Abstract
On September 9, 2009, Ethiopia enacted a highly restrictive biosafety law firmly based on precautionary principles as a foundation for its GMO regulation system. Its drafting process, led by the country’s Environmental Protection Authority, was judged as biased, focusing only on protecting the environment from perceived risks, giving little attention to potential benefits of GMOs. Many of its provisions are very stringent, exceeding those of Cartagena Protocol on Biosafety, while others cannot be fulfilled by applicants, collectively rendering the emerged biosafety system unworkable. These provisions include requirements for advance informed agreement and rigorous socioeconomic assessment in risk evaluation for all GMO transactions, including contained research use—which requires the head of the competent national authority of the exporting country to take full responsibility for GMO-related information provided—and stringent labeling, insurance and monitoring requirements for all GMO activities. Furthermore, there is no provision to establish an independent national biosafety decision-making body(ies). As a result, foreign technology owners that provide highly demanded technologies like Bt cotton declined to work with Ethiopia. There is a fear that the emerged biosafety system might also continue to suppress domestic genetic engineering research and development. Thus, to benefit from GMOs, Ethiopia has to revise its biosafety system, primarily by making changes to some provisions of the law in a way that balances its diverse interests of conserving biodiversity, protecting the environment and enhancing competition in agricultural and other economic sectors.
Introduction
Applications of modern biotechnologies offer huge benefits in agricultural, medical, industrial and environmental sectors throughout the world. In agriculture in particular, crops developed through genetic engineering have a considerable positive impact in the area of crop pest management in many countries. Notwithstanding the great potential for benefits that this technology could bring to society, there is a common understanding within the international community that a balanced and comprehensive approach to biosafety is needed to evaluate the possible adverse effects of these products on the environment and human health. Although Ethiopia has made significant progress in the last decade by using more simple biotechnology applications,Citation1 its approach toward products of genetic engineering, commonly referred to as genetically modified organisms (GMOs), has been skeptical and highly cautious, focusing mostly on avoiding the perceived environmental and socioeconomic risks rather than harnessing the potential benefits. The main reasons for Ethiopia to take this stance on GMOs include the perceived damage to biodiversity, including crop landraces and wild relatives; the assumption that GMOs are incompatible with Ethiopian farming systems, particularly with poor smallholders; the perception that GMOs are a threat to the country’s agricultural exports and concerns about farmers’ dependence on multinational companies for patented seeds.Citation2 However, these claims are strongly contested by supporters of the regulated use of GMOs who see them as potential sources of improved agricultural productivity and environmental sustainability resulting in, for example, reduced application of chemicals.Citation1,Citation2 In general, however, the fact that the country is one of the seven major Vavilov centers of origin and diversity for crops—and concerns over the possible negative impact of GMOs on this biodiversity—dominated the national biosafety debates and continues to largely influence its GMO policy.Citation2
To ensure safe use of GMOs for the benefit of their people, countries have instituted biosafety systems as a mechanism for making informed decisions, taking into account their national interest and international obligations. Ethiopia has played a leading role in international biosafety negotiations, encouraging developing countries to adopt strict regulation of GMOs, firmly based on the precautionary principle.Citation3 At a national level, the country explicitly stated in its environmental policy document that the importation and use of biological material, including those that were genetically engineered, should be under stringent regulations.Citation4 Accordingly, significant domestic or donor-granted resources have been invested in setting up the country’s biosafety framework5. On the contrary, much less attention was given to developing domestic capacity in terms of infrastructure and manpower to initiate genetic engineering activities aimed at safe use of the technology to address priority national problems.Citation1,Citation2 As a result of this imbalance in approach to GMOs, no genetic engineering research or development activity has yet been started in the country. Regarding GMO regulation, the prevailing opinion in Ethiopia, spearheaded by its Environmental Protection Authority (EPA), was that a standalone biosafety law is a prerequisite for initiating any genetic engineering activity, including research. This position contrasts with that of many African countries with permissive GMO policies (e.g., Kenya, Uganda, South Africa and Egypt), which have been using interim biosafety regulations formulated using the existing laws or ministerial decrees to facilitate genetic engineering research long before their respective biosafety laws were in place.
