Abstract
The rapid uptake of biotech crops around the world demonstrates not only strong producer and consumer demand for the technology and its products, but also that where regulatory regimes function effectively and markets are allowed to operate as normal, co-existence between genetically modified (GM) and non-GM supply chains is readily achievable. However, the polarized debate over GMOs within the European Union over the past 15 years has resulted in a highly politicized and progressively impractical approach to the issue of GM crop co-existence, which in itself has become a further barrier to the technology’s development. This article argues that co-existence should not be treated as a pro- or anti-GM issue, and that the aim of co-existence measures should be to permit consumer choice and freedom to operate whatever the production method involved. It suggests that supply chain-based solutions to co-existence, rather than Government prescription, offer the most pragmatic and flexible response to the commercial realities of servicing differentiated market demands.
Keywords: :
Introduction
Co-existence allows farmers to make a practical choice between growing conventional, organic and genetically modified (GM) crops, or any combination, in response to market demand and in accordance with the legal requirements for GM, non-GM, and organic labeling.
At the farm level, the single overriding objective of co-existence arrangements is to minimize unwanted mixing of GM and non-GM crops, so maintaining the integrity of raw material supply chains and safeguarding freedom of choice for consumers.
Since the European Union’s GM food and feed regulationsCitation1 require non-GM crops with accidental GM presence above 0.9% to be labeled as containing GMOs, co-existence may have financial consequences for growers depending on the market situation – ie whether a price differential exists between GM and non-GM products.
But co-existence is above all an economic and market-related issue. It is not concerned with safety to health or the environment, since all GM crops must undergo rigorous, science-based safety assessment prior to their approval for commercial cultivation.
Nor is co-existence a new concept in agriculture. Conventional crop production has long-established practices which enable sexually compatible species to be grown and harvested to meet the quality and purity specifications of a range of different end-markets. No sector of crop production operates to 100% purity and practical thresholds are used to determine a crop’s end-use quality and value according, for example, to its varietal purity or freedom from unwanted material.
Since GM crops are no more or less promiscuous than their non-GM counterparts, these same practices can be applied in the context of GM crop production to maintain segregated supply chains as necessary in response to market demand.
This was the approach advocated by the industry group SCIMAC (Supply Chain Initiative on Modified Agricultural CropsCitation2) when it was established almost 15 years ago as a collaborative initiative of industry organisations spanning the UK farm supply chain.
Combining the UK representative bodies for farmers (NFU), plant breeders (BSPB), technology providers (CPA), seed suppliers and grain merchants (AIC), SCIMAC is founded on a consensus view that access to innovation and new technology in agriculture is vital for future production efficiency, competitiveness, and sustainability. At the same time, SCIMAC membership embraces a range of diverging views and attitudes toward GM crop technology, and the group’s overriding objective is to provide choice and access to new technology without disadvantaging conventional or organic producers.
As an industry grouping, SCIMAC is not involved in regulating the safety of GM crops—that’s the role of policymakers and regulators. Nor is SCIMAC responsible for fixing legal thresholds to distinguish between GM and non-GM products. Again, that’s the job of politicians and regulators.
But should GM crops become available for commercial cultivation in the UK, SCIMAC does represent those sectors of the agricultural supply chain, from plant breeders and seed merchants through to farmers and grain handlers, who will deal with the issue of GM crop co-existence in practice. The primary function of the supply chain is to service the specifications of a range of different market outlets, and to deal with cases where crops are off-specification.
That’s why SCIMAC took the lead in developing a stewardship framework for the on-farm management and co-existence of GM crops in the UK, building on existing industry practices and expertise in areas such as the production of certified seed and industrial, non-food crops.
Guiding Principles on Co-Existence
In developing this program, SCIMAC has observed the following 10 key principles which together underpin the concept of successful co-existence at the farm level:
(1) Co-existence is an economic issue—it is not about safety. All GM crops, and only GM crop cultivars, must undergo rigorous, science-based safety assessment on a case-by-case basis prior to their approval for commercial cultivation. With GM crop safely thus assured, co-existence is concerned with the implications for farmers and their customers of meeting market requirements in the context of a statutory 0.9% GM labeling threshold.
