Abstract
The safety of probiotics is tied to their intended use, which includes consideration of potential vulnerability of the consumer or patient, dose and duration of consumption, and both the manner and frequency of administration. Unique to probiotics is that they are alive when administered, and unlike other food or drug ingredients, possess the potential for infectivity or in situ toxin production. Since numerous types of microbes are used as probiotics, safety is also intricately tied to the nature of the specific microbe being used. The presence of transferable antibiotic resistance genes, which comprises a theoretical risk of transfer to a less innocuous member of the gut microbial community, must also be considered. Genetic stability of the probiotic over time, deleterious metabolic activities, and the potential for pathogenicity or toxicogenicity must be assessed depending on the characteristics of the genus and species of the microbe being used. Immunological effects must be considered, especially in certain vulnerable populations, including infants with undeveloped immune function. A few reports about negative probiotic effects have surfaced, the significance of which would be better understood with more complete understanding of the mechanisms of probiotic interaction with the host and colonizing microbes. Use of readily available and low cost genomic sequencing technologies to assure the absence of genes of concern is advisable for candidate probiotic strains. The field of probiotic safety is characterized by the scarcity of studies specifically designed to assess safety on the one hand contrasted with the long history of safe use of many of these microbes in foods on the other hand.
Introduction and Scope
Probiotics are live microorganisms, which when administered in adequate amounts confer a health benefit on the host.Citation1 Microbes used as probiotics are derived from different genera and species and have been studied for a variety of health and disease endpoints. Currently, both yeast (Saccharomyces cerevisiae) and bacteria are used as probiotics, including lactic acid bacteria (LAB; such as species of Lactobacillus, Streptococcus and Enterococcus), Bifidobacterium, Propionibacterium, Bacillus and Escherichia coli. They may be naturally occurring microbes (as is the case for all used in food), or microbes that have been genetically altered for a specific effect. Although genetically modified microbes were not specifically addressed in this review (assessment on a case-by-case basis is prudent), recommendations for non-recombinant microbes, although not sufficient, would be applicable. Assessment of safety must take into account the nature of the microbe being used, method of administration, level of exposure, health status of users and physiological functions they are called on to perform.
A context for this discussion is an increasing number of well-controlled human studies that have tracked adverse incidents and have provided long-term follow up on some subject groups, providing more data upon which to base safety assessments. Furthermore, as usage of probiotics has expanded and methods to track specific strains have improved, reports of infections and other adverse incidents traced to specific probiotics have surfaced.
Documented correlations between adverse events and probiotic consumption are few considering their widespread use.Citation2–Citation13 With few exceptions, adverse events have been reported primarily in patients with underlying medical conditions. However, the pathogenesis of opportunistic Lactobacillus infections when they do occur is poorly understood. Since microbes used as probiotics are non-pathogenic, it is difficult to identify inherent strain properties that may be related to health risks.Citation14 For instance, the risk of Lactobacillus infection is estimated at about one case per 10 million people over a century of probiotic consumption in France.Citation5 Moreover, the risk of lactobacillemia was considered as ‘unequivocally negligible’, at <1 case per million individuals.Citation15 Further evidence for safety was derived from a retrospective study that showed that increased probiotic consumption of Lactobacillus rhamnosus GG in Finland did not lead to increased cases of Lactobacillus bacteremia.Citation16 Furthermore, clinical studies where certain probiotics have been safely administered to immunocompromised patients (for example, patients with HIV infection), premature infants, elderly and patients with Crohn's disease without any side-effects provide further evidence of poor opportunistic pathogenicity.Citation17,Citation18
However, further investigation is warranted for probiotic use in at-risk human populations such as severely immunocompromised subjects, neonates or hospitalized patients.Citation18 For use in such sensitive populations, the use of appropriate in vitro assays or animal models for the assessment of infectivity should not be precluded. However, any toxicity or infectivity related to ingestion of candidate probiotics might only be evident when pushing the boundaries of current test systems, the results of which may be difficult to put into the context of the human situation.
The aim of this paper is to describe factors that need to be addressed when considering probiotic safety and to consolidate the opinions of the assembled experts on what topics require further research.
Immunological Considerations for Safety
Immunological concerns have been raised regarding safety assessments for probiotics from the perspective of the impact of probiotics on both the immunologically naive and immunologically compromised host. Microbes—both indigenous and transient—impact the immune system. The indigenous intestinal microbiota is known to be pivotal for the development and maintenance of gut physiology and immune homeostasis.Citation19 The gut acts as a highly selective barrier and communication organ between the luminal bacterial environment and the host. The failure of this interaction due to the loss of barrier function or breakdown of tolerance mechanisms is thought to contribute to the development of inflammation-driven metabolic pathologies.
Risks of probiotic supplementation in healthy but immunocompromised humans.
The reported immune stimulating effects of probiotics could be especially beneficial in healthy people with somewhat compromised immune function, such as stressed or aged people, newborns as well as women during pregnancy. These groups are at increased risk of infectious and non-infectious diseases. It was already shown that probiotic supplementation in aged people (>69) resulted not only in an increase of potentially beneficial intestinal bacteria such as bifidobacteria but also in an elevated activation of the non-specific immune response.Citation20 It is unclear whether the observed immune cell activation after probiotic supplementation is sustainable or whether the effects of these short term interventions diminish with time. An indication that effects diminish with time was obtained by a study in Lactobacillus reuteri-reconstituted Lactobacillus-free mice.Citation21 This single bacterial strain induced epithelial cell activation even in the presence of a complex microbiota. Notably, this effect was only transient suggesting the initial activation was part of an adaptation mechanism. The observed activation of the immune system by probiotics could well be due to similar mechanisms preceding tolerance towards the respective bacterial strain.
Although the immunocompromised host might benefit the most from probiotic supplementation, these populations might also be at higher risk for adverse effects such as the development of septic conditions due to their reduced capability for microbial clearance. This is especially true for probiotic strains that may express virulence factors or acquire antibiotic resistance genes via horizontal gene transfer.Citation22 As a consequence, probiotics should be screened for these possibly harmful characteristics in order to ensure their safe use. To date, there are no reports of adverse effects of probiotics in otherwise healthy but immunocompromised humans, but there is certainly a need for long term studies concerning the effectiveness and safety of probiotic supplementation within these populations. Unfortunately, the classical safety measurements such as the determination of toxicity or pathogenicity of probiotic bacteria will always be hampered by the fact that simplified animal models or cell assays will inadequately mimic the complex gene-environment interaction in genetically susceptible human populations that might be at risk for the development of chronic degenerative diseases (autoimmune, atopic, neurodegenerative or metabolic disorders) or complex acute diseases (pancreatitis, sepsis).
Inadequate mechanistic understanding of probiotic activity.
The lack of mechanistic understanding of probiotic activity is a major drawback for the prediction of safety of probiotic intervention. The complex gut-associated microbial ecosystem and nutrition-related factors are the most important environmental triggers for the development and modification of chronic degenerative diseases including immune and metabolic pathologies. In fact, the interaction between the gut microbial ecosystem and the host is considered to be a critical factor in overall health or disease. Whether probiotics will exert protective or harmful, immune-stimulating or immune-suppressing effects on a host is dependent on the interplay between the microbial signals, the genetic make-up of the host and environmental triggers. Many studies have revealed that certain probiotic bacteria stimulate immune cell proliferation and activity as evidenced by the production of cytokines and antibodies, thereby enhancing the effectiveness of the immune response against pathogens. In contrast, certain probiotics are able to reduce chronic inflammation and allergy, two diseases that are due to an overreaction of the immune system, by suppressing effector cells and inducing tolerance mechanisms, as was demonstrated for the treatment of inflammatory bowel diseases,Citation23,Citation24 or prevention of atopic dermatitis.Citation25 Experimental studies with VSL#3, a probiotic mixture of eight different bacterial strains, showed that this combination of strains directly suppress pro-inflammatory immune mechanisms in an animal model of chronic colitis, in which VSL#3 induced TGFβ-bearing regulatory T cells that confer protection to recipient mice after adoptive transfer. These studies suggest that certain strains of probiotics may trigger a protective memory in the adaptive immune compartmentCitation26 that goes beyond their interaction with the epithelial surface. The characterization of specific probiotic structure-function relationships in target populations will limit the risk of inducing converse detrimental effects in the host. Whereas probiotics are in general reported to protect the intestinal barrier, there might be conditions where probiotics not only fail to restore the intestinal barrier but facilitate translocation or induce infections themselves. This is underlined by rare cases reporting the development of sepsis related to probiotic use in diseased patients.Citation13 The reports about negative probiotic effects clearly show that it is necessary to unravel probiotic mechanisms in the context of the intestinal barrier function and host immune function, keeping in mind that effects are most likely specific for each probiotic strain or mixture used.