After signing the Cartagena Protocol on Biosafety to the Convention on Biological DiversityCitation6 (hereafter called the Protocol) on May 24, 2000 and ratifying it on September 22, 2003, Ethiopia started to formulate its national biosafety framework with the assistance of UNEP-GEF.Citation5 The main outcomes of these efforts were the entering into force of the Ethiopian Biosafety LawCitation7 on September 9, 2009, as Proclamation No. 655/2009 and its six accompanying directives (Directive Nos. 1–6/2009)Citation8 issued pursuant to it as implementing guidelines. Various sectors of the society have debated the implications this law will have on national biotechnology development in different national fora and accepted the law with some level of optimism. Some stakeholders, including environmental groups, hailed the law as a mechanism to ensure the safety of the environment, human health and the quality of socio-economic and cultural conditions from the risks arising from GMOs. Similarly, the biotechnology community considered the enactment of this law to be a step forward to fill the legislative and regulatory vacuum that prevented GMO research and development. However, as the provisions of the law were examined in detail and tested for practical applications shortly afterward, it became clear to the proponents of biotechnology that contrary to their expectations, the long-awaited law has several highly restrictive preconditions that acted as disincentives to those parties interested in GMO activities, thereby leading to the institutionalization of an unworkable biosafety system. Although over three years have passed since its enforcement, no attempt has been made to comprehensively examine the biosafety law with respect to the Protocol, international experience with functional biosafety systems and prevailing national trends and interests on using GMOs. Moreover, the current impact and future implications of the legislation on modern biotechnology development in the country was not thoroughly assessed and documented. This paper intends to fill this gap by analyzing information obtained from available legal and technical documents, published reports, personal interactions and global assessments and suggest ways to move forward.
In the next section of this paper, the active actors involved and their role in the biosafety bill drafting process is assessed. In section three, Ethiopia’s yet unsuccessful attempt to access and adopt Bt cotton technology is described. Section four presents the analysis of the main features and provisions of the law that have collectively made it unworkable from the author’s viewpoint. In section five, the main issues arising from the analysis are discussed. The paper then ends up with recommendations and conclusions. The author hopes that this paper will stimulate further debate and dialog on biosafety issues and encourage concerned parties to take necessary actions to enable responsible use of GMOs in the country.
The Biosafety Bill Drafting Process: Did It Take into Account Diverse Views of the Key Stakeholders on GMOs?
A brief examination of the biosafety bill drafting process is believed to provide the reader with background information on how the legislation was developed. In his two consecutive papers based primarily on detailed interviews of participants involved in biosafety bill drafting, Ayele,Citation2,Citation9 inter alia, critically examined the process that led to the drafting of the biosafety bill and ultimately enactment of the law. Accordingly, the Ethiopian Environmental Protection Authority (EPA) took the lead in the implementation of the drafting process. Under its leadership, a national steering committee (SC) comprised of representatives from 33 institutions was established, largely drawn from government organizations and academia.Citation5,Citation9 Representation on the committee outside of government organizations was limited to only two environmental groups and one consumer protection advocacy group.Citation5 No key pro-biotechnology stakeholders were represented, including the transnational private sector technology providers, non-governmental organizations supporting the safe use of biotechnology or farmers’ groups who are potential users of GM technology.Citation5 As a result, there was hardly any debate on biotechnology and biosafety issues with these key stakeholders.Citation2
Recounting the initial stages of the bill drafting process, AyeleCitation9 states, “Major differences emerged before long, among actors within and outside the SC, over the process of developing the draft bill, its content and the proposed location of GMO administration. Although views about genetic engineering varied within and among the organizations, in this instance, it was clear that the major split was between EPA authorities on one hand, and on the other, key representatives to the Committee, namely those drawn from Ethiopian Institute of Agricultural Research (EIAR), Institute of Biodiveristy Conservation (IBC) and Addis Ababa University (AAU). Almost all the EIAR, IBC and AAU scientists interviewed made it clear that their role in the process was at best marginal as (1) legal and technical documents were prepared by EPA lawyers and consultants under its guidance, (2) EPA was made, by default, the competent authority for GMO administration and (3) EPA proposed the adoption of the “protective” principles and criteria of the African Model Law on Safety in Biotechnology, which, according to the interviewees, potentially limit the development of useful modern biotechnologies in the country.”
Hence, it appears that guiding the process to a compromise reflecting the national consensus was not possible, as views of the EPA authorities dominated the process. AyeleCitation9 continues, stating, “Some interviewees suggested that any one organization with “particular interest”—one way or the other—should not lead on the implementation of the biosafety framework nor become a competent authority. The overwhelming view, however, was that whoever champions the process should be competent, work with other actors and seeks to produce a national consensus over the matter.” AyeleCitation2 further describes the exclusion of key stakeholders in the process by stating, “Active proponents of biotechnology, mostly scientists, were hence out of the process early in the development of the bill and there was no room for the participation of the technology providers or potential users.” Based on the analysis, he concludes, “The biosafety rule-making and institutionalization processes were perceived to have failed to find a way through the competing views and concerns over GMOs, leaving sufficiently potent ground for contesting impending decisions on GMO activitiesCitation9.”