(2) The need for co-existence measures will be determined by (differentiated) market demand. If there is no market demand, GM crops will not be grown. The need for co-existence measures will ultimately be determined by prevailing market conditions, and the extent to which customer specifications and price levels differentiate between GM and non-GM crops.
(3) A zero threshold is not achievable, but practical tolerance thresholds work. No sector of agriculture operates to 100% purity. In every crop sector, from certified seed to mainstream commodity production, practical tolerance levels are applied to define a crop’s end-use quality and value according, for example, to its varietal purity or freedom from unwanted material.
(4) Co-existence is not a new concept in farming. The agricultural supply chain is currently able to service a range of market channels with different labeling and/or quality requirements, e.g.:
• certified seed and non-seed (commodity) crops
• malting and feed barley
• sweetcorn and forage maize
In each case, well-established practices are in place to deliver co-existence, including neighbor-to-neighbor communication, separation distances between crops, and careful segregation during harvest, storage and transport.
(5) Experience of growing GM crops in other parts of the world confirms that co-existence is achievable. Where GM crops are grown commercially on a large scale around the world, there is no evidence over the past 15 y of farmers routinely suing each other or claiming compensation. Even in Europe, Spanish farmers are now growing more than 90,000ha of GM maize per year without any specific provision for liability, insurance or compensation, nor any evidence to suggest such measures would be necessary. Indeed there are some farmers in California who grow both organic crops and GM crops (in separate fields, of course).
(6) Co-existence is not a one-way street. Since farming takes place in the open air, co-existence depends on well-established measures to ensure the quality and integrity of crops destined for different market outlets. In practice, this involves mutual co-operation and communication between farmers who share a vested interest in delivering products to meet their customers’ requirements.
(7) GM growers cannot reasonably be expected to bear responsibility for the self-imposed marketing standards of others. GM growers must bear the initial responsibility for delivering co-existence measures in line with any statutory GM labeling threshold. Where voluntary non-GM or “GM-free” marketing standards specify a lower threshold, however, GM growers cannot reasonably be held to account for these additional, self-imposed criteria.
(8) GM crop cultivation introduces no new liability issues. The key to effective co-existence is a sensible, agreed definition of good practice to define the boundaries of negligence and due diligence in respect of GM and non-GM crop production. This is the basis for existing principles of liability. Once GM crops are approved as safe for commercial release and marketing, there are no grounds to suggest they should be treated differently—in liability terms—from other equivalent non-GM products.
(9) No one can predict whether new price differentials will emerge or be sustained between GM and non-GM value chains. Co-existence arrangements must be flexible and responsive enough to challenge the assumption that GM crops will always and inevitably trade at a discount. Where no price differential exists, there may be no potential for economic loss or liability. Equally, a future GM crop may trade at a premium price relative to the equivalent conventional crop, for example if it offers a novel quality trait. Co-existence arrangements must therefore provide for situations in which a GM crop grower seeks to minimize adventitious “contamination” from non-GM sources.
(10) Gene flow data provide a high degree of confidence that any breach of labeling threshold would be extremely rare. Based on the wealth of practical experience and scientific data now available on a crop by crop basis, breach of the 0.9% GM labeling threshold would be extremely rare where all farmers have complied with appropriate, evidence-based co-existence measures. If GM (or non-GM) farmers are at fault through misuse of product or negligence, however, it is clear that they should bear any responsibility—and cost.
Co-Existence in Practice
Many examples exist of farmers and supply chains successfully managing co-existence in a non-biotech context.
At the farm level, measures to support the production of certified seed include: crop-specific thresholds for varietal purity and admixture; use of isolation distances and buffer strips; staggered planting dates and selection of varieties with different flowering times; communication and co-operation between neighboring growers; attention to machinery hygiene and careful sequencing of operations. Importantly, certified seed growers—not their neighbors—are responsible for exercising these measures and assuring the seed purity levels required.
These long-established practices enable seed crops to be consistently and reliably produced to very high levels of varietal purity. Similar measures are applied in other sectors, for example to segregate the production of sweetcorn and forage maize, and to keep food-grade oilseed rape varieties separate from industrial high erucic acid rape (HEAR), which is not suitable for human consumption.
