Abstract
Kashmir valley is a major saffron (Crocus sativus Kashmirianus) growing area of the world, second only to Iran in terms of production. In Kashmir, saffron is grown on uplands (termed in the local language as “Karewas”), which are lacustrine deposits located at an altitude of 1585 to 1677 m above mean sea level (amsl), under temperate climatic conditions. Kashmir, despite being one of the oldest historical saffron-producing areas faces a rapid decline of saffron industry. Among many other factors responsible for decline of saffron industry the preponderance of erratic rainfalls and drought-like situation have become major challenges imposed by climate change. Saffron has a limited coverage area as it is grown as a ‘niche crop’ and is a recognized “geographical indication,” growing under a narrow microclimatic condition. As such it has become a victim of climate change effects, which has the potential of jeopardizing the livelihood of thousands of farmers and traders associated with it. The paper discusses the potential and actual impact of climate change process on saffron cultivation in Kashmir; and the biotechnological measures to address these issues.
Keywords: :
Niche Area of Saffron Cultivation in India
Climate change and environmental degradation have led to increased desertification and soil depletion. This kind of environmental degradation and the exhaustion of natural resources, such as forests and fertile agricultural land, constitute critical obstacles affecting sustainability of agriculture production systems, especially in ecologically fragile zones like the Himalayas. Much of the irrigation water in India and Pakistan originates from Himalayan glaciers. These glaciers are rapidly melting and their summer stream-flow may get significantly reduced within a few decades. Generally speaking, the most drought-vulnerable parts of our planet are the regions between 15° and 30° of the northern and southern latitudes, and the localities on the lee side of mountains.Citation1 The state of Jammu and Kashmir (J&K) is located between 32°17′ to 36°58′ North (latitude) and 73°26′ to 80°30′ East (longitude), encompassing the Western Himalayas and the Karakorum mountains. One of the largest states of the Indian Union, J&K covers an area of 222 236 km2 and includes, besides the famous Kashmir valley, the area of Jammu, Ladakh, Baltistan, Gligit, Hunza, and Nagar. Among these, the Kashmir valley represents one of the major saffron (Crocus sativus L.; Iridaceae) growing areas of the world. The period when saffron was introduced into Kashmir is not precisely known. Evidence from the oldest historical treatise of Kashmir Rajatarangini, written by Kalhana in the 12th century, indicates that saffron was present in Kashmir even before the reign of King Lalitaditya in 750 AD. Saffron is known as “Kum Kum” and “Kesar” in Sanskrit, and “Koung” in the Kashmiri language.Citation2 Even though successful attempts to grow saffron in other parts of J&K state like KargilCitation3 or other areas of India such as Uttar Pradesh and Himachal Pradesh have been reported,Citation4 almost all saffron production is actually limited to Kashmir.
Saffron, originating from the Arabic word “Zafaran” meaning yellow, is a fascinating spice steeped in rich history.Citation5 Its secrets stem from the dried red stigmas which accumulate large amounts of three glucosylated apocarotenoids,Citation6 namely crocin, picrocrocin, and safranal, which, among the more than 150 volatile and aroma yielding compounds, contribute to the color, bitter flavor, and aroma so typical of saffron. Saffron is a high value low volume spice that grows throughout Mediterranean Europe and Western Asia between 10° west and 80° east longitudes and 30 to 50° north latitudes.Citation7 The spice is used as flavoring and coloring agent in food and is a vital part of the dye, perfumery, and flavoring industries. Saffron also has countless biological properties like anticancer, antimutagenic, and antioxidant.Citation8 As a result, saffron fetches the highest price as a spice in the world, at approx. US$ 1100–11 000/kg (http://en.wikipedia.org/wiki/Saffron), depending upon the country of its production.Citation9 Its production is typically favored in countries where labor is cheap, such as Iran and Azerbaijan, but is also produced in countries like Greece, Spain, Argentina, or the USA with global production exceeding 200 tonnesCitation10 and newer areas being brought under its cultivation, viz. China and Japan.
In Kashmir, saffron is grown on uplands (termed in the local language as “Karewas”), which are lacustrine deposits located at an altitude of 1585 to 1677 meters above mean sea level (amsl), under temperate climatic conditions.Citation11 The soils are heavy textured with silty clay loam as the predominant texture in upper horizons and silty clay in lower horizons. These soils are alfisols and are well drained. The soils are calcareous in nature with average calcium carbonate and organic carbon contents of 4.61 and 0.35%, respectively. The soil is slightly alkaline with pH ranging from 6.3 to 8.3 and with electrical conductivity between 0.09 and 0.30 dsm-1.Citation12-Citation14
Kashmir, despite being one of the oldest historical saffron-producing areas, is now facing a rapid decline of saffron industry.Citation15 The total area under this crop in the State in 2012 is 3785 ha with an annual production of 11 t while almost one and half decade back in 1997 the area recorded was 5707 ha with an annual production of 15.95 t (). The lowest productivity of 1.57 kg ha−1 was recorded in 2003–04 due to an acute drought from 1999–2003. According to the data available for 1990–96 the area of saffron cultivation in Kashmir varied in a narrow range of 4036 to 4496 ha, with more or less constant annual production (13.0–14.1 t) and productivity (2.90–3.21 kg ha−1).
Figure 1. The figure shows a sharp decline in saffron area, production and productivity between 1997 and 2006 (decade), and revival since 2010 on account of National Saffron Mission.
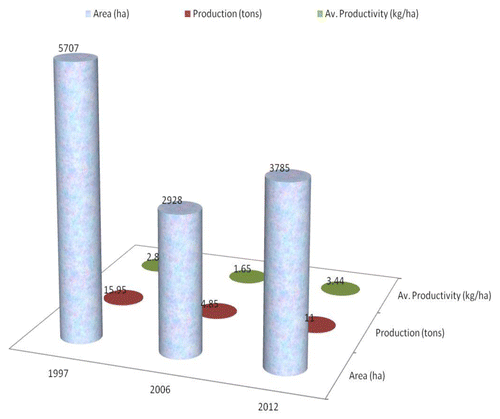
There are many factors responsible for decline of saffron industry in Kashmir viz., the lack of availability of good-quality corms as seed material, poor soil fertility, lack of assured irrigation, infestation by rodents and diseases, poor postharvest management, improper marketing facilities, increased urbanization on saffron land, rampant adulteration, and clandestine smuggling of cheap saffron.Citation2 The latest and most formidable challenge that threatens the existence of saffron industry is the adverse effect of climate change.
