Abstract
Crops genetically engineered to produce insecticidal toxins from the bacterium Bacillus thuringiensis (Bt) manage many key insect pests while reducing the use of conventional insecticides. One of the primary pests targeted by Bt maize in the United States is the western corn rootworm, Diabrotica virgifera virgifera LeConte. Beginning in 2009, populations of western corn rootworm were identified in Iowa, USA that imposed severe root injury to Cry3Bb1 maize. Subsequent laboratory bioassays revealed that these populations were resistant to Cry3Bb1 maize, with survival on Cry3Bb1 maize that was three times higher than populations not associated with such injury. Here we report the results of research that began in 2010 when western corn rootworm were sampled from 14 fields in Iowa, half of which had root injury to Cry3Bb1 maize of greater than 1 node. Of these samples, sufficient eggs were collected to conduct bioassays on seven populations. Laboratory bioassays revealed that these 2010 populations had survival on Cry3Bb1 maize that was 11 times higher and significantly greater than that of control populations, which were brought into the laboratory prior to the commercialization of Bt maize for control of corn rootworm. Additionally, the developmental delays observed for control populations on Cry3Bb1 maize were greatly diminished for 2010 populations. All 2010 populations evaluated in bioassays came from fields with a history of continuous maize production and between 3 and 7 y of Cry3Bb1 maize cultivation. Resistance to Cry34/35Ab1 maize was not detected and there was no correlation between survival on Cry3Bb1 maize and Cry34/35Ab1 maize, suggesting a lack of cross resistance between these Bt toxins. Effectively dealing with the challenge of field-evolved resistance to Bt maize by western corn rootworm will require better adherence to the principles of integrated pest management.
Introduction
The area planted to transgenic crops that produce insecticidal toxins derived from the bacterium Bacillus thuringiensis (Bt) has grown rapidly since their commercialization in 1996.Citation1 Between 2004 and 2010, the area planted to Bt crops more than doubled, increasing from 23 million hectares to 58 million hectares.Citation1,Citation2 Bt crops provide effective control of many key insect pests, and have additional benefits that include reductions in the use of conventional insecticides and regional suppression of pests.Citation3-Citation7 The widespread planting of Bt crops imposes intense selective pressure for pests to evolve resistance, and the evolution of Bt resistance could diminish the benefits these crops provide. Although the majority of insects targeted by Bt crops remain susceptible, several cases of field-evolved resistance to Bt toxins have been documented.Citation8-Citation14 Insect resistance management strategies, including the use of refuges and high-dose events, can act to delay resistance.Citation9,Citation15-Citation18
The western corn rootworm, Diabrotica virgifera virgifera LeConte, is among the most serious pests of maize in the United States and has repeatedly demonstrated its ability to invade new habitats and to develop resistance to pest management practices.Citation19,Citation20 The larvae of this univoltine pest feed on the roots of maize causing structural damage and interfering with the acquisition of water and nitrogen.Citation21,Citation22 Maize plants that are heavily injured by western corn rootworm often lodge (i.e., list) within their rows, which complicates harvest.Citation22 Although native to the United States, the western corn rootworm was restricted to a small portion of the western United States until the 1940s when it expanded its range eastward, reaching the East Coast in the 1990s.Citation19 In the 1990s this pest also became established in Europe and has since colonized maize fields in several countries.Citation19,Citation23,Citation24
Various practices have been applied to manage western corn rootworm including crop rotation (i.e., rotating fields out of maize production for at least one season), insecticide application to the soil to manage larvae, and aerial application of insecticides to manage adults.Citation22 Importantly, cases exist in which the western corn rootworm has developed resistance to all of these tactics.Citation25-Citation28 For example, western corn rootworm colonized eastern Illinois in the mid-1960s and by the mid-1980s resistance to crop rotation was reported in this area.Citation19,Citation25 In Nebraska, western corn rootworm was managed through aerial application of the insecticide methyl-parathion, which was a favored management tactic due in part to resistance of western corn rootworm to soil-applied insecticides.Citation27 Adult management with methyl-parathion subsequently caused the evolution of esterase-mediated resistance in some populations.Citation27,Citation29 Cases of resistance by western corn rootworm to crop rotation and to conventional insecticides raise concerns about resistance to other management tactics including Bt maize.
Evolution of resistance is not unique to western corn rootworm, but rather the western corn rootworm exemplifies a broader pattern among arthropods in which pests respond to the application of insecticides, and other management practices, by developing resistance.Citation30 Any pest management practice that imposes mortality on a pest population will select for resistant genotypes, and in some cases pest susceptibility may be viewed as a non-renewable resource that is expended in the process of managing the pest population. However, the application of insect resistance management can delay, and in some cases may prevent, the evolution of pest resistance.Citation31,Citation32
For Bt crops, pest resistance is managed through the refuge strategy.Citation15,Citation33 Refuges are especially effective when they are coupled with either high-dose events or pyramided events.Citation18,Citation34 The theory behind the refuge strategy predates the development of Bt crops.Citation16,Citation17 However, Bt crops provide options that were not possible with conventional insecticides, such as a consistent high dose of toxin.Citation35 The refuge strategy uses refuges of non-Bt host plants as a source of Bt-susceptible pests that can mate with any resistant pests that develop on Bt crops. The heterozygous progeny of such matings are expected to have lower fitness on the Bt crop than their resistant parent, and it is this reduction in fitness that helps to delay resistance.
Both the inheritance of resistance and the availability of individuals from refuges are critical factors in determining how quickly populations will develop resistance.Citation9,Citation36-Citation38 As the recessive nature of a resistance trait increases, the delays in resistance expected under the refuge strategy become greater, with the longest delays expected for resistance traits that are completely recessive.Citation18 The genetic inheritance of a resistance trait can be predicted based on mortality of susceptible genotypes on a transgenic crop.Citation15,Citation18 In cases where Bt crops produce 25 times more toxin than is needed to kill a susceptible pest and the Bt crop kills greater than 99.99% of susceptible individuals, the crop is termed “high dose” and resistance is expected to be recessive.Citation39 As the survival of susceptible pests on a Bt crop increases, resistance is expected to be more additive in nature, and at a sufficiently high level of survival, resistance may be dominant. In the case of western corn rootworm, experiments in the laboratory and field found that Bt maize kills less than 99.99% of susceptible western corn rootworm, and thus, resistance to Bt maize by the western corn rootworm is not expected to be a recessive trait.Citation40-Citation46 Furthermore, genetic analysis of laboratory strains that were resistant and susceptible to Cry3Bb1 maize revealed that resistance was not recessive.Citation45 The lack of recessive inheritance for resistance traits is expected to increases the risk of resistance in the field.