Beyond the farm-gate, the grain supply chain itself has become increasingly sophisticated in its response to customer demands, with computerized traceability, sampling and testing now in widespread use during handling, storage and onward distribution of crops post-harvest.
The non-GM supply chain also uses dedicated systems to segregate more sensitive or high-value material. Measures range from using physically separate storage and different colored documentation to procuring grain only from a carefully selected grower base or region.
In each case, the level of differentiation in the market place determines the nature—and cost—of the segregation processes applied.
EU Political and Regulatory Context
The primary driver for co-existence and traceability arrangements in an EU context is the statutory requirement to label all food and feed products containing EU-approved GM or GM-derived material above a threshold of 0.9%. This requirement includes GM derivatives such as rapeseed oil and sugar which cannot be distinguished by testing from their non-GM equivalents, hence the requirement for full traceability.
In 2003, the European Commission issued its first recommendation on the issue of GM crop co-existence. This concluded that given the wide variation in geography, farm size, industry structure and cropping patterns across the European Union, the practical detail of co-existence measures should be developed at Member State level.Citation3
However, the recommendation identified a number of guiding principles which Member States should observe in formulating their own national co-existence arrangements. These were broadly sensible and pragmatic, for example advising Member States that:
• the EU-wide GM labeling threshold of 0.9% should provide the reference point for all co-existence measures;
• a farmer’s freedom to choose between GM, non-GM or organic production systems must not be denied;
• Member States should develop practical co-existence measures, specific to crop type and relevant to local conditions;
• measures should build on available agricultural experience and existing segregation practices within the supply chain;
• measures should be cost-effective, proportionate and based on the best available scientific evidence.
The Commission further insisted that in the absence of a lower legal threshold for GM presence in relation to organic production, the general co-existence threshold of 0.9% should be applied equally in respect of non-GM and organic growers.
So far so good, but the response from many Member States was spectacularly insubordinate.
Far from developing co-existence arrangements to promote freedom of choice, the measures proposed or adopted in a number of Member States were specifically designed as a further political barrier to the technology, reinforcing prejudice and stifling access to innovation in those countries.
So, for example, Slovenia developed plans to make agri-environmental payments conditional on not growing GM crops. Ireland tabled proposals for a statutory training and licensing scheme for farmers wishing to grow approved GM crops, while Cyprus sought to ban the cultivation of GM crops in designated environmental protection areas.
There has also been a lack of consensus among EU Member States on the technical measures proposed to support co-existence arrangements. provides an illustration of the separation distances proposed or adopted for cultivating GM maize in different Member States.Citation4
Table 1. Separation distances proposed or adopted for cultivating GM maize in different Member States
Even for the most straightforward technical measures, where evidence of gene flow is well-studied, documented, and backed up by decades of seed-growing experience, there are deviations of up to 30-fold in the isolation distances specified by Member States. Such discrepancies simply cannot be accounted for by variations in farm size, topography or climate. Indeed a number of Member States blatantly defied the European Commission’s original advice that the 0.9% co-existence threshold should apply equally to conventional and organic crops by specifying a requirement for increased separation distances for organic crops—10 times greater in the case of the Netherlands.
Independent analysis of the separation distances proposed in different EU Member States prompted Devos et al.Citation5 to conclude in 2008 that in some cases the measures were unworkable, unscientific, disproportionate and in breach of the co-existence principles set out by the European Commission.
“Based on the analysis of existing cross-fertilization data, it is concluded that some of the currently proposed isolation distances are not in line with the coexistence principles laid down by the European Commission: they are
(1) excessive from a scientific point of view;
(2) difficult to implement in practice;
(3) rarely proportional to the regional heterogeneity in the agricultural landscape; and
(4) not proportional to the farmers' basic economic incentives for coexistence.
Hence, the range of proposed isolation distances cannot simply be explained by different interpretations of available scientific data, possible error intervals and remaining uncertainties inherent in the scientific process. It is argued that other than scientific issues must be at play. One might thus claim that coexistence has become an arena of contending values and visions on the future of agriculture and on the role GM crops might play therein.”