Environmental Stresses Caused by Climate Change
Corm sprouting, flower initiation, and time of flowering are the critical stages that are influenced by environmental fluctuations in terms of temperature and availability of water. Day temperature of 23–25 °C in the month of September is critical for corm sprouting, whereas flowering is initiated when the day temperature reaches to 17 °C with a night temperature around 10 °C.Citation16
Saffron fields in Kashmir are entirely rain-fed. If rains fail, the crop also fails. Although water requirement is low for saffron, water stress affects yield, growth, and development.Citation3,Citation17 The areas receiving 100–150 cm of well distributed rainfall with snow in winter are suitable for saffron cultivation, and rains in September are essential for meeting the water requirement of corms for good flower yields.Citation18 The total rainfall during the saffron growing period is usually sufficient, but its distribution is irregular, and it has now become common for saffron to face water stress. Rainfall of 100–150 mm is considered essential during the pre-flowering stage.Citation19 The flowering is optimum and saffron yields are good only when rains are received at sprouting and pre-flowering stages. Assured irrigation, at pre-sprouting and pre-flowering stages causes a quick activation of buds leading to corm sprouting and flower initiation. Any un-seasonal rains or drop in ambient temperature during October causes serious damage to flowering, leading to heavy reduction in saffron yield (). Flowering occurs between mid-October to mid-November every year, mostly in three flushes in a normal season.
Figure 2. Components of ‘Climate’ interact to create optimal plant growth conditions for Saffron; while their disturbance upsets growth and development, and lowers productivity.
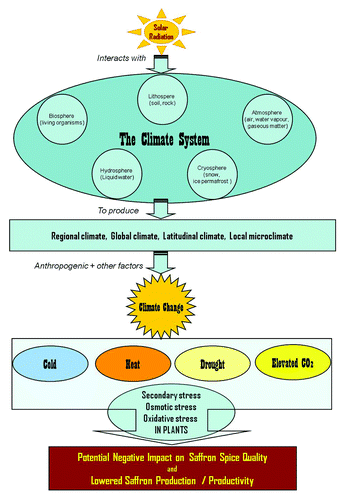
Due to climate change in the last some years the weather has become quite erratic and rains are either scanty or distribution is irregular, thus adversely affecting the critical stage of flowering in saffron. Kashmir faced an acute drought in 1999–2003,Citation20 and during this period productivity reduced from 3.12 kg ha−1 to 1.57 kg ha−1. During 2005, favorable rainfall improved saffron productivity to 2.96 kg ha−1. Saffron is still grown as rainfed crop, and hence vulnerable to scarcity / surplus of water. The surplus of water is as detrimental as the scarcity. Heavy rains over a short period on poorly drained saffron soils pre-dispose saffron corms to rotting fungi like Rhizoctonia crocorum, Phoma crocophila,Citation21 Macrophomina phaseolina,Citation22 Fusarium moniliforme var intermedium, a non-sporulating basidiomycetous fungus,Citation23 Fusarium oxysporum, F. solani, F. equiseti, F. pallidoroseum, Penicillium sp., Mucor sp.,Citation24,Citation25 and Sclerotium rolfsii.Citation26
Corm rot of saffron caused by F. oxysporum and F. solani is considered to be most destructive in Kashmir.Citation24,Citation25 These infections generally take place through the injury of corms. Infected corms possess dark-brown sunken and irregular patches below the corm scales, mostly near root and bud regions. In severe cases the entire corm turns into a black powdery mass. The foliage of infected corms shows symptoms of “die-back.”Citation27 The disease is quite widespread and causes loss of a considerable proportion of the produce every year. For controlling saffron corm rot, the corms to be planted are put in a fungicidal solution containing Carbendazim 50WP (0.1%), Mancozeb 75WP (0.3%) for 5–10 min, and then dried in shade for 10–15 min.Citation27 Similarly, Bavistin and Tecto (0.2% each) dip or drench help in disease control.Citation28 Corm treatment with Carbendazim 50WP (0.2%) or Myclobutanil (10WP) (0.2%) is also effective in reducing the corm rot severity.Citation25 These treatments are effective only for 1st year of planting in a planting cycle of 5–10 y. Even if mother corms are planted after fungicidal treatment, the daughter corms that develop in the later years get easily infected because they cannot be dug out each year to give chemical treatment. Under climate change scenario heavy rainfall over short period along with high temperature have a potential to cause heavy losses to the saffron plantation and yield, because such conditions are conducive for the growth of fungal pathogens causing rot.
Weed infestation
The major weeds found in saffron fields of Kashmir include Euphorbia helioscopia, Lepidium virginicum, Salvia moorcroftiana, Papaver rhoae, Chonspora tanella, Galium tricorne, Tulipa stellata, Erodium cicutarium, Lithospermum arvense, Ranunculus arvensis, Medicago lupilina, Poa bulbosa, Crepis saneta, Filago arvense, Polygonum aviculare, Chenopodium album, Descurainea sophia, among others.Citation29 Weed management would be an important concern in future as climate change may promote the growth of some weeds due to conducive growth conditions for vigorous growth, causing heavy losses to saffron production. Currently, no weed management practices are followed by saffron growers except for harvesting of some weeds as fodder by farm women in May, and cattle grazing by some farmers in August.
Saffron quality
Saffron is economically an important crop as far as its use as spice and medicine is concerned. All these biotic and abiotic stresses not only affect the productivity adversely but may also have negative impact on saffron quality (crocin, picrocrocin, and other bioactive compounds). As such, controlled climate change studies need to be conducted in SPAR (Soil Plant Atmosphere Research) facilities to find the impact of temperature, moisture and CO2 on saffron quality.
