Abstract
When a new student first begins to push flies, an immediate skill that must be learned is sorting the sexes. In Drosophila melanogaster several sexually dimorphic characters can be used to readily distinguish males from females including abdominal pigmentation, male sex combs and genital morphology. Another, often-overlooked, sexual dimorphism is adult abdominal segment number. Externally, adult Drosophila males possess one fewer abdominal segment than females; the terminal pre-genital segment apparently either absent or fused with the next-most anterior segment. Beyond known roles for the homeotic protein Abdominal-B (Abd-B) and the sex-determining transcription factor Doublesex (Dsx) as key regulators of this trait, surprisingly little is known about either the morphogenetic processes or the downstream genetics responsible for patterning these events. We have explored both and found that rapid epithelial reorganization during pupation eliminates a nascent terminal male segment. We found this Abd-B-dependent process results from sex- and segment-specific regulation of diverse developmental targets including the wingless gene and surprisingly, dsx itself.1,2 Here, I review our observations and discuss this trait as a model to explore both dynamics of epithelial morphogenesis as well as the evolution of developmental mechanisms.
Development of the Abdominal Epithelium
Development of the adult Drosophila abdominal epithelium involves a number of genetically tractable processes that make this tissue an amenable model system for investigating wide-ranging questions addressing cell cycle regulation, pattern formation and the evolution of novel character traits. The epithelium is derived from populations of imaginal histoblast cells that are born during embryogenesis and remain quiescent until the onset of pupariation.Citation3 Upon activation, discrete nests of histoblasts proliferate and fuse to form a continuous epithelium, replacing larval epidermal cells that are removed by apoptosis and extrusion.Citation4-Citation6 Individual abdominal segments are patterned by many of the same genes responsible for embryonic segmentation. Histoblast cells within each developing segment retain compartmental identities established during embryogenesis. Dorsally, an anterior and posterior nest express cubitus interruptus (ci) and engrailed (en) respectively, while a single ventral nest is composed of cells separated into distinct anterior and posterior populations.Citation7-Citation9 During larval development a fourth nest, originating from anteriorly fated tracheoblasts, joins the larval epithelium and will generate cells surrounding the spiracle.Citation10 By 24 h APF (after puparium formation) the four nests have fused into a hemisegmental epithelium separated from adjacent segments and contralateral hemisegments by narrow bands of remaining LECs. By 28–30 h APF fusion along the AP and DV axes generates a continuous abdominal epithelium and proliferation continues until the onset of differentiation at approximately 36–40 h APF.Citation1,Citation11 Cell identities along the AP axis of each segment are patterned principally by the transcription factor En and the morphogen Hedgehog (Hh) produced by En expressing cells. In the posterior compartment En and Hh direct cells toward unsclerotized cuticle fate (pleura) while in the anterior compartment opposing Hh gradients direct planar cell polarity and establish discrete cell identities distinguished by unique combinations of pigment, trichomes (fine hairs) and bristle types.Citation7,Citation8,Citation12 In contrast to imaginal discs and embryonic segmentation, Wg does not have a predominant role in abdominal AP patterning.Citation7-Citation9,Citation13 Rather, Wg and Drosophila Epidermal Growth Factor (DER) signaling were found to function in complementary patterns along the DV axis to direct cells away from pleural fate and establish boundaries of the sclerotized anterior cuticle plates; the dorsal tergites and the ventral sternites. However, activated Wg signaling in anterior compartment mitotic clones transforms subpopulation identities suggesting Wg does have a role in AP patterning.Citation14