Use of Probiotics in the Critically Ill
Patients with great potential to benefit from probiotic treatment are critically ill patients or patients who have a reasonable chance of getting into a critically ill state, such as patients who are about to undergo major abdominal surgery. During periods of critical illness, the gut can be hypothesized to be the motor of a pathophysiological state, as gut-barrier dysfunction can initiate and propagate sepsis, systemic inflammatory response syndrome and multi organ failure.Citation27 During critical illness, the equilibrium of the normally symbiotic partnership between the intestinal microbiota, epithelium and immune system is disrupted. In critically ill patients, several factors are involved in the altered composition of the intestinal microbiota including the use of broad-spectrum antibiotics; changes in nutrient availability, gut motility, pH and osmolarity; and release of high levels of stress hormones including catecholamines.Citation28 Mucosal barrier dysfunction provides the opportunity for luminal opportunistic pathogens to translocate through the mucosal barrier, causing a marked pro-inflammatory response, which will increase the risk of systemic inflammatory response syndrome.
Probiotic strains and mixtures are potentially able to bolster this three-way equilibrium. Direct effects include fortification of colonization resistance by secretion of antimicrobial bacteriocins, competitive growthCitation29 and reduction of pathogen adhesion and invasion of epithelial cells.Citation30,Citation31 Furthermore certain probiotics are known to possess anti-apoptotic properties,Citation32,Citation33 and to maintain cytoskeletal integrity and prevent disruption of tight junctions.Citation34,Citation35 But one of the most studied and well known mechanisms of action of probiotics is their immunomodulating capacity. For instance, probiotics have been shown to induce the release of anti-inflammatory cytokines such as interleukin-10,Citation26 to modulate human dendritic cell function,Citation36 and to ameliorate a pro-inflammatory response.Citation37
Probiotics in hospitalized patients.
Critically ill hospitalized patients can be divided in two main categories: patients such as multi trauma or severe acute pancreatitis patients who are in an already critically ill state on admission and patients who will undergo major surgery. Considering the subgroup of patients undergoing abdominal surgery, ten randomized controlled trials have been conducted, which showed various results, ranging from no significant effects up to 93% reduction in post-operative infection rates. Importantly, no adverse effects of probiotics were demonstrated among these surgical patients. Surgical patients with high risk of bacterial infection, for instance after liver transplantation or pancreatic surgery, benefited most from probiotic administration.
Even though the precise extent of the critical illness resulting from the operative procedure cannot be predicted, the timing of the induction of the illness is known, giving room for a possibility to start treatment even before the critical illness occurs. Seven studies have been published that enrolled these high risk patients (). All three studies that used probiotic treatment pre-operatively were able to significantly reduce post-operative bacterial infections, whereas only two of four trials showed any benefit of post operative treatment. Moreover, Sugawara and colleaguesCitation38 demonstrated that consecutive pre-operative and post-operative probiotic treatment is more effective in reducing post-operative infectious complications than post-operative treatment alone.
However, the question of safety of probiotics for critically ill patients is paramount, especially in light of observations of higher mortality in a probiotic intervention group than a control group in a study using critically ill patients with acute pancreatitis.Citation6 In this study, 296 predicted severe acute pancreatitis patients were allocated to receive either a probiotic mixture comprising six strains (four Lactobacillus and two Bifidobacterium) at 1010 CFU/day or placebo, starting within 72 hours of onset of symptoms for 28 days. The incidence of infectious complications was comparable between the groups (probiotics vs. placebo, 30% vs. 28%, respectively). However mortality rates were significantly higher in the probiotic group (16% vs. 6%) as was the incidence of bowel ischemia, which occurred in nine probiotic-treated patients compared to none in the placebo group. Urine analysis demonstrated that in patients whose course of the disease was complicated by multi-organ failure, probiotic treatment resulted in increased levels of intestinal fatty acid binding protein (IFABP), an accurate marker of intestinal mucosal injury resulting from ischemia.Citation39 Furthermore, treatment with probiotics resulted in an overall reduction in bacterial translocation (as gauged by decreased nitric oxide excretion into the urine) compared to placebo-treated patients.Citation40 However sub-group analysis showed that in patients with organ failure bacterial translocation was increased after probiotic treatment, indicating that probiotics had beneficial effects in the moderately ill patients, but deleterious effects in the critically ill.
The most obvious concern with probiotic use in patients with severe barrier dysfunction such as critically ill patients is the risk of sepsis due to the ingested probiotic agent. However in the trial conducted by the Dutch Acute Pancreatitis Study GroupCitation6 no translocation of probiotic bacteria from the lumen into the systemic circulation was found. Therefore bacterial translocation of the probiotic bacteria to the systemic circulation did not contribute to the adverse effects of probiotics in these patients.
Probiotics and endogenous defense systems.
One of the difficulties in assessing the place of probiotics in clinical practice is the limited understanding of their mechanisms of action, especially their effects in patients suffering from or at risk of critical illness. However, the available literature suggests that probiotics may have a safer place in preventive applications in hospitalized patients with peri-operative nutritional support than in treatment. In order to achieve success with such an approach, better understanding of probiotic-host interactions both in healthy and critically ill subjects is needed.
During a critical illness such as severe acute pancreatitis, mucosal barrier dysfunction is thought to be the sequelae of exaggerated and pathological immune responses and is often aggravated by low intestinal perfusion rates and intestinal oxidative stress. Recent experimental work demonstrated that five days of probiotic pre-treatment before induction of acute pancreatitis stimulated glutathione biosynthesis in the ileum, resulting in attenuated acute pancreatitis-induced oxidative mucosal damage and intestinal barrier dysfunction.Citation41,Citation42 In addition, after five days of probiotic supplementation, increased gene expression of antioxidative defense enzymes was demonstrated. Increased expression levels of antioxidative enzymes could be indicative of cellular stress. Administration of probiotics may have caused a minor oxidative assault, such as intracellular accumulation of short-chain fatty acids produced by the bacteria, thereby inducing increased capacity of antioxidant enzymes, preconditioning the mucosa for a major oxidative attack during acute pancreatitis. At first glance, this hypothesis may seem contradictory to the results of the recent placebo-controlled trial by Besselink and colleaguesCitation6 demonstrating increased incidence of bowel ischemia after administration of probiotics in the acute phase of severe acute pancreatitis. However, probiotics administered after the onset of acute pancreatitis may have acted as an extra oxidative burden in an already critically affected redox system,Citation43 thereby, causing increased oxidative stress-induced damage and ischemia. In contrast, probiotics administered before an expected oxidative assault might result in an enhanced antioxidative capacity. This hypothesis is supported by the findings of Sugawara and colleagues,Citation38 who showed that only peri-operative administration of probiotics was able to reduce bacterial infections after hepatectomy while post-operative treatment alone did not seem to be effective. Of note, intravenous antioxidant therapy administered in the early phase of acute pancreatitis showed adverse effects in a recent randomized controlled trial,Citation44 emphasizing the difficulties of targeting oxidative stress in acute pancreatitis.
Besides oxidative stress, an exaggerated pro-inflammatory immune response may also play a role in the development of multi organ failure during critical illness.Citation27 In vitro studies show that certain probiotics can activate an anti-inflammatory defense system. For example, Voltan and colleaguesCitation45 showed that Lactobacillus crispatus downregulated expression of pro-inflammatory genes through production of H2O2-induced peroxisome proliferator.Citation46
In conclusion, the characteristics of the cases of serious adverse events of probiotic treatment reported to date have identified the following risk factors: (1) immune compromised state; (2) impaired intestinal barrier function such as occurs with multi organ failure and severe acute pancreatitis; and (3) central venous catheter. As these factors are involved in critical illness, probiotic treatment is contraindicated for critically ill patients. The Norwegian Scientific Committee for Food Safety evaluated the safety of use of probiotics for hospitalized patients and similarly concluded that probiotics should not be used for critically ill patients, including those with antibiotic-associated diarrhea (AAD), including Clostridium difficile infection.Citation47 Probiotics seem to have a safer application as preventive treatment measures, applied well in advance of the surgical procedure. Even in light of the outcome of the PROPATRIA study,Citation6 probiotics hold promise to prevent complications in patients at risk of becoming critically ill, such as patients scheduled for major abdominal surgery. The safety concerns are paramount in this compromised patient group and use in this application is clearly the purview of a probiotic drug, not food.
