Abstract
Evolution of resistance by pests can reduce the benefits of transgenic crops that produce toxins from Bacillus thuringiensis (Bt) for insect control. One of the world’s most important cotton pests, pink bollworm (Pectinophora gossypiella), has been targeted for control by transgenic cotton producing Bt toxin Cry1Ac in several countries for more than a decade. In China, the frequency of resistance to Cry1Ac has increased, but control failures have not been reported. In western India, pink bollworm resistance to Cry1Ac has caused widespread control failures of Bt cotton. By contrast, in the state of Arizona in the southwestern United States, monitoring data from bioassays and DNA screening demonstrate sustained susceptibility to Cry1Ac for 16 y. From 1996–2005, the main factors that delayed resistance in Arizona appear to be abundant refuges of non-Bt cotton, recessive inheritance of resistance, fitness costs associated with resistance and incomplete resistance. From 2006–2011, refuge abundance was greatly reduced in Arizona, while mass releases of sterile pink bollworm moths were made to delay resistance as part of a multi-tactic eradication program. Sustained susceptibility of pink bollworm to Bt cotton in Arizona has provided a cornerstone for the pink bollworm eradication program and for integrated pest management in cotton. Reduced insecticide use against pink bollworm and other cotton pests has yielded economic benefits for growers, as well as broad environmental and health benefits. We encourage increased efforts to combine Bt crops with other tactics in integrated pest management programs.
Introduction
Crops genetically engineered to produce insecticidal proteins from the bacterium Bacillus thuringiensis (Bt) kill some key pests and help to reduce reliance on insecticide sprays.Citation1,Citation2 Such Bt crops were commercialized in 1996 and covered more than 58 million hectares worldwide in 2010.Citation3 The primary threat to the continued success of Bt crops is evolution of resistance by pests.Citation4,Citation5 Field-evolved (= field-selected) resistance entails a genetically based decrease in susceptibility of a population to a toxin caused by exposure of the population to the toxin in the field.Citation6,Citation7 Although many target pest populations remain susceptible, some degree of field-evolved resistance to Bt crops has been reported in at least seven species of major pests.Citation6-Citation17 Field-evolved resistance to Bt toxins has caused field control failures in some, but not all cases.Citation6-Citation17
Here we focus on resistance to Bt cotton in the pink bollworm (Pectinophora gossypiella), one of the world’s most devastating pests of cotton. First-generation Bt cotton produces only one Bt toxin called Cry1Ac.Citation7 We will use the term “Bt cotton” to refer to such cotton, unless noted otherwise. Monitoring data from three countries reveals three different outcomes for pink bollworm resistance to Bt cotton. The worst outcome has occurred in western India, where pink bollworm evolved resistance resulting in widespread field control failures of Bt cotton in 2009.Citation10,Citation13 The situation is more positive in China, where the frequency of resistance to Cry1Ac increased significantly in 2008–2010 compared with 2005–2007, but the highest percentage of resistant individuals detected in a pink bollworm population during 2010 was 6%.Citation16 The most positive outcome has been documented in the state of Arizona in the southwestern United States, where pink bollworm susceptibility to Cry1Ac has not decreased after 16 y of exposure to Bt cotton. Here we review the success of Bt cotton against pink bollworm in Arizona.
Pink Bollworm and Bt Cotton in Arizona
In Arizona, Bt cotton kills essentially 100% of susceptible pink bollworm larvae.Citation18 Nonetheless, our initial work indicated a high risk of pink bollworm evolving resistance to Bt cotton in Arizona.Citation19 In the lab, selection with Cry1Ac in diet quickly yielded resistance to Bt cotton in several strains of pink bollworm derived from the field.Citation18,Citation20 In Arizona, the larvae of pink bollworm feed almost exclusively on cotton and more than half of the state’s cotton has been planted to Bt cotton since 1997Citation21 (). Thus, the pink bollworm has the genetic potential to evolve resistance and has been under intense selection for resistance in the field.
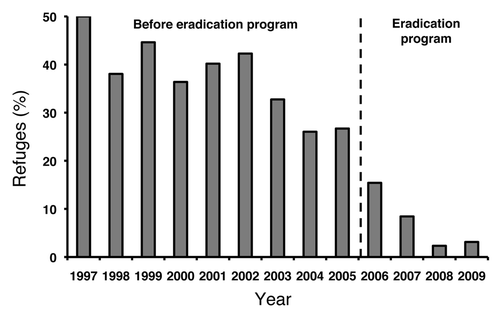
Figure 1. The percentage of cotton planted to non-Bt cotton refuges in Arizona from 1997 to 2009.Citation21
Although pink bollworm evolved resistance to Bt cotton in about nine years in India,Citation13,Citation17 it has remained susceptible in Arizona after 16 years. Below we review the monitoring data demonstrating sustained susceptibility and the key factors delaying pink bollworm resistance to Bt cotton in Arizona, which seem to be refuges of non-Bt cotton from 1996 to 2005, and mass releases of sterile pink bollworm moths from 2006 to 2011. Also, as summarized below, the pink bollworm eradication program using Bt cotton, sterile moth releases and other tactics has dramatically reduced pink bollworm populations and insecticide sprays against this pest in Arizona.
Resistance Monitoring: 1997–2011
We have used three methods for monitoring resistance of pink bollworm to Bt cotton and Bt toxin Cry1Ac: (1) diet bioassays, (2) DNA screening and (3) field efficacy tests. All three methods have demonstrated no net increase in resistance to Cry1Ac or Bt cotton producing Cry1Ac in Arizona field populations of pink bollworm.Citation18,Citation21-Citation23 Results from field efficacy tests conducted from 1997–2005 demonstrated sustained effectiveness of Bt cotton against pink bollworm.Citation18,Citation24 Below we summarize results from our two primary resistance monitoring methods, diet bioassays and DNA screening.