The two years (2008 and 2009) preceding the approval of the biosafety bill into law coincided with the time when EIAR was directed by the Ethiopian government via the Ministry of Agriculture and Rural Development to conduct field experiments on genetically modified (GM) insect resistant (Bt) cotton and to generate and report data on its benefits and drawbacks. It soon became clear from the final draft of the biosafety bill made accessible to the publicCitation5 that the provisions in the forthcoming law would be very restrictive and unlikely to facilitate the planned GMO research and future commercialization. Late efforts to introduce some changes to the bill before its approval into law were not successful. Hence, with few modifications in the process, the bill was ratified on June 7, 2009, by the House of Peoples Representatives with hardly any debate or questionsCitation10 and was eventually enforced on September 9, 2009.Citation7
It is evident from the above account that the bill drafting process failed to accommodate the competing views of the key stakeholders and hence failed to represent the national consensus. In particular, the voice of the biotechnology community—represented mainly by scientists from public research and higher learning institutions who are potential domestic technology developers or importers of technology from abroad—was silenced, as they were excluded or marginalized early on in the process. Moreover, foreign private technology providers and their representatives that are expected to play key roles in future technology access and adoption were not given an opportunity to give their input regarding the emerging biosafety system. It was therefore expected from the outset that the resulting law would be unable to balance the interests of key stakeholders at opposite sides of GMO debate. Thus, finding a middle ground based on national consensus through debate and negotiations between the environmental protection agenda of the country and the country’s need to harness the benefit of GMOs has not been possible.
Ethiopia’s Interest in Bt Cotton Technology and Problems Faced in its Acquisition
EIAR was directed by the government in 2008 to start a field experiment with Bt cottonCitation11 at a time when there was no GMO regulation mechanism in the country. As a first step, EIAR requested and obtained interim permission from the EPA to start the experiment, which was proposed by the draft biosafety bill as a competent national authority. Second, to access the Bt cotton technology, EIAR officially requested in 2008 that the US-based private technology provider, Monsanto, provide Bt cotton varieties for research purposes and collaborate in their implementation. However, Monsanto replied that they could not provide Bt cotton technology to Ethiopia unless the country revised what they considered a stringent and unworkable biosafety system.Citation11 For similar reasons, attempts to strike a technology access and transfer agreement in 2009 between the EIAR and the Chinese Academy of Agricultural ScienceCitation11 that would obtain Chinese Bt cotton technology were also not successful. Hence, at the time of writing of this paper, Ethiopia did not have access to Bt cotton or any other GM technology, even for research purposes.
The Main Features of the Law that Likely Hinder GMO Research, Development and Trade
Provisions exceeding those of Cartagena Protocol on Biosafety
Requirement for advance informed agreement to all GMO transactions (Proclamation Article 5.1–5.3)
The law requires an applicant to obtain Advance Informed Agreement (AIA) after submitting a risk assessment report for all GMO transactions made without distinction. This requirement far exceeds the provisions of the Protocol, in which AIA procedure applies only to the first trans-boundary movement of GMOs intended for introduction into the environment (Protocol Article 7) but not for GMOs destined for contained use or those in transit (Protocol Article 6) or for GMOs to be directly used as food, feed or for processing (Protocol Article 7.4). In addition, the broad definition of GMO that includes all recombinant DNA activities forbids simple and basic research and teaching activities—such as cloning of PCR products or restriction fragments in plasmid vectors and transformation into bacteria—without AIA, even though such activities do not target GMO development.
Application of AIA procedure for all GMO transactions means that the country must treat GMOs with perceived high risk and insignificant risk in the same manner. Most countries with functional biosafety systems prioritize GMO activities based on perceived risk level, giving the most attention to those with higher risk (e.g., those destined for release into the environment) compared with others (e.g., those for contained research use). Hence, lack of the proportionate risk-based review of GMOs, which is hailed as one of the strengths of the ProtocolCitation12 by the Ethiopian biosafety law, is likely to make the regulatory activities very costly, time consuming and inefficient. It should also be noted that there is inconsistency between the Proclamation, the scope of which only covers GMOs as biological entities without any reference to their processed products (Article 2.1) and some of its Directives which include processed products, such as oil, flour, drugs, hormones, etc., in its scope (Directive No. 3, Article 4.1, 4.5). Since there is confusion as to whether processed products of GMOs are regulated under the current law, there is a need to sort out this contradiction as it has serious regulatory implications.
