Abstract
The FGFR pathway triggers a wide range of key biological responses. Among others, the Breathless (Btl, Drosophila FGFR1) receptor cascade promotes cell migration during embryonic tracheal system development. However, how the actin cytoskeleton responds to Btl pathway activation to induce cell migration has remained largely unclear. Our recent results shed light into this issue by unveiling a link between the actin-bundling protein Singed (Sn) and the Btl pathway. We showed that the Btl pathway regulates sn, which leads to the stabilization of the actin bundles required for filopodia formation and actin cytoskeleton rearrangement. This regulation contributes to tracheal migration, tracheal branch fusion and tracheal cell elongation. Parallel actin bundles (PABs) are usually cross-linked by more than one actin-bundling protein. Accordingly, we have also shown that sn synergistically interacts with forked (f), another actin crosslinker. In this Extra View we extend f analysis and hypothesize how both actin-bundling proteins may act together to regulate the PABs during tracheal embryonic development. Although both proteins are required for similar tracheal events, we suggest that Sn is essential for actin bundle initiation and stiffening, while F is required for the lengthening and further stabilization of the PABs.
Parallel Actin Bundles Organize Cortical Cell Protrusions
Parallel actin bundles (PABs) are present in a wide variety of cortical cell protrusions, such as the microvilli, microspikes, filopodia, invadopodia or podosomes. These cortical protrusions are stabilized by tightly packed filaments of actin that possess sufficient rigidity, but yet are flexible enough to be deformed.Citation1 PABs are formed by the addition of actin monomers to the barbed-plus ends of the filaments, which are bundled by the action of cell type specific crosslinkers. The nature and concentration of the actin-bundling proteins determine the degree of packing of the filament bundles,Citation1 thereby determining the specific type of protrusion in each cell type context.
For instance, brush border microvilli are finger-like projections emanating from the apical surfaces of certain absorptive epithelia. They increase the apical membrane extension and the epithelial absorption,Citation2 and they defend the surface area against pathogens.Citation3,4 Each microvillus is supported by a central core bundle of ∼19 actin filaments that are cross-linked by the two actin bundling proteins Fimbrin and Villin.Citation5,6 The core bundle contains also a small amount of a third actin-crosslinker, the Small Espin isoform,Citation7 which appears to accumulate only later during brush border assembly.Citation8
In contrast to the persistent microvilli, the non-protruding microspikes and protruding filopodia, podosomes or invadopodia are transient cell protrusions usually related to cell adhesion, migration or cell-cell interactions. Such protrusive structures, typically at the leading edge of motile cells, are also formed by parallel, unipolar bundles of actin that require the activity of several actin-crosslinkers. Besides motility and adhesion roles, invadopodia (present in many carcinoma cell types) and podosomes also possess the ability to degrade the extracellular matrix (ECM), providing the cell with capacity to extravasate and invade other tissues.Citation9-11 In contrast, filopodia are non-invasive thin finger-like structures with multiple roles in cell motility. Classically, filopodia have been proposed to act as guiding devices that explore the environment ahead of the lamellipodium, serving as sensors that can determine the direction of migration. Additionally, they have more recently been proposed to operate as mechanical devices at sites of signaling and adhesion with the surrounding ECM, generating the traction force required to move the cell body forward,Citation1,12 thus modifying cell morphology and cell function in response to extracellular stimuli.Citation13
The Actin-Bundling Protein Fascin Tightly Packs Actin Bundles
Fascin is the major actin bundling protein present in filopodia. This globular 55KDa protein contains two actin-binding sites that have been highly conserved throughout evolution.Citation14-18 Fascin-family proteins are present in a wide range of cell types, where they tightly pack PABs, providing sufficient strength to overcome the membrane resistance and protrude several microns from the leading edge of the cell.
In humans there are three Fascin isoforms. Fascin-2 and Fascin-3 are retina and testis specific.Citation19,20 In contrast, Fascin-1 is more widely expressed, but importantly it is only present at low levels in specific tissues of healthy adults (i.e., nervous system and highly migratory mesenchymal cellsCitation21,22). Fascin-1 appears clearly upregulated in several carcinoma cell lines, and this upregulation correlates with poor prognosis in cancer patients. It is proposed that Fascin-1 promotes motility and invasiveness in carcinoma cells, as Fascin-1 appears at the front of the tumors,Citation12 stabilizes the F-actin bundles of invadopodia, and potentiates protrusive invasion.Citation9,10 Therefore, Fascin-1 is currently considered a marker for some of the most aggressive cancersCitation23 and has become a potential therapeutic target. The Fascin specific inhibitor Migrastatin (and synthesized analogs) is assayed as a possible oncogenic drug.Citation24,25
Singed is the only Drosophila Fascin Homolog
The Drosophila genome encodes one single protein of the Fascin family, named Singed (sn). sn is expressed in several tissues and structures throughout fly development. During embryonic development sn is expressed and required in blood cells for the organization of microspikes during their migrationCitation26 and in tracheal cells for filopodia organization (see following section). During larval development sn is required in a specific subset of sensory neurons to establish the pattern of the actin-rich terminal branchlets of their dendrites.Citation27 In addition, sn is required during oogenesis and for adult fly bristle formation.
