Abstract
Research projects featuring repetitive phenotypic analysis of insects, such as taxonomic studies, quantitative genetics, and mutant screens, could be greatly facilitated by a simpler approach to scanning electron microscopy (SEM). Here, we have applied low-vacuum SEM to wild type and mutant Drosophila and demonstrate that high quality ultrastructure data can be obtained quickly using minimal preparation. Adult flies, frozen live for storage, were mounted on aluminum stubs with carbon cement and directly imaged, with no chemical treatment or sputter coating. The key imaging parameters were identified and optimized, including chamber pressure, beam size, accelerating voltage, working distance and beam exposure. Different optimal conditions were found for eyes, wings, and bristles; in particular, surface features of bristles were obscured at higher accelerating voltages. The chief difficulties were charging, beam damage, and sample movement. We conclude that our optimized protocol is well suited to large-scale ultrastructural phenotypic analysis in insects.
Introduction
Scanning electron microscopy (SEM) provides higher resolution and magnification than a dissecting microscope while retaining a great depth of field, making it very useful for phenotypic characterization of adult Drosophila. However, for conventional high-vacuum SEM the required sample preparation is time and labor intensive and can introduce artifacts. Both the column and specimen chamber are under high vacuum (< 10−5 Torr = 10−3 Pa) so that primary electrons from the beam and secondary electrons from the specimen are not deflected by air molecules. Thus, specimens generally require chemical fixation, careful dehydration to avoid distorting the structure, and sputter coating with a conductive metal to provide a path for dispersal of beam electrons so that charge doesn't build up on the specimen and interfere with imaging. Dehydration is typically accomplished by critical point drying or by use of dangerous chemicals such as hexamethyldisilazane.Citation1,Citation2
Newer methods, particularly Low-vacuum SEM (LV-SEM) and Environmental SEM (ESEM), provide an alternative for biological SEM.Citation3-Citation8 In LV-SEM, unfixed, hydrated, uncoated specimens are observed by maintaining a high vacuum in the column, to minimize beam interaction with gas, while allowing a lower vacuum (higher pressure) in the specimen chamber (0.1–1 Torr = 10–130 Pa, provided by water molecules). The column and the chamber are separated by pressure-limiting apertures, and differential pumping occurs between the column and the specimen chamber. Imaging is possible at low vacuum because water vapor in the specimen chamber amplifies the secondary electron signal from the specimen while simultaneously neutralizing charge on the specimen. When secondary electrons encounter water vapor molecules, they cause them to produce more secondary electrons that in turn create even more secondary electrons, thus resulting in a cascade of amplified signal. Positively charged water vapor ions that result from secondary electron loss are attracted to the negative charge on the specimen and neutralize it (for reviews, see refs. Citation3–Citation4.).
Goldstein et al.Citation4 have used the analogy of headlights in fog to describe the interaction of the electron beam with gas. An appropriate balance of specimen chamber pressure and gas path length through which the beam travels prevents most of the beam from being scattered, and high-resolution imaging is possible.Citation4 Unless a high vacuum extension cone is placed at the bottom of the column, gas path length is equivalent to working distance. Therefore, with LV-SEM, working distance is involved not just in the usual trade off between resolution (shorter is better) and depth of field (longer is better) that one encounters with high vacuum SEM, but also in the tradeoff between primary electron beam scattering (shorter is better) and amplification of secondary electron signal (longer is better).Citation4,Citation5 Because secondary electron amplification takes time, slower scan rates may be required.Citation4 Disadvantages of imaging uncoated specimens with gas are: that noise increases because some beam electrons are scattered by the gas; that uncoated biological specimens produce fewer secondary electrons (less signal) than do coated ones; that contrast decreases because of a uniform cloud of scattered electrons across the image; and that resolution decreases because the beam penetrates further into uncoated specimens, producing signal from a larger area.Citation3,Citation4,Citation6 Hence, chamber pressure, working distance, and accelerating voltage are critical factors to optimize when imaging with gas.Citation3
ESEM uses a lower vacuum than LV-SEM (1–20 Torr = 130–2600 Pa), and requires an extra pressure-limiting aperture at the bottom of the column. This allows an even higher vapor pressure in the chamber so that water may actually be present around (but not on top of) the specimen while it is being imaged. A Peltier cooling stage may be used with this mode to help prevent dehydration of the specimen. With higher vapor pressure comes more noise, as explained above. ESEM is useful for specimens that are especially susceptible to dehydration or for dynamic experiments (for example see Stabentheiner et al.Citation7) Because the final pressure-limiting aperture restricts the field of view at low magnification, navigation and imaging are less convenient.Citation8 Moreover, ESEM mode is more complicated because it requires control of temperature, pressure, and the rate of change of those variables.Citation6
LV-SEM and ESEM have been used to image diverse specimens from bacteriaCitation9 to plantsCitation7,Citation10 and mammals.Citation8 Fixed and critical point dried flies have been imaged with LV-SEM,Citation11 and ESEM has been used to image flies that were anesthetized and mounted in water-based colloidal carbon glues.Citation12-Citation14 Here we describe LV-SEM imaging of hydrated flies mounted in xylene-based carbon cement. In contrast to ESEM, this approach permits efficient navigation and imaging at both high and low magnification, and avoids the installation of a special pressure-limiting aperture.