Rigorous socioeconomic considerations in risk assessment
Risk, as defined broadly in the Proclamation, includes socioeconomic, cultural and economic conditions of local communities or the country (Proclamation Article 2.9). Additionally, in Article 4.7 of Directive No. 2 of the Proclamation, socioeconomic risks were expanded to include potential effects on employment, market opportunities, substitution of traditional crops and products or indigenous technologies and effects on religious and ethical values, including those affecting other countries. Including such a wide range of socioeconomic issues in risk assessment contrasts with the provisions of the Protocol, whereby only those that have impacts on the conservation and sustainable use of biodiversity are taken into account but only to the extent that they are consistent with other international obligations (Protocol, Article 26.1). Furthermore, Article 15 and Annex III of the Protocol both require that risk assessment be performed in a scientifically sound and transparent manner with no reference to socioeconomic considerations. Hence, to ensure that socioeconomic considerations will not become an obstacle to the research, development, transfer and use of GMOs in Ethiopia, issues to be addressed should be limited to or focus on those specifically described in the Protocol with their application stages in the GMO approval processes clearly defined.
Accuracy of information provided by the applicant (Proclamation Article 8.2)
As a condition for obtaining AIA, the law requires the applicant to provide a statement signed by the head of the competent national authority of the country of export, which assumes full responsibility for the completeness and accuracy of the information provided. However, according to Article 8.1 of the Protocol, the accompanying letter of the exporter (company) can be adequate for this purpose. Similarly, the book Biosafety and the Environment: Introduction to Cartagena Protocol on BiosafetyCitation13 clearly allocates this activity to the exporter. Beyond requiring exporters under its jurisdiction to provide accurate information, it is very unlikely that a government will provide specific signed statements for an applicant on behalf of exporters, which are, in most cases, multinational private companies. Hence, the law puts preconditions in place that are practically impossible to meet by anyone interested in importing GMOs for research, teaching or trade.
The precautionary principle and making or contained use of GMOs (Articles 8.1, 8.2)
The law requires the application of the precautionary principle as a basis for making GMOs in domestic laboratories. In simplified terms, the precautionary principle states that GMOs are potentially dangerous, and thus, their use should be avoided until they are proven safe.Citation14 In the context of the Protocol, the precautionary approach applies only to transboundry movement of GMOs but not for contained use.Citation6 Moreover, most countries which are parties to the Protocol have avoided direct adoption of the precautionary principle in their national biosafety laws because it is criticized as vague, lacking uniform interpretation and judged as an impractical policy.Citation14 Hence, there is a concern in the biotechnology community that applying the precautionary principle to the making of GMOs in a contained domestic laboratory will prevent or suppress local genetic engineering research to improve indigenous organisms such as tef and enset. This concern is based on the fact that the precautionary principle may be interpreted in such a way that requires an applicant seeking permission to carry out genetic engineering research on these organisms under containment to prove that the research is safe before starting the proposed work, which in practice is impossible.
Strict labeling requirements without setting threshold level (Proclamation Articles 7.1–7.4)
The law prohibits the use of the phrase “may contain” modified organisms, the Protocol’s standard for labeling GMOs that are intended for direct use as food, feed and processing (Protocol Art. 18.2a), requiring instead a declaration that a product either does or does not contain GMOs. This contradicts Ethiopia’s legal commitment under the Protocol. Moreover, recent studies in other countries indicated that strict information requirements, particularly for GMO commodities, would impose high costs on exporters and importers alike, thereby acting as a barrier to domestic and international trade (e.g., Gruère and Rosegrant,Citation15 Kinami and GuereCitation16). Furthermore, experience shows that all countries that require mandatory labeling define some threshold for GMO content, ranging from 0.9–5%, with the exception of the likely unapplied 0% standard of China.Citation15 However, there is no indication of threshold level in Ethiopian law or its directives. If the strict labeling requirements for all GMO transactions in Ethiopia are interpreted as 0% tolerance, it will be extremely costly and practically unachievable to Ethiopia’s import and export commodities.