Cooperation between different actin-bundling proteins during PAB formation has been reported in Drosophila. During oogenesis sn plays a role in the process of dumping, by which the nurse cells empty their content into the oocyte. Dumping requires that filopodia-like structures hold the nuclei of the nurse cells in place during the process. In the absence of sn these actin-rich structures are not properly organized and as a result the nuclei block and collapse the ring canals, preventing proper dumping.Citation28 A similar phenotype is observed in the absence of a variety of actin-associated elements, including another actin-crosslinker, named Quail (Qua, Villin-like protein). It was found that sn and qua act in concert in the formation of the actin cables in nurse cells.Citation27 Similarly, during the formation of the neurosensory adult bristles, sn acts in concert with another actin-bundling protein, named Forked (F).Citation29 Bristles are curved and long protrusions that contain multiple parallel actin bundles positioned beneath the plasma membrane.Citation30 During bristle formation sn and f play critical roles in proper organization of this actin-based cytoskeleton, which consists of tightly packed actin bundle modules that are connected end-to-end.Citation31 Whereas in the absence of f the number of actin filaments per bundle decreases, in the absence of sn there are many actin filaments per bundle, but these are not hexagonally packed. Lack of both proteins leads to almost no actin filaments arranged into bundles,Citation30,31 giving rise to very short, thick and twisted bristles.
The Breathless/FGFR Pathway Positively Regulates Singed Tracheal Expression
The tracheal system is the respiratory organ of the fly and an excellent model system for the analysis of cell migration. During tracheal embryonic development, the Branchless (Bnl, a Drosophila FGF homolog)/Breathless (Btl, Drosophila FGFR1) pathway plays a prominent roleCitation32. Bnl was shown to act as a chemoattractant for the tracheal cells,Citation33 which express the FGF Receptor Btl. The cells at the tip of the branches receive the highest levels of Bnl, and thanks to a positive feed-back loop mediated by Ras-MAPK, maximally activate the pathway.Citation34 These tip cells which lead the collective migration of the branches toward the source of Bnl,Citation35 display a high migratory behavior and particular morphological properties. For instance, leading cells extend numerous filopodia which have been proposed to contribute to migration. However, the molecular mechanisms underlying this process are not well understood.Citation36
We have recently found that sn is specifically expressed in the leading cells of the tracheal branches and that this expression is positively regulated by the Btl/FGFR pathway.Citation37 To better understand how sn contributes to cell migration in vivo we have analyzed the requirements for sn in tracheal cells.
Functional Analysis of Singed during Tracheal Development
We focused our analysis on a particular type of tracheal branches, the so-called dorsal branches (DBs).Citation37 Our functional study indicated that sn is required in leading cells, which accumulate high levels of Sn in their cytoplasm and in cellular protrusions. We found that sn is required for the guided and timely migration of DBs, since in sn mutants the DBs are often missguided and their extension is delayed. The two tip cells of the DBs are, under normal conditions, highly specialized and accomplish specific activities. One of them (the fusion cell) mediates the fusion of tracheal branches and the other one (the terminal cell) extends a terminal branch.Citation32 We found that sn is also required for the correct activity of these tip cells, since in sn mutants both branch fusion and terminal branching are compromised ().
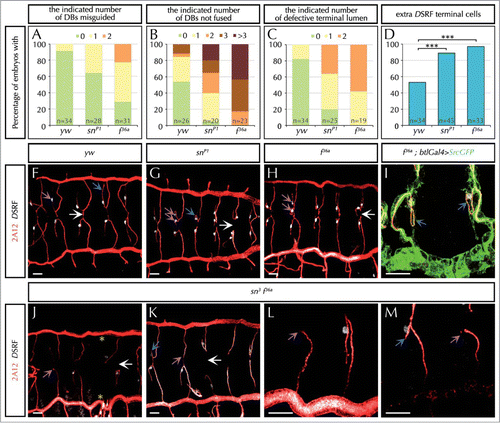
Figure 1. The actin-bundling protein Forked is required during tracheal system development. (A–D) Percentage of embryos with the indicated number (in different colors) of dorsal branches (DBs) misguided (A), unfused DBs (B), terminal intracellular lumen misguided or bifurcated (C), or with extra DSRF-terminal cells (D). n is the total number of fixed embryos analyzed. Note that f mutant embryos (f36 FBal0003950) display stronger defects than sn mutants (snP1. FBal0035641) in all aspects analyzed. ***P < 0,001 analyzed by Fisher's exact test (http://www.langsrud.com/fisher.htm#INTRO). (E–G) Confocal projections of fixed control (yw) (E), snP1 (F) and f36a (G) embryos at the end of embryogenesis stained with the luminal marker 2A12 (red) that labels the tracheal tree and the terminal cell marker DSRF (white). (H) Close-up of two DBs of a f36a; btlGal4 UAS-srcGFP mutant labeled with 2A12, DSRF and GFP (which highlights cell morphology due to the membrane marker Src fused to GFP under the control of the breathless (btl)-Gal4 tracheal driver). (I–L) Confocal projections of fixed sn3f36a embryos (FBst0306268) stained with 2A12 (red) and DSRF (white). (K–L) are close-ups of two DBs of different embryos. White arrows point to the presumptive point of fusion between contralateral DBs at the dorsal midline. Blue arrows point to the intracellular terminal lumen of terminal branches. Note that terminal lumina correctly extends ventrally in control embryos (E), but often turn dorsally (F and H) or hardly extend (J and L) in mutant conditions. Purple arrows point to the individual (E), extra (F-H) or missing (I-L) DSRF-expressing cells of DB in different conditions. Yellow asterisks (I) indicate missing DBs. In all cases yw was used as the control. Scale Bar: 15 μm.