Far from providing a vehicle to support freedom of choice among EU farmers and consumers, the paper suggested that co-existence had now become an intrinsic component of the deeply divisive political debate over GM crops within Europe.
Indeed the creeping politicization of the co-existence issue within Europe was apparent long before Devos et al. completed their review. In December 2005, the European Commission rubber-stamped the Danish government’s plans for dealing with GM crop co-existence and liability.Citation6
Although EU Farm Commissioner at the time, Marian Fischer Boel, had previously served as Denmark’s Agriculture Minister, and had frequently championed the Danish approach as a blueprint for the rest of Europe, the perverse decision to endorse the Danish co-existence model marked a surprising departure from the Commission’s own recommendations set out for Member States just two years earlier.
While practical aspects of the Danish model reflected the guidance that co-existence measures should be science-based and build on existing arrangements at the farm level, the scheme included provision for a €13.40 per hectare tax on GM growers to finance a compensation fund for non-GM producers adversely affected by cross-pollination from neighboring GM crops.
This apparently arbitrary amount—equivalent to 100 DK—was backed up by no supporting information about the assumed price differential between GM and non-GM crops, the predicted frequency with which claims would be made against the compensation fund, or indeed what would happen if there were no price differential.
In fact, this was simply a punitive and politically-motivated tax on innovation—directly at odds with the EU principles of proportionality and non-discrimination—and intended to deter the uptake of GM technology in Denmark.
Rigging the market in this way marked a worrying departure by the Danes from the normal territory of Government regulators in safeguarding consumer health, food safety and the environment.
Worse still, endorsement of the Danish model by the European Commission sent a green light to other Member States that co-existence offered a politically acceptable route to block the development of GM technology, whether by imposing an arbitrary tax on growers or by other means.
The die was indeed cast and a revised set of co-existence recommendations emerged from the European Commission in July 2010.Citation7
Compared with the original recommendation issued in July 2003, Commission Recommendation (2010/C 200/01) of 13 July 2010 on guidelines for the development of national co-existence measures to avoid the unintended presence of GMOs in conventional and organic crops, signed off by Commissioner John Dalli, was an entirely different proposition.
Published in tandem with the European Commission’s proposals to give individual Member States the right to ban the cultivation of EU-approved GM crops on their territories—a 2009 re-election pledge made by EU Commission President Jose Manuel Barroso—this set of recommendations appears also to release the Commission from any position of oversight or supervision in relation to the development of national co-existence strategies.
In the first instance, and with immediate effect, the Commission determined that national or regional co-existence measures could be used by Member States to restrict the cultivation of EU-approved GM crops on all or part of their territories—without any requirement to reference or justify the scientific or technical basis for any co-existence measures adopted.
Critically, the revised recommendation also ditched the Commission’s previous stipulation that the statutory EU labeling threshold of 0.9% for unintended GM presence should be the common basis for co-existence measures, paving the way for a chaotic array of different labeling thresholds and co-existence arrangements across the EU.
SCIMAC described the 2010 recommendation as ill-conceived and divisive,Citation8 representing a complete U-turn on the Commission’s previous advice which stipulated that co-existence measures must be science-based and proportionate, respecting individual farmers’ freedom to choose between GM, conventional and organic crop production.
Specifically, SCIMAC highlighted the following seven key objections to the Commission’s new approach:
(1) Disrupts the single market. The Commission’s approach threatens to undermine and disrupt the integrity of the EU internal market by allowing different Member States or regions to impose arbitrary and unscientific criteria to restrict the cultivation of approved GMOs, including the application of varying threshold levels for GM presence below the 0.9% statutory EU labeling requirement.
(2) Establishes a two-tier market disadvantaging the EU food chain. Allowing Member States to disregard the established 0.9% GM labeling threshold in setting their own national cultivation rules could open up a two-tier market, in which imported GM crops circulate freely while cultivation of the same crops can be restricted within the EU, placing Europe’s farmers, food industry and consumers at a competitive disadvantage.