Issues of concerns apecific to the proclamation
Absence of independent national body(ies) to guide in decision making
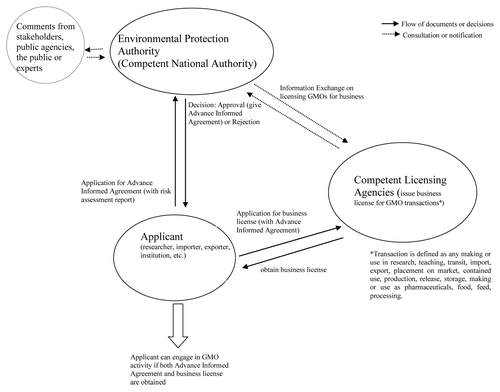
The biosafety law vests all implementation and decision-making powers in the EPA, which assumes the role of national competent authority (). Although the proclamation indicates that decision making will incorporate expert opinions and stakeholders’ comments, as well as consult with public agencies (Proclamation Article 14.1), it does not specify how the experts or stakeholders are selected or which public agencies are to be involved. Moreover, there is no mechanism to ensure that the EPA incorporates the comments made by these bodies. International experience (e.g., RaoCitation17 and JaffeeCitation18) shows that to encourage autonomous, accurate and transparent decision making processes, biosafety laws or regulations in most countries establish a national biosafety committee, authority or similar body(ies). These bodies typically take on an advisory role and are comprised of representatives from relevant governmental and nongovernmental organizations, as well as experts from the private sector. In most cases, the competent national authority is obliged to consult this body and make its recommendation a basis for decision making. Furthermore, biosafety laws of many countries (e.g., Kenya, South Africa and India) establish an appeal board to hear pleas from applicants who feel aggrieved by the decisions made by their competent national authority on GMOs. The Ethiopian law has no provision to establish a similar independent national biosafety advisory body or to address appeals. These absences raise huge concerns about the accuracy of data evaluation, including risk assessment reports, transparency and justness of the decisions made by the competent national authority.
The need to furnish an unspecified guarantee and insurance coverage by the applicant
Article 5.3 of the Proclamation indicates that, as a condition for giving any AIA, the authority may require an applicant to furnish a guarantee, which is to be sufficient to meet his obligation. There is some concern that the lack of specification and clarity concerning the type of guarantee will create room for subjective decisions by the authority. Additionally, there is a requirement for anyone involved in transport, storage and processing of any GMO to provide adequate insurance to cover possible harm caused (Directives Nos. 5& 6). These requirements are likely to discourage those interested in GMO activities, including research.
Duration of validity of authorization and post-release monitoring
When a GMO activity is approved, the period of validity of the authorization is not indicated in the proclamation or its directives. Knowledge of the period of validity for commercial release (which is usually 10 y) is critically important for the technology providers because they must assess whether they can get a return on their investment in the stated period of time before deciding to invest in the country. Furthermore, after release into the environment and commercialization of GM plants, an applicant or his heir is required to monitor and present an annual report to the EPA for at least 150 y for trees, 30–150 y for perennial crops and at least 30 y for annual crops (Directive No. 3). It is difficult to imagine that a foreign technology owner will commit itself to monitoring and reporting for such long period of time, and hence choose to invest in Ethiopia.
Discussion
It can be seen from the above account that the EPA-led process to draft the bill failed to accommodate the views of some key stakeholders in the development of the biosafety system. In particular, despite their initial participation, scientists from public institutions, who are potential developers and initial adopters, were excluded early on in the process, unable to make any significant contributions to the bill. Similarly, private multinational technology providers who own proprietary biotechnology products were not involved in the process directly or indirectly. Instead of reconciling the differing views on GMOs, a one-sided biosafety bill with highly restrictive requirements that cannot be fulfilled by GMO proponents was prepared and approved made into law. Furthermore, contrary to the rich experience of many countries with functional biosafety systems, no independent or advisory bodies, such as a national biosafety committee or appeal system, were established. Altogether, applicants are likely to be discouraged from pursuing GMO activities in the country for a number of reasons. These reasons include the stringent preconditions they cannot comply with, the high possibility of compliance cost, lengthy processing time, unpredictable prospects for commercialization and overall lack of trust in the system. Consequently, technology providers who judged the system as unworkable have thus far refused to invest in the country, denying the country access to highly demanded technologies like Bt cotton.
One may argue that the Protocol is a compromise instrument and that it permits countries to take more stringent measures to protect their environment. However, it should also be noted that the Protocol clearly recognizes “the great potential of modern biotechnology for human wellbeing” and states that it creates an enabling environment for the countries “to derive maximum benefit from the potential that biotechnology can offer, while minimizing possible risk to the environment and human health”Citation6. Moreover, the Protocol requires a country’s domestic regulatory system to be consistent with its objectives and provisions (Protocol Articles 2.4, 9.3) and calls for the need to reconcile the respective needs of environmental protection and trade with respect to biotechnology. Therefore, selectively adopting the protective provisions of the Protocol in the law in a prohibitive way that denies the country the benefits of modern biotechnology does not concur with the objectives of the Protocol. From this viewpoint, it can be argued that the current Ethiopian biosafety system is inconsistent with the Protocol.