A second factor that will affect resistance evolution is the availability of insects from refuges to mate with insects that experience selection on a Bt crop. For western corn rootworm, both the willingness of farmers to plant refuges and the patterns of pest movement increase the risk of this pest developing resistance to Bt maize. A survey of farmers found that approximately 25% were not in compliance with EPA regulations in terms of either the size or location of refuges.Citation47 Furthermore, the western corn rootworm has low rates of infield dispersal. Measurements of infield movement suggest that adult western corn rootworm typically disperse less than 40 m per day.Citation21,Citation48 In cases where large fields are planted to Bt maize, the dispersal of insects from a block (i.e., structured) refuge is likely to be uneven with a higher density of insects near the refuge, thus diminishing the effectiveness of a block refuge.
In 2009, Iowa populations of western corn rootworm were found that had evolved resistance to Cry3Bb1 maize in the field.Citation13 Western corn rootworm were sampled from fields that had severe root injury to Cry3Bb1 maize. Furthermore, these populations were sampled from fields that had a history of continuous maize cultivation, and a history of cultivation of Cry3Bb1 maize. In all fields sampled, the type of Bt maize planted produced Cry3Bb1 and fields had been planted to this type of Bt maize for at least three, and as many as six, consecutive seasons. Rootworm populations from these problem fields had three times higher survival on Cry3Bb1 maize in laboratory bioassays compared with control fields, which were not associated with management problems.Citation13 Subsequent field experiments in two of these problem fields found no difference in survival of western corn rootworm between Cry3Bb1 maize and non-Bt maize, and higher root injury to Cry3Bb1 maize than any other treatment tested except maize that had no protection against larval rootworm.Citation49
Here we report the results of field visits conducted in Iowa during 2010 and of subsequent laboratory bioassays. Similar to data from 2009, we visited fields in response to notification from growers, assessed the type of maize and level of root injury, and sampled western corn rootworm adults from these fields to generate eggs for laboratory bioassays. The results of this study highlight the need to incorporate Bt maize into a broader integrated pest management strategy in order to preserve the efficacy of this technology and its associated benefits.
Results
In 2010, we visited 14 fields (). With the exception of S3, we visited these fields in response to notifications from farmers and crop consultants of rootworm injury to Bt maize in 2010. Seven of the 2010 populations had evidence of severe rootworm feeding, with greater than 1 node of roots injured per plant, while the other seven fields had lower levels of root injury ().
Table 1. Fields visited in Iowa in 2010
From the 14 fields visited in 2010, we were able to obtain sufficient eggs to run bioassays for seven populations ( and and ). All seven of these fields had a history of continuous maize cultivation, with only two of the seven fields planted to soybeans since 2003, and at most only 1 y of soybeans grown in either of these two fields ( and ). Fields sampled in 2010 had been planted to Cry3Bb1 maize for at least 3 y and up to 7 y ( and ). Six of the seven fields were planted to Cry3Bb1 maize in 2010 () and injury to Cry3Bb1 maize among these fields ranged from 0.48 to 2.57 nodes (). Field S2 was planted to Cry34/35Ab1 maize in 2010 and had an average node injury of 0.45; this field also had a history of 5 y of Cry34/35Ab1 maize along with 3 to 6 y of Cry3Bb1 maize ( and 3). For field S3, which corresponded to field P1 in Gassmann et al.Citation13 we sampled roots of maize pyramided with Cry3Bb1 and Cry34/35Ab1, and observed an average root injury score of 0.02.
Figure 1. Distribution of (A) fields sampled in Iowa for use in bioassays and (B) control sites within the United States. For (B), Iowa is highlighted in gray.
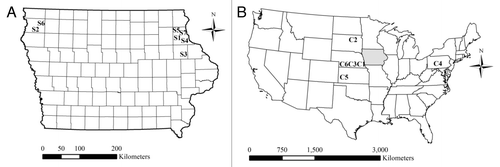
Table 2. Detailed field history for populations used in bioassays in 2010ab
Table 3. Date sampled, root injury, corrected survival and field history for 2010 populations
For survival on Cry3Bb1 maize, there was a significant interaction between maize type and population type (f = 44.83; df = 1.11; p < 0.0001) (). No difference in survival was present between control populations and 2010 populations on non-Bt maize. However, on Cry3Bb1 maize, survival was 11 times higher and significantly greater for 2010 populations than control populations (survival on Cry3Bb1 maize for 2010 populations ÷ survival on Cry3Bb1 maize for control populations = 0.2680 ÷ 0.0234 = 11.4) (). Additionally, we did not detect a significant difference for survival of 2010 populations on non-Bt maize and Cry3Bb1 maize ().
Figure 2. Survival of western corn rootworm on (A) Cry3Bb1 maize and (B) Cry34/35Ab1 maize. Bar heights represent sample means and error bars are the standard error of the mean. Letters indicate pairwise differences among means.
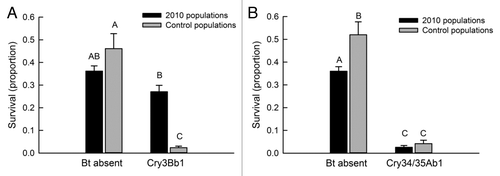
A significant interaction also was present for survival on Cry34/35Ab1 (f = 5.15; df = 1.11; p = 0.04). However, the pattern of survival was substantially different from Cry3Bb1 maize (). Survival on Cry34/35Ab1 maize did not differ between 2010 populations and control populations, and for both population types, survival was significantly lower on Cry34/35Ab1 maize than on non-Bt maize. On non-Bt maize survival was significantly higher for control populations than 2010 populations ().