Singed is Required for the Organization of the Actin Cytoskeleton in the Leading Cells
To better understand these effects we analyzed in detail sn requirements at single cell resolution. We found clear and reproducible defects in filopodia and morphology of sn mutants tip cells. While in control embryos DB tip cell filopodia were long, straight and stiff and the terminal cells extended organized cell fronts, in sn mutants filopodia were curved and bent with an apparent flaccid aspect and the cell fronts were typically irregular (). To better characterize these defects we quantified filopodia number and length in fixed tissue and compared control and sn mutants. We detected around 17 filopodia per cell, typically long (∼4,4 μm) and conspicuously straight in appearance in control embryos. sn mutants displayed a slight but stastistically significant lower number of filopodia but with a normal length (∼13,5 filopodia/terminal cell) ().
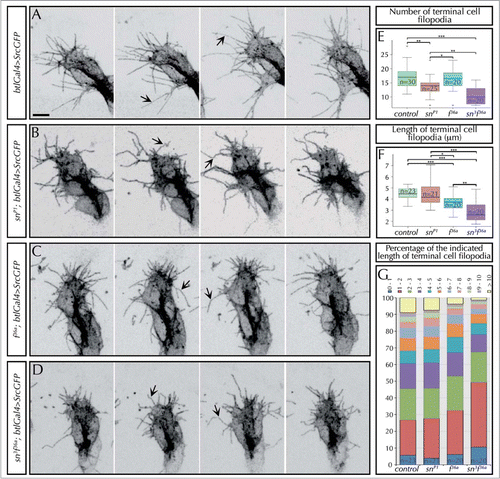
Figure 2. Singed and Forked control the number, length and stiffness of tracheal filopodia. (A–D) Stills from in-vivo movies taken approximately every 10 s of embryos carrying btlGal4 > SrcGFP (which allows to clearly visualize the morphology and dynamics of filopodia) in an otherwise wild-type (A), snP1 (B), f36a (C) or sn3f36a (D) backgrounds. The images show the tip of DBs of stage 14–15 embryos. Arrows point to straight (in control, A) or curved filopodia (in sn, f or snf mutants). Note also the presence of conspicuously shorter filopodia in f or snf mutants. Scale Bar: 5 μm. (E) Quantification of the number of filopodia in the terminal cells of DBs of fixed embryos at stage 16 carrying btlGal4 > SrcGFP in a control or, snP1, f36a and sn3f36a mutant background. Note the decrease in the number of filopodia in sn mutants, as well as sn f double mutants. *P < 0,05; **P < 0,01; ***P < 0,001 were obtained using two-tail t test of R-Commander software. n = number of terminal cells analyzed. (F) Quantification of the length (in microns) of filopodia in the terminal cells of DBs of fixed embryos at stage 16 carrying btlGal4 > SrcGFP in a control or, snP1, f36a and sn3f36a mutant background. f terminal cell filopodia are significantly shorter than control and sn mutant ones. sn f terminal cell filopodia are much shorter than the previous cases. *P < 0,05; **P < 0,01; ***P < 0,001 were obtained using two-tail t test of R-Commander software. n = number of terminal cells analyzed. (G) Distribution of the length of filopodia of terminal cells (represented in different color/micron) in control, snP1, f36a and sn3f36a fixed embryos at stage 16. Note that in sn f mutants almost 50% of filopodia are shorter than 2 μm and hardly any filopodia extend longer than 10 μm. Typically, long filopodia (> 10 μm) are associated with the ventral cytoplasmic extension that generates the terminal branch. n = number of terminal cells analyzed. In all cases btlGal4 > SrcGFP was used as control.