Diet bioassays.
From 1997 to 2008, we monitored resistance to Cry1Ac in Arizona using diet bioassays as follows: Each year, we tested an average of 2,730 larvae that were the progeny of individuals collected in cotton fields from an average of 12 sites statewide. The progeny of field-collected pink bollworm from each site were reared and tested separately. Because pink bollworm larvae were scarce in most Bt cotton fields, nearly all of the sampled fields that provided enough individuals to produce sufficient progeny for testing in bioassays were non-Bt cotton fields. For example, non-Bt cotton fields accounted for 9 of 10 fields yielding progeny that were tested with bioassays in 1997, 10 of 10 fields in 1998, and 11 of 13 fields in 1999 (mean = 92% non-Bt cotton fields).Citation18 Neonates were tested individually for 21 d on wheat germ diet without toxin (control) or on diet with a diagnostic concentration (10 ∝g Cry1Ac per ml diet), which kills susceptible homozygotes and heterozygotes, but not resistant homozygotes.Citation18 Based on the observed recessive inheritance of resistance at the diagnostic concentration, the Cry1Ac resistance allele frequency for each site was estimated as the square root of the frequency of survivors after adjustment for control mortality.Citation18 We calculated the 95% confidence interval for each yearly statewide mean resistance allele frequency using the bootstrap method with 10,000 repetitions.Citation18
The results from diet bioassays show that the resistance allele frequency was 0.16 (95% confidence interval = 0.05 to 0.26) in 1997, the second year Bt cotton was grown widely in Arizona (). This turned out to be the highest resistance allele frequency measured in Arizona pink bollworm populations in 15 y of monitoring (1997–2011). Although the resistance allele frequency estimated from diet bioassays fluctuated from 1998 to 2008, it never reached the level detected in 1997 (). Moreover, no resistant individuals were detected with diet bioassays in 2007 or 2008 (n = 3,602 larvae tested),Citation21 the most recent years in which we collected enough pink bollworm from field populations to enable testing of their progeny with diet bioassays.
Figure 2. Pink bollworm resistance allele frequency (with 95% confidence intervals) in Arizona from 1997 to 2008 estimated from laboratory diet bioassays with Cry1Ac.Citation21
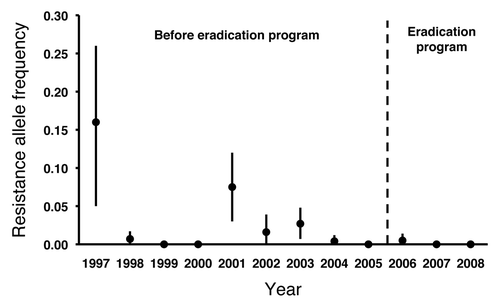
The estimated resistance allele frequency for pink bollworm in 1997 was stunning at the time, in part because it was so high compared with estimates from other pests targeted by Bt crops. This estimate for pink bollworm is 100 times higher than the frequency of resistance to Cry1Ac in field populations of Heliothis virescens estimated before Bt cotton was commercializedCitation25 and no major alleles for resistance to Cry1Ab in Bt corn were detected in extensive screening of field populations of Ostrinia nubilalis.Citation26,Citation27 Nonetheless, the estimate for pink bollworm in 1997 does not provide evidence of field-evolved resistance, because it was the first estimate and thus did not demonstrate a decrease in susceptibility. Despite considerable effort, neither members of our research team nor others have identified reasons to discount the relatively high estimated resistance allele frequency for pink bollworm in 1997.
We do not know why the frequency of resistance was so high in 1997, but we have speculated about various possibilities.Citation23 We infer that one or more key factors influencing resistance changed between 1997 and 1998, causing the observed decline in resistance frequency between these two years ().Citation23 One factor that might have changed is the concentration of Cry1Ac in Bt cotton. In 1997, some problems with Bt cotton were observed in the field, but it is not clear if these were caused by moderate concentrations of Cry1Ac or putative Bt cotton bolls that produced no Cry1Ac. If Bt cotton grown during 1996 and 1997 produced enough Cry1Ac to kill homozygous susceptible larvae but not enough to kill most heterozygous larvae, non-recessive inheritance of resistance could have quickly raised resistance allele frequency.Citation23 If increased Cry1Ac concentration in 1998 and thereafter killed all or nearly all heterozygous larvae, this shift to recessive inheritance could have caused decreases in resistance.Citation23 Another possibility is that the effects of fitness costs and incomplete resistance, which varied substantially in experiments, increased after 1997. Such changes could be associated with changes in agronomic practices, weather or other environmental factors.Citation23 A third possibility is a decreased effect from 1997 to 1998 of one or more factors other than Bt cotton favoring the resistance alleles.Citation23
Because our relatively high initial estimate of resistance allele frequency in pink bollworm has produced some confusion in the literature, we review some additional details about the estimate and three resistant strains generated from the pink bollworm collected from the field in 1997. Five of the 10 Arizona cotton field populations sampled in 1997 yielded survivors of exposure to the diagnostic concentration of Cry1Ac.Citation18 Of the five sample sites where resistance was detected, four were non-Bt cotton fields and one was a Bt cotton field. Excluding the data from the lone Bt cotton field, the estimated resistance allele frequency is 0.14, which does not differ significantly from the estimate of 0.16 noted above, which is based on data from all 10 sample sites.