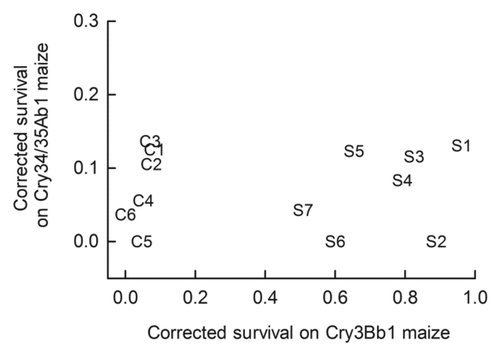
A second set of complementary analyses were conducted using survival on Bt maize that was corrected for survival on the accompanying non-Bt near isoline ( and ). Corrected survival on Cry3Bb1 maize was significantly greater for 2010 populations than control populations (f = 102.61; df = 1.11; p < 0.0001). By contrast, corrected survival did not differ between control populations and 2010 populations on Cry34/35Ab1 maize (f = 0.04; df = 1.11; p = 0.85). Additionally, there was no significant correlation between survival on Cry34/35Ab1 maize and survival on Cry3Bb1 maize (r = 0.05; df = 11; p = 0.87), indicating a lack of cross resistance between these Bt toxins (). Corrected survival on Cry3Bb1 maize was significantly greater for S2 than control populations (t = 37.6; df = 5; p < 0.001).
Table 4. Location, corrected survival and date taken into culture for control populations
Figure 3. Correlation analysis for survival of populations on Cry3Bb1 maize and Cry34/35Ab1 maize. Symbols correspond to and .
For bioassays with Cry3Bb1 maize vs. non-Bt maize a significant interaction between population type and hybrid type was present for the proportion of first and third instar larvae (). Larval development was delayed on Cry3Bb1 maize relative to non-Bt maize for control populations, however, developmental delays were minimal for 2010 populations (). For control populations, there was a significantly higher proportion of first instar larvae and a significantly lower proportion of third instar larvae on Cry3Bb1 maize compared with non-Bt maize, but these differences were not present for 2010 populations. The pattern of larval development on Cry34/35Ab1 maize did not differ between larvae from control populations and 2010 populations, and development was significantly delayed on Cry34/35Ab1 maize (). For both control populations and 2010 populations, there was a significantly higher proportion of first and second instar larvae and a significantly lower proportion of third instar larvae on Cry34/35Ab1 maize compared with non-Bt maize. Of the 72 bioassay cups checked for pupae at the completion of the bioassay, none yielded any pupae, indicating that larvae were not pupating in the bioassay containers.
Table 5. Proportion of insects in each larval instar by population type and maize hybrid
Discussion
We found that western corn rootworm from continuous maize fields with a history of cultivation of Cry3Bb1 maize had significantly higher survival and faster development on Cry3Bb1 maize in laboratory bioassays than did control populations that did not experience selection for Cry3Bb1 resistance in the field (). By contrast, no difference in survival or developmental rate on Cry34/35Ab1 maize was detected between 2010 populations and control populations. In some cases, resistance to Cry3Bb1 maize was associated with severe root injury in the field ( and ). Additionally, we did not detect any correlation among populations for survival on Cry3Bb1 maize and Cry34/35Ab1 maize, indicating a lack of cross resistance between these Bt toxins. These data are consistent with Gassmann et al.Citation13 in which a history of selection on Cry3Bb1 maize in the field resulted in resistance to Cry3Bb1 maize but did not confer cross resistance to Cry34/35Ab1 maize.
Of all fields visited in 2010, seven of 14 fields were planted to Cry3Bb1 maize and had an average node injury rating of greater than 1.0 (). This level of injury is higher than other published accounts of injury for commercialized Cry3Bb1 maize hybrids.Citation55-Citation57 Averaging across several studies, Dun et al.Citation58 found a 17.9% reduction in yield for each node of roots lost to feeding by western corn rootworm, indicating that in seven of the fields visited in 2010 farmers may suffer substantial reductions in yield.
Fields sampled in 2010 had the common feature of having been planted to Cry3Bb1 maize for at least 3 y and as many as 7 y (). The appearance of resistance after three to seven generations of selection is consistent with laboratory selection using Cry3Bb1 maize. Oswald et al.Citation59 found that western corn rootworm strains exposed to incremental selection (increasing by 12 or 24 h of exposure to Cry3Bb1 maize per generation) displayed approximately double the survival on Cry3Bb1 maize after four generations of selection and approximately four times greater survival after eight generations of selection. For intensely selected strains (those exposed to Cry3Bb1 maize for at least 14 d per generation), resistance to Cry3Bb1 maize was measured after seven generations of selection and was found to be approximately four times higher than unselected strains. Meihls et al.Citation45 also found a rapid response to selection on Cry3Bb1 maize. After three generations in which larvae were continuously exposed to Cry3Bb1 maize, larval survival did not differ on Cry3Bb1 maize and non-Bt maize. By contrast, the unselected control colony had significantly lower survival on Cry3Bb1 maize than non-Bt maize.Citation45 After six generations of selection, larval survival and survival to adulthood did not differ between non-Bt maize and Cry3Bb1 maize for the selected strain but was significantly lower on Cry3Bb1 maize for the unselected strain.Citation45
Because of the similarity between laboratory selection experiments with Cry3Bb1 maize and results observed in the field here and in Gassmann et al.,Citation13,Citation49 data from laboratory selection experiments with other Bt traits targeting western corn rootworm may be helpful in predicting durability in the field. Lefko et al.Citation60 found that under continuous selection on Cry34/35Ab1 maize, resistance levels increased but fluctuated considerably for the initial four to six generations of selection. However, after five to seven generations of selection, resistance remained stable in two replicated lines and selected lines displayed survival that was at least 10 times greater than observed during the first generation of selection.Citation60 Simulation modeling also predicted a plateau in the response to selection following six generations of selection.Citation60 Under laboratory selection on mCry3a maize, Meihls et al.Citation61 found significantly greater survival after four generations of selection, a pattern that remained after populations were exposed to an additional three and seven generations of selection. Because these selection experiments were conducted without an infusion of unselected (i.e., refuge) insects, the data may be viewed as a worst case scenario. However, given the low rate of pest dispersal and lack of compliance in refuge planting, this worst case scenario will likely be realized in some fields.Citation21,Citation47,Citation48 Thus, for Bt maize with only one rootworm active Bt toxin that is not a high dose event, continuous use of the same toxin for three to five growing seasons may lead to resistance in some cases.
Currently, the mechanism of field-evolved resistance to Cry3Bb1 maize in western corn rootworm is unknown. Analysis of resistance ratios (LC50 of resistant ÷ LC50 of susceptible) for laboratory selected strains of western corn rootworm found a resistance ratio of 22 for a Cry3Bb1-selected strain and of 15.4 for a mCry3A-selected strain.Citation45,Citation61 These values are lower than those found for some Bt-resistant Lepidoptera. For example, resistance ratios between 520 and 1,700 were found for strains of pink bollworm, Pectinophora gossypiella, with resistance to Cry1Ac cotton.Citation62 Because Cry3Bb1 maize is not a high dose event, and some susceptible insects can survive on Bt plants, relatively small resistance ratios (e.g., 10 to 20) may substantially enhance survival on Bt maize. This hypothesis is consistent with the bioassay data reported here, in which field-selected populations had 11 times higher survival on Cry3Bb1 maize than control populations.