As during embryogenesis, superimposed on these patterning events are regional activities of the Hox proteins Ultrabithorax (Ubx), Abdominal-A (Abd-A) and Abdominal-B (Abd-B) which promote unique morphology among the seven abdominal segments A1-A7.Citation15,Citation16 For example, in A1 Ubx promotes the absence of a sternite, reduced tergite size and unique bristle morphology and pigmentation.Citation17 In the posterior abdomen (A5-A7) Abd-B functions in collaboration with sex-specific Dsx isoforms to generate male specific pigmentation and modified cuticle morphology.Citation15 The terminal segment A7 presents the most striking of these modifications as female hemitergites do not fuse dorsally and retain a characteristic triangular shape while male A7 is superficially absent generating neither tergite nor sternite (). Male specific pigmentation and the roles of Abd-B and Dsx in promoting this trait have been extensively studied as a model for mechanisms of morphological evolution.Citation18-Citation22 Sex-specific pigmentation is under strong sexual selection and as such has diverged rapidly within the family Drosophilidae. In contrast, reduction of male posterior segments is deeply fixed within the monophyletic clade Cyclorrhapha which diverged an estimated 145myaCitation23 and reflects a trend among the aerially acrobatic higher Diptera (Brachycera) toward reduced body size. Although Abd-B and Dsx have long been known to control this trait in DrosophilaCitation17,Citation24 no studies have investigated either the morphogenesis of male reduction or the genetic events regulated by these transcription factors. Here I summarize our observations that show reduction of Drosophila male A7 occurs through rapid epithelial reorganization driven largely by male-specific Abd-B and Dsx dependent Wg repression as well as segment-specific, but not sex-specific, modulation of apoptosis.Citation1 I additionally review our more recent data that show not only is Dsx a requisite Abd-B co-regulator of this trait, but it is also positively regulated downstream of Abd-B. Finally I discuss this trait as a model to investigate the evolution of a complex morphological process.
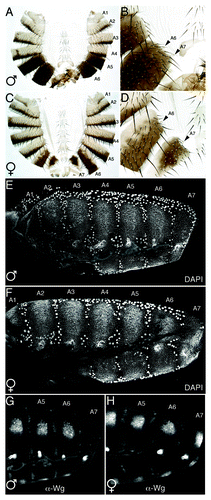
Figure 1. Morphogenesis of the terminal abdominal segment. Adult Drosophila males externally lack the terminal abdominal segment A7. (A and C) Whole abdominal cuticle preparation of adult male and female dissected longitudinally along the dorsal axis. Segment tergites are numbered. Note a robust, but characteristically modified, female A7 is present (C), but males lack both tergite and sternite (A). (B and D) Higher magnification of same cuticles framing the posterior-most tergites. Arrows indicate spiracle pits. A single spiracle pit is associated with each female tergite (D) however in males the A7 spiracle has moved anteriorly and is associated with the A6 tergite (E and F) Despite its absence in adults, male A7 undergoes substantial proliferation during the first 28 h of pupation. By 24 h APF, male A7 histoblasts (small nuclei) have proliferated and formed a continuous segmental epithelium (E). (G and H) Abd-B and Dsx mediated repression of Wg is a major contributor of the ultimate reduction of male A7. The same pupae shown in E-F stained with an anti-Wg antibody show absence of Wg from male A7 (G).
Reduction of the Terminal Male Segment
Prior to our investigation, the only genetic alterations known to affect male A7 development modify the function of either Abd-B or Dsx. Abd-B loss of function alleles cause homeotic transformations including A7 toward A6 identity producing a robust male A7 segment.Citation17 Similar transformations are observed when the Hox co-factor Extradenticle is absent and upon overexpression of the Hox antagonist Bric-á-brac (Bab).Citation18,Citation25 dsx loss of function flies have an intersex phenotype with XX and XY individuals exhibiting similar sexually intermediate characters.Citation24 In both sexes, A7 retains the characteristic triangular morphology of females but is reduced in size compared with wild type A7. The differences in A7 morphology between Abd-B and dsx mutants suggest that while dsx functions cooperatively with Abd-B to promote male A7 reduction Abd-B also regulates dsx-independent processes that contribute to this trait. As the dsx null A7 is smaller than wild type female A7 this suggests Abd-B and Dsx co-govern proliferation, and Abd-B governs processes that eliminate A7 for which Dsx-F (the female specific isoform) is an antagonist.