Use of Probiotics in Patients with Inflammatory Bowel Disease
Inflammatory bowel diseases (IBD) are chronic inflammatory conditions of the intestinal tract that affect children and adults and cause significant morbidity and occasional mortality. Inflammatory diseases of the bowel include Crohn disease (CD) and ulcerative colitis (UC). Probiotics have been investigated for effectiveness in reducing active disease or extending remission in both CD and UC. Additionally, they have been tested as agents to extend disease-free periods in patients suffering from pouchitis, an inflammation of a pouch created surgically after colon removal. No benefit of various probiotic preparations was observed with studies on induction of remission, prevention of recurrence of CD following active disease, or for prevention of post-operative recurrence.Citation48–Citation50 Evidence for extending remission for patients with pouchitis was found,Citation51,Citation52 but a study by another research group did not find the same product to be effective.Citation53 A systematic review did not find any benefit to combining probiotics with standard therapy for remission of CD in mild to moderate disease.Citation54 There was some evidence for a slight decrease in severity of active UC observed in four studies using a single Bifidobacterium strain, an Escherichia coli strain and two multi-strain probiotic products.Citation54 Since that time there have been additional studies that reported some benefit (including small pediatric trials one of which was of open label design); all subjects enrolled in these trials had mild to moderate disease.Citation55–Citation58
Use of probiotics in cases of active disease, where mucosal ulceration and exposure of the submucosa is present, could carry an increased risk of bacterial translocation and secondary bacteremia. Indeed, sepsis with a Lactobacillus rhamnosus strain was reported in a patient with severe colitis.Citation59 Thus, caution in severely ill patients much the same as in other very ill patients is warranted before consideration of administration of probiotics in active UC.
Use of Probiotics in Healthy, Term Infants
Newborns enter this world with a sterile gut. However, they are immediately exposed to a contaminated environment and natural bacterial colonization begins early on the first day of life. Palmer and colleaguesCitation60 tracked the process of bacterial colonization of the full-term newborn by analyzing stools using a microarray developed to detect and quantify the RNA gene sequences of most currently recognized taxonomic groups of bacteria. They found that bacterial colonization was specific to each infant and was unstable over the first few weeks of life. This initial period of flux and instability was resolved by one year of life when the bacterial communities, although marked by a degree of temporal and individual variability, resembled adult-type patterns. By 7–10 days of age, most healthy full-term breast-feeding neonates are colonized with a heterogeneous bacterial microbiota including Lactobacillus, Escherichia coli and Enterococcus, but with a clear cut predominance of Bifidobacterium. Among premature infants, Gewolb and colleaguesCitation61 showed that Bifidobacterium and Lactobacillus could be found in feces of only 5% of infants at one month of age, but these results remain to be confirmed with DNA-based detection techniques. The process of newborn colonization is driven by exposure to the maternal vaginal tract, skin, rectum and the surrounding environment (hospital maternity wards, neonatal units, home deliveries). Other factors include mode of parturition and whether the infant is breast-fed or bottle-fed.Citation62
Changes in the gastrointestinal tract environment have been shown to correlate actions in specific signaling pathways in the colonic epithelium, which has been proposed as a mechanism by which organisms associated with the mature intestine come to dominate the adult intestinal microbiota.Citation63 It has been suggested that signals from the newly developing microbiota are causally related and trigger differentiation in the gut of newly hatched zebrafish.Citation64 There is significant literature on the role of the microbiota on the development of the gut-associated immune system leading to clinical studies of probiotic organisms as treatment for a variety of diseases in neonates as well as its use in infant formula.
Risk of infections.
The addition of probiotic bacteria to the diet of healthy, term babies must consider the risk that ingestion of live microbes could lead to infection. Typically, the probiotics suggested for use in infants are species of Lactobacillus or Bifidobacterium, although PropionibacteriumCitation65 and S. thermophilusCitation66 are also used. Lactobacillus are found in approximately 0.1–0.2% of positive blood cultures from patients of all ages but sepsis or deep tissue infections resulting from ingestion of probiotics are rare.Citation67 However, case reports exist in the literature of serious systemic infections with ingestion of probiotic bacteria. Most of the reported infections from bacterial probiotics used in infants are related to L. rhamnosus strains. Two representative examples include L. rhamnosus GG sepsis in a complicated post-operative period following repair of a double-outlet right ventricle and pulmonary stenosis and a report of two infants with short gut syndrome.Citation3,Citation12 For these cases the clinical blood isolate and supplemental probiotic underwent DNA fingerprinting by repetitive element sequence-based polymerase chain reaction or pulsed field electrophoresis (PFGE) of chromosomal DNA digests confirming identical band patterns.
There are also case reports of serious infections by organisms generally presumed to be non-pathogenic in infants. For instance, case reports have been published documenting Bifidobacterium breve meningitis and Pediococcus sepsis in infants with gastroschisis.Citation68,Citation69 Although these cases were not associated with probiotic usage, they do highlight the concept that even presumed non-pathogenic organisms might cause infection in at-risk populations.
One of the issues involving the widespread administration of probiotics to infants is the difficulty in defining infants that are in a greater at-risk category. Of note is a study undertaken to evaluate the efficacy of probiotic administration in reducing nosocomial infection in the pediatric intensive care unit setting. Rising concern about the use of L. rhamnosus GG and an interim analysis revealing a trend (but statistically non-significant) toward increased nosocomial infections in those subjects receiving L. rhamnosus GG compared to placebo led to premature closure of the study.Citation70 Thus, there remains a significant need to better define at-risk infant groups to include level of prematurity, degree of underweight for very low birth weight infants, the minimum safe level for total white blood counts and neutrophils, the structural defects that place infants at risk (such as defects in heart or gut), immune and non-immune protective defects and whether alterations to the intestinal barrier function are a significant threat. Inflammation of the intestinal mucosa as observed in infants with short bowel syndrome may be an additional risk factor.
Growth.
For infant formula studies, growth is a primary determinant of safety. In their study with growth as a primary outcome, Saavedra and colleaguesCitation66 studied a formula containing Bifidobacterium animalis subsp. lactis Bb-12 and Streptococcus thermophilus TH-4 in 131 healthy infants aged 6 months and older. They found no difference in growth between infants taking a formula with the two different probiotic bacteria and those infants on an identical formula that did not contain the probiotic microbes. Another study involved 201 healthy 4- to 10-month-old infants recruited from multiple day-care centers. They were fed formula with either B. animalis subsp. lactis Bb-12 or L. reuteri SD 2112 and investigators did not detect any differences when examining growth compared to an identical formula without added probiotics.Citation71 In another study with primary outcome being growth for infants receiving at least 50% of their feeds from formula, 120 infants between birth and 2 months of age were randomized either to receive their formula unchanged or with L. rhamnosus GG.Citation72 The authors of this study reported that the study group receiving the probiotic group grew better but this result is controversial.Citation73 Other research in which no differences in growth were detected between infants receiving probiotic-supplemented formula and controls include studies with L. reuteri ATCC 55730;Citation74 with L. rhamnosus LPR, Bifidobacterium longum BL888, and Lactobacillus paracasei ST11;Citation75 with B. animalis subsp. lactis CNCM I-3446;Citation76 with L. rhamnosus GG;Citation77 with L. rhamnosus GG and LC705, B. breve Bb99, and Propionibacterium freudenreichii subsp. shermanii;Citation78 with B. longum BL999;Citation79 with L. paracasei subsp. paracasei CRL-431 and B. animalis subsp. lactis Bb-12;Citation80 and with L. rhamnosus HN001 or B. animalis subsp. lactis HN019.Citation81,Citation82 Thus, there is no evidence of compromised growth for infants receiving probiotic containing formula with strains studied. Noteworthy is a decades-old studyCitation83 in which newborn infants in their first three months receiving a formula containing 0.35 to 0.5 grams of lactic acid to 100 grams of milk formula for 10-day periods had significant problems with growth.