From the initial bioassays of progeny from the 10 sample sites, we pooled 169 survivors of exposure to 1, 3.2 and 10 ∝g Cry1Ac per ml diet to start the Arizona pooled resistant strain (AZP-R). After four generations of rearing on diet without toxin followed by selection at the diagnostic concentration of Cry1Ac, the concentration of Cry1Ac killing 50% of larvae (LC50) was 300 times higher for AZP-R compared with the susceptible APHIS-S strain.Citation18 This rapid response to selection confirms that resistance alleles were not rare in the field populations of pink bollworm sampled in 1997. Moreover, two additional resistant strains (MOV97-R and SAF97-R) were started with pink bollworm collected in 1997 from Mohave in western Arizona and Safford in eastern Arizona, respectively.Citation20 As with AZP-R, selection of each of these strains quickly produced high levels of resistance to Cry1Ac.Citation20
We discovered that each of the three lab-selected resistant strains had a distinct profile in terms of the frequency of three mutant cadherin alleles (r1–r3) linked with resistance to Cry1Ac.Citation20,Citation28 Consistent with their origins and supporting the hypothesis that resistance alleles were not rare in the field populations sampled in 1997, all three cadherin resistance alleles occurred in AZP-R, while only two cadherin resistance alleles were in each of the two strains started from single sample sites (r1 and r3 in MOV97-R and r1 and r2 in SAF97-R).Citation20,Citation28
DNA screening.
In screening DNA from more than 9,000 pink bollworm larvae and adults collected from the field in the southwestern United States during 2001–2011, we did not detect any of the three mutant cadherin alleles that are linked with resistance to Cry1Ac in lab-selected strains of pink bollwormCitation21,Citation22 (unpublished data). We used polymerase chain reaction (PCR) primers that specifically amplify the three mutant cadherin alleles linked with resistance to Cry1Ac in the three lab-selected resistant strains of pink bollworm from Arizona mentioned above (AZP-R, SAF97-R, MOV97-R) and in a fourth, independently derived lab-selected resistant strain (APHIS-98R, which had r1–r3) from Arizona.Citation28-Citation30
Compared with our bioassays, this DNA screening has several advantages and an important disadvantage.Citation22,Citation29 Unlike bioassays, which do not distinguish heterozygotes from homozygous susceptible pink bollworm larvae, DNA screening can detect single resistance alleles in heterozygotes. Also, whereas bioassays require live larvae for testing, DNA screening can be done with properly preserved dead individuals or groups of individuals of any life stage. However, while bioassays can detect resistance caused by any mechanism, our DNA screening could detect only the three known mutant cadherin alleles and thus would miss resistance caused by other cadherin mutations or mutations in other genes.
The lack of resistance alleles detected by DNA screening could reflect three scenarios that are not mutually exclusive: (1) the three mutant cadherin alleles occurred in the samples screened but were not detected, (2) resistance in the samples was conferred by mutations other than the three known mutant cadherin alleles and (3) resistance was extremely rare. The first factor probably had little or no effect, because we included extensive controls to check our methods, including “blind” positive controls.Citation22 For example, in tests conducted from 2001 to 2005, DNA screening detected 97% of positive controls (n = 100) that were not known to the person performing the PCR testing.Citation22 The effect of the second factor is more difficult to exclude because we cannot rule out the possibility of alternative mechanisms of resistance. Indeed, we recently isolated a fourth mutant cadherin allele that is linked with resistance to Cry1Ac in a strain of pink bollworm that was selected from a susceptible strain from Arizona.Citation31,Citation32 In addition, many cadherin resistance alleles have been found in Helicoverpa armigera.Citation33 Nonetheless, unlike the fourth cadherin resistance allele in pink bollworm and the first cadherin resistance allele identified from Heliothis virescens,Citation34,Citation35 the three cadherin alleles for which we screened were not rare in field populations during 1997.Citation18,Citation20,Citation28 Most importantly, independent data from bioassays and field efficacy testing are consistent with the third interpretation: resistance of pink bollworm to Cry1Ac remained rare.
Refuges Delay Resistance: 1996–2005
The refuge strategy has been the predominant approach for delaying pest resistance to Bt crops worldwide.Citation5,Citation7,Citation8 The concept underlying this strategy is that refuges of non-Bt host plants near Bt crops will promote survival of susceptible pests. Ideally, most of the rare resistant pests emerging from the Bt crop will mate with the relatively abundant susceptible pests from the refuges. If resistance to the Bt crop is inherited as a recessive trait, the Bt crop will kill the heterozygous progeny produced by such matings between homozygous susceptible moths from the refuge and homozygous resistant moths emerging from the Bt crop. Retrospective analyses of global resistance monitoring data suggest that when the assumptions of the refuge strategy apply, refuges can substantially delay resistance to Bt crops.Citation6-Citation8,Citation36 A large-scale field test incorporating eight years of data also shows that refuges of unsprayed cotton delayed whitefly resistance to the insecticide pyriproxyfen.Citation37 We found four factors that probably delayed pink bollworm resistance to Bt cotton in Arizona from 1996 to 2005: abundant refuges of non-Bt cotton, recessive inheritance of resistance, fitness costs associated with resistance and incomplete resistance.Citation23
Refuges.
Growers planted abundant refuges of non-Bt cotton in Arizona from 1996 to 2005. The statewide percentage of cotton acreage planted to non-Bt cotton was > 50% in 1996, the first year Bt cotton was planted commercially, and > 25% every year from 1996 to 2005 (). A more detailed spatial analysis for 1998–2003 showed an average of > 86% compliance with the size and distance requirements for non-Bt cotton refuges in the six counties of Arizona that produced > 95% of the state’s cotton.Citation38 Furthermore, the abundance of susceptible moths was higher in locations compliant with the size and distance requirements than in non-compliant locations.Citation39
Recessive inheritance.
Results from many tests on Bt cotton plants showed substantially increased survival of larvae from lab-selected resistant strains compared with susceptible strains, yet the F1 hybrid larvae from crosses between resistant and susceptible strains did not survive on Bt cotton.Citation18,Citation20,Citation28,Citation40,Citation41 Thus, inheritance of resistance to Bt cotton was recessive, which is expected to enhance the success of the refuge strategy.