Analyses of wild type adult cuticles reveal that male A7 is not completely absent. In the lateral pleura, adjacent to the dorsal tergite of each female abdominal segment and male A1-A5, lies a bilateral pair of trachea spiracle pits. However, two spiracle pairs are associated with male A6 suggesting A7 either only partially develops or fuses with A6 during pupation (). We developed a dissection and imaging protocol that preserves abdominal epithelial morphologyCitation26 and imaged fixed pupae with nuclei fluorescently labeled to monitor the fate of male A7 histoblasts.Citation1 We confirmed that histoblast nests in male A7 third instar larvae have similar cell numbers compared with more anterior segments,Citation3 therefore the absence of adult cuticle does not result from a failure to establish or maintain histoblast populations. Additionally, by 24hr APF histoblasts nests in all segments, including male A7, have undergone extensive proliferation and are fused into individual segment epithelia (). While the absence of adult male A7 cannot be attributed to a failure of histoblast proliferation we found A7 mitotic counts in both sexes are significantly lower compared with more anterior segments and male A7 counts are more than 2-fold lower than female A7. In support of a role for Abd-B in suppressing cell division we recently found that A7 Abd-B null clones in either sex exhibit increased proliferation compared with surrounding wild type tissue (unpublished data).
To track the fate of male A7 we monitored expression of GFP driven by En-GAL4 to mark posterior compartments. Rapidly, between 28 and 40 h APF, anterior A7 (aA7) cell number decreases such that by 42hr APF few, if any, aA7 cells remain and the posterior compartments of A6 and A7 are tightly juxtaposed. Strikingly, we observed during this process that pA6 gradually buckles around the anterior-fated A7 spiracle and passes this structure before merging with pA7. As a result the A7 spiracle pit is repositioned and becomes associated with aA6. Therefore, although a nascent male A7 segment develops during early pupation it is rapidly eliminated prior to the onset of differentiation. We initially hypothesized that male-specific programmed cell death in combination with decreased A7 proliferation may be sufficient to drive this elimination. We found no clear male-specific pattern of apoptosis, however cell death is enhanced surrounding all spiracle pits and is significantly enriched around A7 spiracles of both sexes. We used a Dsx -GAL4 driver, which is strongly expressed in all abdominal histoblasts, to block apoptosis during pupation through ectopic expression of the baculovirus anti-apoptotic gene p35.Citation27 Importantly, this GAL4 transgene is inactive in the LECs where apoptosis has been shown necessary to promote proper histoblast proliferation and expansion.Citation4 Reducing histoblast apoptosis in the pupal abdomen does not promote widespread defects in cuticle formation suggesting histoblast apoptosis does not play a major role in development of adult abdominal segments. Interestingly, in adult males of this genotype there is excess pleural tissue posterior to the A6 tergite suggesting male aA7 is not completely eliminated. However this A7 tissue does not develop tergite or sternite cuticle plates.
Therefore, mechanisms in addition to regulating proliferation and apoptosis must be involved in male A7 reduction. Insight into this came from our observations of dynamic En-driven GFP expression. During the 12-h period when male aA7 is eliminated we found that pA6 expands and is several cell diameters wider than in more anterior segments or female A6. By co-labeling these GFP expressing pupae with an anti-En antibody we found that during reduction the posterior-most pA6 En positive nuclei do not co-express GFP. Our interpretation of these data are that during pupation the fate of the anterior-most a7A cells is transformed toward posterior identity; they begin to express engrailed de novo and become a part of pA6. A similar fate transformation has been observed during embryonic posterior spiracle morphogenesis.Citation28 Another possible interpretation of these results is that the non-GFP, En-positive cells at the male A6/A7 border are not transformed aA7 cells, but instead are true pA6 cells which in more anterior segments and female A6 express En at undetectable levels. Such a scenario would require an Abd-B/Dsx-M mediated mechanisms of enriching en expression in pA6. It is also possible that these cells reflect the early embryonic lineage of the anterior-most anterior compartment cells. In early embryos these cells initially express en but this expression decays as Wg expression, necessary for en maintenance, is refined within the anterior compartment.Citation29 As such, the dynamics of en expression we have observed may occur at all segment borders but are enhanced at A6/A7 in an Abd-B/Dsx-M dependent manner. Accordingly, we do observe similar non co-labeled cells, though far fewer and only sporadically, in more anterior segments of both sexes. Addressing these possibilities through lineage analyses is challenged by the fact that no reliable characters distinguish the anterior-most A compartment cells from the posterior-most P compartment cells in the adult abdominal cuticle.Citation7
The sex-specific modulation of en compelled us to investigate expression of other segmentation genes, or their protein products, with previously characterized roles in adult abdominal segmentation.Citation7,Citation8,Citation11,Citation12,Citation16 Strikingly, only Wg shows sexually dimorphic expression; it is strongly repressed in developing male A7 and this repression is dependent on both Abd-B and Dsx (). We propose that, regardless of the mode of En expression dynamics, the absence of Wg in male A7 promotes these modifications and contributes to the loss of cells from aA7. More rigorous analyses, perhaps employing ectopic Wg expressing clones in male A7, will be necessary for further clarification.