A functional and practical biosafety legal framework is essential for a technology developer or provider to invest in a country.Citation18,Citation19 Such a system strikes a balance between the development of biotechnology, protection of the environment and safeguarding the interests of consumers. In so doing, it addresses the major concerns of technology providers, namely the cost and time associated with moving a product through a regulatory process and on to market prospects after commercialization of the product.Citation20 The resulting biosafety system in Ethiopia does not take these key issues into account and hence cannot encourage investment by foreign technology providers. As already predicted by some analysts before enactment of the law,Citation2 the biosafety system faced serious problems at early stages of its implementation, as exemplified by the country’s inability to access Bt cotton in the last few years due to restrictive provisions of the law. This experience from Ethiopia underlines the importance of actively incorporating private or public technology providers in the development of a national biosafety system and shows that technology providers will not invest in a country unless they are convinced that the biosafety system is workable, regardless of the potential market.
The reasons put forward by GMO critics, including EPA authorities, to justify the highly precautious position toward GMOs in EthiopiaCitation2 are being contested by proponents as exaggerated or based on inadequate information. Regarding concern on damage to biodiversity, Ethiopia is the center of origin or diversity for some crops but not for cotton, maize or soybean, the major GM crops commercialized globally, and the adoption of which are of national interest at the moment. Thus, using the generic concept “damage to biodiversity” as a reason to discourage selective use of genetic engineering products is misleading. For example, it would be unreasonable to deprive Ethiopian farmers the access to the benefit of safe use of GM maize while Mexico, the center of origin and diversity of maize, is conducting field trials with the aim of cultivating the crop.Citation21 The claim about the incompatibility of GM crops with small scale farmers has also been proven wrong. A recent report indicates that over 90% of the 17.3 million beneficiaries of GM crops in 2012 were smallholder and resource-poor farmers in developing countries,Citation22 indicating that GM crops are highly compatible with their systems. As to the possible export risk, studies show that there will be insignificant or negligible export risk to Ethiopia, if the country decides to plant GM cotton or maize, as the export to Europe is negligible.Citation23,Citation24 Similarly, experience from other countries (e.g., Knight et al.Citation25) shows that the presence of GM crops in Ethiopia is unlikely to create a negative perception for the export of non-GM crops like coffee.Citation2 On the positive side, there is compelling evidence showing that GM crops have delivered substantial socio-economic and environmental benefits since their commercialization 17 y ago.Citation22 Hence, finding a common ground to maximize the benefit of this technology, while at the same time minimizing its effect on the environment using science-based and responsible regulatory systems, will benefit the country far more than imposing prohibitive preconditions.
The huge potential of GMOs in addressing some of the major biotic development constraints in Ethiopia has been previously described.Citation1 For instance, being aware of global yield gains of GM crops, the average of which over many years is as high as 48.5% for Bt cotton in India and 30% for Bt maize in Argentina,Citation26 Ethiopia’s private sector large scale farmers have showed keen interest in adopting GM crops, particularly Bt cotton, Bt maize and herbicide tolerant (RR) soybean.Citation11 In the last five years, the sector has been applying enormous pressure on the government to create a favorable environment for importing these technologies. Nevertheless, over three years have passed since the biosafety law was enacted, and no GMO import has yet been authorized, even for research purposes. This means that Ethiopian farmers will indefinitely continue to miss the proven benefits of GM crops, thereby being less competitive in the international market. Ethiopia therefore has to take proactive measures to create an environment that encourages investment by foreign technology owners, while at the same time strengthening its domestic research and development efforts on GMOs by allocating adequate public funds and encouraging donor investment. Central to these efforts is building an accommodative and workable biosafety system that takes into account the interest of key stakeholders, including potential private technology providers from abroad, public research institutions and farmers’ groups.