To better characterize sn requirements we analyzed actin organization in tracheal leading cells. A first analysis of wild type embryos revealed the technical difficulties to visualize actin rearrangements due to the small size of leading cells and to the fast development of the tracheal system. Nevertheless, an analysis with high temporal and spatial resolution allowed us to visualize the organized movement of tracks of actin toward the cell edge during migration, forming the lamellipodia. We also detected non-protruding actin bundles and actin-pools, which accumulated toward the direction of migration, fusion or terminal cell elongation during stages 14 and 15. In addition, we also detected bundles of actin perpendicular to the membrane and protruding beyond the cell edge, forming the filopodia. In contrast, lack of sn generated a disorganized movement of actin pools that barely formed a continuous lamellipodia in the deformed cell edge, neither long and thick bundles of intracellular actin. Therefore we concluded that sn acts in tracheal leading cells as a link between the Bnl/FGF signal and the actin cytoskeleton.Citation37
Forked is Required for Tracheal Formation
As already mentioned, most cortical cell protrusions based on actin-bundles require at least two actin-crosslinkers to maximise the bundle packing.Citation38 This is true for the neurosensory bristles (with f and sn) and filopodia-like cables of nurse cells (with qua and sn) in Drosophila, for microvilli (with Fimbrin and Villin), and also for hair cell stereocilia (with Fimbrin, Espin and FascinCitation38,39) (for a review see ref.Citation40). Hence, we investigated the possible requirements of other actin cross-linkers in tracheal development.
qua (villin-like) single mutants did not show reproducible tracheal phenotypes. When tested in the absence of sn, we did not detect any clear genetic interaction.
In contrast, f mutant embryos displayed tracheal defects that were similar, and even more penetrant, than the ones found in sn mutants. In particular, we detected a high-percentage of DB missguidances (in 71% of f mutants, compared with 36% of sn mutant embryos or 9% in the wild type) (). The fusion of the contralateral DBs at the end of embryogenesis was also compromised in all f embryos analyzed (in 100% of f embryos, vs. 58% of control embryos, and 95% of sn mutants) ( and white arrow in ). Lumen guidance inside the terminal cells was also strongly affected in f mutants (100% of f mutants displayed missguided terminal lumina, compared with 16% in the control, and 80% in sn mutants) ( and blue arrows in ). Occasionally, this terminal lumen was also shorter (), in spite of an apparent normal extension of the terminal cell. Finally, we also found an excess of terminal-DSRF positive cells (in 97% of f mutants compared with 53% of control embryos, and 89% of sn mutants) ( and purple arrow in ).
Given the similarities between sn and f tracheal requirements, and the fact that both are actin-bundling proteins, we speculated that F would also contribute to organize the actin cytoskeleton. To evaluate this possibility we analyzed tip cell morphology and filopodia and compared the results to sn mutants. In vivo analysis of f mutants using the Src membrane marker confirmed that the cell fronts of the leading cells were as defective and irregular as in sn mutants () and that when filopodia were long enough, these also seemed weakly bent and curved. However, we also observed that f filopodia were shorter than those of control and sn mutants (). These differences in filopodia defects between sn and f mutants, suggested differences in the molecular and cellular mechanisms of Sn and F. To confirm this observation we analyzed and quantified filopodia parameters in fixed tissue. Interestingly, we found that f mutants have a normal number of filopodia but shorter than in the control (∼3,6 μm vs. ∼4,4 μm of control terminal filopodia) or sn mutants, while sn mutants have less filopodia but with a normal length (). These results show that although these two genes are required for filopodia formation, they act differently.
Singed and Forked Interact during Filopodia Formation
To better characterize the contribution of sn and f to filopodia formation, we also analized tracheal filopodia in sn f double mutants. In-vivo analysis showed extremely irregular cell shapes with apparently inefficient cell fronts from where only a few and short filopodia protrude (). To have a more accurate measure of these defects we quantified filopodia parameters in fixed embryos and compared them to those of sn or f single mutants. This analysis corroborated that the number and length of filopodia was differently affected in the three mutant conditions: Sn affects the number of filopodia, f mutants display shorter filopodia, and sn f mutants extend shorter and fewer filopodia ().
We also analyzed the consequences of sn f absence at the tracheal tissue level in double mutants. We detected a synergisitic genetic interaction between the two genes as new tracheal phenotypes were observed (). sn f double mutants lacked some branches, in particular some DBs (), and also lacked DSRF-expressing terminal cells in the formed DBs (). This lack of terminal cells is particularly intriguing since each mutant on its own produced an increase in the number of terminal cells (). We can propose different explanations for such observations.Citation37 On the one hand, we speculate that terminal cells cannot receive the Bnl signal and correctly respond to it by expressing DSRF due to delayed migration (caused by inefficient filopodia) or to an impaired reception of the signal (in case the tracheal filopodia act as chemosensitive organelles by carrying Btl receptorsCitation41). Alternatively, the abnormal specification of the terminal cells could be due to the defective actin-rearrangements, as it has been described that actin can modulate the expression of the terminal determinant DSRF.Citation42,43 In this case, the disorganized actin-cytoskeleton in terminal cells could also explain the short intracellular lumen inside the correctly specified terminal cells found in sn f mutants (blue arrows in ).