Beyond a potential role in modulating en expression, Wg is a potent mitogen and is necessary for abdominal cuticle differentiationCitation13,Citation30; therefore its absence in male A7 leads to logical conclusions concerning reduced proliferation in this segment and the absence of adult cuticle. Importantly, ectopic wg restores male A7 tergite and sternite, though weakly as compared with either Abd-B or dsx mutants. It is possible that simultaneously blocking apoptosis may enhance A7 rescue, however this experiment leads to early pupal lethality and could not be assayed. We also considered it likely that Abd-B and Dsx also differentially regulate other developmental pathways. For example, DER signaling is required for dorsolateral and ventral cuticle fate.Citation11 Consistent with this, we found activation of DER signaling, through overexpression of the ligand Vein, also partially restores male A7. A role for modification of DER signaling was recently confirmed and found to be associated with Abd-B/Dsx dependent repression of the ligand Spitz (Spi).Citation31 Because both Wg and DER signaling have mitogenic functions, the dramatic decrease in male A7 proliferation is likely due to the combined repression of these two pathways. Furthermore, it was shown that male A7-specific cell extrusion plays a significant role in the elimination of histoblast cells from the developing epithelium. This process is regulated downstream of Abd-B and Dsx-M through positive regulation of extramacrocheate (emc) which encodes an HLH transcription factor.Citation31 These analyses suggest that emc, in turn, promotes elevated expression of the myosin light chain regulatory subunit spaghetti-squash (sqh) promoting basal extrusion of male A7 histoblasts. This process is similar to extrusion of LECs from the larval abdominal epithelium which has been shown to require myosin-dependent apical constriction.Citation4
Our observations, combined with other studies, suggest a model in which Abd-B and Dsx-M cooperatively repress wg and spi in male A7, promoting decreased proliferation and preventing cuticle differentiation. Additionally, Abd-B and Dsx-M promote elevated A7 levels of emc leading to segment-specific activation of epithelial cell extrusion. Independent of Dsx, we propose Abd-B enhances a segmentally reiterated pattern of spiracle-associated apoptosis. The mechanism of cell death regulation is less clear and would require collaboration with some unidentified factor restricted to the spiracle perimeter. Together, the combination of reduced proliferation, aA7 transformation, enriched A7 apoptosis and cell extrusion are sufficient to completely eliminate the nascent male A7 segment. In females, subtle modification to the regulation of these processes by Dsx-F, acting either in combination with or antagonistically to Abd-B activity, likely sculpts the unique morphology of the female terminal segment. We have recently revised our model to include an additional level of regulatory complexity. We found that not only does Dsx function cooperatively with Abd-B to control these morphogenetic events, but dsx is itself regulated downstream of Abd-B.Citation2 Similar observations were subsequently reported independently.Citation31 During early pupation, when proliferation and apoptosis promote sex-specific A7 epithelial morphogenesis, Dsx expression is restricted to A7 of both sexes under positive regulation by Abd-B. Although Dsx is expressed within similar domains in male and female pupae, Dsx-M protein levels are approximately 2.5 fold higher than Dsx-F. Appropriate levels of Dsx-F are clearly necessary for proper morphogenesis as we find that overexpression of Dsx-F during pupation leads to A7 tergite enlargement (unpublished data).