Long-term colonization and immunologic effects.
Generally speaking, probiotics have not been found to establish intestinal colonization for long periods of time.Citation84 However, exposure of younger infants may lead to long-term colonization. For instance, in a study evaluating the colonization of infants whose mothers' ingested L. rhamnosus strain GG during late pregnancy, but stopped at the time of delivery, all four vaginally delivered infants had the probiotic detectable in the stool for six months, three for one year and in one infant until two years of age.Citation85 In a larger study with a primary outcome of prevention of atopic dermatitis at two years of age, L. rhamnosus GG or placebo was administered to mothers for 4 weeks before delivery, and either directly to the infant (N = 38) or to the breast-feeding mother (N = 25) for 6 months.Citation77 Stools collected 6 months later showed between 20% and 26% (depending on method to detect L. rhamnosus GG) remained positive for this organism.Citation86 Although the study was complicated by a high incidence of the organisms in the controls in a country with high ingestion of this probiotic, these studies suggest a predisposition for some infants to be more protracted carriers of this probiotic strain than others. In addition, an increasing incidence of diseases involving the immune system over the last several decades has been thought to be a consequence of too few antigenic challenges to the developing immune system due to the increased sterility of the environment.Citation87 However, administration of one or a few single probiotic strains in high numbers does not represent a natural approach to colonization and raises the possibility of long-term adverse unintended consequences such as increased allergic manifestations reported in three studies following administration of Lactobacillus to infants:
Kalliomaki and colleaguesCitation77 followed a cohort of individuals whose mothers and/or the infants received L. rhamnosus GG for a six-month period following birth to evaluate the prevention of atopic dermatitis. The seven year follow-upCitation88 reported that whereas the cumulative risk for developing eczema was lower in the infants administered probiotic, the risk for allergic rhinitis and the more serious asthma was increased in the probiotic group.
Kopp and colleaguesCitation89 conducted a study with similar design with one significant difference; breastfeeding mothers, not infants, directly received L. rhamnosus GG until the infants were 3 months of age, at which time all infants were fed the probiotic or placebo. Only five non-breast fed infants directly received the intervention before the age of 3 months. In this study, increased wheezing bronchitis was observed in participants that received L. rhamnosus GG compared to the control group.
Taylor and colleaguesCitation90 conducted a study of similar design but with a different probiotic. Lactobacillus acidophilus LAVRI-A1 was administered directly to newborns for the first 6 months of life. There was no effect on prevention of atopic dermatitis but an increased rate of sensitization was observed in the probiotic group. However, a follow-up after the third year of life found that “[the tested strain] did not have any long-term effects on allergic outcomes. Specifically, the higher rates of sensitization that were previously seen at 1 year of age in the probiotic group were no longer apparent in the third year of life”.Citation91
Conclusions on safety of probiotics for full-term infants.
The use of infant formulas containing Lactobacillus, Bifidobacterium and/or S. thermophilus is currently allowable in parts of Asia, Europe and in the United States. Numerous studies have been conducted on full-term and premature infants, with no short-term, serious adverse events being reported. However, long-term effects have only rarely been measured and some question remains about the ability to identify at-risk infants at the time of birth. Furthermore, newborn infants are microbiologically and immunologically naïve, and as such are a vulnerable population. Immune effects, as well as other endpoints, can be expected to be strain-specific, so safety studies should be done for specific strains in infants and not extrapolated from data on taxonomically related strains. Metabolic activity such as the ability to produce D-lactic acid must be evaluated and disclosed to preclude use by infants with known underlying defects or conditions. The presence of any antigenic material (such as milk proteins) that may be contained in the probiotic product should be disclosed on labelling. The use of probiotics for the treatment or prevention of disease should be under the supervision of physicians. Further studies are warranted on the long-term consequences of manipulation of the intestinal microbiota by administration of single or limited number of bacteria in high-concentration probiotic supplements for prolonged periods of time. Any widespread administration of such infant foods requires careful and comprehensive post-marketing surveillance to monitor for unintended consequences.
Use of Probiotics in Premature Infants
Short term effects.
The intestinal bacterial colonization of preterm neonates differs both temporally and qualitatively from full term infants. Because neonatal intensive care is characterized by prolonged separation from the mother, delayed enteral feeding, a relatively sterile environment (incubators) and frequent use of broad-spectrum antibiotics, the natural colonization process tends to be both delayed and impaired. Preterm neonates are colonized by fewer bacterial strains, their microbiota is more likely to be populated by pathogenic bacteria, and bifidobacteria are undetectable in the intestinal microbiota during the first 1–2 weeks after birth and do not predominate until after the third week of life.Citation92
The immature intestine is further characterized by impaired mucosal barrier function leading to increased gut permeability. Normally, at this stage of immature development, the intestine is protected by a sterile fetal environment. However, when thrust prematurely into the hostile outside world, and exposed to potentially pathologic bacteria before its barrier function is fully developed, the leaky intestine becomes extremely susceptible to microbial invasion, which may culminate in necrotizing enterocolitis (NEC), a disease with a mortality of 20–50% and significant long term morbidity in survivors.
Several studies,Citation93–Citation96 provide compelling evidence that prophylactic administration of certain Lactobacillus, Bifidobacterium and S. thermophilus probiotics in this population reduces both the incidence and severity of NEC, thereby conferring short term health benefits to premature neonates. To date over 2,000 premature neonates have been exposed to prophylactic probiotics in different prospective studies and no adverse short term effects have been noted. For example, Dani and colleaguesCitation97 administered 109 CFU/day L. rhamnosus GG to 295 very low birth weight infants and there were no Lactobacillus infections. Similarly, in a multicenter, randomized controlled study involving 434 very low birth weight infants, the study group was administered two probiotic strains (one L. acidophilus and one Bifidobacterium bifidum) in either breast milk or breast milk and formula twice daily for six weeks. No episodes of sepsis from either Lactobacillus or Bifidobacterium were evidenced during this study.Citation94 This history of safe consumption of some probiotics has generally been considered proof of short term safety for this vulnerable population of infants but, as always, proof of safety of a specific strain requires study of that strain rather than extrapolation from related strains.
In summary, preterm infants represent a unique situation. The aberrant fecal colonization resulting from prematurity provides an important rationale for the use of probiotics in these high risk neonates, which is different from any other population. In this population, probiotics offer an opportunity to expose the at-risk neonatal intestine to microbes not associated with pathologies thereby reducing the threat from exposure to less innocuous colonizers and subsequent development of NEC.
Long term effects.
Nevertheless, precisely because we are intervening at a stage of developmental immaturity, the potential for a given treatment to result in a long term impact is magnified. However, no cohorts from probiotic intervention studies conducted with premature infants have been followed long-term with regard to the impact of probiotics on colonizing microbiota, leaving a gap in the direct observation of any long-term effects.
Our understanding of the interaction between the intestinal microecology and the development of the intestinal innate immune system is only beginning to evolve. Likewise, our knowledge of the interaction of neonatal exposures and the development of subsequent atopic phenomena is insufficient. Hypotheses have been advanced suggesting that changes in the microbial environment of the gastrointestinal tract brought about by probiotics given during infancy may play a role in diseases that manifest later in childhood such as allergies, atopy, and perhaps even autoimmune disorders such as type 1 diabetes. The increasing prevalence of these diseases in certain geographic locations has fueled interest in this area as a focus of research.
Studies of gnotobiotic animals show that a normal microbiota is needed for intestinal angiogenesis,Citation98 for the development of normal immune functionCitation99 and normal intestinal epithelial development,Citation100 and for normal gut motility and peristalsis.Citation101 Conversely, introduction of normal microbes or even of only one species of a commensal bacterium to gnotobiotic animals restores intestinal function, mucosal proliferation and immune development, and improves barrier function.Citation102 This potential to impact intestinal development would be expected to have both short and long-term implications.