Adoption of a new imaging protocol requires a large up-front investment: the labor required to assess and optimize each variable. This article is intended to provide a starting point and roadmap for potential adopters of LV-SEM who wish to image adult insects. We find image quality is exquisitely sensitive to pressure, spot size, and especially accelerating voltage, and so these parameters were investigated using several different fly structures. We also demonstrate the utility of LV-SEM in characterizing novel mutant phenotypes, and define some of the potential drawbacks. For our specimens LV-SEM presents an effective compromise between the extensive sample preparation required for high vacuum SEM and the more complex imaging conditions of ESEM. Once we determined the appropriate parameters, we were able to image large numbers of unprocessed flies with ease using LV-SEM.
Results
We identified five important machine parameters for Drosophila LV-SEM: chamber pressure, working distance, accelerating voltage, spot size, and scan speed. Here we show how image quality varies across a range of values for each parameter. In some cases, varying a parameter can lead to somewhat subtle changes that are hard to discern in a compressed image file; therefore we provide the full TIFF figures and the corresponding raw, unprocessed image files at our website, bio.illinoisstate.edu/facilities/microscopy.shtml. Several sample variables were also investigated: mounting medium, mounting geometry, and sample lifetime under both vacuum and beam exposure. The optimal conditions are summarized in . While the optima were determined for our instrument, the general principles described should hold true for other SEMs (see Discussion).
Table 1. Optimized conditions for Drosophila LV-SEM using the FEI Quanta 450
Mounting adult flies on the stub
The mounting medium needs to secure the fly to the EM stub without introducing artifacts or damage, and provide strong electrical grounding, while allowing the fly to be positioned at the appropriate viewing angle. Several options were tested. To avoid all exposure to chemicals, we found the fly's abdomen can be opened and the hemolymph used as “glue” to affix the body to the stub. While this yielded reasonable images, it was difficult to position the fly, and the attachment was easily broken. Silver paint was tested, but found to lack the viscosity needed to position the fly. The silver paint solvent also wicks up from the stub to the sample, potentially causing artifacts. Conductive adhesive tabs proved to be ineffective, allowing the fly to detach easily. Finally, we used conductive carbon cement (CCC), which bridges the gap in viscosity between silver paint and tapes. CCC provided the consistency required to properly position samples for imaging, but the solvent did not visibly wick up the sample (see Fig. S1A and Materials and Methods). CCC sets in ~1 min, providing secure attachment and electrical grounding.
Scan speed
The scan “speed” is measured in µsec, but this actually refers to a dwell time in µsec per pixel; larger values mean slower speeds. As with other scanning methods, there is a tradeoff: slower scanning provides greater signal/noise, but more energy is put into the sample, and more damage may result. The image is focused and composed at high speeds (~3 µsec), then the final image is scanned at a slower speed to reduce noise. Scan speed also affects collection time, which may be a consideration for higher-throughput work: for a 1024 × 884 pixel image, collection times ranged from 15 sec (10 µsec setting) to 44 sec (40 µsec setting.) By zooming an image until pixels are visible, the decline in noise from a 10 µsec to a 40 µsec scan is apparent (Fig. S1B–E). A 30 µsec setting represents a good compromise among speed, noise and sample damage.
