 18:395 - 400; http://dx.doi.org/10.1038/nsmb.1988; PMID: 21297636
- Deal RB, Henikoff S. The INTACT method for cell type-specific gene expression and chromatin profiling in Arabidopsis thaliana. Nat Protoc 2011; 6:56 - 68; http://dx.doi.org/10.1038/nprot.2010.175; PMID: 21212783
- Schmitz RJ, Zhang X. High-throughput approaches for plant epigenomic studies. Curr Opin Plant Biol 2011; 14:130 - 6; http://dx.doi.org/10.1016/j.pbi.2011.03.010; PMID: 21470901