In summary, in the case of Lactobacillus and Bifidobacterium probiotic administration to premature infants at-risk for developing NEC must be considered from the perspective of risk and benefit. Since the typical condition of the premature neonate is one of developmental intestinal deprivation, and since no adverse effects have been reported to date on probiotic intervention trials with this susceptible group, and since prevention of NEC has been achieved through probiotic intervention, the debate may be shifted from whether or not it is safe to give probiotics to the premature neonate to whether it is safe not to give probiotics to premature neonates. The premature neonatal population is unique vis a vis administration of probiotics because normal gut microbial colonization has not yet developed, potentially contributing to severe pathology. Some tested strains of Lactobacillus and Bifidobacterium probiotics appear to have short term health benefits in this population and no major short term adverse effects. It is also true, however, that the potential for long term probiotic-related adverse effects has not yet been completely defined.
Microbiological and Metabolic Issues Related to Safety
Identification.
Although sometimes overlooked as a safety criterion, correct assessment of taxonomic identity should be scheduled as early as possible in the screening process for new probiotic candidate strains.Citation103–Citation105 Correct identification of probiotic strains to the species level is essential for safety assessment as it allows a linkage to potentially relevant, species-related scientific and technological information, including data on growth conditions, metabolic characteristics and genomic information. In addition, strains must be identified to the strain level.Citation103 The ability to identify a specific probiotic strain among other probiotics or members of the native microbiota is essential for strain selection and characterization, assessments of strain stability throughout the manufacturing process, for proper description of material used in human intervention studies, efficient tracking of the probiotic through the host, and for post-market surveillance including matching of strains isolated from any suspected infections.
The majority of probiotic microorganisms for human use belong to the taxonomically complex LAB and bifidobacteria. For the identification of probiotic LAB or Bifidobacterium strains, the use of phenotypic tests or commercial identification systems such as API,Citation105,Citation106 are inadequate for species level resolution. Furthermore, typing methods (such as pulsed-field gel electrophoresis and randomly amplified polymorphic DNA analysis) are primarily useful for differentiation of individual strains, but are otherwise unsuitable for species recognition. At present, the inappropriate use of identification methods is considered to be the major cause of mislabeling of probiotic products reported worldwide.Citation107,Citation108 Inconsistencies in the microbial identification for commercial products with probiotic claims affects their potential efficacy and safety record, and are likely to have a negative impact on consumer trust. There are examples of such misattribution of products labeled as Bacillus subtilis but actually containing other species.Citation109,Citation110
Triggered by the limitations of phenotypic approaches and the increasingly important role played by molecular tools in the field of bacterial systematics, a range of DNA methods either based on fingerprinting or on sequencing has been used for the identification of probiotic bifidobacteria and LAB.Citation111 Fingerprinting methods that have been most commonly used for this purpose include amplified fragment length polymorphism,Citation106,Citation112 repetitive DNA element (rep)-PCRCitation106,Citation107 and ribotyping.Citation113,Citation114 The major advantage offered by several of these methods is that they yield information not only at the species level but in some cases even up to the strain level. However, fingerprinting methods usually require specialized equipment, up-to-date databases with reliable reference material and trained personnel that may not be readily available in all companies producing probiotic products. In addition, DNA fingerprints are less exchangeable and reproducible among laboratories compared to sequencing data. Depending on the method and group of organisms in question, this may hamper the verification of identification results between independent expert laboratories.
Due to the growing availability of whole bacterial genome sequences, sequence-based identification approaches have in recent years been intensively explored for bacterial taxa commonly used as probiotics. In the hands of probiotic producers, the use of partial or complete sequencing of the 16S rRNA gene has become a standard method for species identification of their cultures. However, there are potential drawbacks to this approach, such as the presence of unreliable or poorly documented sequence entries in public sequence databases (EMBL/GenBank/DDBJ) or the low taxonomic resolution to separate closely related (sub) species such as those belonging to the Lactobacillus plantarum group and the Lactobacillus paracasei group or within the species Bifidobacterium animalis and Bifidobacterium longum.Citation105 Compared to 16S rRNA, the use of single-copy protein-coding (housekeeping) genes pheS and rpoA for lactobacilliCitation115 and atpD, tuf, groEL and recA for bifidobacteriaCitation116 generally offers a higher discriminatory potential. The application of these molecular markers has a large potential in the probiotic field because they are easily accessible through outsourcing services, although data interpretation by taxonomic experts remains crucial to ensure reliability of the identification result. Another approach common in the bacterial systematics literature is the combination of information from 16S and one or two additional genes, a technique that was recently shown to help discriminate among related strains from B. subtilis and closely related species.Citation117 Due to advances in DNA sequencing technology significantly increasing capacity and speed at reduced cost, it is expected that more and more probiotic candidate strains will be identified through whole-genome sequencing. Yet, an important challenge lies downstream of this approach at the level of data analysis, data interpretation and interchangeability of techniques and conclusions.
Colonization.
The relevance of long-term or permanent colonization as a safety criterion for probiotics should not be generalized across all hosts, all probiotic strains or all possible colonization sites. As previously discussed in this paper, the administration of probiotics to mothers of healthy infants can lead to long-term colonization of six months and longer,Citation85,Citation86 but few data are available on colonization and persistence when the probiotic is directly administered to full-term or preterm infants. Furthermore, even though most studies focus on colonization of intestinal mucosa or feces, there are many potential niches along the gastrointestinal tract, from the mouth to the rectum, and the vaginal tract, which should be considered. This is especially important with increased focus on development of probiotics specifically for activity in the mouth, throat, stomach, skin and vaginal tract.
The reported evidence for long-term colonization in the gastrointestinal tract of adults is sparse. Although the design of prolonged monitoring studies in healthy subjects or patient groups who take a specific probiotic on a regular basis seems relatively straightforward, there are several factors that may complicate the outcome. First, the long-term ability of a probiotic strain to colonize the intestinal tract depends on its ability to survive through and grow in the gastrointestinal tract. For some probiotic strains such as Lactobacillus casei Shirota, it has been shown that a more permanent colonization may be hindered by the low rate of cell division.Citation118 In the mouse intestine, the average doubling times of the Shirota strain estimated from the residual fluorescent levels of surface-adhered cells were 4.10, 4.78, 4.56 and 5.59 days in the duodenum, jejunum, ileum and colon, respectively. Lee and colleaguesCitation118 estimated that the strain would need a much shorter average doubling time of 1.03 to 2.04 days to colonize the various sections of the mouse intestinal tract more permanently. Also, any assessment of intestinal colonization in the most representative way would require jejunal or rectal mucosal biopsies rather than fecal samples. Alander and colleaguesCitation119 compared both sample types in their study on the persistence of L. rhamnosus GG in human volunteers undergoing routine diagnostic colonoscopy. Based on the finding that seven out of the 21 subjects scored positive in biopsies but negative in fecal samples for the presence of strain GG, the authors concluded that the study of fecal samples alone is not sufficient in evaluating colonization by a probiotic strain. In critically ill patients, however, the collection of rectal biopsies to monitor probiotic colonization may hold inherent risks and will rarely be approved by ethical committees for large patient populations.Citation120 Consequently, the use of animal models may currently be the safest way to study long-term colonization.Citation105 Nevertheless, interpretation of probiotic safety data from animal models remains controversial. It has been shown that the route and dose of administration for Bacillus strains in mice may highly influence the outcome of toxicity tests,Citation121 and it is to be expected that these and other pharmacological parameters may also affect the outcome of long-term colonization studies in animal models.
Antibiotic resistance and transferability.