Spot size
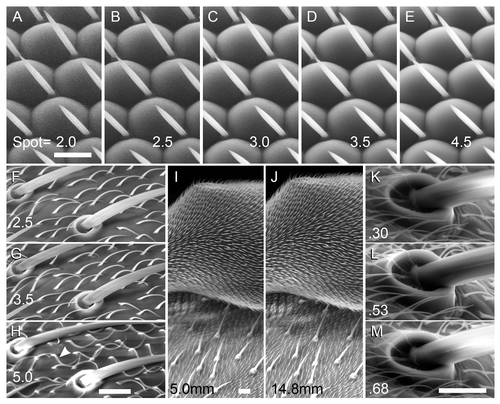
The spot size (beam diameter), controlled by magnetic focusing, has major effects on noise, resolution and sample damage. Increasing the spot size is known to reduce resolution, since the signal is derived from a larger area, but this effect is weak at the magnifications used here (2500× or less). More significantly, we note a strong increase in signal/noise as spot size was raised from 2.0 to 4.5; images with spot sizes of 2.0–2.5 are unacceptably noisy (). At higher spot sizes, sample damage begins to become obvious. At a spot size of 5.0, hairs immediately singe upon focusing and scanning, there is an increased incidence of charging, and resolution begins to visibly decline (). Therefore, a spot size of 3.5 is recommended up to ~2000×, since it keeps both noise and sample damage low, and there is no obvious loss of resolution. Smaller spot sizes may improve the resolution at high magnification, if some noise is tolerable.
Figure 1. Effects of spot size, working distance, and pressure on LV-SEM images. Bar = 10 µm for each set of images. The contrast was adjusted post-collection in this and the remaining figures, except where noted (see Materials and Methods). (A–E) Effect of spot size on the w1118 eye; the same region is shown in each panel; shot at 1000× magnification. Spot sizes were 2.0 (A); 2.5 (B); 3.0 (C); 3.5 (D); and 4.5 (E) as indicated. Resolution is slightly better at lower spot sizes, but the signal is lower and noise more apparent. Spot = 3.5 balances good resolution with low noise and sample damage. (F-H) Thoracic bristles of w1118 were scanned at 2000× and 15 kV, with spot sizes 2.5 (F), 3.5 (G), and 5.0 (H) as indicated. The same region is shown in each panel. Using spot = 5.0 leads to singed hairs (arrowhead) and charging on the bristle sockets, problems not observed with spot = 3.5. (I–J) Increased working distance (WD) provides increased depth of field. Focus is set on the upper surface of a w1118 haltere in both panels, taken at 500×. Note the underlying thorax has better focus in (J) (WD = 14.8 mm) than in (I) (WD = 5.0 mm). (K–M) Variation in chamber pressure, using orbital bristles taken at 2500× and shown with original contrast. The same bristle socket is shown at 0.30 (K), 0.53 (L), and 0.68 Torr (M).
Working distance
The working distance (WD) is measured from the sample to the final condenser lens, and is easily changed by moving the stage in the Z direction. A 10 mm WD provided a good starting point to balance depth of field and resolution in adult flies. Depth of field increases with WD,Citation15 and this was especially noticeable at moderate magnifications (~200–500×; ). Increasing the WD to 10–15 mm helped to keep structures such as the head or thorax in focus through their depth. A shorter working distance (~8 mm) provided slightly better resolution for details imaged at high-magnification (~1000× or more).
Chamber pressure
For the instrument used here, the low-vacuum mode offers a range from ~0.1–1.0 Torr in the chamber. This is on the order of only 1/1000 atmospheric pressure, but nonetheless we found major changes in brightness and noise over this range. At the low end of the range (~0.30 Torr), there is less amplification of secondary electrons by water vapor, and the signal is weak; however, noise remains low. At these low pressures, relatively fragile structures such as the eye were more likely to collapse via dehydration, and charging built up more rapidly since there were fewer water molecules to dissipate the charge. At higher pressures (~0.80 Torr), both brightness and noise increased substantially (and S2). This high signal was difficult to control, as small adjustments to the brightness dial drastically altered the image. A pressure of 0.53 Torr was the best option to balance dehydration and charging with excessive signal amplification by water vapor.
Accelerating voltage
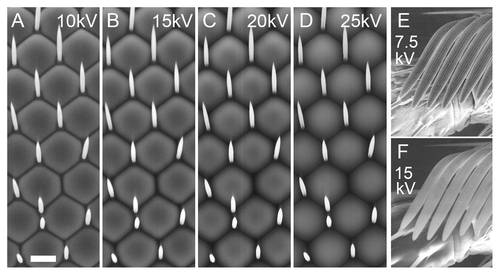
Interestingly, beam strength has radical effects on the qualitative appearance of the image. In general, images appear “flatter” when accelerating voltage is set to 10 kV, look most like conventional high-vacuum SEM at 12–15 kV, and begin to look overly “soft” at higher voltages. For example, in , the valleys between ommatidia darken from 10–20 kV, and then at 25 kV, the darker tops/centers of the ommatidia invert to a lighter tone, giving a translucent appearance. Other tissues behave differently; in particular, the bristles became overly charged and lost detail at higher energies. The longitudinal striations of macrochaetae and sex comb bristles were obvious at 7.5–10 kV (as they are in conventional SEM),Citation16 but they were not resolved at higher voltages (Figs. S3 and ). We imaged mutant eye tissue whose ommatidial bristles are larger than normal (described below), and these also appeared too bright at 15 kV, and too low-contrast at 10 kV, with 12.5 kV a good compromise (Fig. S3E–H). We recommend using 15 kV for general imaging, and dropping the voltage to 12.5, 10 or 7.5 kV if important bristle details are not visible (see also wing imaging, below).