(3) Threatens the market for conventional seed. Allowing Member States to disregard the established 0.9% GM labeling threshold will effectively impose a de facto EU threshold level of 0.1% or below for GM presence in conventional seed. This is likely to result in damaging cost implications for seed supply to the EU, particularly as the number of GM crops and traits in commercial cultivation globally is predicted by the EU’s own Joint Research Centre to increase significantly.Citation9
(4) Undermines free-market principles. In relation to GM crop authorization, the role of Government is to protect the safety of consumers and the environment. It is contrary to fundamental free market principles to impose arbitrary, opinion-based, or politically-motivated criteria to the GM crop approvals process. Such intervention in the market place is entirely inappropriate for national Governments, and by denying producer and consumer choice would undermine the basis of the EU as a free trading economy.
(5) Abandons EU legal principles and WTO obligations. The Commission’s proposals conflict directly with the European Union’s own guiding legal principles of non-discrimination, proportionality and practicality, and disregard internationally recognized obligations which stipulate that trade restrictions must have a scientific basis, and be justified in terms of human health or environmental safety.
(6) Discourages research and innovation. The proposals send a negative signal of the EU’s attitude toward agricultural science and technology, offering no long-term certainty for the outputs of future research activity within the EU. Ultimately the consequence of such a move would be to deter research investment, stifle innovation and block access to the tools EU farmers may need to help address the major challenges facing 21st century agriculture.
(7) Establishes a damaging precedent for agricultural science and technology. By abandoning the established legal principles of science-based authorization according to health and environmental safety, the proposals set a potentially damaging precedent beyond GM crops for the future regulation and application of modern, science-based agriculture generally within the EU.
SCIMAC warned that the Commission was set to create an EU-wide charter for discrimination against the products of agricultural biotechnology at a time when European agriculture needed access to all available tools to address the major challenges of food security and climate change.
GM Co-Existence Research Flourishes
The constipated regulatory regime within the EU has, however, provided fertile ground for plant scientists, economists, lawyers and social scientists to secure funding to research and debate the practical, biological, legal and socio-economic implications of GM crop co-existence.
Multi-partner research projects funded by the European Union include Co-Extra (GM and non-GM supply chains: their CO-Existence and TRAceabilityCitation10), SIGMEA (Sustainable Introduction of GMOs into European AgricultureCitation11), TRANSCONTAINER (Developing efficient and stable biological containment systems for genetically modified plantsCitation12), and PRICE (Practical Implementation of Co-existence in EuropeCitation13).
In addition, the Seville-based European Co-Existence BureauCitation14 was established by the European Commission in 2008 to provide a forum for Member State experts to discuss and develop advisory guidelines for the co-existence of GM and non-GM crops.
However, while the outputs of these multi-million euro studies have certainly reinforced the body of existing knowledge and peer-reviewed literature on the issue of co-existence, they have revealed nothing earth-shatteringly new or which might challenge the 10 key principles set out earlier.
As farmers and supply chains around the world are demonstrating on a daily basis, market-based solutions, rather than Government prescription, offer the most pragmatic and flexible response to the commercial realities of servicing differentiated customer demands.
Where GM crops are part of a functioning regulatory and trading environment, their integration and co-existence with existing production systems has scarcely proved an insurmountable barrier—expansion of the global GM crop area since 1996 makes it the most rapidly adopted crop technology on record, according to ISAAA.Citation15
Indeed growth in the US biotech crop area over the past 16 years has coincided with a 4-fold increase in certified US organic cropland,Citation16 yet we do not receive reports of neighboring farmers routinely taking each other to court or claiming economic damages. This in itself speaks volumes about the real-time success of co-existence at the farm level, given the United States’ reputation as the world’s most litigious society. As well, to date, no US organic grower has lost organic certification due to inadvertent presence of GM material.
There have, of course, been high-profile lawsuits in North America in which co-existence issues have been wrongly implicated by individual farmers as the basis for straightforward patent infringement.
The most notorious is the case of Canadian canola farmer Percy Schmeiser who was successfully sued by Monsanto for patent violation after unlicensed Roundup Ready GM canola was found growing on his farm. Schmeiser claimed the biotech plants in his fields got there by accident—blown in on the wind or carried in by birds. But when tests revealed that 95 to 98 per cent of his 1,030 acre canola crop was made up of Roundup Ready canola, the trial judge concluded that “none of the suggested sources could reasonably explain the concentration or extent of Roundup Ready canola of a commercial qualityCitation17.”