Fitness costs.
Fitness costs occur when fitness in refuges is lower for insects with resistance alleles than insects without resistance alleles.Citation42,Citation43 Thus, fitness costs select against resistance in refuges. A series of experiments on non-Bt cotton plants showed costs that lower the fitness of resistant individuals compared with susceptible individuals.Citation23,Citation41 Like resistance to Bt cotton, these costs were generally recessive, which means they occurred in resistant individuals, but not in F1 hybrid progeny from crosses between resistant and susceptible adults.
Based on several experiments conducted on non-Bt cotton plants, the mean estimated fitness was 0.46 for resistant pink bollworm relative to susceptible pink bollworm (fitness defined as 1).Citation23 This indicates a 54% fitness cost, which reflects lower larval survival on non-Bt cotton plants for resistant than susceptible strains and lower overwintering survival for resistant than susceptible strains.Citation23,Citation44 This estimate does not include fitness costs affecting other life history traits such as the ability of males to compete for mates or first-male paternity when females mate with more than one male.Citation45,Citation46
Incomplete resistance.
Incomplete resistance occurs when resistant insects suffer a disadvantage on Bt crop plants relative to non-Bt crop plants, which reduces the selective advantage of resistant insects on Bt crop plants.Citation23,Citation41 Analysis of data from experiments on plants indicated that the net reproductive rate of resistant pink bollworm was 0.35 on Bt cotton relative to non-Bt cotton (defined as 1).Citation23 This incorporates effects of incomplete resistance on larval survival, the proportion of adults that are female and fecundity; all were lower on Bt cotton than non-Bt cotton.Citation23 Egg hatching rate was similar for progeny of individuals that were reared on Bt and non-Bt cotton.Citation46 Taking into account the effects of fitness costs and incomplete resistance, the estimated mean fitness of resistant pink bollworm on Bt cotton was only 0.16 (0.46 × 0.35) relative to susceptible pink bollworm on non-Bt cotton.
Modeling.
To project the potential rate of evolution of pink bollworm resistance to Bt cotton in Arizona under the refuge strategy, we incorporated into mathematical models the experimental results summarized above on refuge percentage, inheritance of resistance, fitness costs and incomplete resistance.Citation23 This modeling confirmed the importance of each of these four factors. The modeling results also show that for a substantial set of realistic values for the key factors, resistance was not expected to increase.
Sterile Insect Releases Delay Resistance and Suppress Populations: 2006–2011
A large collaborative team has developed and deployed a multi-tactic eradication program in Arizona that uses sterile insect releases instead of refuges to delay pink bollworm resistance to Bt cotton.Citation21 This approach for delaying resistance relies on the idea that rare resistant pink bollworm moths emerging from Bt cotton will mate primarily with sterile moths, yielding no fertile progeny. Computer simulations show that this tactic works in principle, even if pest resistance is inherited as a dominant trait.Citation21 The US Environmental Protection Agency (EPA) convened a Scientific Advisory Panel to review the proposed eradication program.Citation48 The EPA followed the Panel’s recommendation and decided to allow Arizona cotton growers to plant up to 100% Bt cotton producing either one toxin (Cry1Ac) or two toxins (Cry1Ac and Cry2Ab).Citation48
The eradication program was introduced in phases in Arizona, starting in 2006 and moving from east to west. During the nine years before the program began, the percentage of cotton acreage planted to non-Bt cotton in Arizona ranged from about 50% in 1997 to 27% in 2005 (mean = 37%). With the onset of sterile releases, the statewide percentage of non-Bt cotton dropped to 15% in 2006, 8% in 2007, 2% in 2008 and 3% in 2009 ().Citation21 The mean percentage of non-Bt cotton was 7% from 2006 to 2009 and large areas of the state had only Bt cotton with no refuges. The percentage of Arizona’s Bt cotton producing two toxins (Cry1Ac and Cry2Ab) increased from 11% in 2005 to 79% in 2009, and was close to 100% in 2010, after the registration for Bt cotton producing only Cry1Ac expired in 2009.Citation7,Citation21
During May to October of each year from 2006 to 2009, about 2 billion sterile pink bollworm moths were released from airplanes into Arizona’s cotton fields. Releases were made two to three times per week in all cotton fields statewide. An average of approximately 25,000 sterile moths were released per ha of cotton each year. Because higher moth emergence was expected in non-Bt cotton fields, the release rate was about ten times higher in non-Bt cotton fields (4,000 moths per ha per week) than in Bt cotton fields (400 moths per ha per week).