Figure 2. Effect of accelerating voltage on the w1118 eye and the sex comb of the male foreleg. All panels were shot at 1000×; bar = 10 µm for all. A-D, w1118 eye; the same region is shown in each panel. Voltages were 10 (A); 15 (B); 20 (C); and 25 kV (D). Appearance of ommatidia changes substantially as voltage varies; the 15 kV image most resembles a conventional high-vacuum SEM image. (E–F) the striations of the sex comb bristles are clearly seen at 7.5 kV (E), but not at 15 kV (F).
Examples of phenotypic analysis with LV-SEM
shows the performance of LV-SEM on a range of wild type adult structures. High quality images were obtained for the whole fly (), male genitalia (a key species diagnostic; ), the wing margin (), tarsal claw (), mechanosensors on the ventral thorax (), and mouthparts (); also sex comb () and eyes (Fig. S4A). We observed sn mutant males in order to determine whether LV-SEM replicates previous results from conventional SEM. The sn bristles are gnarled and have irregular striations, unlike wild type (), and match the known ultrastructure (compare with Fig. 6 of Cant et al.Citation17)
Figure 3. Examples of wild type structures at varying magnifications in w1118 flies. (B, D and E) were scanned in large format (2048 pixel width). Beam = 15 kV for (A, B, E and F). (A) Whole female mounted in CCC; bar = 1 mm; shot at 20×. (B) Male genitalia (ventral); bar = 50 µm; shot at 250×. (C) Male wing margin (distal is up); bar = 10 µm; shot at 500× and 7.5 kV. (D) Male tarsal claw and pulvillus; bar = 10 µm; shot at 2000× and 10 kV. (E) Sensory hair bed on the ventral anterior side of the thorax near the head (male; anterior is up). Bar = 10 µm; shot at 1500×. (F) Proboscis; bar = 50 µm; shot at 340× and spot = 3.0.
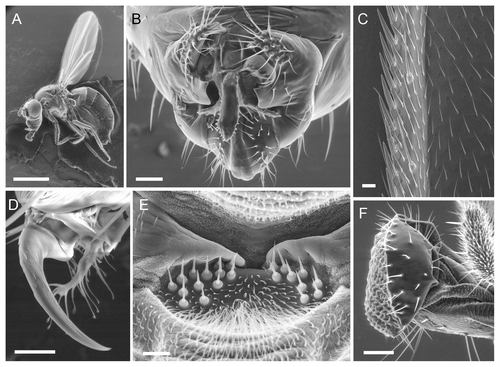
Figure 4. Analysis of mutant phenotypes using LV-SEM. Bars = 10 µm for each pair of images. (A–B) orbital bristles from dorsal head of w1118 (A) and sn (B) males shot at 750×, 10 kV. (C–D) retina of w1118 (C) and GMR-GAL4 > Mi[Hto-WP]QYE (D), shot at 1000× and 2048 pixel width. (E–F) Equivalent regions of w1118 (E) and ms1096w-GAL4 > Mi[Hto-WP]VRC male wings (F), shot at 1500×, 7.5 kV. Note multipronged wing hairs in (F). (G–H) Wing surface with L3 vein and campaniform sense organ (center), from w1118 (G) and ms1096w-GAL4 > Mi[Hto-WP]NAW (H), shot at 1000×, 7.5 kV. In (H), note the hairs are eliminated from this region of the wing, and the cuticle is abnormal.