Omics Approaches for Understanding Effect of Climate Change Stresses on Saffron Biology
Saffron is a triploid species with 3n = 24, x = 8 chromosomes. Its triploid nature allows for vegetative multiplication, but not regular sexual reproduction. This is because meiosis and gamete development in triploids are irregular, resulting in many anomalies in sporogenesis and gametophyte development. Saffron stigmas and corms are characterized by the presence of antifungal saponinsCitation30 and defense proteins capable of binding to chitin and chitin oligosaccharides. A new class of defense chitinase namely Safchi A has been isolated from saffron.Citation31,Citation32 Different phenolic compounds (pyrogallic acid, kaempferol, p-coumaric acid, and gallic acid) involved in stress responses have also been identified.Citation33,Citation34 Peroxidase, catalase, and superoxide dismutase activities have been detected in saffron corms in different developmental stages,Citation35,Citation36 and genomic approaches have enabled the identification of partial sequence homologs for these enzymes. Bioinformatics analysis of corm and stigma libraries from Crocus has identified several genes associated with defense responses.Citation37
Although a lot of literature can be found on botanical aspects of saffron, not much information is available on ecophysiological aspect. Several environmental parameters affect flower induction in saffron among which temperature seems to play a pivotal role.Citation38,Citation39 Flower induction requires an incubation of the corms at high temperature (23–27 °C), followed by a period of exposure at moderately low temperature (17 °C) for flower emergence. There are evidences which show the critical importance of light and temperature in biological activities of plants including regulatory effects on dormancy period, vegetative and generative growth particularly flowering habit.Citation40,Citation41 Transcriptome analysis of saffron plants subjected to different photoperiod and temperature regimes can throw light on the genes that get up or downregulated and might lead to the identification of novel regulators which influence or lead to saffron flower initiation in a particular agroclimatic condition.Citation42 How these differences and similarities translate into gene expression can be known using DNA microarray technology and bioinformatics tools. The huge database generated by physiological, agronomic, transcriptomics, proteomics, metabolomics based studies can then be analyzed by computational biology approach to find target genes for improving resilience of saffron to climate change.Citation43 This knowledge may also be used to specifically tailor saffron plants for new geographical areas with adaptability to specific climatic conditions. The problems due to the challenges of climate change to saffron production can be mitigated by use of ‘alternative’ sustainable technologies coupled with modern biotechnological approaches. Adoption of such technologies can be promoted by strong policy decisions.Citation44
Microbial Associations for Enhancing Resilience
Changes in rainfall patterns affect soil surface temperatures and moisture availability. This can influence crop establishment, crop quality, and the total saffron production. To cushion crop production against these adversaries, symbiotic fungi can come to our rescue ().
Figure 3. Biotechnological approaches for building resilience and mitigating the negative effect of stresses imposed by climate change.
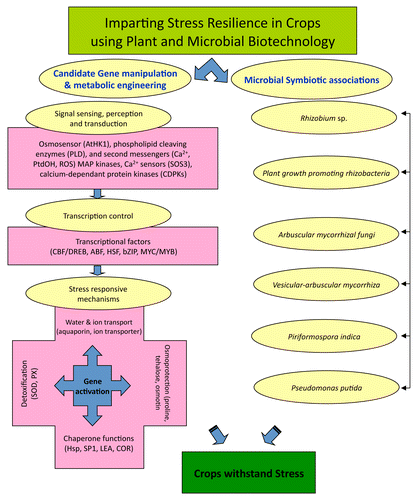
During the last two decades, the mycorrhizal technology has been used to improve growth of a number of micropropagated horticultural crops as well as to enable host plants to tolerate or withstand the impairing effects of abiotic and biotic stresses.Citation45-Citation49 Among these soil microorganisms, the most abundant and effective are rhizobia, plant growth promoting rhizobacteria (PGPR), and arbuscular mycorrhizal fungi (AMF).Citation50
Rhizobium sp
The beneficial effects of rhizobial symbiosis on plant growth and yield under water stress conditions are bacterial-genotype specific. Swaine et al.Citation51 reported that a strain of Bradyrhizobium elkanii isolated from a drought environment was more tolerant to in vitro osmotic stress than strains isolated from wet environments. Mnasri et al.Citation52 found that Phaseolus vulgaris plants inoculated with a salt-tolerant nitrogen-fixing bacterial strain (Ensifer meliloti) were more tolerant to drought than those inoculated with a salt-sensitive bacterial strain (Rhizobium tropici). Rhizobial symbiosis has been associated with regulation of root hydraulic properties.Citation53 A diminution in the expression of an aquaporin gene in the roots of soybean plants inoculated with the Bradyrhizobium japonicum under both control and water-deprived conditions was observed. At the same time, it is known that some aquaporins may transport ammonia or ammonium, and hence they could be involved in the transport of nitrogen compounds between the nodules and the host plant.Citation54,Citation55
Plant growth promoting rhizobacteria (PGPR)
Plant growth promoting rhizobacteria (PGPR) represent about 2–5% of total rhizobacterial community. These are an indispensable part of rhizosphere biota which can stimulate the growth of the host.Citation56 PGPRs are candidates for establishing in soil ecosystem as these are highly adaptability in a wide variety of environments, growth rate is faster and have capacity to metabolize a wide range of natural and xenobiotic compounds.Citation57 PGPR colonize roots of plant and promote plant growth and development through a variety of mechanisms such as production of phytohormones, suppression of deleterious organisms, activation of phosphate solubilization, and promotion of the mineral nutrient uptake.Citation58,Citation59 IAA (indoleacetic acid) is a product of l-tryptophan metabolism in bacteria and is prevalent in the rhizosphere because of the rich supplies of substrates exuded from the roots.Citation60,Citation61 Phosphate solubilizing bacteria (PSBs) inhabiting the rhizosphere are promising biofertlizers as these are known for secretion of organic acids and phosphates to solubilise insoluble phosphate to soluble forms.Citation62 PGPR’s also secrete Siderophores, in response to iron deficiency, which help in the transportation of ferric iron into plant cells from insoluble forms. They also improve antagonism against phytopathogensCitation63 and in induction of systemic resistance in plants.Citation64
Sharaf-Eldin et al.Citation65 studied the effect of Bacillus subtilis FZB24 on saffron corms under ex vitro conditions in Egypt and reported that inoculation of B. subtilis FZB24 significantly increased leaf length, flowers per corm, weight of the first flower stigma, total stigma biomass, and significantly decreased the time required for corms to sprout and the number of shoots. In India (Kashmir) Parray et al.Citation66 isolated the associated rhizobacteria from the roots of the flowering corms of saffron on Laureia Bertani (LB) agar medium. A total of 06 isolates of rhizobacteria were selected and were identified. The rhizobacterial strains were identified as Acinetobacter lwofii, Acinetobacter hemolyticus, Bacillus subtilis, Psuedomonas ssp, Pantoea ssp., and Klebsella ssp. The B. subtilis, Pantoea ssp. and A. lwofii were categorized as high IAA producers. The B. subtilis was best strain for IAA production and shows efficient prospect for enhancing the growth and development of corms. Pseudomonas ssp. was found to be highly efficient in terms of phosphate solubilisation production followed by B. subtilis. The study also focused on the screening of rhizobacterial strains for their potential to produce siderophore compounds. The highest percentage for siderophore production was for Psuedomonas ssp. (62%) followed by B. subtilis (59%), Pantoea ssp. (52%), A. lwofii (45%), and Klebsella ssp. (31%); however, A. hemolyticus did not show any activity. In another study, Ambardar and VakhluCitation67 reported that saffron rhizosphere is dominated by c-proteobacteria characterized by Pseudomonas and Acinetobacteria genera. Saffron rhizobacteria and bulk soil bacteria were categorized into 16 different bacterial species with Bacillus aryabhattai (WRF5-rhizosphere; WBF3, WBF4A, and WBF4B-bulk soil) common to both rhizosphere as well as bulk soil. Pseudomonas sp. in rhizosphere and Bacillus and Brevibacterium sp. in the bulk soil were the predominant genera respectively. Three species of Pseudomonas isolated from the Saffron rhizosphere are specific to saffron rhizosphere, as P. koreensis WRF6 and P. kilonensis WRF3 have not been reported from any other rhizosphere and P. tremae have been isolated only from coffee seedlings. The isolated rhizobacteria were screened for plant growth promotion activity like siderophore, indole acetic acid production, and phosphate solubilization. 50% produced siderophore and 33% were able to solubilize phosphate whereas all the rhizobacterial isolates produced indole acetic acid. From the results, it can be concluded that the bacterial consortia have some important plant growth promoting traits that can be used as biofertilizers and application of these rhizobacterial strains may provide some benefit to saffron growers by speeding corm growth (earlier shoot emergence) and increasing stigma biomass.