A Model for Development and Evolution
Morphogenesis of the Drosophila abdominal epithelium requires organized control of diverse cellular events. This tissue has been developed as a model to explore the coordination of cell cycle regulation, apoptosis and differentiation in an expanding epithelial population.Citation4-Citation6 Additionally, this system has provided valuable insight into epithelial axial patterning and planar cell polarity.Citation7-Citation9,Citation14,Citation16,Citation32 Our focus on the terminal abdominal segment is complimentary to these two lines of investigation and highlights the synergy of these varied processes and their coordinated regulation by homeotic and sex-determination pathways.
Our investigations also shed light on the evolution of this novel morphology. Male posterior segment reduction and modifications to corresponding female segments (usually as a retractable ovipositor) is a characteristic trait, if not a synapomorphy, of Cyclorrhapha. Though many non-Cyclorrhaphan flies have evolved variations in segment number and morphology, most retain the ancestral phenotype of sexually monomorphic abdominal segment number. Therefore, our observations present testable hypotheses about the evolution of posterior segment morphology within the Cyclorrhapha. Considering male segment reduction specifically, we predict that the patterning function and requirement for cuticle differentiation of Wg and EGF signaling are conserved through the insects. In support of this, a requirement for Wg in tergite differentiation has been shown in the milkweed bug O. fasciatus, a hemimetabolous insect.Citation33 Therefore, within the Cyclorrhaphan diptera we hypothesize that this trait evolved through regulatory changes that brought wg, spi and emc under transcriptional regulation downstream of Abd-B and Dsx. Comparative expression studies will be needed to confirm this prediction. Whether Abd-B and Dsx expression are conserved throughout the Diptera is an important question that must also be addressed. Abd-B expression in the posterior abdomen is likely conserved as I have previously shown that its embryonic expression domains are conserved between Drosophila and basal dipterans.Citation34 Whether dsx, for which the production and function of sex-specific isoforms is deeply conserved, was ancestrally an Abd-B target in the abdomen will provide important clues about the stepwise evolution of this trait. The two transcription factors are co-expressed in Drosophila genitalia and likely have conserved co-regulatory functions. Through the evolution of Abd-B regulated dsx abdominal expression, these developmental modules may have been co-opted into morphogenesis of the posterior abdomen. Alternatively, dsx may have ancestrally been expressed in the abdomen and been required for more subtle morphogenetic processes. Selection for male segment reduction would then have brought wg, spi, emc and apoptosis under dual regulation of these proteins.
This raises the question of what selection pressure promoted male segment reduction and how it has remained a rigidly fixed trait within the Cyclorrhapha? Reduction in body size is a trend within the suborder Brachycera, which includes the Cyclorrhapha. Therefore, segment reduction may be viewed as an extreme example of this trait. However, it is also possible that male segment reduction evolved as a necessary adaptation for another Cyclorrhaphan synapomorphy, genital circumversion.
Peculiar among Diptera, the male genitalia of Cyclorrhapha undergo a 360° rotation during pupation or immediately after eclosion. This process, while retaining the dorsoventral orientation of the genitalia, produces abdominal ventroflexion allowing mating to occur with males positioned dorsally, rather than tail-to-tail as in most Diptera. Reduction of male posterior segmentation probably allows necessary abdominal flexion during mating and is therefore under strong stabilizing selection.
Acknowledgments
I thank all members of my lab, past and present, for their contributions to this work. I am especially indebted to W. Wang for his hard work and determination. I thank G. Struhl for insightful discussions and interpretations on segment compartment fate transformation. This work was supported by a grant from the National Science Foundation (IOS-0919891).