From our analysis of single and double mutants we draw several conclusions. First, since we find that in the absence of both sn and f filopodia can still form, we suggest that another actin-bundling protein may also participate in the process. Second, we find that while the morphogenetic defects found in sn and f mutants are very similar, and though both proteins are proposed to act as actin cross-linkers, our analysis at the cellular level reveals that they affect filopodia formation differently, suggesting that they play different molecular roles. In the following section we elaborate on this aspect and propose a model of how each protein can contribute to filopodia organization.
Model for Singed and Forked Function in Filopodia Formation
Tilney, Guild and colleagues put forward a model for Sn and F during actin organization in Drosophila bristle formation. They proposed that in a first step a yet unidentified actin cross-linker forms small and disordered actin bundlets. Subsequently, F aggregates them into loosely ordered modules. In a third step F also facilitates Sn entry into the bundles, and Sn in turn displaces F and tightly organizes the bundles into hexagonally packed structures that become straight and stiff. Finally, the different actin modules that form each bundle are glued by actin filaments that are held by F (for a review see Ref.Citation44). However, filopodia are not formed by actin modules. This opens the question of what is the exact role of each actin cross-linker in the organization of PAB during filopodia formation.
Here, we propose a model for the role of each protein during filopodia formation based on our results. We propose that Sn acts in the first step of filopodia formation while F acts later facilitating filopodia elongation. In addition, the binding of each protein along the PAB stabilizes the bundle. Together both proteins control the correct number, shape, stiffness and length of the filopodia.
The fact that in sn mutants the number of filopodia decreases may indicate that sn is required at the initial steps of filopodia formation, in the region of the lamellipodia or the lamellae. This is in agreement with several observations we made, including the cytoplasmic localization of both the active and inactive bundling forms of Sn, and the disorganization of the cytoplasmic actin-cytoskeleton in the mutants (including the partial loss of cytoplasmic microspikes or actin-bundles).Citation37 Previously published data also supports this hypothesis: 1) the recruitment or activation of fascin to the VASP clustered barbed ends of the filament precursors (called Λ-precursors) initiate filament bundling and allow the growth of the nascent filopodiumCitation45,46; 2) sn is required for the formation of cytoplasmic actin cables such as those of the nurse cellsCitation26,28; 3) sn is also found in branched filaments in the lamellipodia, not only in parallel bundles.Citation47
In contrast to sn, f mutant filopodia are shorter. This would fit with a model in which F facilitates actin monomer addition, to compensate for actin monomer elimination at the minus end during treadmilling, either by acting directly on actin polymerization or by stabilizing the bundles thereby preventing depolymerization. In agreement with this hypothesis it is interesting to point out the similarity of F to the Espin family of actin-bundling proteins. In particular, there is a 39% homology between F and a 66aa peptide at the Espin C-terminus, which corresponds to the actin-bundling module (ABM) and to the ankyrin-repeat motive.Citation48 The ABM is necessary and sufficient for the lengthening of the parallel actin-bundles in microvilli. Mechanistically, it is suggested that Espin provides to the initial short microvillar PABs a net barbed-end elongation of treadmilling actin filaments, giving rise to longer bundles without joining shorter modules.Citation7 Additionally it has been reported that the magnitude of the PAB lengthening is dependent on the level of espin expression.Citation49 It is also possible that F has an uncapping function in filopodia, in such a way that in its absence the filaments are capped at their barbed ends and then the constant actin depolymerisation leads to filopodia dismantling.
Taken together we propose that during tracheal filopodia formation Sn acts first (before F, in contrast to bristle formation) probably just after Ena/VASP initiate the filopodia precursorsCitation46 to initiate the first bundling of the actin filaments and to generate the first packing of the PABs. Thus, in the absence of sn less filopodia form, and as the bundles are not tightly organized, these filopodia are wavy and bent. F acts later on the initial and short filaments of actin, providing a maintained barbed-end elongation of actin, allowing for filopodia length increase. Hence, f mutant filopodia are shorter, in agreement with shorter microvilli, stereocillia and bristles in espin and f mutants.Citation7,49,50 These reports also showed that f and espin mutants have a decreased number of filaments giving rise to thinner protrusions. Our imaging resolution level does not allow us to address this aspect in tracheal filopodia; however, fewer actin filaments could contribute to the weak bending of filopodia observed. In sn f double mutants the first actin-bundling step would be compromised. Later, those PABs that successfully form would soon have an actin treadmilling displaced toward filament de-polymerisation and final reabsorption. Thus, only very few and short filaments would form under these conditions.
In summary, we propose that Sn and F together are necessary to bundle and elongate several filaments of actin and give them enough strength to protrude and elongate several microns from the cell edge generating straight and long filopodia. Stiff filopodia provide the cell with the trailing force to move forward and to change shape in response to the signals at play.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank members of the Llimargas and Casanova labs for helpful discussions and S. Araújo for critically reading the manuscript.