Arbuscular mycorrhizal fungi (AMF)
About 90% of vascular plants establish symbiosis with arbuscular mycorrhizal fungi (AMF).Citation68 The association between an AMF and a plant makes the host plant more tolerant to drought in terms of plant growth.Citation69-Citation71 These beneficial effects have been attributed to the expression of some genes related to drought stress like late embryogenesis abundant proteins (LEA), ∆Citation1-pyrroline 5 carboxylate synthase (P5CS). Porcel et al.Citation72,Citation73 found that both genes increased their expression under drought conditions more in non-AM plants than in AM ones. Similarly some superoxide dismutase isoforms from lettuce are specifically upregulated by drought conditions in AMF-colonized plants.Citation74 Generally AMF-colonized plants have higher activities of several antioxidant enzymes than non-colonized ones,Citation75 but it depends on the enzyme activity, plant organ, and the AMF genotype involved.Citation76,Citation77
Vesicular-Arbuscular Mycorrhiza (VAM)
Many studies have shown that drought resistance of crops could be improved by vesicular-arbuscular mycorrhiza (VAM).Citation78-Citation83 VAM fungi inoculation could slow down the reduction of chlorophyll and increase the drought resistance of plants by promoting the defense response of protective enzyme system in host plants.Citation49 Some researches showed that the formation of VAM could enhance the activity of antioxidant enzymes of SOD, POD, CAT, and H+-ATPase in host plants and reduce the content of MDA and electrical conductivity of plasma membrane significantly,Citation84,Citation85 but it is still not clear about the mechanism of VAM to enhance the drought resistance of plants.
Since most arbuscular vesicular-mycorrhizal fungi are obligate biotrophes in nature, they are difficult to culture in vitro, and this is a major obstacle to their practical use.Citation86 A traditional method of producing large amounts of inoculum is to culture the fungi on roots in soil,Citation87 but this method is very tedious and time-consuming.
Piriformospora indica
Piriformospora indica (Hymenomycetes, Basidiomycota) is a wide-host root-colonizing endophytic fungus which allows the plants to grow under extreme physical and nutrient stress. This novel multifunctional symbiotrophic fungal endophyte was discovered from the Great Indian Desert of Western Rajasthan in India.Citation88 The fungus was found to colonize roots of the desert plants which were growing in extreme conditions of water scarcity. Subsequently, it was established that the fungus promotes acquisition of drought tolerance in plants.Citation89,Citation90 In contrast with arbuscular mycorrhizal fungi, it can be easily cultivated in axenic culture where it produces chlamydospores.Citation91-Citation93 It is easily cultivated on a number of synthetic and complex media.Citation94,Citation95 Being the only cultivable endophyte that colonizes roots, this fungus provides a model organism for the study of beneficial plant–microbe interactions and a new tool for improving plant production systems.Citation88,Citation96,Citation97
P. indica has a vast geographical distribution and is reported from Asia, South America and Australia. The fungus has a huge potential for biotechnological applications, especially under changing climate scenario because it functions as a biofertilizer in nutrient-deficient soils; as a bioprotector against biotic and abiotic stresses; as a bioregulator for plant growth development and enhanced seed production; and as a bio-agent for the hardening of tissue-culture-raised plants.Citation98
Pseudomonas spp
Pseudomonas pudita and P. flouresence, the common PGPR of most of the plants, are absent in Saffron rhizosphere though P. koreensis WRF6, which shows maximum production of the indole acetic acid (28.5 lg/ml) is comparable to the other common growth promoting Pseudomonads e.g., P. putida (24.08 mg/l) and P. fluorescens (31.6 mg/l).Citation67 The inoculation of saffron corms with P. koreensis WRF6 may provide protection against the adverse effect of temperature rise on saffron plants, in a manner similar to that observed in sorghum and pearl millet. It has been found that seed inoculation with stress-tolerant strain of Pseudomonas putida helped sorghum and pearl millet seedlings survive at 50 °C up to 21 d, whereas the controlled seedlings could survive only up to 10 d (DARE-ICAR Report 2008–09; http://www.icar.org.in). This remarkable protective effect was mediated through induction of the synthesis of novel high molecular weight proteins in the leaves, which were not found in the controlled seedlings. Inoculation also reduced the oxidative stress in seedlings exposed to high temperature (50 °C) as evidenced by significantly lower oxidative enzyme activity in treated seedlings. The introduced organism successfully entered into the roots and induced the physiological changes at the whole plant level as confirmed by electron micrography (DARE-ICAR Report 2008–09).
Genetic Modification and Genome Engineering
Five primary environmental factors are critical for plants to germinate, grow, and reproduce, and saffron is no exception. These factors are carbon dioxide, sunlight, water, optimal temperature range, and nutrients. Of these, the first four are directly related to climate and vary spatially, diurnally, and seasonally. In the context of global climate change these factors behave erratically and, therefore, need to be considered foremost while designing saffron plants adapted to changing climatic conditions. Tolerance to heat, drought, water-logging, salinity, and frost; resistance to pests and diseases; water-use and nitrogen-use efficiency are the most important traits for adaptation to climate change.Citation1 Genetic modification techniques are becoming increasingly feasible due to improved techniques and demystification of the various side effects of transgenic technology.Citation99,Citation100 An array of genes, available for use in both cisgenic and transgenic approaches, can be used for the development of varieties with better resilience to vagaries of climate (). Clustered regulatory interspaced short palindromic repeats (CRISPRs) and CRISPR-associated (Cas) systems is another emerging technique that can be employed to introduce useful genome modifications through genome engineering of saffron. These are revolutionizing the field of genome editing.Citation101 With their highly flexible but specific targeting, CRISPR-Cas systems can be manipulated and redirected to become powerful tools for genome editing.