Survival on non-Bt maize in bioassays suggests that fitness costs may be associated with field-evolved resistance to Cry3Bb1 maize, because in one of two cases tested, 2010 populations displayed significantly lower survival on non-Bt maize than control populations (). However, this effect also may have resulted from control populations undergoing selection for survival in laboratory conditions, because they had been kept in laboratory culture for an average of 13 generations (). Fitness costs often accompany Bt resistance, although few data are currently available for western corn rootworm.Citation13,Citation63 Analysis of data on Bt resistance across insect taxa by Gassmann et al.Citation63 found that the magnitude of fitness costs was positively correlated with resistance ratio, with more resistant strains suffering greater fitness costs. As such, if relatively small resistance ratios are associated with Bt resistance in western corn rootworm, then fitness costs may be less than those observed for insects with high levels of Bt resistance, such as pink bollworm.
While many fields studied here were planted at least in part to Cry3Bb1 maize in 2010, field S2 was planted to Cry34/35Ab1 maize (). Although resistance to Cry3Bb1 was found in S2 (see Results), that field had not been planted to Cry3Bb1 maize since 2008, which raises the possibility that once selected, Bt resistance might persist in a population. In a laboratory-selected strain, resistance was found to remain at a similar level after six generations without selection.Citation45 Parallels may be present with resistance to the insecticide aldrin, which took several generations to build within populations of western corn rootworm but has since persisted for more than 20 y in the absence of selection.Citation64
While resistance to Cry3Bb1 was associated with three or more years of cultivation for Cry3Bb1 maize, not all fields experience severe rootworm injury to Cry3Bb1 maize (). This is to be expected because root injury in the field will be a function of resistance and pest abundance.Citation65 Levels of severe root injury to Bt maize are expected only when resistant pests reach a sufficiently high abundance in the field. Additionally, survival of western corn rootworm larvae, and subsequent root injury, may be affected by field conditions such as soil type and degree of soil saturation.Citation66 This too will influence whether or not a Bt-resistant population imposes severe root injury in the field.
Because Bt toxins targeting corn rootworm are not present in high-dose events, pyramiding multiple Bt toxins is likely the optimal insect resistance management strategy.Citation34 However, for fields in which resistance is present to one Bt toxin, the benefits of pyramiding likely will be diminished. Computer modeling suggests that for Bt crops containing events that are less than high dose, the durability of pyramids is contingent on the frequency of resistance alleles at the time the pyramid is deployed. For example, RoushCitation34 found that sequential deployment vs. pyramiding provided similar delays in resistance once resistance allele frequencies exceeded ca. 0.01, but at lower frequencies of resistance alleles, pyramiding Bt events provided longer delays in resistance than sequentially releasing Bt events. Similarly, Gould et al.Citation67 reported that an order of magnitude increase in resistance allele frequency (i.e., from 0.001 to 0.01) to an order of magnitude decrease in the time until a pest population developed resistance to a pyramid. Thus, for Bt events that are less than high dose, releasing events initially as pyramids rather than single events followed by pyramids is a better resistance management strategy.
Better use of integrated pest management in conjunction with robust insect resistance management will be essential for maintaining the viability of Cry3Bb1 maize and likely all types of Bt maize. A common pattern observed among fields in this study and in 2009 was continuous maize cultivation and continuous use of Cry3Bb1 maize.Citation13 Pest susceptibility to management tactics is often a non-renewable resource that is expended in the process of managing a pest. However, the rate at which this occurs will be driven, in part, by how frequently a management practice is applied. By applying a greater diversity of practices such as crop rotation, cultivation of different Bt events and use of non-Bt maize with soil insecticides, selection for resistance to any single Bt toxin will be diminished. Bt maize for management of western corn rootworm is a valuable tool, but both laboratory and field data show that there are limits to the durability of this technology. Better incorporation of Bt maize into integrated pest management for western corn rootworm is likely the best management option to deal with future challenges.
Methods
During the summer of 2010, fields were visited in Iowa in response to grower reports of rootworm injury to Bt maize targeting corn rootworm (). One of the fields (Field S3 in and , and ) was visited to follow-up on a field that had been sampled in 2009 and was reported as field P1 in Gassmann et al.Citation13 In 2010 S3 was planted to a combination of Cry3Bb1 maize and pyramided maize with Cry3Bb1 and Cry34/35Ab1. Three additional problem fields described in Gassmann et al.Citation13 were not included in this study. The reason for these omissions was that one had been rotated to soybeans (field P2 in ref. Citation13), and consequently did not allow for survival of larval western corn rootworm. A second field could not be visited for logistical reasons (field P3 in ref. Citation13). A maize field that was ca. 0.75 Km from P4 in Gassmann et al.Citation13 was sampled (root injury to Cry3Bb1 maize = 0.52 ± 0.15 nodes, mean ± standard deviation, n = 5 roots), but we obtained only 400 eggs and could not run bioassays.
Upon visiting a field, samples of the predominant rootworm species were collected, which was western corn rootworm in all cases. For each population used in bioassays, we sampled between 91 and 429 adult western corn rootworm per field (mean = 200; standard deviation = 115). Data on the history of crops grown in the field was gathered by interviewing farmers, crop consultants, and local extension personnel.
We sampled five roots from each field. Roots were not sampled at random but were sampled to confirm the presence of rootworm feeding. When possible roots were sampled from a portion of the field in which plants displayed rootworm injury, which included plants that were lodged (i.e., listing) and goosenecked (i.e., plants that were bent at base in an attempt to grow upright after becoming lodged). Our sampling methodology was to walk into a field at least 10 m and scan a row for approximately 5 sec. If a plant was noticeably goose necked or lodged, that plant was sampled. If all plants appeared the same, a plant was selected haphazardly. Any plants that displayed stunted or lacked a full sized ear were not sampled. After digging a root, we moved approximately 10 m toward the interior of the field, and sampled another plant following the same methodology. For all plants sampled, the Bt toxin that the farmer thought was present in the maize (i.e., Cry3Bb1, Cry34/35Ab1 or a pyramid of the two) was confirmed by ELISA with a kit (Envirologix, Portland, Maine).