![Figure 4. Analysis of mutant phenotypes using LV-SEM. Bars = 10 µm for each pair of images. (A–B) orbital bristles from dorsal head of w1118 (A) and sn (B) males shot at 750×, 10 kV. (C–D) retina of w1118 (C) and GMR-GAL4 > Mi[Hto-WP]QYE (D), shot at 1000× and 2048 pixel width. (E–F) Equivalent regions of w1118 (E) and ms1096w-GAL4 > Mi[Hto-WP]VRC male wings (F), shot at 1500×, 7.5 kV. Note multipronged wing hairs in (F). (G–H) Wing surface with L3 vein and campaniform sense organ (center), from w1118 (G) and ms1096w-GAL4 > Mi[Hto-WP]NAW (H), shot at 1000×, 7.5 kV. In (H), note the hairs are eliminated from this region of the wing, and the cuticle is abnormal.](/cms/asset/bf63d76c-2ca5-4137-9062-ce0d349f4672/kfly_a_10920525_f0004.gif)
To assess performance with novel phenotypes, we used lines from our Minos-based overexpression/protein trapping system called Hostile takeover. Individual protein trap lines called QYE, TRG, VRC, and NAW were imaged in combination with appropriate GAL4 drivers (see Materials and Methods). GMR-GAL4 > QYE eyes have a fuzzy appearance by light microscopy, and this was borne out by LV-SEM: the retina is converted into an epithelium with hairs on most cells, never seen in wild type eyes, and the ommatidial bristles are replaced by significantly larger socketed bristles. The ommatidia are apparent, but lack definition (; S3E–H). For GMR-GAL4 > TRG, the retina is smooth and irregularly studded with ommatidial bristles, and the eye comes to a point ventrally instead of being rounded (Fig. S4). In combination with a wing driver (ms1096w), two lines were found to yield wing defects that could not be fully characterized without SEM analysis (see below for wing imaging conditions). In ms1096w-GAL4 > VRC flies, the hairs were often converted from long, thin projections to branched and tufted structures that were several-fold thicker at the base (). In ms1096w-GAL4 > NAW flies, the wing surface appears defective by dissecting scope; LV-SEM elucidated the basis of the problem. The hairs are largely missing, although the sense organs appear normal (). Also, the cuticle is uneven and the cell remnants bulge outward, giving a tiled appearance not seen in wild type.
Conditions for imaging the wing blade
Adult wings are of particular interest since they are often used for phenotypic screening, genetic interaction analysis, and studies of intra- and interspecific variation.Citation18,Citation19 Wings did not image well under the conditions that are optimal for eyes. The wing hairs, as well as some veins, became charged and could appear quite bright by LV-SEM (much like conventional SEMCitation20). We found that lower accelerating voltages (7.5–10 kV) improved the image by limiting charging and excessive contrast (). To offset the resulting increase in noise, the scan speed setting was slowed from a 30 to a 40 µsec dwell time.
Next, we noted that with LV-SEM, the CCC mounting medium was “visible” underneath the wing blade (Fig. S5A–B). Apparently, the region not touching the CCC charges more than the region in contact with the CCC. Thus, for a set of images to be consistent, one must image regions that are entirely in contact with the CCC, or entirely away from it. Imaging the blade with space underneath was preferable, since the CCC's texture made the blade appear mottled (Fig. S5B). Figure S5C shows a method for mounting the wing in this position, using drops of CCC only at the proximal and distal tips of the wing.
Troubleshooting LV-SEM artifacts
We observed several imaging artifacts related to LV-SEM, and we discuss them here in an effort to make them easier to identify and minimize.
Sample motion and beam damage
Since the samples were unfixed and uncoated, they were prone to movement during a scan and between scans. The sample is subjected to a complex combination of forces. Dehydration of muscles can drive rotation of the head, bending of limbs, etc. Beam exposure causes heating and breakdown of the cuticle, which may lead to shifting of bristles and hairs, and eventually warping or collapse of tissue. These various movements precluded the use of integration or averaging two images together for noise reduction, since successive images usually differed in some details and could not be accurately superimposed.-Motion could also produce a warped image of a structure, as it moved a bit between each scan line. Under our optimized conditions (), there was usually minimal movement, allowing for reproducible scans (e.g., and S3), but in practice, motion should always be monitored from scan to scan.
Repeated scanning of the same area, or use of large spot size, should be avoided as they damage fine structures such as hairs. shows the same region before and after scanning at a spot size of 5; note the hairs have been “singed” by the beam. Using a zoomed focusing window also accelerates damage and should be avoided.
Charging
Charging of limbs and bristles is common in conventional SEM, and also observed in LV-SEM. However, charging is less confined to one entire structure (e.g., antenna, leg segment) in LV-SEM, since the water vapor can dissipate the charge. Charging is most likely to be seen on isolated or poorly grounded structures, for example the distal tarsal segments and tarsal claw ().