Conclusion
Using biotechnology, agronomic practices that increase carbon sequestration can become easy to adopt and as such render additional benefits too, like increased root biomass, soil organic matter, water and nutrient retention capacity, and, hence, increased saffron productivity. Enhancing carbon sequestration capacity in degraded saffron lands through organic means could thus have direct environmental, economic and social benefits for local people, with consequent improvement in their livelihood and food security status.
Establishment of symbiotic relationships between microorganisms and plants has shown promising potential for enhancing tolerance of plants to disease and drought. The beneficial effect of mycorrhization on plant drought tolerance is caused by the development of superior root system, enhanced water conducting capacity, increased uptake of macro, micro, and immobile nutrients, resulting in better carbon dioxide assimilation and higher photosynthetic rates. Among the promising bacteria that can be included into the saffron management practices Pseudomonas ssp. and Bacillus ssp. are particularly important owing to their beneficial influence. Among the symbiotic fungi, Piriformospora indica and Sebacina vermifera should be studied as these have been shown to mimic the capabilities of AMF and are axenically cultivable on a number of synthetic and complex media.
Data generated by physiological, agronomic, transcriptomics, proteomics, metabolomics studies can help find target candidate genes for improving resilience of saffron to climate change. Modern Genetic modification techniques including CRISPR can be used to create saffron plants that possess inbuilt tolerance to biotic and abiotic stresses, and hence make these plants resilient to some adverse effects of climate change.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I am personally thankful to Professors Azra N Kamili and Raies Qadri, University of Kashmir and Dr Meenakshi Munshi, Department of Biotechnology, GoI for their selfless support. The suggestions given by Drs Autar K Mattoo, Dilip Lakashman, and Jeffrey S. Buyer, during my visit to Sustainable Agricultural Systems Lab, Beltsville, Maryland, USDA have provided insight to this paper. The efforts of Professors Shafiq A Wani and FA Nehvi of SKUAST-K have been vital in addressing the problems faced by saffron farmers of J&K, (India) and hence necessitate a special mention here.
References
- Husaini AM, Tuteja N. Biotech crops: imperative for achieving the millenium development goals and sustainability of agriculture in the climate change era. GM Crops Food 2013; 4:1 - 9; http://dx.doi.org/10.4161/gmcr.22748; PMID: 23160541
- Husaini AM, Hassan B, Ghani MY, Teixeira da Silva JA, Kirmani NA. Saffron (Crocus sativus Kashmirianus) cultivation in Kashmir: Practices and problems. In: Husaini AM (Ed) Saffron. Functional Plant Science and Biotechnology 2010; 4 (Special Issue 2), 108-115.
- Munshi AM, Wani SA, Tak GM. Improved cultivation practices for saffron. In: Proceedings of Seminar-cum-Workshop on Saffron (Crocus sativus), June 14, 2001. SKUAST-K, India, 2002; pp 83-88.
- Dhar AK, Mir GM. Saffron in Kashmir-VI: A review of distribution and production. J Herbs Spices Med Plants 1997; 4:83 - 90; http://dx.doi.org/10.1300/J044v04n04_09
- Caiola MG, Canini A. Looking for Saffron’s (Crocus sativus L.) Parents. In: In: Husaini AM (Ed) Saffron. Functional Plant Science and Biotechnology 2010; 4 (SI-2):1-14.
- Gómez-Gómez L, Rubio-Moraga Á, Ahrazem O. Understanding Carotenoid Metabolism in Saffron Stigmas. Unravelling Aroma and Colour Formation. Funct. Plant Sci Biotechnol. 2010; 4:56 - 63
- Yasmin S, Nehvi FA. Saffron as a valuable spice: A comprehensive review. African Journal of Agricultural Research 2013; 8:234 - 42
- Premkumar K, Ramesh A. Anticancer, Antimutagenic and Antioxidant Potential of Saffron: An Overview of Current Awareness and Future Perspectives. Funct Plant Sci Biotechnol 2010; 4:91 - 7
- Anastasaki EG, et al. In Proceedings of 3rd International Symposium on Saffron: Forthcoming Challenges in Cultivation, Research and Economics, Krokos, Kozani, Greece, 2009.
- Husaini AM, ed. Saffron, Global Science Books, Ltd, UK, 2010, p. 135.
- Kanth RH, Khanday BA, Tabassum S. Crop weather relationship for saffron production. In: Nehvi FA, Wani SA (Eds) Saffron Production in Jammu and Kashmir, Directorate of Extension Education. SKUAST-K, India, 2008; 170-188.
- Nazir NA, Khitrov NB, Chizhikova NP. Statistical evaluation of soil properties which influence saffron growth in Kashmir. Eurasian Soil Sci 1996; 28:120 - 38
- Ganai MRD, Wani MA, Zargar GH. Characterisation of saffron growing soils of Kashmir. Applied Biological Research 2000; 2:27 - 30
- Ganai MRD. Nutrient status of saffron soils and their management. In: Proceedings of Seminar-cum-Workshop on Saffron (Crocus sativus), June 14, 2001. SKUAST-K, India, 2002; 51-54.
- Husaini AM, Bhat MA, Kamili AN, Mir MA. Kashmir saffron in crisis. Curr Sci 2013; 104:686 - 7
- Nehvi FA, Lone AA, Allai BA, Yasmin S. Impact of climate change on saffron industry of Jammu and Kashmir. Crop Improvement 2010; 37:203
- Hassan B, Shah MH. Increased sustainability and yield of saffron in Kashmir. In: Proceedings of Seminar-cum-Workshop on Saffron (Crocus sativus), June 14, 2001. SKUAST-K, India, 2002; 55-58.
- Srivastava RP. Cultivation of saffron in India. Fertilizer News 1963; 8:9 - 16
- Kamili AS, Nehvi FA, Trag AR. Saffron - a legendary crop of Kashmir Himalaya. Journal of Himalayan Ecology and Sustainable Development 2007; 2:1 - 12
- Alam A. Status and prospects of mechanisation in saffron cultivation in Kashmir. Acta Hortic 2007; 739:383 - 8
- Madan CL, Kapoor BM, Gupta US. Saffron. Econ Bot 1967; 20:377 - 85; http://dx.doi.org/10.1007/BF02904059
- Thakur RN, Singh C, Kaul BL. First report of corm rot in Crocus sativus L. Indian Phytopathology 1992; 45:278 - 82
- Dhar AK. Bio-ecology and control of corm rot of saffron (Crocus sativus L.). MSc thesis, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, India, 1992; 109 pp.
- Wani A. Studies on corm rot of saffron (Crocus sativus L.). PhD thesis, Sher-e-Kashmir University of Agricultural Sciences and Technology of Kashmir, India, 2004; 108 pp.
- Ahmad M, Sagar V. Integrated management of corm/tuber rot of saffron and Kalazeera. Horticulture Mini Mission-1, Indian Council for Agricultural Research (ICAR), India, 2007; 22 pp.