Western corn rootworm adults were brought to the laboratory and each population was held in an individual cage (18 cm × 18 cm × 18 cm L × W × H) (Megaview Science, Taiwan) in a growth chamber (25°C; 16/8 L/D). Cages contained maize leaf tissue and an artificial diet (western corn rootworm diet, Bio-Serv, Frenchtown, New Jersey), as food for the rootworm adults, and a water source provided by a 1.5% agar solid. Adults were provided with an oviposition substrate that consisted of moist, finely sieved soil (< 180 ∝m) placed in a Petri dish (diameter = 10 cm). Eggs obtained from each population were placed separately in 45 mL plastic cups containing moistened sieved soil, sealed in a plastic bag, and placed in a cold room at 6°C for at least 5 mo to break diapause. Eggs were held in a cold room until their removal for bioassays. For fields visited in 2010 (), we conducted bioassays on all populations from which we obtained at least 3,000 eggs, which was the minimum number needed to ensure sufficient replication of the bioassay across all maize hybrids evaluated.
For control populations, we obtained eggs from the United States Department of Agriculture’s North Central Agricultural Research Laboratory (NCARL) in Brookings, South Dakota ( and ). All control populations were diapausing strains of western corn rootworm that were brought into the laboratory prior to 2003, which is the year that Bt maize was commercialized for management of western corn rootworm. Thus, control populations represent field populations that never experienced selection for resistance to Bt maize. Eggs of control populations were sent from NCARL to Iowa State University in diapause and upon arrival at Iowa State University were placed in a cold room at 6°C for later use in bioassays.
For both 2010 populations and control populations, neonate larvae were obtained for bioassays by removing eggs from 6°C after at least 5 mo had passed. Following exposure to cold, eggs were stored for 1 week at 25°C. Eggs were then washed from the soil using a screen with 250 ∝m openings and then placed atop moistened sieved soil held in a 10 cm Petri dish. Neonate larvae began hatching approximately 1 week thereafter.
Neonate larvae from 2010 populations and from control populations were evaluated for their survival on transgenic maize following Gassmann et al.Citation13 We used two transgenic maize hybrids, each of which contained a unique Bt toxin that targets western corn rootworm. The hybrid DKC 6169 (DeKalb Brand, Monsanto Company) produced Cry3Bb1 and the hybrid 2T789 (Mycogen Brand, Dow AgroSciences, Indianapolis, Indiana) produced Cry34/35Ab1. For both hybrids, we also measured survival of rootworm on a near isogenic hybrid that lacked a gene for a rootworm active Bt toxin but otherwise was genetically similar to its respective Bt hybrid. In the case of Cry3Bb1 maize, the non-Bt hybrid was DKC 6172 (DeKalb) and for Cry34/35Ab1 maize the non-Bt hybrid was 2T777 (Mycogen). Prior to their use in bioassays, all maize seeds were soaked for one hour in a 10% bleach solution during which they were stirred every 15 min. Seeds were then rinsed 10 times with deionized water and allowed to dry for at least 24 h. Although seeds did not receive any type of seed treatment, they were bleached to remove any traces of insecticide that may have been present.
Maize plants used in bioassays were grown in the greenhouse (16/8 L/D) in 1 L containers following Gassmann et al.Citation13 with one plant per container. Once plants had five fully formed leaves, they were used in bioassays. For bioassays, plants were first trimmed to a height of 20 cm with two to three leaves (trimmed to 8 cm long) remaining on the plant. Recently hatched larvae (less than 24 h old) were removed from the soil’s surface within their Petri dish using a fine brush and then placed at the base of a maize plant on a root that had been exposed by moving away a small amount of soil. Each plant received 11.85 ± 0.54 larvae (mean ± standard deviation). Although the goal was to place 12 larvae in each cup, in some cases an insufficient number of larvae was available. Cups containing plants, soil and larvae were placed in an incubator (I41LL with light and humidity control, Percival Scientific, Perry, Iowa) for 17 d (25°C, 65% RH, 16/8 L/D) and plants were watered as needed. A length of 17 d was chosen because it provided sufficient time for the majority (ca. 75%) of larvae on non-Bt maize to reach the third and final instar (see ).Citation50 Maize plants remained in their original 1 L containers throughout the bioassay. Bioassays were conducted between February and September, 2011 and bioassays alternated between 2010 populations and control populations during the entire testing period.
After 17 d in an incubator, bioassay cups were removed and the above-ground tissue of the maize plant excised. The soil, containing roots and larvae, was then removed from the 1 L plastic container and placed on a Berlese funnel for 4 d to extract larvae from the soil. Rootworm larvae were collected in 15 mL glass vials containing 10 mL of 85% ethanol. The total number of larvae recovered from each bioassay container was counted. For all larvae, we used a microscope (Leica MZ6) with digital camera and image analysis software (Motic Images Inc.) to measure width of larval head capsules and then determined larval instar based on the scale of Hammack et al.Citation51 To ensure that larvae were not pupating in bioassay cups, we carefully inspected the soil of three bioassay cups for each of the four maize hybrids evaluated (DCK 6169, DCK 6172, 2T777 and 2T789) for three of 2010 populations and three of control populations (n = 72 bioassay cups). Individual bioassays were randomly selected and the soil carefully checked for pupae immediately after removal from a Berlese funnel.
The average sample sizes per population were 11.00 ± 2.18 (mean ± standard deviation) bioassay cups for Cry3Bb1 maize and for its non-Bt near isoline, and 11.00 ± 2.18 bioassay cups for Cry34/35Ab1 maize and for its non-Bt near isoline. In all, there were seven 2010 populations and six control populations, and a total of 572 bioassay cups were run for the entire experiment. Rootworm survival per bioassay cup was calculated as the proportion of larvae recovered after 17 d divided by the number of neonate larvae placed into that cup. For larvae recovered from each bioassay cup, we calculated the proportion in each of the three larval instars.
Data analysis.
All data analyses were conducted using SAS Enterprise Guide 4.2.Citation52 Data on the proportion of rootworm surviving per bioassay cup and the proportion of insects in each larval instar were analyzed with a mixed model analysis of variance (ANOVA) (PROC MIXED in SAS). Data were analyzed separately for Cry34/35Ab1 maize and Cry3Bb1 maize. Data were transformed by the arcsine of the square root to ensure normality of the residuals. Fixed effects in the model included maize type (Bt maize vs. non-Bt maize) and population type (control population vs. 2010 population). Random factors in the analysis were population nested within population type, and the interaction of maize type with population nested within population type. When a significant interaction between maize type and population type was present, pairwise comparisons were made among least-squares means using the PDIFF option in PROC MIXED with an experimentwise error rate of p < 0.05.