References
- Wang W, Kidd BJ, Carroll SB, Yoder JH. Sexually dimorphic regulation of the Wingless morphogen controls sex-specific segment number in Drosophila. Proc Natl Acad Sci U S A 2011; 108:11139 - 44; http://dx.doi.org/10.1073/pnas.1108431108; PMID: 21690416
- Wang W, Yoder JH. Hox-mediated regulation of doublesex sculpts sex-specific abdomen morphology in Drosophila. Dev Dyn 2012; 241:1076 - 90; http://dx.doi.org/10.1002/dvdy.23791; PMID: 22488883
- Madhavan MM, Madhavan K. Morphogenesis of the epidermis of adult abdomen of Drosophila. J Embryol Exp Morphol 1980; 60:1 - 31; PMID: 6796636
- Ninov N, Chiarelli DA, Martín-Blanco E. Extrinsic and intrinsic mechanisms directing epithelial cell sheet replacement during Drosophila metamorphosis. Development 2007; 134:367 - 79; http://dx.doi.org/10.1242/dev.02728; PMID: 17166923
- Ninov N, Manjón C, Martín-Blanco E. Dynamic control of cell cycle and growth coupling by ecdysone, EGFR, and PI3K signaling in Drosophila histoblasts. PLoS Biol 2009; 7:e1000079; http://dx.doi.org/10.1371/journal.pbio.1000079; PMID: 19355788
- Bischoff M, Cseresnyés Z. Cell rearrangements, cell divisions and cell death in a migrating epithelial sheet in the abdomen of Drosophila. Development 2009; 136:2403 - 11; http://dx.doi.org/10.1242/dev.035410; PMID: 19542353
- Struhl G, Barbash DA, Lawrence PA. Hedgehog acts by distinct gradient and signal relay mechanisms to organise cell type and cell polarity in the Drosophila abdomen. Development 1997; 124:2155 - 65; PMID: 9187142
- Struhl G, Barbash DA, Lawrence PA. Hedgehog organises the pattern and polarity of epidermal cells in the Drosophila abdomen. Development 1997; 124:2143 - 54; PMID: 9187141
- Kopp A, Duncan I. Control of cell fate and polarity in the adult abdominal segments of Drosophila by optomotor-blind. Development 1997; 124:3715 - 26; PMID: 9367427
- Pitsouli C, Perrimon N. Embryonic multipotent progenitors remodel the Drosophila airways during metamorphosis. Development 2010; 137:3615 - 24; http://dx.doi.org/10.1242/dev.056408; PMID: 20940225
- Kopp A, Blackman RK, Duncan I. Wingless, decapentaplegic and EGF receptor signaling pathways interact to specify dorso-ventral pattern in the adult abdomen of Drosophila. Development 1999; 126:3495 - 507; PMID: 10409497
- Kopp A, Muskavitch MA, Duncan I. The roles of hedgehog and engrailed in patterning adult abdominal segments of Drosophila. Development 1997; 124:3703 - 14; PMID: 9367426
- Shirras AD, Couso JP. Cell fates in the adult abdomen of Drosophila are determined by wingless during pupal development. Dev Biol 1996; 175:24 - 36; http://dx.doi.org/10.1006/dbio.1996.0092; PMID: 8608866
- Lawrence PA, Casal J, Struhl G. Towards a model of the organisation of planar polarity and pattern in the Drosophila abdomen. Development 2002; 129:2749 - 60; PMID: 12015301
- Lewis EB. A gene complex controlling segmentation in Drosophila. Nature 1978; 276:565 - 70; http://dx.doi.org/10.1038/276565a0; PMID: 103000
- Kopp A, Duncan I. Anteroposterior patterning in adult abdominal segments of Drosophila. Dev Biol 2002; 242:15 - 30; http://dx.doi.org/10.1006/dbio.2001.0529; PMID: 11795937
- Bender W, Akam M, Karch F, Beachy PA, Peifer M, Spierer P, et al. Molecular Genetics of the Bithorax Complex in Drosophila melanogaster. Science 1983; 221:23 - 9; http://dx.doi.org/10.1126/science.221.4605.23; PMID: 17737996