- Kalha CS, Gupta V, Gupta D. First report of sclerotial rot of saffron caused by Sclerotium rolfsii in India. Plant Dis 2007; 91:1203 - 6; http://dx.doi.org/10.1094/PDIS-91-9-1203B
- Ghani MY. Corm rot disease of saffron and its management. In: Proceedings of Seminar-cum-Workshop on Saffron (Crocus sativus), June 14, 2001. SKUAST-K, India, 2002; pp 107-112.
- Sud AK, Paul YS, Thakur BR. Corm rot of saffron and its management. Journal of Mycology and Plant Pathology 1999; 29:380 - 2
- Pir FA, Nehvi FA, Singh KN, Hassan B, Khanday BA, Mir ZA. Saffron weed flora of Kashmir. In: Nehvi FA, Wani SA (Eds) Saffron Production in Jammu and Kashmir, Directorate of Extension Education. SKUAST-K, India, 2008; pp 189-200.
- Hosseinzadeh H, Younesi HM. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol 2002; 2:7; http://dx.doi.org/10.1186/1471-2210-2-7; PMID: 11914135
- Castillo R, Gomez G, Fernandez JA. SafchiA is a new class of defence chitinase from saffron (Crocus sativus L.). Acta Hortic 2007; 739:195 - 202
- López RC, Gómez-Gómez L. Isolation of a new fungi and wound-induced chitinase class in corms of Crocus sativus. Plant Physiol Biochem 2009; 47:426 - 34; http://dx.doi.org/10.1016/j.plaphy.2009.01.007; PMID: 19246207
- Nk C, Koul KK, Farooq S. Phenolic compounds in corm of saffron crocus (Crocus sativus L.) during bud development. Plant Physiol Biochem 1986; 13:78 - 81
- Ebrahimzadeh H, Abrishamchi P, Noori-Daloii MR. Study on ontogenetic changes in Crocus sativus by study phenolics and phenol oxidases in the corm and bud tissues. Journal of Sciences of the Islamic Republic of Iran 1997; 8:81 - 5
- Keyhani E, Keyhani J. Hypoxia/anoxia as signaling for increased alcohol dehydrogenase activity in saffron (Crocus sativus L.) corm. Ann N Y Acad Sci 2004; 1030:449 - 57; http://dx.doi.org/10.1196/annals.1329.056; PMID: 15659829
- Keyhani E, Ghamsari L, Keyhani J, Hadizadeh M. Antioxidant enzymes during hypoxia-anoxia signaling events in Crocus sativus L. corm. Ann N Y Acad Sci 2006; 1091:65 - 75; http://dx.doi.org/10.1196/annals.1378.055; PMID: 17341603
- D’Agostino N, Pizzichini D, Chiusano ML, Giuliano G. An EST database from saffron stigmas. BMC Plant Biol 2007; 7:53; http://dx.doi.org/10.1186/1471-2229-7-53; PMID: 17925031
- Molina RV, Garcia-Luis A, Coll V, Ferrer C, Valero M, Navarro Y, Guardiola JL. Flower formation in the saffron crocus (Crocus sativus L.)- The role of temperature. Acta Hortic 2004; 650:39 - 47
- Molina RV, Valero M, Navarro Y, Garci’a-Luis A, Guardiola JL. Low temperature storage of corms extends the flowering season of saffron (Crocus sativus L.). J Hortic Sci Biotechnol 2005; 80:319 - 26
- Milyaeva EL, Azizbekova NSH. Cytophysiological changes in the course of development of stem apices of saffron crocus. Soviet Plant Physiology 1978; 25:227 - 33
- Halevy AH. Recent advances in control of flowering and growth habit of geophytes. Acta Hortic 1990; 266:35 - 42
- Husaini AM, Wani SA, Sofi P, Rather AG, Mir JI. Bioinformatics for Saffron (Crocus sativus L.) Improvement. Communications in Biometry and Crop Science 2009; 4:1 - 6
- Husaini AM, Ashraf N. Understanding Saffron biology using bioinformatics tools. In: Husaini AM (Ed) Saffron. Functional Plant Science and Biotechnology 2010; 4 (Special Issue 2):31-37.
- Husaini AM, Kamili AN, Wani MH, Teixeira da Silva JA, Bhat GN. Sustainable Saffron (Crocus sativus Kashmirianus) Production: Technological and Policy Interventions for Kashmir. In: Husaini AM (Ed) Saffron. Functional Plant Science and Biotechnology 2010; 4 (Special Issue 2):116-127.
- Guillemin JP, Gianinazzi S, Trouvelot A. Screening of arbuscular endomycorrhizal fungi for establishment of micropropagated pineapple plants. Agronomie 1992; 12:831 - 6; http://dx.doi.org/10.1051/agro:19921016
- Hooker JE, Gianinazzi S, Vestberg M, Barea JM, Atkinson D. The application of arbuscular mycorrhizal fungi to micropropagation systems: an opportunity to reduce chemical inputs. Agr Sci Finland 1994; 3:227 - 32
- Dodd JC. The role of arbuscular mycorrhizal fungi in agro-and natural ecosystems. Outlook Agric 2000; 29:55; http://dx.doi.org/10.5367/000000000101293059
- Borkowska B. Growth and photosynthetic activity of micropropagated strawberry plants inoculated with endomycorrhizal fungi (AMF) and growing under drought stress. Acta Physiol Plant 2002; 24:365 - 70; http://dx.doi.org/10.1007/s11738-002-0031-7
- Yin B, Wang Y, Liu P, Hu J, Zhen W. Effects of vesicular-arbuscular mycorrhiza on the protective system in strawberry leaves under drought stress. Front Agric China 2010; 4:165 - 9; http://dx.doi.org/10.1007/s11703-010-0109-8
- Barea JM, Pozo MJ, Azcón R, Azcón-Aguilar C. Microbial co-operation in the rhizosphere. J Exp Bot 2005; 56:1761 - 78; http://dx.doi.org/10.1093/jxb/eri197; PMID: 15911555
- Swaine E, Swaine M, Killham K. Effects of drought on isolates of Bradyrhizobium elkanii cultured from Albizia adianthifolia seedlings of different provenances. Agrofor Syst 2007; 69:135 - 45; http://dx.doi.org/10.1007/s10457-006-9025-6
- Mnasri B, Aouani ME, Mhamdi R. Nodulation and growth of common bean (Phaseolus vulgaris) under water deficiency. Soil Biol Biochem 2007; 39:1744 - 50; http://dx.doi.org/10.1016/j.soilbio.2007.01.030
- Porcel R, Aroca R, Azcón R, Ruiz-Lozano JM. PIP aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. Plant Mol Biol 2006; 60:389 - 404; http://dx.doi.org/10.1007/s11103-005-4210-y; PMID: 16514562