Horizontal banding due to chamber pressure fluctuation
As an image is scanned, small fluctuations in chamber pressure cause horizontal bands of differing brightness to appear in the image. When the pressure increases momentarily, the scanned region is brighter, while a decrease in pressure results in darker bands (not shown). This occurs because the amplification of secondary electrons by water vapor varies steeply with pressure. We recommend that if banding is observed, the operator should note whether it correlates with chamber pressure variations as displayed on-screen.
Discussion
For imaging adult Drosophila, LV-SEM is a strong alternative option to conventional SEM and ESEM, providing excellent resolution and contrast, and manageable noise and sample damage. The central advantage to our protocol is its speed and simplicity: there are no fixation, dehydration, or coating steps. Personnel can be trained in a few minutes to prepare stubs for examination, and high-quality mounts can be achieved after minimal practice. Once the SEM is set using the parameters described here, the bulk of the time for an experiment is spent in pumping down the chamber, finding and focusing on the sample, adjusting brightness/contrast, and scanning. The unfixed flies resist distortion for a variable amount of time under vacuum, but most of them remain usable for an hour or more, and one can easily acquire 20–30 images in that time. Overall, one could reasonably reach a throughput of 50–100 individual flies per day, opening up the possibility of using SEM for genetic screens or other large-scale experiments.
The main drawback to LV-SEM is the possibility of sample damage, especially at magnifications over ~400×. The main forms of damage we encountered are (1) hairs/bristles becoming “singed” by the beam (bending, collapsing or shriveling; ), and (2) warping of the cuticle, e.g., collapse of the eye or haltere. Two factors alleviate this problem. First, it is fairly easy to assess sample damage: one can compare subsequent scans to the initial one, and warping can often be observed during a scan as it overwrites the pre-scan on the monitor. Second, the time to damage is quite variable, and with a few tries, one can usually find a region that survives several scans if needed (e.g., ). Distortion of the sample is not unique to LV-SEM; it may also occur during dehydration steps of conventional SEM.
Here, we searched for optimal conditions for LV-SEM on flies. For spot size and pressure, we found single values that work well across a range of imaging conditions (). For accelerating voltage, however, different optima were found for eyes (15 kV) and for easily charging structures such as bristles and wings (7.5–12.5 kV). Because beam penetration of the specimen is more pronounced at higher voltages, signal from beneath the surface obscures the surface signal, and surface features such as bristle striations are poorly resolved at 15kV (). It is notable that the optimal values for each variable tend to fall in the middle of the available range, rather than at the extremes. This is not surprising since in digital imaging, optimization usually involves a tradeoff between two or more conflicting goals, such as low noise vs. sample preservation. This should make our results generalizable to most other recent SEMs, which can operate in the same pressure and voltage ranges. The image series in and and S1–S3 can be used as a guide to find the corresponding optimal values on another SEM system.
We found that LV-SEM can yield rich phenotypic data and is well suited to mutant analysis. Our images of sn bristles closely match those obtained with conventional SEM.Citation17 Further, LV-SEM gave new insights into several phenotypes we had recovered from gain-of-function genetic screens for UAS-bearing inserts. Overexpression of bonus (a regulator of p53)Citation21 in the retina using the QYE insert led to remodeling of the retinal epithelium, with apparent conversion of cells on the periphery of the ommatidia into more typical epithelial cells that make hairs. The VRC insert drives expression of steppke, which encodes a guanine exchange factor of the cytohesin family.Citation22 Ectopic steppke expression allowed wing hairs to grow in tufts; this indicates a role for steppke in spatial control of actin organization, which underlies hair morphology.Citation20 Finally, the insert NAW defines a locus that can reprogram the wing epithelium, eliminating wing hairs and altering the texture of the cuticle. Hopefully the ease of this method will promote broader use of EM to explore mutant phenotypes at the ultrastructural level.
Materials and Methods
Genetics
Adult w1118 (stock from S. Benzer) were used as a standard since this mutation is in the background of most transgenic stocks. Females were used unless noted. The singed (sn) [FBgn0003447] bristle examples are from FM7c, sn/Y males. The other mutant examples carry GAL4 drivers paired with Minos-based UAS-driven protein traps, raised at 25°C. The GAL4 driver stocks were GMR-GAL4 (retina-specific) [FBti0002994] or ms1096w-GAL4 (wing-specificCitation23) [FBti0072884]. The protein trap lines were made using the Minos[Hostile takeover] (Mi[Hto]) vector (K.E. in prep.; the vector is described in GenBank #JN049642). Mi[Hto-WP]QYE is an insert that drives expression of bonus [FBgn0023097]; Mi[Hto-WP]VRC is an insert that drives expression of steppke [FBgn0086779]; Mi[Hto-WP]NAW is an insert on chromosome 2 whose target gene is not known; Mi[Hto]TRG is an insert on chromosome 3 whose target gene is not known.