The Federal Court’s 2001 judgment was upheld in 2002 by the Canadian Federal Court of Appeal, and again in 2004 by the Canadian Supreme Court. All three judgments concluded that the level of GM could not have occurred adventitiously, and that Schmeiser had knowingly infringed Monsanto’s patented technology to end up with over 1,000 acres of GM canola which would otherwise have cost him $15,000.
Farm-Level Experience Shows that Co-Existence Can Work
Practical experience of growing GM crops at the farm scale within the UK and more widely within Europe confirms that effective co-existence between GM and non-GM crops can be achieved without compromising the interests of either GM or non-GM producers.
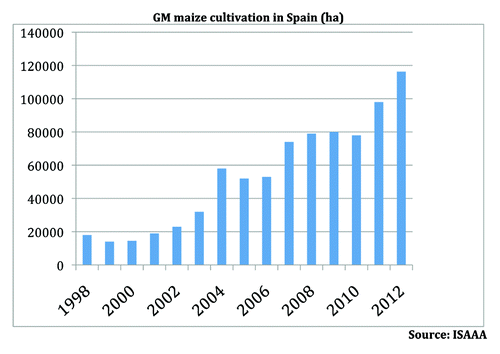
In Spain, for example, the area of insect-resistant GM maize under commercial cultivation has increased steadily from just under 20,000 ha in 1998 to more than 100,000 ha today, accounting for some 30 per cent of the country’s total maize production ().
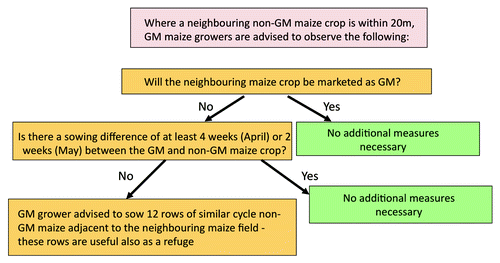
Co-existence arrangements, developed by the Spanish Seed Producers’ Association (ANOVE), take the form of good practice guidance supplied with all bags of GM seed.
Based around a 20 m separation distance from neighboring non-GM crops, growers are advised to complete a simple decision-tree process in respect of their GM cropping plans ().
In certain situations, additional protocols have also been established by farming co-operatives to maintain adventitious GM presence below 0.9% for farmer members.
Since maize can be sown in Spain from early March to late May, GM and non-GM producers also consult on varietal selection, sowing and flowering intervals as part of the co-existence process.
Commercial experience of GM crop cultivation in Spain, Europe’s largest producer of GM crops, suggests that co-existence is working well, with no reported lawsuits filed between neighboring farmers, no (validated) reports of economic damage caused by adventitious GM presence within the supply chain, and no requirement identified for dedicated insurance products or compensation mechanisms.
This empirical evidence is backed up by the findings of co-existence studies conducted by public and independent research institutes in Spain.
For example, in 2005 the Spanish Ministry of Agriculture (MAPA) conducted a study of GM presence in organic maize in Aragon, where GM maize accounted for 29% of the region’s total production. No samples were found to exceed the 0.9% EU labeling threshold.Citation18
A similar survey conducted by MAPA in 2004 of maize destined for human consumption in Extremadura, the main area where maize is grown for human consumption, found no samples exceeded the 0.9% threshold while the average GM presence among 192 samples analyzed was 0.015%.Citation19
Although no commercial cultivation of GM crops has yet taken place in the UK, a field-scale, multi-year research program (known as the GM Crop Farm-Scale Evaluations) from 1999–2003 represents the largest scale cultivation of herbicide tolerant GM crops anywhere in the EU.
Over a four year period, more than 280 whole field sites—representative of UK farming conditions and ranging in size from 2 to 10 hectares—hosted commercial-scale trials of five different GM crops: forage maize, autumn-sown oilseed rape, spring-sown oilseed rape, sugar beet, and fodder beet.