- Tyerman SD, Niemietz CM, Bramley H. Plant aquaporins: multifunctional water and solute channels with expanding roles. Plant Cell Environ 2002; 25:173 - 94; http://dx.doi.org/10.1046/j.0016-8025.2001.00791.x; PMID: 11841662
- Uehlein N, Fileschi K, Eckert M, Bienert GP, Bertl A, Kaldenhoff R. Arbuscular mycorrhizal symbiosis and plant aquaporin expression. Phytochemistry 2007; 68:122 - 9; http://dx.doi.org/10.1016/j.phytochem.2006.09.033; PMID: 17109903
- Vessey JK. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003; 255:571 - 86; http://dx.doi.org/10.1023/A:1026037216893
- Bhattacharyya PN, Jha DK. Plant growth-promoting rhizobacteria (PGPR): emergence in agriculture. World J Microbiol Biotechnol 2012; 28:1327 - 50; http://dx.doi.org/10.1007/s11274-011-0979-9; PMID: 22805914
- Glick B. The enhancement of plant growth by freeliving bacteria. Microbiology 1995; 41:109 - 17
- Lalande R, Bissonnette N, Coutlee D, Antoun H. Identification of rhizobacteria from maize and determination of their plant-growth promoting potential. Plant Soil 1989; 115:7 - 11; http://dx.doi.org/10.1007/BF02220688
- Strzelczyk E, Pokojska-Burdziej A. Production of auxins and gibberellin like substances by mycorrhizal fungi, bacteria and actinomycetes isolated from soil and mycorhizosphere of pine (PinussilvestrisL.). Plant Soil 1984; 81:185 - 94; http://dx.doi.org/10.1007/BF02197150
- Lynch JM. Origin, nature and biological activity of aliphatic substances and growth hormones found in soil. In: Soil Organic Matter and Biol. Activity. Eds. Vaughan D and Malcom RE. Martinus Nijhoff/Dr. W. Junk Publishers. Dordrecht, Boston, Lan Lancaster. 1985; pp. 151-174.
- Ahemad M, Khan MS. Functional aspects of plant growth rhizobacteria: Recent advancements. Insight Microbial 2011; 1:39 - 54; http://dx.doi.org/10.5567/IMICRO-IK.2011.39.54
- Chincholkar SB, Chaudhari BL, Rane MR. Microbial Siderophores: State of art. In: Microbial Siderophores. Chincholkar, S. B. and Varma, A. (eds.) Springer Verlag, Germany. 2007; pp. 233-242.
- De Meyer G, Capieau K, Audenaert K, Buchala A, Métraux JP, Höfte M. Nanogram amounts of salicylic acid produced by the rhizobacterium Pseudomonas aeruginosa 7NSK2 activate the systemic acquired resistance pathway in bean. Mol Plant Microbe Interact 1999; 12:450 - 8; http://dx.doi.org/10.1094/MPMI.1999.12.5.450; PMID: 10226378
- Sharaf-Eldin M, Elkholy S, Fernández JA, Junge H, Cheetham R, Guardiola J, Weathers P. Bacillus subtilis FZB24 affects flower quantity and quality of saffron (Crocus sativus). Planta Med 2008; 74:1316 - 20; http://dx.doi.org/10.1055/s-2008-1081293; PMID: 18622904
- Parray JA, Kamili AN, Reshi ZA, Hamid R, Qadri RA. Screening of beneficial properties of rhizobacteria isolated from Saffron (Crocus sativus L) rhizosphere. Afr J Microbiol Res 2013; 7:2905 - 10
- Ambardar S, Vakhlu J. Plant growth promoting bacteria from Crocus sativus rhizosphere. World J Microbiol Biotechnol 2013; 29:2271 - 9; http://dx.doi.org/10.1007/s11274-013-1393-2; PMID: 23749248
- Gai JP, Christie P, Feng G, Li XL. Twenty years of research on community composition and species distribution of arbuscular mycorrhizal fungi in China: a review. Mycorrhiza 2006; 16:229 - 39; http://dx.doi.org/10.1007/s00572-005-0023-8; PMID: 16284782
- Ruiz-Lozano JM. Arbuscular mycorrhizal symbiosis and alleviation of osmotic stress. New perspectives for molecular studies. Mycorrhiza 2003; 13:309 - 17; http://dx.doi.org/10.1007/s00572-003-0237-6; PMID: 12690537
- Wu Q-S, Xia R-X. Arbuscular mycorrhizal fungi influence growth, osmotic adjustment and photosynthesis of citrus under well-watered and water stress conditions. J Plant Physiol 2006; 163:417 - 25; http://dx.doi.org/10.1016/j.jplph.2005.04.024; PMID: 16455355
- Bolandnazar S, Aliasgarzad N, Neishabury MR, Chaparzadeh N. Mycorrhizal colonization improves onion (Allium cepa L.) yield and water use efficiency under water deficit condition. Sci Hortic (Amsterdam) 2007; 114:11 - 5; http://dx.doi.org/10.1016/j.scienta.2007.05.012
- Porcel R, Azcón R, Ruiz-Lozano JM. Evaluation of the role of genes encoding for Δ1-pyrroline-5-carboxylate synthetase (P5CS) during drought stress in arbuscular mycorrhizal Glycine max and Lactuca sativa plants. Physiol Mol Plant 2004; 65:211 - 221; http://dx.doi.org/10.1016/j.pmpp.2005.02.003
- Porcel R, Azcón R, Ruiz-Lozano JM. Evaluation of the role of genes encoding for dehydrin proteins (LEA D-11) during drought stress in arbuscular mycorrhizal Glycine max and Lactuca sativa plants. J Exp Bot 2005; 56:1933 - 42; http://dx.doi.org/10.1093/jxb/eri188; PMID: 15911559
- Ruiz-Lozano JM, Collados C, Barea JM, Azcón R. Cloning of cDNAs encoding SODs from lettuce plants which show differential regulation by arbuscular mycorrhizal symbiosis and by drought stress. J Exp Bot 2001; 52:2241 - 2; PMID: 11604465
- Wu QS, Zou YN, Xia RX. Effects of water stress and arbuscular mycorrhizal fungi on reactive oxygen metabolism and antioxidant production by citrus (Citrus tangerine) roots. Eur J Soil Biol 2006; 42:166 - 72; http://dx.doi.org/10.1016/j.ejsobi.2005.12.006
- Lambais MR, Ríos-Ruiz WF, Andrade RM. Antioxidant responses in bean (Phaseolus vulgaris) roots colonized by arbuscular mycorrhizal fungi. New Phytol 2003; 160:421 - 8; http://dx.doi.org/10.1046/j.1469-8137.2003.00881.x