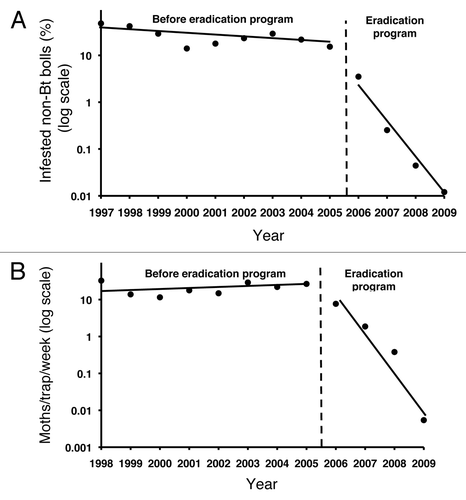
Pink bollworm populations in Arizona have declined dramatically since the eradication program began in 2006 (). In 2009, only two pink bollworm larvae were found in 16,600 bolls of non-Bt cotton screened statewide. This yields an infestation rate of 0.00012, which is a 99.9% decline from the infestation rate of 0.15 in 2005. Likewise, the number of wild male pink bollworm moths caught per trap per week in Bt cotton fields dropped from 26.7 in 2005 to 0.0054 in 2009, a 99.98% decrease. The decrease in pink bollworm populations during the eradication program was much steeper than the decline observed with the planting of Bt cotton before the eradication program began in Arizona and the declines in other target pests associated with planting of Bt crops in other regions.Citation2,Citation49-Citation54
Figure 3. Pink bollworm abundance in Arizona before and during the eradication program.Citation21 (A) Larval infestation of non-Bt cotton bolls from 1997 to 2009. Linear regression shows that the slope, which indicates the decrease in infestation per year, was 18 times steeper from 2006 to 2009 (-0.81, r2 = 0.97, p = 0.012) than from 1997 to 2005 (-0.044, r2 = 0.42, p = 0.059). (B) Wild male pink bollworm moths trapped in Bt cotton fields from 1998 to 2009. Linear regression shows that the slope, which indicates the change in moths trapped per year, was significantly negative from 2006 to 2009 (-1.0, r2 = 0.92, p = 0.04), but did not differ significantly from zero from 1998 to 2005 (0.017, r2 = 0.071, p = 0.52).
While pink bollworm populations declined dramatically, insecticide sprays to control this pest also plummeted.Citation21 The mean number of sprays per field per year targeting pink bollworm in Arizona was 2.7 from 1990 to 1995, which fell to 0.64 from 1996 to 2005 with use of Bt cotton, before the eradication program. During the eradication program, this mean decreased to 0.14 in 2006, 0.013 in 2007, 0.0029 in 2008 and 0 in 2009.
The average annual cost of pink bollworm to Arizona cotton growers, including yield losses and insecticide sprays, was $18 million for 1990 to 1995, $5.4 million from 1996 to 2005 and only $172,000 for 2006 to 2009.Citation55 By using Bt cotton as part of a comprehensive integrated pest management program, Arizona growers also greatly reduced insecticide sprays against all cotton pests, including those not killed by Bt cotton, saving a cumulative total of $200 million from 1996 to 2009.Citation55,Citation56
Conclusions
The sustained susceptibility of pink bollworm to Cry1Ac in Arizona contrasts with less positive outcomes in India and China.Citation10,Citation13,Citation16 In India, refuges of non-Bt cotton have been required, but apparently compliance has been low.Citation17,Citation57 In China, non-Bt cotton refuges have not been required.Citation16 This lack of refuges may be a key factor promoting faster evolution of pink bollworm resistance in these two countries compared with the United States. Wan et al. suggest that another factor accelerating pink bollworm resistance in India and China may be a lower concentration of Cry1Ac in Bt cotton in these countries compared with the United States, which could increase survival of heterozygotes and thus increase the dominance of resistance, but this hypothesis has not been tested. Farmers planted Bt cotton in India before it was officially approved in 2002.Citation58 MonsantoCitation59 speculated that Cry1Ac concentration is lower in the unapproved Bt cotton than approved Bt cotton in India, and that early use of unapproved Bt cotton contributed to the resistance problem. However, the unapproved Bt cotton came to light in India in 2001 because of its efficacy against bollworms and remained in wide use after approved Bt cotton seed became available.Citation58
In Arizona, Bt cotton has had a superb positive impact. It has been a key tool for suppressing pink bollworm, a major cotton pest. The sustained susceptibility of pink bollworm to Bt cotton has provided a cornerstone for the pink bollworm eradication program and for integrated pest management in cotton. The reduction in insecticide use against pink bollworm and other cotton pests has provided economic benefits for growers, as well as environmental and health benefits. Elimination of sprays of broad-spectrum insecticides targeting pink bollworm adults, particularly chlorpyrifos and methyl parathion, has been especially beneficial.
The objective of the pink bollworm eradication program is to eliminate this pest from all cotton-growing regions of the US and adjacent areas of northern Mexico.Citation60,Citation61 This binational program, funded by cotton growers and the US Department of Agriculture, has benefitted from a strong grower commitment, public investment in sterile insect technology, a well-developed infrastructure for monitoring pink bollworm resistance and population density, virtually 100% efficacy of Bt cotton against pink bollworm, and this pest’s nearly exclusive dependence on cotton in Arizona. While great progress has been achieved, eradication of pink bollworm remains challenging as this invasive pest is resilient and can move long distances.
Because several tactics were implemented simultaneously in the eradication program in Arizona, including reduction in non-Bt cotton refuge percentage, increased percentage of Bt cotton producing two toxins, sterile moth releases and early season pheromone treatments to disrupt mating in non-Bt cotton fields, we cannot conclusively attribute the suppression of pink bollworm to any single tactic. Moreover, we do not know if the success of this program can be replicated with other pests or even with pink bollworm in other parts of the world. Nonetheless, our results support the idea that Bt crops are likely to be most useful when combined with other tactics for integrated control of pests. Much research on delaying resistance to Bt crops has focused on the size and spatial configuration of refuges and the number and type of toxins used in plants. We encourage more effort to combine Bt crops with other tactics in integrated pest management programs.
Acknowledgements
We thank two anonymous reviewers for their comments that helped to improve the paper. Preparation of this paper was supported by USDA Agriculture and Food Research Initiative Grant 2008-35302-0390.
Disclosure of Potential Conflicts of Interest
Research reported in this article was supported by organizations that may gain or lose financially through its publication: Arizona Cotton Growers Association, Arizona Cotton Research and Protection Council (ACRPC), Bayer CropScience, Cotton Foundation, Cotton Inc. Dow AgroSciences, Monsanto and National Cotton Council. L.A. and R.T.S. are independent contractors paid by ACRPC. L.L. and M.W. are employees of ACRPC. T.J.D. is an employee of Bayer CropScience. B.E.T. is a coauthor of a patent application on engineering modified Bt toxins to counter pest resistance, which is related to research described by Tabashnik et al. (Nature Biotechnology 2011; 29:1128–31).