Funding
P.O-R. is supported by a FPI fellowship from Ministerio de Ciencia e Innovación. This work was supported by funds from the Ministerio de Ciencia e Innovación to M.L (BFU2006–09515/BMC, BFU2009–09041) and from Programme Consolider 2007 (CSD2007–00008) project.
References
- Mogilner A, Rubinstein B. The physics of filopodial protrusion. Biophys J 2005; 89:782-95; PMID:15879474; http://dx.doi.org/10.1529/biophysj.104.056515
- Heintzelman MB, Mooseker MS. Assembly of the intestinal brush border cytoskeleton. Curr Top Dev Biol 1992; 26:93-122; PMID:1563281; http://dx.doi.org/10.1016/S0070-2153(08)60442-1
- Zhou D, Mooseker MS, Galán JE. An invasion-associated Salmonella protein modulates the actin-bundling activity of plastin. [Internet]. Proc Natl Acad Sci U S A 1999; 96:10176-81; PMID:10468582; http://dx.doi.org/10.1073/pnas.96.18.10176
- Iizumi Y, Sagara H, Kabe Y, Azuma M, Kume K, Ogawa M, Nagai T, Gillespie PG, Sasakawa C, Handa H. The enteropathogenic E. coli effector EspB facilitates microvillus effacing and antiphagocytosis by inhibiting myosin function. Cell Host Microbe 2007; 2:383-92; PMID:18078690; http://dx.doi.org/10.1016/j.chom.2007.09.012
- Brown JW, McKnight CJ. Molecular model of the microvillar cytoskeleton and organization of the brush border. PLoS One 2010; 5:e9406; PMID:20195380; http://dx.doi.org/10.1371/journal.pone.0009406
- Mooseker MS. Actin binding proteins of the brush border. [Internet]. Cell 1983; 35:11-3; PMID:6313218; http://dx.doi.org/10.1016/0092-8674(83)90202-7
- Loomis PA, Zheng L, Sekerková G, Changyaleket B, Mugnaini E, Bartles JR. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J Cell Biol 2003; 163:1045-55; PMID:14657236; http://dx.doi.org/10.1083/jcb.200309093
- Bartles JR, Zheng L, Li A, Wierda A, Chen B. Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J Cell Biol 1998; 143:107-19; PMID:9763424; http://dx.doi.org/10.1083/jcb.143.1.107
- Li A, Dawson JC, Forero-Vargas M, Spence HJ, Yu X, König I, Anderson K, Machesky LM. The actin-bundling protein fascin stabilizes actin in invadopodia and potentiates protrusive invasion. Curr Biol 2010; 20:339-45; PMID:20137952; http://dx.doi.org/10.1016/j.cub.2009.12.035
- Machesky LM, Li A. Fascin: Invasive filopodia promoting metastasis. Commun Integr Biol 2010; 3:263-70; PMID:20714410; http://dx.doi.org/10.4161/cib.3.3.11556
- Quintavalle M, Elia L, Condorelli G, Courtneidge SA. MicroRNA control of podosome formation in vascular smooth muscle cells in vivo and in vitro. J Cell Biol 2010; 189:13-22; PMID:20351064; http://dx.doi.org/10.1083/jcb.200912096
- Hashimoto Y, Skacel M, Adams JC. Roles of fascin in human carcinoma motility and signaling: prospects for a novel biomarker? Int J Biochem Cell Biol 2005; 37:1787-804; PMID:16002322; http://dx.doi.org/10.1016/j.biocel.2005.05.004
- Khurana S, George SP. The role of actin bundling proteins in the assembly of filopodia in epithelial cells. Cell Adh Migr 2011; 5:409-20; PMID:21975550; http://dx.doi.org/10.4161/cam.5.5.17644
- Kane RE. Preparation and purification of polymerized actin from sea urchin egg extracts. [Internet]. J Cell Biol 1975; 66:305-15; http://www.ncbi.nlm.nih.gov/pubmed/7202107; PMID:1095598; http://dx.doi.org/10.1083/jcb.66.2.305
- Otto JJ, Kane RE, Bryan J. Formation of filopodia in coelomocytes: localization of fascin, a 58,000 dalton actin cross-linking protein. [Internet]. Cell 1979; 17:285-93; http://www.ncbi.nlm.nih.gov/pubmed/378407; PMID:378407; http://dx.doi.org/10.1016/0092-8674(79)90154-5
- Bryan J, Edwards R, Matsudaira P, Otto J, Wulfkuhle J. Fascin, an echinoid actin-bundling protein, is a homolog of the Drosophila singed gene product. [Internet]. Proc Natl Acad Sci U S A 1993; 90:9115-9; http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=47512&tool=pmcentrez&rendertype=abstract; PMID:8415664; http://dx.doi.org/10.1073/pnas.90.19.9115