- Wu QS, Xia RX, Zou YN. Reactive oxygen metabolism in mycorrhizal and non-mycorrhizal citrus (Poncirus trifoliata) seedlings subjected to water stress. J Plant Physiol 2006; 163:1101 - 10; http://dx.doi.org/10.1016/j.jplph.2005.09.001; PMID: 17032615
- Sylvia DM, Williams SE. Vesicular-arbuscular mycorrhizae and environmental stress. ASA Spec Publ 1992; 54:101 - 24
- Al-Karaki G. Benefit, cost and water-use efficiency of arbuscular mycorrhizal durum wheat grown under drought stress. Mycorrhiza 1998; 8:41 - 5; http://dx.doi.org/10.1007/s005720050209
- Li M, Jiang DF, Meng XX, Liu RJ, Li XL. Effects of arbuscular mycorrhizal fungi on growth, yield and quality of Phaseolus vulgaris L. in field. Chinese J Eco-Agr 1999; 7:43 - 6
- Liang J, Zhang Y, Jia XZ, Lv Q, Zhang XY. Effects of ectomycorrhizae on growth and resistance of poplar. J Nanjing Forest Univ 2003; 27:39 - 43
- Lu JY, Mao YM, Shen LY, Peng SQ, Li XL. Effects of VA mycorrhizal fungi inoculated on drought tolerance of wild jujube (Zizyphus spinosus Hu) seedlings. Acta Hort Sin 2003; 30:29 - 33
- Zhang HS, He XL. Effect of AM fungal on the protective system in leaves of Artemisia ordosica under drought stress. Biotechnol Bull 2007; 3:129 - 33
- Wu QS, Xia RX. Research and application on vesicular-arbuscular mycorrhiza of fruit frees. Plant Physiol Co 2003; 39:536 - 40
- Wu QS, Zou YN, Wang GY. Effect of inoculation with arbuscular mycorrhizal fungal on citrus under water stress conditions. [Nat Sci Edit] J Yangtze Univ 2007; 4:18 - 21
- Srivastava D, Kapoor R, Srivastava SK, Mukerji KG. Vesicular arbuscular mycorrhiza-an overview. In: Mukerji KG (ed) Concepts in mycorrhizal research. Kluwer Academic Publisher, Netherlands, 1996; pp 1-39.
- Mosse B, Hepper C. Vesicular-arbuscular mycorrhizal infections in root organ cultures. Physiol Plant Pathol 1975; 5:215 - 23; http://dx.doi.org/10.1016/0048-4059(75)90088-0
- Verma S, Varma A, Rexer KH, Hassel A, Kost G, Sarbhoy A, Bisen P, Btehorn B, Franken P. Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 1998; 90:896 - 903; http://dx.doi.org/10.2307/3761331
- Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Hückelhoven R, Neumann C, von Wettstein D, et al. The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci U S A 2005; 102:13386 - 91; http://dx.doi.org/10.1073/pnas.0504423102; PMID: 16174735
- Sherameti I, Tripathi S, Varma A, Oelmüller R. The root-colonizing endophyte Pirifomospora indica confers drought tolerance in Arabidopsis by stimulating the expression of drought stress-related genes in leaves. Mol Plant Microbe Interact 2008; 21:799 - 807; http://dx.doi.org/10.1094/MPMI-21-6-0799; PMID: 18624643
- Pekan-Berghöfer T, Shahollari B, Giong PH, Hehl S, Markert C, Blanke V, Kost G, Varma A, Oelmüller R. Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant-microbe interactions and involves early plant protein modifications in the endoplasmic reticulum and at the plasma membrane. Physiol Plant 2004; 122:465 - 77; http://dx.doi.org/10.1111/j.1399-3054.2004.00424.x
- Oelmüller R, Pekan-Berghöfer T, Shahollari B, Trebicka A, Sherameti I, Varma A. MATH domain proteins represent a novel protein family in Arabidopsis thaliana, and at least one member is modified in roots during the course of a plant-microbe interaction. Physiol Plant 2005; 124:152 - 66; http://dx.doi.org/10.1111/j.1399-3054.2005.00505.x
- Shahollari B, Varma A, Oelmüller R. Expression of a receptor kinase in Arabidopsis roots is stimulated by the basidiomycete Piriformospora indica and the protein accumulates in Triton X-100 insoluble plasma membrane microdomains. J Plant Physiol 2005; 162:945 - 58; http://dx.doi.org/10.1016/j.jplph.2004.08.012; PMID: 16146321
- Hill TW, Kafer E. Improved protocols for Aspergillus minimal medium: trace element and minimal medium salt stock solutions. Fungal Genet Newsl 2001; 48:20 - 1
- Pham GH, Singh AN, Malla R, Kumari R, Prasad R, Sachdev M, Rexer K-H, Kost G, Luis P, Kaldorf M, et al. Interaction of Piriformospora indica with diverse microorganisms and plants. In: Varma A, Abbott L, Werner D, Hampp R (eds) Plant surface microbiology. Springer-Verlag New York, 2004; pp 237-265.
- Varma A, Savita Verma, Sudha NS, Sahay N, Butehorn B, Franken P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microbiol 1999; 65:2741 - 4; PMID: 10347070
- Das A, Varma A. Symbiosis: The art of living. In: Varma A, Kharkwal AC (eds) Symbiotic fungi: Soil biology. Springer-Verlag, Berlin, 2009; pp 1-28.
- Oelmüller R, Sherameti I, Tripathi S, Varma A. Piriformospora indica, a cultivable root endophyte with multiple biotechnological applications. Symbiosis 2009; 49:1 - 17; http://dx.doi.org/10.1007/s13199-009-0009-y
- Husaini AM, Rashid Z, Mir RU, Aquil B. Approaches for gene targeting and targeted gene expression in plants. GM Crops 2011; 2:150 - 62; http://dx.doi.org/10.4161/gmcr.2.3.18605; PMID: 22179193
- Husaini AM, Abdin MZ, Parray GA, Sanghera GS, Murtaza I, Alam T, Srivastava DK, Farooqi H, Khan HN. Vehicles and ways for efficient nuclear transformation in plants. GM Crops 2010; 1:276 - 87; http://dx.doi.org/10.4161/gmcr.1.5.14660; PMID: 21844685
- Belhaj K, Chaparro-Garcia A, Kamoun S, Nekrasov V. Plant genome editing made easy: targeted mutagenesis in model and crop plants using the CRISPR/Cas system. Plant Methods 2013; 9:39; http://dx.doi.org/10.1186/1746-4811-9-39; PMID: 24112467