References
- Cattaneo MG, Yafuso C, Schmidt C, Huang CY, Rahman M, Olson C, et al. Farm-scale evaluation of the impacts of transgenic cotton on biodiversity, pesticide use, and yield. Proc Natl Acad Sci U S A 2006; 103:7571 - 6; http://dx.doi.org/10.1073/pnas.0508312103; PMID: 16675554
- National Research Council. The Impact of Genetically Engineered Crops on Farm Sustainability in the United States. Washington DC: National Academy Press 2010.
- James C. Global status of commercialized biotech/GM crops. ISAAA. Brief No 42. Ithaca, NY 2010.
- Tabashnik BE. Evolution of resistance to Bacillus thuringiensis.. Annu Rev Entomol 1994; 39:47 - 79; http://dx.doi.org/10.1146/annurev.en.39.010194.000403
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu Rev Entomol 1998; 43:701 - 26; http://dx.doi.org/10.1146/annurev.ento.43.1.701; PMID: 15012402
- Tabashnik BE, Gassmann AJ, Crowder DW, Carriére Y. Insect resistance to Bt crops: evidence versus theory. Nat Biotechnol 2008; 26:199 - 202; http://dx.doi.org/10.1038/nbt1382; PMID: 18259177
- Tabashnik BE, Van Rensburg JB, Carrière Y. Field-evolved insect resistance to Bt crops: definition, theory, and data. J Econ Entomol 2009; 102:2011 - 25; http://dx.doi.org/10.1603/029.102.0601; PMID: 20069826
- Carrière Y, Crowder DW, Tabashnik BE. Evolutionary ecology of insect adaptation to Bt crops. Evol Appl 2010; 3:561 - 73; http://dx.doi.org/10.1111/j.1752-4571.2010.00129.x
- Van Rensburg JBJ. First report of field resistance by stem borer, Busseola fusca (Fuller) to Bt-transgenic maize. South African J Plant and Soil 2007; 24:147 - 51
- Bagla P. India. Hardy cotton-munching pests are latest blow to GM crops. Science 2010; 327:1439; http://dx.doi.org/10.1126/science.327.5972.1439; PMID: 20299559
- Downes S, Parker T, Mahon R. Incipient resistance of Helicoverpa punctigera to the Cry2Ab Bt toxin in Bollgard II cotton. PLoS One 2010; 5:e12567; http://dx.doi.org/10.1371/journal.pone.0012567; PMID: 20830203
- Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, Bing JW, et al. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol 2010; 103:1031 - 8; http://dx.doi.org/10.1603/EC10040; PMID: 20857709
- Dhurua S, Gujar GT. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag Sci 2011; 67:898 - 903; http://dx.doi.org/10.1002/ps.2127; PMID: 21438121
- Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW. Field-evolved resistance to Bt maize by western corn rootworm. PLoS One 2011; 6:e22629; http://dx.doi.org/10.1371/journal.pone.0022629; PMID: 21829470
- Zhang H, Yin W, Zhao J, Jin L, Yang Y, Wu S, et al. Early warning of cotton bollworm resistance associated with intensive planting of Bt cotton in China. PLoS One 2011; 6:e22874; http://dx.doi.org/10.1371/journal.pone.0022874; PMID: 21857961
- Wan P, Huang Y, Wu H, Huang M, Cong S, Tabashnik BE, et al. Increased frequency of pink bollworm resistance to Bt toxin Cry1Ac in China. PLoS One 2012; 7:e29975; http://dx.doi.org/10.1371/journal.pone.0029975; PMID: 22238687
- Tabashnik BE, Carrière Y. Resistance to transgenic crops and pest outbreaks. In: Barbosa P, Letourneau D, Agrawal A, (Eds). Insect Outbreaks Revisited, Blackwell Publishing Ltd. 2012; p 341-354.
- Tabashnik BE, Patin AL, Dennehy TJ, Liu YB, Carrière Y, Sims MA, et al. Frequency of resistance to Bacillus thuringiensis in field populations of pink bollworm. Proc Natl Acad Sci U S A 2000; 97:12980 - 4; http://dx.doi.org/10.1073/pnas.97.24.12980; PMID: 11087854
- Carrière Y, Dennehy TJ, Pedersen B, Haller S, Ellers-Kirk C, Antilla L, et al. Large-scale management of insect resistance to transgenic cotton in Arizona: can transgenic insecticidal crops be sustained?. J Econ Entomol 2001; 94:315 - 25; http://dx.doi.org/10.1603/0022-0493-94.2.315; PMID: 11332820
- Tabashnik BE, Biggs RW, Higginson DM, Henderson S, Unnithan DC, Unnithan GC, et al. Association between resistance to Bt cotton and cadherin genotype in pink bollworm. J Econ Entomol 2005; 98:635 - 44; http://dx.doi.org/10.1603/0022-0493-98.3.635; PMID: 16022286
- Tabashnik BE, Sisterson MS, Ellsworth PC, Dennehy TJ, Antilla L, Liesner L, et al. Suppressing resistance to Bt cotton with sterile insect releases. Nat Biotechnol 2010; 28:1304 - 7; http://dx.doi.org/10.1038/nbt.1704; PMID: 21057498
- Tabashnik BE, Fabrick JA, Henderson S, Biggs RW, Yafuso CM, Nyboer ME, et al. DNA screening reveals pink bollworm resistance to Bt cotton remains rare after a decade of exposure. J Econ Entomol 2006; 99:1525 - 30; http://dx.doi.org/10.1603/0022-0493-99.5.1525; PMID: 17066779
- Tabashnik BE, Dennehy TJ, Carrière Y. Delayed resistance to transgenic cotton in pink bollworm. Proc Natl Acad Sci U S A 2005; 102:15389 - 93; http://dx.doi.org/10.1073/pnas.0507857102; PMID: 16227430
- Dennehy TJ, Unnithan GC, Harpold V, Carrière Y, Tabashnik BE. Susceptibility of Southwestern Pink Bollworm to Bt toxins Cry1Ac and Cry2Ab2 in 2005. In Arizona Cotton Report (P-151) (Cooperative Extension, Agricultural Experiment Station, The University of Arizona, Tucson, USDA 2007) <http://cals.arizona.edu/pubs/crops/az1437/az14373a.pdf>.