Recommendations and Conclusions
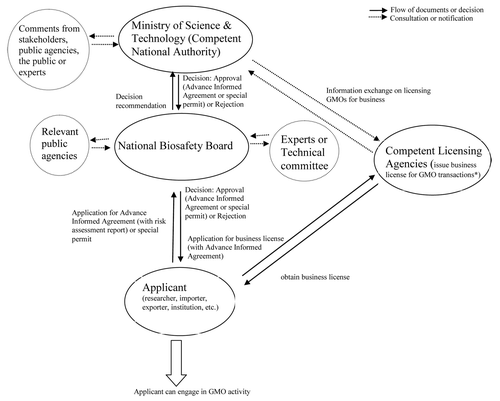
Given the current situation, the best way forward for Ethiopia is to revise its current biosafety system to make it workable. A crucial step in the right direction would be to make changes to the provisions in the law and its implementing directives that make the current biosafety system non-functional, which would include addressing many of the shortcomings discussed above. These efforts should also be backed by enabling an institutional arrangement such as the establishment of an autonomous biosafety advisory body like the National Biosafety Board, comprised of senior experts in disciplines relevant to genetic engineering and biosafety, as well as representatives of relevant government ministries and other key stakeholders, including private sector. The board could include key government departments—like the EPA or the Ministries of Agriculture, Health and Trade—and be hosted and overseen by the Ministry of Science and Technology, which would be a competent national authority and would have the power to make final decisions on biosafety issues based on the board’s recommendations in consultation with key stakeholders (). Such an institutional arrangement would be expected to avoid the excessive influence of departments with direct interest on GMO and biosafety issues like the Ministries of Agriculture, Health or Trade and the EPA in decision making processes, thereby building trust in the system. The EPA could continue to function as a national focal point for the Protocol. Similar institutional arrangements have proved to be successful in facilitating GMO research, development and regulation in countries like Kenya and Ethiopia can learn from such experiences. Since contained use is typically done for research and teaching purposes, which are the basis for the development of genetic engineering in the country, a special permit mechanism that does not require that rigorous risk assessment procedures be put in place. Delays in taking steps to render the current biosafety system workable would not only continue to deny Ethiopian farmers the benefits of useful biotechnologies that will significantly contribute to national development but will also hinder the domestic capacity to conserve and sustainably use the country’s rich biodiversity using modern biotechnologies. This could result in undesirable situations like what happened in southern Brazil, where GM soybean smuggled across the border from Argentina was illegally planted.Citation27 Sudan, a neighboring country to Ethiopia, has already commercialized Bt cotton,Citation22 and there is a high chance that Kenya, which recently legalized commercialization of GMOs,Citation21 will place on the market in 2014. Thus, to the extent that it is important to consider the risks associated with adopting GMOs, it is equally important to thoroughly assess the possible socioeconomic consequences of not adopting GM technologies in Ethiopia.
Figure 2. Suggested regulatory framework for approval of GMO activities in Ethiopia. *Upon revision of the law, GMO transactions that need Advance Informed Agreement are expected to be limited to those intended for environmental release. For contained use, special permit mechanisms will be in place
Parallel to making efforts to create a workable biosafety system, Ethiopia should also work toward selecting potentially useful biotechnologies from abroad and negotiating the modalities of partnerships with private technology providers for accessing, further developing for national use (e.g., introgression into local varieties) and commercializing them. Likewise, the multinational private technology providers should show willingness to engage in technology access negotiations with Ethiopia for research and/or commercialization purposes for the benefit of both sides. Donor-funded organizations like African Agricultural Technology Foundation and the Program for Biosafety Systems that have good track records in other African countries can play a useful role in mediating technology access negotiations and facilitating efficient and responsible development and safe use of agricultural biotechnologies, respectively, in Ethiopia.
In conclusion, Ethiopia has to work toward revising its biosafety system to facilitate active participation of foreign technology providers and local researchers in the biotechnology sector so that it can harness the maximum benefits from developments in modern biotechnology. For this to happen, the current legal and administrative challenges related to regulatory issues must be corrected, and progressive policy dialog among the key stakeholders must be promoted. Putting in place a balanced, science-based and responsible biosafety system that encourages foreign investment and domestic innovation is a necessity if Ethiopia is to effectively advance its diverse interests of conserving biodiversity, protecting the environment and enhancing competitiveness in agricultural and other sectors of the economy.
Acknowledgments
The author is grateful to Dr Melaku Alemu and Dr Gemechu Keneni for their useful comments on the manuscript.
Disclosure of Potential Conflicts of Interest
The arguments, recommendations and conclusions in this paper are those of the author and not necessarily of the Ethiopian Institute of Agricultural Research.
References
- Abraham A. Agricultural biotechnology research and development in Ethiopia. Afr J Biotechnol 2009; 8:7196 - 204
- Ayele S. Biotechnology and biodiversity debates and policies in Africa. IKD Working Paper No. 29. The Open University. 2008; 19 pp.
- Egziabher GTB. Balancing biosafety, trade and economic development interests in the implementation of the Cartagena Protocol: A developing country perspective. pp. 33-34. In: Cartagena Protocol on Biosafety: From negotiation to implementation, Secretariat on the Convention of Biological Diversity, CBD News, Special Report, 2003.
- Environmental Protection Authority. Environmental Policy. The Government of the Federal Democratic Republic of Ethiopia, Environmental Protection Authority in Collaboration with the Ministry of Economic Development and Cooperation, April 2, 1997. Addis Ababa, Ethiopia.
- Environmental Protection Authority. Biosafety Framework (Final Draft). The Government of the Federal Democratic Republic of Ethiopia, Addis Ababa, Ethiopia, 2007.
- Convention on Biological Diversity. Cartagena Protocol on Biosafety to the Convention on Biological Diversity. Text and annexes Montréal. Secretariat of the Convention on Biological Diversity, 2000; 30 pp.