Antibiotic resistance expression and transferability of antibiotic resistance determinants from fed probiotic strains to commensal microbiota in vivo are important components of the safety assessment of bacteria used as probiotics. Assuming that the probiotic in question does not present a risk of infectivity, the primary concern with the presence of antibiotic resistance genes is that probiotic strains should not function as a potential source of antibiotic resistance genes to less innocuous microbial members of the intestinal microbial ecosystem. In vivo transfer to potentially more harmful microbes (commensal or transient) is a theoretical concern which is extremely complex to assess in practice. Recently, the vancomycin resistance gene, vanA, was transferred in an animal model from an Enterococcus strain to L. acidophilus, providing evidence that transfer can take place in vivo.Citation22
Currently, there is not a harmonized approach worldwide to this issue. This topic has been addressed by the European Union-funded “Biosafety Assessment of Probiotics used for Human Consumption” (PROSAFE) and “Assessment and Critical Evaluation of Antibiotic Resistance Transferability in Food Chain” (ACE-ART) projects, the Joint International Organization for Standardization-International Dairy Federation (ISO-IDF) Action Team on Probiotics and other research projects.Citation105,Citation122–Citation126 One of the most crucial aspects in communications concerning antibiotic resistance in probiotic or nutritional cultures is to separate intrinsic resistance from acquired resistance. In the latter category, it is furthemore important to distinguish resistance caused by random genetic changes on chromosomal genes (such as topoisomomerase inactivation leading to quinolone resistance or ribosomal changes leading to rifampicin resistance) from those more likely to be transmissible (such as vancomycin or tetracycline resistance elements that frequently reside on genetically mobile elements such as plasmids or transposons). While the mechanisms and frequently even specific genes are widely studied in pathogenic bacteria, there is almost no published literature that examines the mechanisms of antibiotic resistance in bacterial genera that do not contain pathogens.
In the PROSAFE project, the MICs of 16 antimicrobials representing all major classes were determined for 473 taxonomically well-characterized LAB isolates encompassing the genera Lactobacillus, Pediococcus and Lactococcus.Citation123 It was found that breakpoints for phenotypic antibiotic resistance assessment must be obtained at species or species group level, and as a result, tentative epidemiological cut-off values of 13 antibiotics were determined for up to 12 LAB species. Six probiotic and nutritional cultures of Lactobacillus that displayed phenotypic resistance to oxytetracycline and/or erythromycin possessed erm(B) and/or tet(W), tet(M) or unidentified members of the tet(M) group. In vitro intra- and interspecies filter-mating experiments failed to show transfer of resistance determinants. Three probiotic Lactobacillus strains were highly resistant to streptomycin, but no corresponding resistance gene could be detected by PCR. As one of the important conclusions from the PROSAFE project, the finding of acquired resistance genes in isolates intended for probiotic or nutritional use highlights the importance of antimicrobial susceptibility testing in documenting the safety of commercial LAB.
In the ACE-ART project, a collection of 1,579 strains mainly from food origin was amassed from species of Lactobacillus (n = 843), Bifidobacterium (n = 467), Lactococcus (n = 178) and Streptococcus thermophilus (n = 91). Methodology was shown to be of paramount relevance (growth medium, inoculum size, incubation time) and methods for all four bacterial groups were developed, based on previous work by Klare and co-workers.Citation122 A limited number of antibiotics were tested including ampicillin, tetracycline, erythromycin, clindamycin, gentamicin, streptomycin and, only for Bifidobacterium, vancomycin. To reliably establish species-specific breakpoints, it was the aim to collect a sufficient number of genotypically unique strains (approximately 50 was judged to be the target number) of each species to reflect possible variations in biological source and/or geographical origin. However, not all tested species were represented by a large number of strains. Findings from phenotypic susceptibility tests indicated that molecular characterization using PCR detection or microarrays must be done when the resistance phenotype for a particular strain falls outside the breakpoint defined for the to which it species it belongs. In most cases, erm and tet resistance determinants were found in strains displaying a high MIC value for the particular antibiotic, corresponding with a resistant phenotype.Citation127 Of these, the most frequently identified genes included tet(W), tet(M), tet(S) and erm(B). However, the correspondence between phenotype and genotype was not always straightforward, since 22 isolates with a susceptible phenotype seemed to harbor tet or erm genes. Also in the opposite way, in at least 15 phenotypically resistant strains no corresponding resistance gene could be identified by microarray analysis in which case resistance may be due to mutations in intrinsic genes and is not linked to transferable resistance genes.
Although the PROSAFE and ACE-ART projects represent significant progress toward developing the needed construct for generating and interpreting information on antibiotic resistance among bacteria intended for human food use, some questions remain unanswered. Remaining research needs include:
The range of antibiotics being tested must be expanded to include all those relevant to human clinical applications.
Not enough strains are available for some species to reliably establish the norm for inherent resistances for a species. Additional strains would make conclusions more robust.
The risk of transfer of antibiotic resistance determinants from a fed probiotic strain to commensal microbiota in vivo has not been assessed experimentally. However, it should be emphasized that an antibiotic resistant strain of Lactobacillus, Bifidobacterium or S. thermophilus is not a pathogen.
It has been suggestedCitation15 that probiotics for human use should be susceptible to at least two clinically relevant antibiotics. However, since clinical susceptibility is not always predicted by in vitro testing, the link between in vitro antibiotic susceptibility and in vivo susceptibility must be better understood.
An additional concern is the use of distributions of data points from collections of isolates to establish breakpoints for antibiotic resistance testing. This practice, while accepted by the European Food Safety Authority for animal feed applicationsCitation128 differs from those used to establish breakpoints used by clinical laboratories in the United States. For clinical applications, antibiotic resistance breakpoint testing is less likely to be a wild-type cutoff value (derived from diverse diagnostic laboratory collections) and is more likely to be a clinical value (derived from a clinical field trial, or a pharmacokinetic/pharmacodynamic cutoff value) based on the relationship between achievable drug concentrations at the site of infection and the dynamics of the antimicrobial activity of the drug. Values used by clinical laboratories are generally those published by the Clinical and Laboratory Standards Institute.Citation129
As a practical example, it is interesting to consider the FDA reaction to the presence of genes for tetracycline resistance in the commercial probiotic strain, B. animalis subsp. lactis Bb-12,Citation130 (in the opinion text, the taxonomically incorrect species name B. lactis was used). A partial tet(W) gene was found residing on the chromosome of Bb-12. However, the FDA did not question the conclusion of the manufacturer's expert panel that this did not impact the assertion that this strain was GRAS for its intended use as an ingredient in infant formula. This conclusion of the panel was based on the following rationale:
A likely mechanism for transfer of this gene was not found
The likelihood of transfer of tet(W) from Bb-12 to other microorganisms is considered very low
Tetracycline resistance is relatively common among lactic acid bacteria used in food
The tet(W) gene is relatively common among human commensal gut bacteria
Strain Bb-12 is commonly used in a number of food products in the U.S. and other developed countries with no known adverse events attributed to its tetracycline resistance
Consumption of Bb-12 would not compromise clinical use of tetracycline, since tetracycline is not recommended in children younger than 8-years-old.
Genetic stability/genetic transfer.
The genetic stability of a probiotic strain reflects the susceptibility to genomic rearrangements in the course of its natural evolution. These rearrangements may reflect small variations introduced at specific or random positions of the genome through mutations, deletions and insertions, but may also be linked to larger structural variations resulting from homologous recombination (vertical inheritance) and horizontal gene transfer events. Although this is a highly relevant issue in order to ensure that specific health-promoting characteristics and functionalities are not affected during long-term preservation and production, surprisingly few studies have reported comprehensive data documenting the genetic stability of commonly used probiotic strains.
Ideally, a rigorous assessment of a strain's genetic stability requires the availability of its whole-genome sequence. In the absence of this information, however, the use of molecular typing techniques probably provides the best estimate of genetic stability at the individual strain level. This estimate can be based on genotypic comparisons between re-isolates of a given probiotic strain, such as re-isolations before and after gastrointestinal passage or throughout the course of production. Within the European Union-PROSAFE project, the genotypic and phenotypic stability of a probiotic candidate strain, L. rhamnosus PRSF-L477, was assessed in a randomized, placebo-controlled, double-blind, parallel-group study of 36 healthy volunteers.Citation105 PFGE analysis was used to track strain PRSF-L477 in fecal samples of nine subjects. Based on the highly identical if not indistinguishable PFGE profiles exhibited by the original strain and its fecal re-isolates, the investigators concluded that PRSF-L477 did not undergo major genomic rearrangements thus indicating its genetic stability during healthy gastrointestinal tract passage. Deposit of all commercial probiotic strains into an internationally recognized culture collection will provide an enduring source of reference material for confirmation of genetic stability.