- Duh FM, Latif F, Weng Y, Geil L, Modi W, Stackhouse T, Matsumura F, Duan DR, Linehan WM, Lerman MI, et al. cDNA cloning and expression of the human homolog of the sea urchin fascin and Drosophila singed genes which encodes an actin-bundling protein. [Internet]. DNA Cell Biol 1994; 13:821-7; http://www.ncbi.nlm.nih.gov/pubmed/8068206; PMID:8068206; http://dx.doi.org/10.1089/dna.1994.13.821
- Jansen S, Collins A, Yang C, Rebowski G, Svitkina T, Dominguez R. Mechanism of actin filament bundling by fascin. J Biol Chem 2011; 286:30087-96; PMID:21685497; http://dx.doi.org/10.1074/jbc.M111.251439
- Saishin Y, Ishikawa R, Ugawa S, Guo W, Ueda T, Morimura H, Kohama K, Shimizu H, Tano Y, Shimada S. Retinal fascin: functional nature, subcellular distribution, and chromosomal localization. [Internet]. Invest Ophthalmol Vis Sci 2000; 41:2087-95; PMID:10892848
- Tubb B, Mulholland DJ, Vogl W, Lan Z-J, Niederberger C, Cooney A, Bryan J. Testis fascin (FSCN3): a novel paralog of the actin-bundling protein fascin expressed specifically in the elongate spermatid head. [Internet]. Exp Cell Res 2002; 275:92-109; PMID:11925108; http://dx.doi.org/10.1006/excr.2002.5486
- De Arcangelis A, Georges-Labouesse E, Adams JC. Expression of fascin-1, the gene encoding the actin-bundling protein fascin-1, during mouse embryogenesis. Gene Expr Patterns 2004; 4:637-43; PMID:15465486; http://dx.doi.org/10.1016/j.modgep.2004.04.012
- Zhang F-R, Tao L-H, Shen Z-Y, Lv Z, Xu L-Y, Li E-M. Fascin expression in human embryonic, fetal, and normal adult tissue. J Histochem Cytochem 2008; 56:193-9; PMID:17998567; http://dx.doi.org/10.1369/jhc.7A7353.2007
- Tan VY, Lewis SJ, Adams JC, Martin RM. Association of fascin-1 with mortality, disease progression and metastasis in carcinomas: a systematic review and meta-analysis. BMC Med 2013; 11:52; PMID:23442983; http://dx.doi.org/10.1186/1741-7015-11-52
- Majchrzak K, Lo Re D, Gajewska M, Bulkowska M, Homa A, Pawłowski K, Motyl T, Murphy PV, Król M. Migrastatin analogues inhibit canine mammary cancer cell migration and invasion. PLoS One 2013; 8:e76789; PMID:24116159; http://dx.doi.org/10.1371/journal.pone.0076789
- Chen L, Yang S, Jakoncic J, Zhang JJ, Huang X-Y. Migrastatin analogues target fascin to block tumour metastasis. Nature 2010; 464:1062-6; PMID:20393565; http://dx.doi.org/10.1038/nature08978
- Huelsmann S, Ylänne J, Brown NH. Filopodia-like actin cables position nuclei in association with perinuclear actin in Drosophila nurse cells. Dev Cell 2013; 26:604-15; PMID:24091012; http://dx.doi.org/10.1016/j.devcel.2013.08.014
- Cant K, Knowles BA, Mahajan-Miklos S, Heintzelman M, Cooley L. Drosophila fascin mutants are rescued by overexpression of the villin-like protein, quail. J Cell Sci 1998; 111:213-21; PMID:9405306
- Cant K, Knowles BA, Mooseker MS, Cooley L. Drosophila singed, a fascin homolog, is required for actin bundle formation during oogenesis and bristle extension. [Internet]. J Cell Biol 1994; 125:369-80; PMID:8163553; http://dx.doi.org/10.1083/jcb.125.2.369
- DeRosier DJ, Tilney LG. How actin filaments pack into bundles. [Internet]. Cold Spring Harb Symp Quant Biol 1982; 46:525-40; PMID:6955098; http://dx.doi.org/10.1101/SQB.1982.046.01.049
- Lewis G, Tilney MS, Guild GM. F actin bundles in Drosophila bristles. I. Two filament cross-links are involved in bundling. J Cell Biol 1995; 130:629-38; PMID:7622563; http://dx.doi.org/10.1083/jcb.130.3.629
- Tilney LG, Connelly P, Smith S, Guild GM. F-actin bundles in Drosophila bristles are assembled from modules composed of short filaments. [Internet]. J Cell Biol 1996; 135:1291-308; PMID:8947552; http://dx.doi.org/10.1083/jcb.135.5.1291
- Ghabrial A, Luschnig S, Metzstein MM, Krasnow MA. Branching morphogenesis of the Drosophila tracheal system. Annu Rev Cell Dev Biol 2003; 19:623-47; PMID:14570584; http://dx.doi.org/10.1146/annurev.cellbio.19.031403.160043
- Sutherland D, Samakovlis C, Krasnow MA. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 1996; 87:1091-101; PMID:8978613; http://dx.doi.org/10.1016/S0092-8674(00)81803-6