- Kopp A, Duncan I, Godt D, Carroll SB. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 2000; 408:553 - 9; http://dx.doi.org/10.1038/35046017; PMID: 11117736
- Williams TM, Selegue JE, Werner T, Gompel N, Kopp A, Carroll SB. The regulation and evolution of a genetic switch controlling sexually dimorphic traits in Drosophila. Cell 2008; 134:610 - 23; http://dx.doi.org/10.1016/j.cell.2008.06.052; PMID: 18724934
- Jeong S, Rokas A, Carroll SB. Regulation of body pigmentation by the Abdominal-B Hox protein and its gain and loss in Drosophila evolution. Cell 2006; 125:1387 - 99; http://dx.doi.org/10.1016/j.cell.2006.04.043; PMID: 16814723
- Jeong S, Rebeiz M, Andolfatto P, Werner T, True J, Carroll SB. The evolution of gene regulation underlies a morphological difference between two Drosophila sister species. Cell 2008; 132:783 - 93; http://dx.doi.org/10.1016/j.cell.2008.01.014; PMID: 18329365
- Wittkopp PJ, True JR, Carroll SB. Reciprocal functions of the Drosophila yellow and ebony proteins in the development and evolution of pigment patterns. Development 2002; 129:1849 - 58; PMID: 11934851
- Wiegmann BM, Yeates DK, Thorne JL, Kishino H. Time flies, a new molecular time-scale for brachyceran fly evolution without a clock. Syst Biol 2003; 52:745 - 56; PMID: 14668115
- Hildreth PE. Doublesex, A recessive gene that transforms both males and females of Drosophila into intersexes. Genetics 1965; 51:659 - 78; PMID: 14330702
- González-Crespo S, Morata G. Control of Drosophila adult pattern by extradenticle. Development 1995; 121:2117 - 25; PMID: 7635057
- Wang W, Yoder JH. Drosophila pupal abdomen immunohistochemistry. J Vis Exp 2011; •••:e3139; PMID: 21988937
- Hay BA, Wolff T, Rubin GM. Expression of baculovirus P35 prevents cell death in Drosophila. Development 1994; 120:2121 - 9; PMID: 7925015
- Merabet S, Hombria JC-G, Hu N, Pradel J, Graba Y. Hox-controlled reorganisation of intrasegmental patterning cues underlies Drosophila posterior spiracle organogenesis. Development 2005; 132:3093 - 102; http://dx.doi.org/10.1242/dev.01889; PMID: 15930099
- Bejsovec A, Martinez Arias A. Roles of wingless in patterning the larval epidermis of Drosophila. Development 1991; 113:471 - 85; PMID: 1782860
- Neumann CJ, Cohen SM. Distinct mitogenic and cell fate specification functions of wingless in different regions of the wing. Development 1996; 122:1781 - 9; PMID: 8674417
- Foronda D, Martín P, Sánchez-Herrero E. Drosophila Hox and Sex-Determination Genes Control Segment Elimination through EGFR and extramacrochetae Activity. PLoS Genet 2012; 8:e1002874; http://dx.doi.org/10.1371/journal.pgen.1002874; PMID: 22912593
- Lawrence PA, Casal J, Struhl G. Cell interactions and planar polarity in the abdominal epidermis of Drosophila. Development 2004; 131:4651 - 64; http://dx.doi.org/10.1242/dev.01351; PMID: 15329345
- Angelini DR, Kaufman TC. Functional analyses in the milkweed bug Oncopeltus fasciatus (Hemiptera) support a role for Wnt signaling in body segmentation but not appendage development. Dev Biol 2005; 283:409 - 23; http://dx.doi.org/10.1016/j.ydbio.2005.04.034; PMID: 15939417
- Yoder JH, Carroll SB. The evolution of abdominal reduction and the recent origin of distinct Abdominal-B transcript classes in Diptera. Evol Dev 2006; 8:241 - 51; http://dx.doi.org/10.1111/j.1525-142X.2006.00095.x; PMID: 16686635