- Federal Democratic Republic of Ethiopia. Biosafety Proclamation No. 655/2009. Federal Negarit Gazetta of Federal Democratic Republic of Ethiopia, 15th Year No. 63, Addis Ababa, 9th September, 2009.
- Environmental Protection Authority, The Government of the Federal Democratic Republic of Ethiopia, Directive No. 01/2009, Directive No. 02/2009, Directive No. 03/2009, Directive No. 04/2009, Directive No. 05/2009 and Directive No. 06/2009 issued in accordance with Article 22 of the Biosafety Proclamation No. 655/2009. January 2008, Addis Ababa, Ethiopia.
- Ayele S. The legitimation of GMO governance in Africa. Sci Public Policy 2007; 34:239 - 49; http://dx.doi.org/10.3152/030234207X213931
- Zenebe W. Overlooked bill makes big waves for international aid. Addis Fortune [Newsletter]. Volume 10, No. 495. November 1, 2009 issue. Available on www.addisfortune.agenda.htm
- Teklewold A, Assefa S. Biotechnology in the Agricultural Research Institutes of Ethiopia: A tool of hope to advance the prime economic sector. pp. 114-132. In: Seyoum E, Nigussie M, Cherinet G, Zeweldu T (eds.). Proceedings of the First National Biotechnology and Biosafety Workshop of Ethiopia. 1-4 February 2010, Ministry of Agriculture and Rural Development, Addis Ababa, Ethiopia.
- Jaffe G. Implementing the Cartagena Biosafety Protocol through National biosafety regulatory systems: an analysis of key unresolved Issues. Journal of Public Affairs 2005; 5:299 - 311; http://dx.doi.org/10.1002/pa.30
- Convention on Biological Diversity. Biosafety and the Environment. An Introduction to the Cartagena Protocol on Biosafety. Secretariat of the Convention on Biological Diversity/United Nations Environmental Program. 2003; 15 pp.
- Puttagunta PS. The precautionary principle in the regulation of genetically modified organisms. Health Law Rev 2001; 9:10 - 7; PMID: 15706712
- Gruère GP, Rosegrant MW. Assessing the implementation effects of the biosafety protocol’s proposed stringent information requirements for genetically modified commodities in countries of the Asia Pacific Economic Cooperation. Review of Agricultural Economics 2008; 30:214 - 32; http://dx.doi.org/10.1111/j.1467-9353.2008.00401.x
- Kimani V, Gruère G. Implications of import regulations and information requirements under the Cartagena Protocol on Biosafety for GM commodities in Kenya. AgBioForum 2010; 13:222 - 41
- Rao SR. Status of Regulation of Genetically Engineered Products in Selected Countries – An Analysis. Asia Biotechnology and Development Review 2003; 6:23 - 38
- Jaffe G. Comparative analysis of the national biosafety regulatory systems in East Africa. IFPRI Discussion Paper 146, January 2006, 81pp.
- Vosges M. Regulation of GMO activities in South Africa: Experience from a technology developer. Workshop proceedings report on: GMOs for African Agriculture: Challenges and Opportunities. pp. 141-146. ISBN: 978-0-9814159-7-0; 2010.
- Pray CE, Bengali P, Ramaswani B. The cost of regulation: The Indian Experience. Quarterly Journal of International Agriculture 2005; 44:267 - 89
- James C. Global status of commercialized biotech/GM crops: ISAAA Brief No. 43. ISAAA: Ithaca, NY, 2011.
- James C. Global status of commercialized Biotech/GM crops: ISAAA Brief No. 44. ISAAA: Ithaca, NY; 2012.
- Paarlberg R. Are genetically modified (GM) crops a commercial risk for Africa?. Int. J. Technology and Globalization 2006; 2:81 - 92
- Paarlberg R, Wafula D, Minde I, Wakhungu JW. Commercial export risks from approval of genetically modified (GM) crops in the COMESA/ASARECA region. /Robert Paarlberg,-Nairobi, Kenya: ACTS Press ISBN 9966-41-144-5; 2006.
- Knight JG, Mather DW, Holdsworth DK. Impact of genetic modification on country image of imported food products in European markets. Perceptions of Channel Members. Food Policy 2005; 30:385 - 98; http://dx.doi.org/10.1016/j.foodpol.2005.05.001
- Brookes G, Barfoot PGM. Crops: Global socioeconomic and environmental impacts 1996-2009. PG Economics Ltd. Dorchestere, UK; 2011.
- Pelaez V. State of exception in the regulation of genetically modified organisms in Brazil. Sci Public Policy 2009; 36:61 - 71; http://dx.doi.org/10.3152/030234209X403235