For each population, rootworm survival on Bt maize was corrected for survival on non-Bt maize. We calculated corrected survival on Bt maize as the complement of corrected mortality based on Abbott’s correction.Citation53 For each population, survival on Bt maize in each bioassay cup was adjusted for average survival on the non-Bt near isoline. For Cry3Bb1 maize and Cry34/35Ab1 maize, the average corrected survival for each population was compared between 2010 populations and control populations using a one-way ANOVA (PROC ANOVA). We tested the correlation between corrected survival on Cry3Bb1 maize and Cry34/35Ab1 maize using the mean value for each population (PROC CORR). Corrected survival on Cry3Bb1 maize for S2 vs. control populations was compared with a t-test.Citation54
Acknowledgements
We thank Dow AgroSciences and Monsanto for providing maize seed for bioassays. Dr Wade French provided eggs for control populations. This research was supported by the US Department of Agriculture through grants 2009-33120-20256, 2010-33522-21673 and 2011-34103-30620.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- James C. Global Status of Commercialized Biotech/GM Crops: 2010. ISAAA Brief No. 42. Ithaca, New York: ISAAA 2010.
- James C. Global Status of Commercialized Biotech/GM Crops: 2004. ISAAA Brief No. 32. Ithaca NY 2004.
- Hutchison WD, Burkness EC, Mitchell PD, Moon RD, Leslie TW, Fleischer SJ, et al. Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 2010; 330:222 - 5; http://dx.doi.org/10.1126/science.1190242; PMID: 20929774
- Tabashnik BE, Sisterson MS, Ellsworth PC, Dennehy TJ, Antilla L, Liesner L, et al. Suppressing resistance to Bt cotton with sterile insect releases. Nat Biotechnol 2010; 28:1304 - 7; http://dx.doi.org/10.1038/nbt.1704; PMID: 21057498
- Romeis J, Shelton AM, Kennedy GG, eds. Integration of Insect-Resistant Genetically Modified Crops within IPM Programs. New York: Springer 2008.
- Carrière Y, Ellers-Kirk C, Sisterson MS, Antilla L, Whitlow M, Dennehy TJ, et al. Long-term regional suppression of pink bollworm by Bacillus thuringiensis cotton. Proc Natl Acad Sci U S A 2003; 100:1519 - 23; http://dx.doi.org/10.1073/pnas.0436708100; PMID: 12571355
- Huang J, Hu R, Rozelle S, Pray C. Insect-resistant GM rice in farmers’ fields: assessing productivity and health effects in China. Science 2005; 308:688 - 90; http://dx.doi.org/10.1126/science.1108972; PMID: 15860626
- van Rensburg JBJ. First report of field resistance by stem borer, Busseolafusca (Fuller) to Bt-transgenic maize. S Afr J Plant Soil 2007; 24:147 - 51
- Tabashnik BE, Gassmann AJ, Crowder DW, Carriére Y. Insect resistance to Bt crops: evidence versus theory. Nat Biotechnol 2008; 26:199 - 202; http://dx.doi.org/10.1038/nbt1382; PMID: 18259177
- Storer NP, Babcock JM, Schlenz M, Meade T, Thompson GD, Bing JW, et al. Discovery and characterization of field resistance to Bt maize: Spodoptera frugiperda (Lepidoptera: Noctuidae) in Puerto Rico. J Econ Entomol 2010; 103:1031 - 8; http://dx.doi.org/10.1603/EC10040; PMID: 20857709
- Downes S, Parker T, Mahon R. Incipient resistance of Helicoverpa punctigera to the Cry2Ab Bt toxin in Bollgard II cotton. PLoS One 2010; 5:e12567; http://dx.doi.org/10.1371/journal.pone.0012567; PMID: 20830203
- Dhurua S, Gujar GT. Field-evolved resistance to Bt toxin Cry1Ac in the pink bollworm, Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae), from India. Pest Manag Sci 2011; 67:898 - 903; http://dx.doi.org/10.1002/ps.2127; PMID: 21438121
- Gassmann AJ, Petzold-Maxwell JL, Keweshan RS, Dunbar MW. Field-evolved resistance to Bt maize by western corn rootworm. PLoS One 2011; 6:e22629; http://dx.doi.org/10.1371/journal.pone.0022629; PMID: 21829470
- Zhang H, Yin W, Zhao J, Jin L, Yang Y, Wu S, et al. Early warning of cotton bollworm resistance associated with intensive planting of Bt cotton in China. PLoS One 2011; 6:e22874; http://dx.doi.org/10.1371/journal.pone.0022874; PMID: 21857961
- Gould F. Sustainability of transgenic insecticidal cultivars: integrating pest genetics and ecology. Annu Rev Entomol 1998; 43:701 - 26; http://dx.doi.org/10.1146/annurev.ento.43.1.701; PMID: 15012402
- Georghiou GP, Taylor CE. Genetic and biological influences in the evolution of insecticide resistance. J Econ Entomol 1977; 70:319 - 23; PMID: 874142
- Comins HN. The development of insecticide resistance in the presence of migration. J Theor Biol 1977; 64:177 - 97; http://dx.doi.org/10.1016/0022-5193(77)90119-9; PMID: 556789
- Tabashnik BE, Gould F, Carrière Y. Delaying evolution of insect resistance to transgenic crops by decreasing dominance and heritability. J Evol Biol 2004; 17:904 - 12, discussion 913-8; http://dx.doi.org/10.1111/j.1420-9101.2004.00695.x; PMID: 15271091
- Gray ME, Sappington TW, Miller NJ, Moeser J, Bohn MO. Adaptation and invasiveness of western corn rootworm: intensifying research on a worsening pest. Annu Rev Entomol 2009; 54:303 - 21; http://dx.doi.org/10.1146/annurev.ento.54.110807.090434; PMID: 19067634
- Metcalf RL. Forward. In: Krysan JL, Miller TA, Eds. Methods for the Study of Pest Diabrotica. New York: Springer-Verlag 1986; 7-15.