The Farm-Scale Evaluations (FSE) provided a unique opportunity to apply and monitor the co-existence guidelines developed by SCIMAC within a realistic farming context.
The SCIMAC guidelines build on existing principles of good agricultural practice and closely mirror the proven system operated for more than 40 y to control the production of certified seed crops. All aspects of on-farm operations are covered, from seed storage and planting procedures through to crop separation distances, machinery hygiene, volunteer control and record-keeping.
These co-existence guidelines were applied and independently audited at all FSE sites. With no loss of organic or non-GM status throughout the trials process, this experience has indicated that the protocols developed by SCIMAC are workable in practice and robust in safeguarding the integrity of GM and neighboring non-GM crops.
To support this conclusion, SCIMAC conducted a questionnaire surveyCitation20 of all growers involved in the trials. Feedback suggests that the vast majority (94%) found the guidelines straightforward to follow, and in line with other management practices on the farm.
In addition, an independent audit of farmers’ compliance with the SCIMAC guidelines was conducted at all FSE sites by ADAS Consulting Ltd. The audit process, involving on-farm and telephone checks, focused on eight critical control points throughout the production process, from seed storage and planting to separation distances, harvesting procedures and record-keeping.
In its audit summary report,Citation21 ADAS confirmed that there were no incidents of non-compliance at these critical control points over the entire period of the trials process.
Overall, this experience confirms that practical measures to deliver co-existence between GM and non-GM crops can be managed effectively at the farm level, and need not represent a significant departure from current best practice within the industry.
Conclusion
Over time, the political and scientific context which frames the EU policy debate over farm-level co-existence between GM and non-GM crops has lost sight of the practical and commercial realities involved.
Co-existence is not a new concept in agriculture, and well-established practices exist throughout the farm supply chain to produce, segregate, store and deliver harvested crops to specified levels of quality and purity.
In each case, the level of market differentiation determines the nature and extent of the segregation procedures applied.
Once cleared for marketing, GM crops introduce no new considerations—biological, agronomic, legal, or socio-economic—which cannot be addressed through these established supply chain mechanisms.
The politically-motivated use of disproportionate or discriminatory co-existence measures by EU Governments to block the adoption of approved GM crops, and the complicity of the European Commission in providing a legal framework for Member States to do so, marks a serious departure from the norms of national and international governance.
The wider implications of this approach—for the single market, for international trade, for European farming competitiveness, and for future research investment within the EU—could be extremely damaging and far-reaching. By establishing the basis for future innovation to be blocked or restricted without scrutiny or justification, the Commission risks consigning European agriculture to a technological backwater at a time when farmers in other countries around the world are scaling up their adoption of advanced biotech crops and traits.
Co-existence should be a question of choice, not prejudice, and a supply chain-based approach—for example based on the guiding principles developed by the cross-industry SCIMAC group in the UK—offers the most pragmatic and flexible solution to servicing differentiated market demands.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Regulation (EC) No 1829/2003 of the European Parliament and of the Council of 22 September 2003 on genetically modified food and feed [Internet]. EUR-Lex: c2003 [cited 2013 July 15]. Available from: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:32003R1829:EN:NOT
- SCIMAC. (Supply Chain Initiative on Modified Agricultural Crops) [Internet]. SCIMAC: c2013 August 29 [cited 2013 July 15]. Available from: www.scimac.org.uk