Mounting Samples for SEM
The mounting medium was Leit-C Conductive Carbon Cement (CCC), manufactured by Plano (EM Sciences #12664). Frozen adult flies were mounted shortly after removal from the freezer. In some cases, live anaesthetized flies were used. Live flies, or flies frozen live and then thawed, have pliable joints, which facilitates the positioning of the fly on the stub. In contrast, flies become brittle when dehydrated or air-dried before mounting. CCC was resuspended and dabbed onto an aluminum pin stub (Pella 16111) with an applicator stick; the fly was placed in the CCC droplet with fine forceps and positioned within ~1 min. After ~1 min the CCC solidified and the fly could not be repositioned. If the CCC stock became too viscous in the bottle, xylene was added to dilute it to the original viscosity. Up to four adults were affixed to each stub (Fig. S1). A stub can be stored in a humid chamber for a few hours, but here all samples were mounted just before imaging to reduce dehydration.
SEM
Images were collected on the FEI Quanta 450 tungsten environmental scanning electron microscope (ESEM) in low-vacuum mode with the large field secondary electron detector. The scan settings are described in Results. Chamber pressure was 0.53 Torr unless noted. Images were collected at 1024 × 884 pixels unless stated.
Image processing
Images were processed in Adobe Photoshop. In some cases, global adjustments to contrast were made using Photoshop Curves function. Any adjustment was applied to the entire image. The rationale is as follows: brightness/contrast are quite unstable during imaging (i.e., they change radically with changes to other parameters) and need constant adjustment during scanning. This is effectively similar to making these adjustments during post-processing in Photoshop; that is, brightness/contrast are not inherent features of the sample. It is nearly impossible to keep brightness/contrast steady for a series of images, and this variation distracts from the relevant changes to image quality, or comparisons of mutant to wild type, that are the subject of the study. Thus, unless stated, the images in a series were adjusted so that brightness/contrast are relatively uniform across successive scans. All the original, unprocessed images from the SEM that we used to compose each montage figure are available for inspection at our website bio.illinoisstate.edu/facilities/microscopy.shtml.
| Abbreviations: | ||
| CCC | = | Leit-C Conductive Carbon Cement |
| ESEM | = | environmental scanning electron microscopy |
| kV | = | kilovolts |
| LV-SEM | = | low-vacuum scanning electron microscopy |
| WD | = | working distance |
Additional material
Download Zip (4.2 MB)Acknowledgments
Supported by NSF DBI-0923448 and NIH GM62185. Stocks obtained from Bloomington Drosophila Stock Center, Indiana Univ.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Supplemental Materials
Supplemental Materials may be found here: www.landesbioscience.com/journals/fly/article/20525
References
- Nation JL. A new method using hexamethyldisilazane for preparation of soft insect tissues for scanning electron microscopy. Stain Technol 1983; 58:347 - 51; PMID: 6679126
- Araujo JC, Téran FC, Oliveira RA, Nour EAA, Montenegro MAP, Campos JR, et al. Comparison of hexamethyldisilazane and critical point drying treatments for SEM analysis of anaerobic biofilms and granular sludge. J Electron Microsc (Tokyo) 2003; 52:429 - 33; PMID: 14599106
- Stokes DJ. Recent advances in electron imaging, image interpretation and applications: environmental scanning electron microscopy. Philos Transact A Math Phys Eng Sci 2003; 361:2771 - 87; http://dx.doi.org/10.1098/rsta.2003.1279; PMID: 14667297
- Goldstein JI, Newbury DE, Echlin P, Joy DC, Lyman CE, Lifshin E, Sawyer L, Michael JR. Scanning Electron Microscopy and X-Ray Microanalysis. New York, NY: Springer Science + Business Media, LLC, 2003, pp 220-240.
- Chandler DE, Roberson RW. Bioimaging: Current Concepts in Light and Electron Microscopy. Sudbury, MA: Jones & Bartlett Publishers, 2009, p 201.