- Spencer JL, Hibbard BE, Moeser J, Onstad DW. Behaviour and ecology of the western corn rootworm (Diabrotica virgifera virgifera LeConte). Agric For Entomol 2009; 11:9 - 27; http://dx.doi.org/10.1111/j.1461-9563.2008.00399.x
- Levine E, Oloumi-Sadeghi H. Management of diabroticite rootworms in corn. Annu Rev Entomol 1991; 36:229 - 55; http://dx.doi.org/10.1146/annurev.en.36.010191.001305
- Miller N, Estoup A, Toepfer S, Bourguet D, Lapchin L, Derridj S, et al. Multiple transatlantic introductions of the western corn rootworm. Science 2005; 310:992; http://dx.doi.org/10.1126/science.1115871; PMID: 16284172
- Kiss J, Edwards CR, Berger HK, Cate P, Cean M, Cheek S, et al. Monitoring of western corn rootwom (Diabrotica virgifera virgifera LeConte) in Europe 1992–2003. In: Vidal S, Kuhlmann U, Edwards CR, Eds. Western Corn Rootworm: Ecology and Management. Cambridge: CAB International 2005.
- Levine E, Oloumi-Sadeghi H. Western corn rootworm (Coleoptera: Chrysomelidae) larval injury to corn grown for seed production following soybeans grown for seed production. J Econ Entomol 1996; 89:1010 - 6
- Ball HJ, Weekman GT. Insecticide resistance in the adult western corn rootworm in Nebraska. J Econ Entomol 1962; 55:439 - 41
- Meinke LJ, Siegfried BD, Wright RJ, Chandler LD. Adult susceptibility of Nebraska western corn rootworm (Coleoptera: Chrysomelidae) populations to selected insecticides. J Econ Entomol 1998; 91:594 - 600
- Spencer JL, Levine E, Isard SA, Mabry TR. Movement, dispersal and behaviour of western corn rootworm adults in rotated maize and soybean fields. In: Vidal S, Kuhlmann U, Edwards CR, Eds. Western Corn Rootworm: Ecology and Management Cambridge: CABI Publishing 2005; 121-44.
- Zhou X, Scharf ME, Meinke LJ, Chandler LD, Siegfried BD. Immunological assessment of an insecticide resistance-associated esterase in the Western corn rootworm. Arch Insect Biochem Physiol 2005; 58:157 - 65; http://dx.doi.org/10.1002/arch.20040; PMID: 15717320
- Metcalf RL. The ecology of insecticides and the chemical control of insects. In: Kogan M, Ed. Ecological Theory and Integrated Pest Management Practices. New York: Wiley 1986; 251-97.
- Carrière Y, Crowder DW, Tabashnik BE. Evolutionary ecology of insect adaptation to Bt crops. Evol Appl 2010; 3:561 - 73; http://dx.doi.org/10.1111/j.1752-4571.2010.00129.x
- Carrière Y, Tabashnik BE. Reversing insect adaptation to transgenic insecticidal plants. Proc Biol Sci 2001; 268:1475 - 80; http://dx.doi.org/10.1098/rspb.2001.1689; PMID: 11454291
- EPA. Plant Incorporated Protectants. Accessed at http://www.epa.gov/oppbppd1/biopesticides/pips/.2007.
- Roush RT. Two-toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not?. Philos Trans R Soc Lond B Biol Sci 1998; 353:1777 - 86; http://dx.doi.org/10.1098/rstb.1998.0330
- Roush RT. Bt-transgenic crops: just another pretty insecticide or a chance for a new start in resistance management?. Pestic Sci 1997; 51:328 - 34; http://dx.doi.org/10.1002/(SICI)1096-9063(199711)51:3<328::AID-PS650>3.0.CO;2-B
- Storer NP. A spatially explicit model simulating western corn rootworm (Coleoptera: Chrysomelidae) adaptation to insect-resistant maize. J Econ Entomol 2003; 96:1530 - 47; http://dx.doi.org/10.1603/0022-0493-96.5.1530; PMID: 14650529
- Onstad DW, Guse CA, Spencer JL, Levine E, Gray ME. Modeling the dynamics of adaptation to transgenic corn by western corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol 2001; 94:529 - 40; http://dx.doi.org/10.1603/0022-0493-94.2.529; PMID: 11332850
- Gassmann AJ, Stock SP, Sisterson MS, Carrière Y, Tabashnik BE. Synergism between entomopathogenic nematodes and Bacillus thuringiensis crops: integrating biological control and resistance management. J Appl Ecol 2008; 45:957 - 66; http://dx.doi.org/10.1111/j.1365-2664.2008.01457.x
- EPA. Final Report of the FIFRA Scientific Advisory Panel Subpanel on Bacillus thuringiensis (Bt) Plant-Pesticides and Resistance Management. Accessed at http://www.epa.gov/scipoly/sap/meetings/1998/february/finalfeb.pdf.1998.
- Binning RR, Lefko SA, Millsap AY, Thompson SD, Nowatzki TM. Estimating western corn rootworm (Coleoptera: Chrysomelidae) larval susceptibility to event DAS-59122-7 maize. J Appl Entomol 2010; 134:551 - 61
- Hibbard BE, Meihls LN, Ellersieck MR, Onstad DW. Density-dependent and density-independent mortality of the western corn rootworm: impact on dose calculations of rootworm-resistant Bt corn. J Econ Entomol 2010; 103:77 - 84; http://dx.doi.org/10.1603/EC09277; PMID: 20214371
- Oyediran IO, Hibbard BE, Clark TL. Western corn rootworm (Coleoptera: Chrysomelidae) beetle emergence from weedy Cry3Bbl rootworm-resistant transgenic corn. J Econ Entomol 2005; 98:1679 - 84; http://dx.doi.org/10.1603/0022-0493-98.5.1679; PMID: 16334339
- Oyediran IO, Higdon ML, Clark TL, Hibbard BE. Interactions of alternate hosts, postemergence grass control, and rootworm-resistant transgenic corn on western corn rootworm (Coleoptera: Chrysomelidae) damage and adult emergence. J Econ Entomol 2007; 100:557 - 65; http://dx.doi.org/10.1603/0022-0493(2007)100[557:IOAHPG]2.0.CO;2; PMID: 17461083
- Petzold-Maxwell JL, Jaronski ST, Gassmann AJ. Tritrophic interactions among Bt maize, an insect pest and entomopathogens: effects on development and survival of western corn rootworm. Ann Appl Biol 2012; 160:43 - 55; http://dx.doi.org/10.1111/j.1744-7348.2011.00515.x
- Meihls LN, Higdon ML, Siegfried BD, Miller NJ, Sappington TW, Ellersieck MR, et al. Increased survival of western corn rootworm on transgenic corn within three generations of on-plant greenhouse selection. Proc Natl Acad Sci U S A 2008; 105:19177 - 82; http://dx.doi.org/10.1073/pnas.0805565105; PMID: 19047626
- Hibbard BE, Frank DL, Kurtz R, Boudreau E, Ellersieck MR, Odhiambo JF. Mortality impact of Bt transgenic maize roots expressing eCry3.1Ab, mCry3A, and eCry3.1Ab plus mCry3A on western corn rootworm larvae in the field. J Econ Entomol 2011; 104:1584 - 91; http://dx.doi.org/10.1603/EC11186; PMID: 22066188
- Jaffe G. Complacency on the Farm: Significant Noncompliance with EPA’s Refuge Requirements Threatens the Future Effectiveness of Genetically Engineered Pest-protected Corn. Washington, DC: Center for Science in the Public Interest 2009.