Alternatively, indirect evidence for genetic stability may also be obtained from genotypic screening of collections of probiotic cultures belonging to a single taxonomic group but collected from various geographical origins. Following this approach, a subset of 118 L. rhamnosus isolates mostly from human fecal or clinical originCitation131 included 14 commercial strains that were received from 10 different producers or distributors of probiotic products. The integrated use of AFLP and PFGE fingerprinting revealed that some of these probiotic strains were indistinguishable from one another, suggesting that several companies are using duplicate cultures of the same probiotic strain. Furthermore, some human isolates were indistinguishable from a particular probiotic strain, suggesting that some of these isolates may be re-isolations of commercial strains that display a high genotypic stability. Likewise, a study of probiotic products claimed to contain bifidobacteria revealed that the number of genotypically unique Bifidobacterium strains used as commercial probiotics was surprisingly low in view of the great range of products analyzed.Citation107 Among a set of 39 B. animalis subsp. lactis isolates recovered from 32 different products, only four strain types could be distinguished by PFGE. The large majority of these products (n = 30) were found to contain PFGE type I, thus indicating that a single strain exhibiting a highly stable PFGE fingerprint was used in a broad range of dairy-based and lyophilized probiotic products worldwide. Besides their relevance in the scope of microbial product quality or epidemiological surveys, the findings shown in these studies indirectly suggest that Lactobacillus and Bifidobacterium strains used in probiotic applications tend to show a high genotypic stability under different production and/or host passage conditions. Clearly, more direct and in-depth evidence for this hypothesis needs to be gathered from long-term studies for commonly used probiotic strains, ideally using a series of different molecular typing methods or, ultimately, comparative genomics. Complete genome sequencing of Lactobacillus delbrueckii subsp. bulgaricus, a LAB member applied worldwide in yogurt production, revealed that the genome of this organism is in a phase of rapid reductive evolution as a possible result of its adaptation from a plant-associated habitat to a milk environment through the loss of superfluous functions and protocooperation with S. thermophilus.Citation132 In the same way, comparative genomics could also determine the potential of probiotic strains to acquire and/or transfer DNA elements during host passage and long-term colonization, which is a research area that has remained virtually unexplored.
Viability.
One challenge faced by the probiotic manufacturing industry is the delivery of live, viable microorganisms. Maintaining viability is related to physiological characteristics of the strain as well as delivery format. For example, dairy products have an inherently shorter shelf-life than freeze-dried probiotic products in the form of powders and pills. Even within dried formats, levels of oxygen, moisture and storage temperature play an important role in stability. Inactivated microbes, although not considered probiotics, have been investigated for beneficial effects and were considered to be a possible solution to overcome stability problems. But live, viable probiotics appear to have superior efficacy to heat-inactivated microbes for both in vitro and clinical studies, largely dispelling this notion.Citation32,Citation133,Citation134 However, it should be recognized that the presence of dead or injured microbes in commercial products is unavoidable, as some death occurs during storage of products and the standard approach to maintaining the target dosage of live probiotic entails addition of surplus probiotics to account for any death occurring during storage.
Interestingly, some studies have revealed adverse effects resulting from ingestion of heat-killed probiotics. In a study comparing one group that ingested live, viable L. rhamnosus GG with another group ingesting heat-killed L. rhamnosus GG for the prevention of allergies in infants, Kirjavainen and colleaguesCitation135 reported increased gastrointestinal symptoms and diarrhea in those ingesting the formula with the heat-killed product, which led the Review Ethics Board to prematurely discontinue the study. In another study,Citation136 micronutrients were combined with either a heat-inactivated L. acidophilus probiotic strain or placebo and the impact on the prevalence of diarrhea in an at-risk group of children was assessed. The benefits of the micronutrients were negated by the addition of heat-inactivated probiotics. The prevalence of diarrhea in children receiving the combination of micronutrients and heat-killed preparation was the same as placebo with both being worse than micronutrients given alone.Citation136 Thus, the importance of ingestion of viable probiotics is important not only from an efficacy point of view but possibly from an association of dead microbes with adverse effects. Products comprising heat-killed microbes should not be made available to infants until any adverse effects from such products are better understood.
Pathogenicity/toxicogenicity.
Factors that could play a role in the virulence of a microorganism might include adhesion mechanisms allowing adherence to mammalian cells, mucin degradation providing metabolites for growth, and bile salt hydrolase activity enhancing survival in the gut environment. However, these factors also contribute to the survival of microorganisms in the mammalian gut, and as such are characteristics of much of the natural microbiota. Therefore, they are not a priori meaningful measures of virulence. Furthermore, in vitro measurements of these factors are not necessarily good predictors of in vivo activity.
For bacterial groups for which no known pathogenic members are known, it is difficult to assess what potential virulence factors might exist. No genes associated with pathogenicity have been identified in Lactobacillus or Bifidobacterium species used as probiotics. Although several reports of sepsis in at-risk individuals have been linked to lactobacilli, there is no conclusive evidence from analysis of clinical isolates that would indicate any species-related properties that would favor infection in at-risk populations.Citation14 Asahara and colleaguesCitation137 have suggested that resistance to host innate defence mechanisms should be considered in the safety assessment of Lactobacillus strains, but research in this area is limited.
On the contrary, numerous virulence factors in enterococci have been reported, including hemolysin, gelatinase or DNAse activities, or the presence of structural genes cylL, ace, asal and esp.Citation138,Citation139 However, there is no consensus on a method or procedure for reliably differentiating pathogenic from non-pathogenic strains. Pathogenic species of streptococci produce hemolysins, and a surface enolase that binds and activates human plasminogen.Citation140 Strains of Bacillus species licheniformis, pumilus and subtilis have occasionally been reported as causative agents in food poisoning. For Qualified Presumption of Safety (QPS) status, EFSA requires demonstration of the absence of Bacillus toxins by PCR based methods (Hbl and Nhe genes), and by cytotoxicity assays.Citation110 If a probiotic strain under investigation belongs to a bacterial genus where pathogenic species are known, then it is important to demonstrate the absence of associated virulence factors.
D-lactic acid production.
Human metabolism produces the L(+)-isomer of lactic acid. D(−)-lactate presence in humans is a consequence of bacterial production of D(−)-lactate directly or indirectly from L(+)-lactate through a bacterial DL-lactate racemase.Citation141 Not all probiotic species possess the ability to directly produce D(−)-lactate, but some Lactobacillus species do possess the racemase enzyme and, thus, can convert L(+)-lactate to D(−)-lactate.Citation142 Whereas normally lactobacilli constitute a very small fraction of the microbial population of the human intestinal tract at all ages,Citation143 the same is not true for those suffering from D-lactic acidosis. Bongaerts et al. showed that in children with short bowel syndrome and acidosis that the majority of fecal flora is lactic-acid producing lactobacilli and D-lactic acid blood concentrations are high at the time of symptoms.Citation144 Human cells metabolize and excrete D(−)-lactate poorly with the very young newborn and neonate being at particular risk, due to the lack of full renal excretory capability and decreased barrier function of the intestinal tract. If excessive build-up of D(−)-lactate occurs, metabolic acidosis may result, the early clinical effects of which can be difficult to detect. While clinical cases of D-lactic acidosis have not been observed in healthy infants, the possibility cannot be excluded, particularly in light of the difficulty of diagnosis of sub-clinical cases.
One small study evaluated otherwise older healthy infants and the safety of ingesting a probiotic capable of D(−)-lactate production. Twenty-four six-month-old infants were randomly selected from a larger group of subjects receiving 108 CFU of L. reuteri ATCC 55730 since birth as part of a double blind, multi-center trial for the prevention of allergy. Comparison of blood levels of D(−)-lactate between those receiving placebo and the probiotic were similar at the six-month time age of the infants.Citation145 Studies in newborns and infants younger than six months most at risk of this problem are lacking.
Most patients reported with D-lactic acidosis have been those with short gut syndrome as occurs following mesenteric thrombosis, mid-gut volvulus or Crohn's disease.Citation146,Citation147 Administration of probiotics to such individuals has been associated with the development of D-lactic acid associated encephalopathy.Citation148 Other patients who have developed this problem include those who have undergone intestinal bypass surgery and patients with small-bowel bacterial overgrowth.Citation146,Citation147 One common feature among these patients is excessive carbohydrate exposure to D(−)-lactate producing bacteria. For patients developing this D-lactic acidosis, re-colonization with bacteria that are not D(−)-lactate producers has proven beneficial.Citation149 Taken together, administration of D(−)-lactate producing probiotics should be carefully considered in patients at risk of developing D-lactic acidosis, such as those with previous bowel surgery and subsequent short gut syndrome and in the very young newborn or neonate until appropriate safety data become available for specific probiotic strains.