- Kirk SE, Skepper JN, Donald AM. Application of environmental scanning electron microscopy to determine biological surface structure. J Microsc 2009; 233:205 - 24; http://dx.doi.org/10.1111/j.1365-2818.2009.03111.x; PMID: 19220687
- Stabentheiner E, Zankel A, Pölt P. Environmental scanning electron microscopy (ESEM)--a versatile tool in studying plants. Protoplasma 2010; 246:89 - 99; http://dx.doi.org/10.1007/s00709-010-0155-3; PMID: 20446004
- Muscariello L, Rosso F, Marino G, Giordano A, Barbarisi M, Cafiero G, et al. A critical overview of ESEM applications in the biological field. J Cell Physiol 2005; 205:328 - 34; http://dx.doi.org/10.1002/jcp.20444; PMID: 15965928
- Nishimura M, Wada M, Akiba T, Yamada M. Scanning electron microscopy of food-poisoning bacterium Bacillus cereus using a variable-pressure SEM. J Electron Microsc (Tokyo) 2003; 52:153 - 9; PMID: 12868586
- Zheng T, Waldron KW, Donald AM. Investigation of viability of plant tissue in the environmental scanning electron microscopy. Planta 2009; 230:1105 - 13; http://dx.doi.org/10.1007/s00425-009-1009-0; PMID: 20183923
- Price DM, Jin Z, Rabinovitch S, Campbell SD. Ectopic expression of the Drosophila Cdk1 inhibitory kinases, Wee1 and Myt1, interferes with the second mitotic wave and disrupts pattern formation during eye development. Genetics 2002; 161:721 - 31; PMID: 12072468
- Iyengar B, Roote J, Campos AR. The tamas gene, identified as a mutation that disrupts larval behavior in Drosophila melanogaster, codes for the mitochondrial DNA polymerase catalytic subunit (DNApol-gamma125). Genetics 1999; 153:1809 - 24; PMID: 10581287
- Settle M, Gordon MD, Nadella M, Dankort D, Muller W, Jacobs JR. Genetic identification of effectors downstream of Neu (ErbB-2) autophosphorylation sites in a Drosophila model. Oncogene 2003; 22:1916 - 26; http://dx.doi.org/10.1038/sj.onc.1206240; PMID: 12673197
- Moyer KE, Jacobs JR. Varicose: a MAGUK required for the maturation and function of Drosophila septate junctions. BMC Dev Biol 2008; 8:99; http://dx.doi.org/10.1186/1471-213X-8-99; PMID: 18847477
- Bozzola JJ, Russell LD. Electron Microscopy: Principles and Techniques for Biologists. 2nd ed. Sudbury, MA: Jones & Bartlett Publishers, 1999, pp 211-212.
- Tilney LG, Connelly PS, Ruggiero L, Vranich KA, Guild GM, Derosier D. The role actin filaments play in providing the characteristic curved form of Drosophila bristles. Mol Biol Cell 2004; 15:5481 - 91; http://dx.doi.org/10.1091/mbc.E04-06-0472; PMID: 15371540
- Cant K, Knowles BA, Mooseker MS, Cooley L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. J Cell Biol 1994; 125:369 - 80; http://dx.doi.org/10.1083/jcb.125.2.369; PMID: 8163553
- Cruz C, Glavic A, Casado M, de Celis JF. A gain-of-function screen identifying genes required for growth and pattern formation of the Drosophila melanogaster wing. Genetics 2009; 183:1005 - 26; http://dx.doi.org/10.1534/genetics.109.107748; PMID: 19737745
- Edwards KA, Doescher LT, Kaneshiro KY, Yamamoto D. A database of wing diversity in the Hawaiian Drosophila. PLoS ONE 2007; 2:e487; http://dx.doi.org/10.1371/journal.pone.0000487
- Guild GM, Connelly PS, Ruggiero L, Vranich KA, Tilney LG. Actin filament bundles in Drosophila wing hairs: hairs and bristles use different strategies for assembly. Mol Biol Cell 2005; 16:3620 - 31; http://dx.doi.org/10.1091/mbc.E05-03-0185; PMID: 15917291
- Allton K, Jain AK, Herz HM, Tsai WW, Jung SY, Qin J, et al. Trim24 targets endogenous p53 for degradation. Proc Natl Acad Sci U S A 2009; 106:11612 - 6; http://dx.doi.org/10.1073/pnas.0813177106; PMID: 19556538
- Fuss B, Becker T, Zinke I, Hoch M. The cytohesin Steppke is essential for insulin signalling in Drosophila.. Nature 2006; 444:945 - 8; http://dx.doi.org/10.1038/nature05412; PMID: 17167488
- Park H, Edwards K. “Marker removal” screen to generate an improved wing disc GAL4 driver. Drosoph Inf Serv 2004; 87:96 - 9