- Spencer JL, Mabry TR, Vaughn TT. Use of transgenic plants to measure insect herbivore movement. J Econ Entomol 2003; 96:1738 - 49; http://dx.doi.org/10.1603/0022-0493-96.6.1738; PMID: 14977111
- Gassmann AJ. Field-evolved resistance to Bt maize by western corn rootworm: predictions from the laboratory and effects in the field. J Invertebr Pathol 2012; 110:287 - 93; http://dx.doi.org/10.1016/j.jip.2012.04.006; PMID: 22537837
- Nowatzki TM, Lefko SA, Binning RR, Thompson SD, Spencer TA, Siegfried BD. Validation of a novel resistance monitoring technique for corn rootworm (Coleoptera: Chrysomelidae) and event DAS-59122-7 maize. J Appl Entomol 2008; 132:177 - 88; http://dx.doi.org/10.1111/j.1439-0418.2008.01270.x
- Hammack L, Ellsbury MM, Roehrdanz RL, Pikul JLJ Jr.. Larval sampling and instar determination in field populations of northern and western corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol 2003; 96:1153 - 9; http://dx.doi.org/10.1603/0022-0493-96.4.1153; PMID: 14503586
- SAS. SAS Enterprise Guide 4.2. Cary: SAS Institute Inc. 2008.
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol 1925; 18:265 - 7
- Sokal RR, Rohlf FJ. Biometry. New York: WH Freeman and Company 1995.
- Coulter JA, Nafziger ED, Janssen MR, Pedersen P. Response of Bt near-isoline corn hybrids to plant density. Agron J 2010; 102:103 - 11; http://dx.doi.org/10.2134/agronj2009.0217
- Ma BL, Meloche F, Wei L. Agronomic assessment of Bt trait and seed or soil-applied insecticides on control of corn rootworm and yield. Field Crops Res 2009; 111:189 - 96; http://dx.doi.org/10.1016/j.fcr.2008.12.006
- Gray ME, Steffey KL, Estes RE, Schroeder JB. Responses of transgenic maize hybrids to variant western corn rootworm larval injury. J Appl Entomol 2007; 131:386 - 90; http://dx.doi.org/10.1111/j.1439-0418.2007.01171.x
- Dun Z, Mitchell PD, Agosti M. Estimating Diabrotica virgifera virgifera damage functions with field trial data: applying an unbalanced nested error component model. J Appl Entomol 2010; 134:409 - 19; http://dx.doi.org/10.1111/j.1439-0418.2009.01487.x
- Oswald KJ, French BW, Nielson C, Bagley M. Selection for Cry3Bb1 resistance in a genetically diverse population of nondiapausing western corn rootworm (Coleoptera: Chrysomelidae). J Econ Entomol 2011; 104:1038 - 44; http://dx.doi.org/10.1603/EC10312; PMID: 21735927
- Lefko SA, Nowatzki TM, Thompson SD, Binning RR, Pascual MA, Peters ML, et al. Characterizing laboratory colonies of western corn rootworm (Coleoptera: Chrysomelidae) selected for survival on maize containing event DAS-59122-7. J Appl Entomol 2008; 132:189 - 204; http://dx.doi.org/10.1111/j.1439-0418.2008.01279.x
- Meihls LN, Higdon ML, Ellersieck M, Hibbard BE. Selection for resistance to mCry3A-expressing transgenic corn in western corn rootworm. J Econ Entomol 2011; 104:1045 - 54; http://dx.doi.org/10.1603/EC10320; PMID: 21735928
- Tabashnik BE, Biggs RW, Higginson DM, Henderson S, Unnithan DC, Unnithan GC, et al. Association between resistance to Bt cotton and cadherin genotype in pink bollworm. J Econ Entomol 2005; 98:635 - 44; http://dx.doi.org/10.1603/0022-0493-98.3.635; PMID: 16022286
- Gassmann AJ, Carrière Y, Tabashnik BE. Fitness costs of insect resistance to Bacillus thuringiensis.. Annu Rev Entomol 2009; 54:147 - 63; http://dx.doi.org/10.1146/annurev.ento.54.110807.090518; PMID: 19067630
- Parimi S, Meinke LJ, French WB, Chandler LD, Siegfried BD. Stability and persistence of aldrin and methyl-parathion resistance in western corn rootworm populations (Coleoptera: Chrysomelidae). Crop Prot 2006; 25:269 - 74; http://dx.doi.org/10.1016/j.cropro.2005.04.017
- Tabashnik BE, Van Rensburg JBJ, Carrière Y. Field-evolved insect resistance to Bt crops: definition, theory, and data. J Econ Entomol 2009; 102:2011 - 25; http://dx.doi.org/10.1603/029.102.0601; PMID: 20069826
- Toepfer S, Kuhlmann U. Natural mortality factors acting on western corn rootworm populations: a comparison between the United States and Central Europe. In: Vidal S, Kuhlmann U, Edwards CR, Eds. Western Corn Rootworm: Ecology and Management. Wallingford: CABI Publishing 2005; 95-119.
- Gould F, Cohen MB, Bentur JS, Kennedy GG, Van Duyn J. Impact of small fitness costs on pest adaptation to crop varieties with multiple toxins: a heuristic model. J Econ Entomol 2006; 99:2091 - 9; http://dx.doi.org/10.1603/0022-0493-99.6.2091; PMID: 17195678