- Ohshiro T, Emori Y, Saigo K. Ligand-dependent activation of breathless FGF receptor gene in Drosophila developing trachea. [Internet]. Mech Dev 2002; 114:3-11; PMID:12175485; http://dx.doi.org/10.1016/S0925-4773(02)00042-4
- Ribeiro C, Ebner A, Affolter M. In vivo imaging reveals different cellular functions for FGF and Dpp signaling in tracheal branching morphogenesis. [Internet]. Dev Cell 2002; 2:677-83; PMID:12015974; http://dx.doi.org/10.1016/S1534-5807(02)00171-5
- Petit V, Ribeiro C, Ebner A, Affolter M. Regulation of cell migration during tracheal development in Drosophila melanogaster. [Internet]. Int J Dev Biol 2002; 46:125-32; PMID:11902673
- Okenve-Ramos P, Llimargas M. Fascin links Btl/FGFR signalling to the actin cytoskeleton during Drosophila tracheal morphogenesis. Development 2014; 141:929-39; PMID:24496629; http://dx.doi.org/10.1242/dev.103218
- Tilney LG, Tilney MS, DeRosier DJ. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu Rev Cell Biol 1992; 8:257-74; PMID:1476800; http://dx.doi.org/10.1146/annurev.cb.08.110192.001353
- Shin J-B, Longo-Guess CM, Gagnon LH, Saylor KW, Dumont RA, Spinelli KJ, Pagana JM, Wilmarth PA, David LL, Gillespie PG, et al. The R109H variant of fascin-2, a developmentally regulated actin crosslinker in hair-cell stereocilia, underlies early-onset hearing loss of DBA/2J mice. J Neurosci 2010; 30:9683-94; PMID:20660251; http://dx.doi.org/10.1523/JNEUROSCI.1541-10.2010
- Bartles JR. Parallel actin bundles and their multiple actin-bundling proteins. [Internet]. Curr Opin Cell Biol 2000; 12:72-8; PMID:10679353; http://dx.doi.org/10.1016/S0955-0674(99)00059-9
- Roy S, Hsiung F, Kornberg TB. Specificity of Drosophila cytonemes for distinct signaling pathways. Science 2011; 332:354-8; PMID:21493861; http://dx.doi.org/10.1126/science.1198949
- Miralles F, Posern G, Zaromytidou AI, Treisman R. Actin dynamics control SRF activity by regulation of its coactivator MAL. Cell 2003; 113:329-42; PMID:12732141; http://dx.doi.org/10.1016/S0092-8674(03)00278-2
- Somogyi K, Rørth P. Evidence for tension-based regulation of Drosophila MAL and SRF during invasive cell migration. [Internet]. Dev Cell 2004; 7:85-93; PMID:15239956; http://dx.doi.org/10.1016/j.devcel.2004.05.020
- Tilney LG, DeRosier DJ. How to make a curved Drosophila bristle using straight actin bundles. [Internet]. Proc Natl Acad Sci U S A 2005; 102:18785-92; PMID:16357198; http://dx.doi.org/10.1073/pnas.0509437102
- Svitkina TM, Bulanova EA, Chaga OY, Vignjevic DM, Kojima S, Vasiliev JM, Borisy GG. Mechanism of filopodia initiation by reorganization of a dendritic network. J Cell Biol 2003; 160:409-21; PMID:12566431; http://dx.doi.org/10.1083/jcb.200210174
- Winkelman JD, Bilancia CG, Peifer M, Kovar DR. Ena/VASP Enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. [Internet]. Proc Natl Acad Sci U S A 2014; 111:4121-6; PMID:24591594; http://dx.doi.org/10.1073/pnas.1322093111
- Tseng Y, Fedorov E, McCaffery JM, Almo SC, Wirtz D. Micromechanics and ultrastructure of actin filament networks crosslinked by human fascin: a comparison with alpha-actinin. J Mol Biol 2001; 310:351-66; PMID:11428894; http://dx.doi.org/10.1006/jmbi.2001.4716
- Bartles JR, Zheng L, Li A, Wierda A, Chen B. Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. [Internet]. J Cell Biol 1998; 143:107-19; PMID:9763424; http://dx.doi.org/10.1083/jcb.143.1.107
- Sekerková G, Richter C-P, Bartles JR. Roles of the espin actin-bundling proteins in the morphogenesis and stabilization of hair cell stereocilia revealed in CBA/CaJ congenic jerker mice. PLoS Genet 2011; 7:e1002032; PMID:21455486; http://dx.doi.org/10.1371/journal.pgen.1002032
- Tilney LG, Connelly PS, Ruggiero L, Vranich KA, Guild GM. Actin filament turnover regulated by cross-linking accounts for the size, shape, location, and number of actin bundles in Drosophila bristles. Mol Biol Cell 2003; 14:3953-66; PMID:14517310; http://dx.doi.org/10.1091/mbc.E03-03-0158