- Gould F, Anderson A, Jones A, Sumerford D, Heckel DG, Lopez J, et al. Initial frequency of alleles for resistance to Bacillus thuringiensis toxins in field populations of Heliothis virescens.. Proc Natl Acad Sci U S A 1997; 94:3519 - 23; http://dx.doi.org/10.1073/pnas.94.8.3519; PMID: 11038613
- Andow DA, Alstad DN. F2 screen for rare resistance alleles. J Econ Entomol 1998; 91:572 - 8
- Andow DA, Olson DM, Hellmich RL, Alstad DN, Hutchison WD. Frequency of resistance to Bacillus thuringiensis toxin Cry1Ab in an Iowa population of European corn borer (Lepidoptera: Crambidae). J Econ Entomol 2000; 93:26 - 30; http://dx.doi.org/10.1603/0022-0493-93.1.26; PMID: 14658507
- Morin S, Biggs RW, Sisterson MS, Shriver L, Ellers-Kirk C, Higginson D, et al. Three cadherin alleles associated with resistance to Bacillus thuringiensis in pink bollworm. Proc Natl Acad Sci U S A 2003; 100:5004 - 9; http://dx.doi.org/10.1073/pnas.0831036100; PMID: 12695565
- Morin S, Henderson S, Fabrick JA, Carrière Y, Dennehy TJ, Brown JK, et al. DNA-based detection of Bt resistance alleles in pink bollworm. Insect Biochem Mol Biol 2004; 34:1225 - 33; http://dx.doi.org/10.1016/j.ibmb.2004.08.003; PMID: 15522618
- Tabashnik BE, Liu YB, Unnithan DC, Carrière Y, Dennehy TJ, Morin S. Shared genetic basis of resistance to Bt toxin Cry1ac in independent strains of pink bollworm. J Econ Entomol 2004; 97:721 - 6; http://dx.doi.org/10.1603/0022-0493(2004)097[0721:SGBORT]2.0.CO;2; PMID: 15279243
- Fabrick JA, Forlow Jech L, Henneberry TJ. Novel pink bollworm resistance to the Bt toxin Cry1Ac: Effects on mating, oviposition, larval development and survival. J Insect Sci 2009; 9:8; http://dx.doi.org/10.1673/031.009.2401; PMID: 19611255
- Fabrick JA, Tabashnik BE. Similar genetic basis of resistance to Bt toxin Cry1Ac in Boll-selected and diet-selected strains of pink bollworm. PLoS One 2012; 7:e35658; http://dx.doi.org/10.1371/journal.pone.0035658; PMID: 22530065
- Zhao J, Jin L, Yang Y, Wu Y. Diverse cadherin mutations conferring resistance to Bacillus thuringiensis toxin Cry1Ac in Helicoverpa armigera.. Insect Biochem Mol Biol 2010; 40:113 - 8; http://dx.doi.org/10.1016/j.ibmb.2010.01.001; PMID: 20079435
- Gahan LJ, Gould F, Heckel DG. Identification of a gene associated with Bt resistance in Heliothis virescens.. Science 2001; 293:857 - 60; http://dx.doi.org/10.1126/science.1060949; PMID: 11486086
- Gahan LJ, Gould F, López JD Jr., Micinski S, Heckel DG. A polymerase chain reaction screen of field populations of Heliothis virescens for a retrotransposon insertion conferring resistance to Bacillus thuringiensis toxin. J Econ Entomol 2007; 100:187 - 94; http://dx.doi.org/10.1603/0022-0493(2007)100[187:APCRSO]2.0.CO;2; PMID: 17370827
- Huang F, Andow DA, Buschman LL. Success of the high-dose/refuge resistance management strategy after 15 years of Bt crop use in North America. Entomol Exp Appl 2011; 140:1 - 16; http://dx.doi.org/10.1111/j.1570-7458.2011.01138.x
- Carrière Y, Ellers-Kirk C, Hartfield K, Larocque G, Degain B, Dutilleul P, et al. Large-scale, spatially-explicit test of the refuge strategy for delaying insecticide resistance. Proc Natl Acad Sci U S A 2012; 109:775 - 80; http://dx.doi.org/10.1073/pnas.1117851109; PMID: 22215605
- Carrière YC, Ellers-Kirk C, Kumar K, Heuberger S, Whitlow M, Antilla L, et al. Long-term evaluation of compliance with refuge requirements for Bt cotton. Pest Manag Sci 2005; 61:327 - 30; http://dx.doi.org/10.1002/ps.1039; PMID: 15714465
- Carrière Y, Dutilleul P, Ellers-Kirk C, Pedersen B, Haller S, Antilla L, et al. Sources, sinks and the zone of influence of refuges for managing insect resistance to Bt crops. Ecol Appl 2004; 14:1615 - 23; http://dx.doi.org/10.1890/03-5268
- Liu YB, Tabashnik BE, Dennehy TJ, Patin AL, Bartlett AC. Development time and resistance to Bt crops. Nature 1999; 400:519; http://dx.doi.org/10.1038/22919; PMID: 10448853
- Carrière Y, Ellers Kirk C, Biggs RW, Nyboer ME, Unnithan GC, Dennehy TJ, et al. Cadherin-based resistance to Bt cotton in hybrid strains of pink bollworm: fitness costs and incomplete resistance. J Econ Entomol 2006; 99:1925 - 35; http://dx.doi.org/10.1603/0022-0493-99.6.1925; PMID: 17195656