- Commission Recommendation of 23 July 2003 on guidelines for the development of national strategies and best practices to ensure the co-existence of genetically modified crops with conventional and organic farming [Internet]. Brussels (Belgium); Commission of the European Communities: c2003 July 23 [cited 2013 July 15. Available from: http://ecob.jrc.ec.europa.eu/documents/Recommendation_2003_556.pdf
- Data sourced from: Communication from the Commission to the Council and the European Parliament - Report on the implementation of national measures on the coexistence of genetically modified crops with conventional and organic farming [Internet]. Brussels (Belgium): Commission of the European Communities: c2006 Sept 3 [cited 2013 July 15]. Available from: http://ecob.jrc.ec.europa.eu/documents/COM_2006_104final.pdf
- Devos, et al. Coexistence of genetically modified (GM) and non-GM crops in the European Union. A review. Agron Sustain Dev 2009; 29:11 - 30; http://dx.doi.org/10.1051/agro:2008051
- EC allows Danes to compensate for GM crop mixing [Internet]. Checkbiotech: cited 2005 Nov 25 [cited 2013 July 15]. Available from: http://greenbio.checkbiotech.org/news/ec_allows_danes_compensate_gm_crop_mixing
- Commission Recommendation. (2010/C 200/01) of 13 July 2010 on guidelines for the development of national co-existence measures to avoid the unintended presence of GMOs in conventional and organic crops [Internet]. European Commission: c2010 July 3 [cited 2013 July 15]. Available from: http://ecob.jrc.ec.europa.eu/documents/CoexRecommendation.pdf
- SCIMAC concerned over EU plans on GM crop co-existence [Internet]. SCIMAC: c2010 July [cited 2013 July 15]. Available from: http://www.scimac.org.uk/july2010.html
- Joint Research Centre. Global pipeline of new GM crops database [Internet]. Brussels (Belgium): Commission of the European Communities: c2003 July 23 [cited 2013 July 15]. Available from: http://ipts.jrc.ec.europa.eu/publications/pub.cfm?id=2199
- Co-Extra [Internet]. Versailles (France): Co-Extra: c2006 [cited 2013 July 15]. Available from: http://www.coextra.eu/
- SIGMEA [Internet]. Therival-Grignon (France): INRA: c2013 [cited 2013 July 15]. Available from: http://www7.inra.fr/sigmea
- TRANSCONTAINER Project. Containing GMOs [Internet]. European Commission Research – Biodiversity: c2007 Dec 6 [cited 2013 July 15]. Available from: http://ec.europa.eu/research/biosociety/food_quality/projects/132_en.html
- PRICE. Practical Implementation of Coexistence in Europe [Internet]. Wageningen (The Netherlands): EC Seventh Framework Programme: c2013 [cited 2013 July 15]. Available from: http://price-coexistence.com/
- European Co-existence Bureau [Internet]. Seville (Spain): European Commission Joint Research Centre: c2013 [cited 2013 July 15]. Available from: http://ecob.jrc.ec.europa.eu/
- Global GM Crop Plantings Increase 100-fold from 1996 [Internet]. Manila (Philippines): International Service for the Acquisition of Agri-Biotech Applications (ISAA): c2013 Feb 20 [cited 2013 July 15]. Available from: http://www.isaaa.org/resources/publications/briefs/44/pressrelease/
- USDA Economic Research Service data [Internet]. Washington, D.C. (US): United States Department of Agriculture: c2012 July 5 [cited 2013 July 15]. Available from: http://www.ers.usda.gov/data-products/organic-production.aspx#.UdrTxvmR98E
- Decisions of the Federal Court of Canada. 2001 FCT 256 [Internet]. Ontario (Canada): Federal Court of Canada: c2001 March 29 [cited 2013 July 15]. Available from: http://decisions.fct-cf.gc.ca/en/2001/2001fct256/2001fct256.html
- The Spanish experience with co-existence after eight years of cultivation of GM maize [Internet]. Vienna (Austria): Spanish Plants Varieties Office: c2006 April [cited 2015 July 15]. Available from: http://ec.europa.eu/agriculture/events/vienna2006/presentations/ortega_en.pdf
- The Spanish experience with co-existence after eight years of cultivation of GM maize [Internet]. Vienna (Austria): Spanish Plants Varieties Office: c2006 April [cited 2015 July 15]. Available from: http://ec.europa.eu/agriculture/events/vienna2006/presentations/ortega_en.pdf
- FSE Grower Survey [Internet]. SCIMAC: c2013 August 29 [cited 2013 July 15]. Available from: http://www.scimac.org.uk/files/FSEQuestionnaire2.ppt
- Audits of GM-HT crops within the Farm-Scale Evaluation trial [Internet]. Wolverhampton (UK): ADAS Consulting Ltd: c2003 April [cited 2013 July 15]. Available from: http://www.scimac.org.uk/files/ADAS_2003.pdf