- Carrière Y, Tabashnik BE. Reversing insect adaptation to transgenic insecticidal plants. Proc Biol Sci 2001; 268:1475 - 80; http://dx.doi.org/10.1098/rspb.2001.1689; PMID: 11454291
- Gassmann AJ, Carrière Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis.. Annu Rev Entomol 2009; 54:147 - 63; http://dx.doi.org/10.1146/annurev.ento.54.110807.090518; PMID: 19067630
- Carrière Y, Ellers-Kirk C, Patin AL, Sims MA, Meyer S, Liu YB, et al. Overwintering cost associated with resistance to transgenic cotton in the pink bollworm (Lepidoptera: Gelechiidae). J Econ Entomol 2001; 94:935 - 41; http://dx.doi.org/10.1603/0022-0493-94.4.935; PMID: 11561855
- Higginson DM, Nyboer ME, Biggs RW, Morin S, Tabashnik BE, Carrière Y. Evolutionary trade-offs of insect resistance to Bt crops: fitness cost affecting paternity. Evolution 2005; 59:915 - 20; PMID: 15926701
- Carrière Y, Showalter AM, Fabrick JA, Sollome J, Ellers-Kirk C, Tabashnik BE. Cadherin gene expression and effects of Bt resistance on sperm transfer in pink bollworm. J Insect Physiol 2009; 55:1058 - 64; http://dx.doi.org/10.1016/j.jinsphys.2009.07.013; PMID: 19666026
- Liu YB, Tabashnik BE, Dennehy TJ, Patin AL, Sims MA, Meyer SK, et al. Effects of Bt cotton and crylac toxin on survival and development of pink bollworm (Lepidoptera: Gelechiidae). J Econ Entomol 2001; 94:1237 - 42; http://dx.doi.org/10.1603/0022-0493-94.5.1237; PMID: 11681689
- US EPA October 24–26, 2006: Evaluation of the Resistance Risks from Using 100% Bollgard and Bollgard II Cotton as Part of a Pink Bollworm Eradication Program in the State of Arizona. http://www.epa.gov/scipoly/sap/meetings/2006/102406_mtg.htm
- Carrière Y, Ellers-Kirk C, Sisterson M, Antilla L, Whitlow M, Dennehy TJ, et al. Long-term regional suppression of pink bollworm by Bacillus thuringiensis cotton. Proc Natl Acad Sci U S A 2003; 100:1519 - 23; http://dx.doi.org/10.1073/pnas.0436708100; PMID: 12571355
- Adamczyk JJ, Hubbard D. Changes in populations of Heliothis virescens (F.) (Lepidoptera: Noctuidae) and Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) in the Mississippi Delta from 1986 to 2005 as indicated by adult male pheromone traps. J Cotton Sci 2006; 10:155 - 60
- Micinski S, Blouin DC, Waltman WF, Cookson C. Abundance of Helicoverpa zea and Heliothis virescens in pheromone traps during the past twenty years in Northwestern Louisiana. Southwest Entomologist 2008; 33:139 - 49; http://dx.doi.org/10.3958/0147-1724-33.2.139
- Storer NP, Dively GP, Herman RA. Landscape effects of insect-resistant genetically modified crops. In Integration of Insect-Resistant Genetically Modified Crops within IPM Programs, (Romeis J, Shelton AM, Kennedy GG, Eds.) Springer, The Netherlands 2008; 273-302.
- Wu KM, Lu YH, Feng HQ, Jiang YY, Zhao JZ. Suppression of cotton bollworm in multiple crops in China in areas with Bt toxin-containing cotton. Science 2008; 321:1676 - 8; http://dx.doi.org/10.1126/science.1160550; PMID: 18801998
- Hutchison WD, Burkness EC, Mitchell PD, Moon RD, Leslie TW, Fleischer SJ, et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 2010; 330:222 - 5; http://dx.doi.org/10.1126/science.1190242; PMID: 20929774
- Ellsworth PC, Fournier A, Smith TD. Cotton Insect Losses (The University of Arizona, Cooperative Extension 2010). <http://cals.arizona.edu/crops/cotton/insects/cil/cil.html>.
- Naranjo SE, Ellsworth PC. Fourteen years of Bt cotton advances IPM in Arizona. Southwest Entomologist 2010; 35:439 - 46; http://dx.doi.org/10.3958/059.035.0329
- Stone GD. Biotechnology and the political ecology of information in India. Hum Organ 2004; 63:127 - 40
- Herring RJ. Stealth seeds: Bioproperty, biosafety, biopolitics. J Dev Stud 2007; 43:130 - 57; http://dx.doi.org/10.1080/00220380601055601
- Monsanto. Cotton in India 2010. http://www.monsanto.com/monsanto_today/for_the_record/india_pink_bollworm.asp.
- Antilla L, Liesner L. Program advances in the eradication of pink bollworm Pectinophora gossypiella in Arizona cotton. In Proceedings, Beltwide Cotton Conferences, National Cotton Council of America, Nashville TN 2008; 1162-8.
- Grefenstette B, El-Lissy O, Staten RT. Pink Bollworm Eradication Plan in the US. (USDA 2009). <http://www.aphis.usda.gov/plant_health/plant_pest_info/cotton_pests/downloads/pbw-erad-plan2-09.pdf>.