Biogenic amine production.
Biogenic amines such as histamine and tyramine are low molecular weight organic molecules that are present in many foods but also produced in high amounts by microorganisms through the activity of amino acid decarboxylases. Ingestion of high amounts of biogenic amines can be mistaken for allergic reactions due to the similarity of the signs and symptoms including facial flushing, sweating, rash, burning taste in the mouth, diarrhea and cramps with severe reactions including respiratory distress, swelling of the tongue and throat and blurred vision.Citation150 One of the better-recognized presentations is that of ingestion of fish from the family Scombridae (tuna) although the accumulation of histamine and adverse events can be prevented through constant cool temperature control.Citation150 Although some species of Lactobacillus are capable of forming biogenic amines, there is great variability in this ability. The addition of probiotic organisms to foods should take biogenic amine producing capacity into account if opportunity for production and substrate availability are conducive to such activity.Citation151,Citation152 To date, there are no reported cases of such potentially harmful compounds found in fermented milk prepared with lactobacilli.Citation17
Presence of unlabeled microorganisms.
Microbiological evaluation of retail probiotic products has revealed the presence of microorganisms not indicated on the label.Citation107,Citation108,Citation153–Citation155 The presence of these microbes suggests either inadequate attention to production parameters and a failure in quality control procedures, or an intention to deceive consumers, substituting less expensive or more production-friendly microbes for labeled ones, presumably for financial gain. Avenues for contamination of the production process are numerous. A commercial product that untruthfully labels the contents in the product is illegal. Furthermore, it is impossible to assess the safety of such a product as the identity of the strains in the product are not known or characterized.
In addition to microbiological hazards with labels that inaccurately describe contents, excipients used in formulation of final products could also be problematic. For instance, some children receiving probiotics have developed anaphylactic reactions from cow's milk protein allergens present in the probiotic product.Citation156,Citation157 There are culture methods to grow lactic acid bacteria that effectively eliminate residual cow milk protein allergens. While it is unclear whether such methods are sufficient to exempt products from food allergen labeling requirements, unless the probiotic product specifically states that it is allergen-free, allergic individuals who want to use probiotic products should actively seek assurance from manufacturers that a given product does not contain an offending allergen.
Measurement of Safety: Limitations of Existing Models
Standard acute, subchronic and chronic oral toxicological studies performed with certain currently-used probiotics have not demonstrated any adverse effects even at very high doses over a long period of time.Citation158,Citation159 It has proved difficult to establish infection after oral administration of probiotic bacteria, even when tested at doses 10,000 times higher than those normally consumed by humans.Citation160 Adverse effects might be detected only when pushing the boundaries of test systems, raising the question of the very relevance of these systems.
Monitoring toxicity in vivo.
The following are in vivo measures that are of use in when evaluating toxicity in vivo.
Evaluation of general health status of the animals during the in-life phase. Any possible infection-induced changes in physiological functions could be evaluated by measurements of blood parameters such as blood cell counts (leukocytes and red cells, absolute and differential), and also hemato-biochemistry markers such as glucose concentration and glutamic-oxalacetic transaminase activity. Serum α-amyloid protein can be used as a plasma marker of sepsis.Citation160 C-reactive protein could also be measured as a general indicator of inflammation.Citation161 However, serum α-amyloid protein and C-reactive protein are probably best-suited for murine models. Plasma malondialdehide concentration could also be determined in order to evaluate changes in lipid peroxidation, following an oxidative stress subsequent to bacteraemia.Citation160,Citation162 This latter analysis can also be carried out at the end of the in-life phase.
Blood cultures for assessment of bacterial translocation. The presence of administered probiotics in blood culture (as measured by culturing and/or PCR-based methods) is of clinical relevance as an indicator of potential translocation and bacteraemia.Citation4,Citation161 In animal models, this analysis could also be carried out at the end of the in-life phase. This could be done in liver, spleen, blood and/or other potential target organs/tissues. Sampling of tissues or organs should be carried out carefully and in sterile conditions to avoid undesired cross-contamination. Detection of the administered probiotic directly from a human study subject (or patient), is also an important finding. Such detection requires availability of reliable methods for culture and identification (e.g., by PCR) of the organism from blood, stool and any other clinical samples appropriate for the study. However, such methods (such as methods for recovery of fastidious anaerobic probiotic bacteria) are not necessarily available in clinical laboratory settings.
Splenic weight index. This index is a ratio of the spleen weight to the body weight of the animal and follows a curvilinear progression. Alterations of the splenic weight index could be used as indicators of infections, and comparisons should be carried out between groups of equal body weights and between animal of the same gender.Citation163
Weight-to-length ratio. A change in the weight-to-length ratio of the intestine could be a sign of intestinal inflammation.Citation160 Used alone, the clinical relevance of this parameter is rather limited, and an assessment of the weight-to-length ratio in the intestine should be complemented by a macroscopic examination at necropsy, followed by a subsequent histopathology evaluation.
Additional markers. Total liver glutathione concentration and plasma malondialdehide concentration could be determined in order to evaluate changes in the antioxidant defence and lipid peroxidation, respectively, as a possible consequence of bacteraemia.Citation160,Citation162 Serum lactate and fecal calprotectin as a biomarker for intestinal inflammationCitation164 could also be considered satisfactory indicators of general health status.
Measuring infectivity in vitro or in animal models.
Adhesion. Pathogens often have a high affinity for proteins such as collagen, fibrinogen and mucus, as these give them access to host tissues.Citation165 However, discriminating between mucosal adhesion as a normal feature of probiotics or commensal microorganisms as opposed to a virulence factor of pathogens remains to be further investigated.
In vitro evaluation of the nitric oxide pathway. It has been suggested that nitric oxide and related reactive nitrogen intermediates exert bacteriostatic and microbiocidal effects and play an important role in the killing of bacteria by macrophages.Citation166 As resistance to this pathway is thought to allow the bacteria to escape the phagocytic activity of innate immunity,Citation137 this ability should also be assessed.
Routes of administration in the safety assessment of probiotics. The route of administration may play a major role in safety assessment of probiotics. Indeed, it was shown that mice treated orally with 5 × 108 CFU L. salivarius CECT5713/animal were healthy and survived daily administration after 28 days. Yet intraperitoneal administration caused a significant translocation of the bacteria to the liver and spleen, a transient inflammatory process, but without any behavioral changes, and no illness or death observed in the injected mice.Citation160 In contrast, experimental sepsis following intraperitoneal administration of the same dose of a pathogenic strain of E. coli was lethal to mice at 2 days after inoculation.Citation167 Oral administration of probiotics appeared to be by far the safest route and outlined the very low infectivity of LAB and absence of safety issues for current uses in healthy populations.
Animal models for infectivity. Due to the low pathogenicity of the Lactobacillus genus, specialized in vitro and/or animal models are needed for the assessment of potential infectivity. One approach is to consider pushing the animal models beyond their normal physiological states (for example, immunosuppressed or severely debilitated hosts, and by using unusual routes of administration) to allow the detection of any potential pathogenicity of the candidate probiotic strain. Although various animal models are available, few have been reviewed and validated for the purposes of the safety assessment of probiotics. Animal models that have been reviewed for this purpose in the European Union PROSAFE project were the experimental endocarditis rat and immunocompromised mouse models.Citation168
The rat model of experimental endocarditis is of interest because it is generally well accepted and shown to mimic the human pathophysiology of infective endocarditis.Citation169 Thus, this animal model may serve as a standard for the assessment of the infectivity potency of a new probiotic strain, as results can be compared to reference negative and positive controls.Citation170 One such negative control could be the poorly infective L. lactis.Citation105 In this model it was shown that lactobacilli were between 100 and 10,000-fold less infective than the two most common endocarditis pathogens (Staphylococcus aureus and Streptococcus viridans) in human pathology.Citation105,Citation170 In addition, for a particular Lactobacillus strain, infectivity could be different between clinical and probiotic isolates,Citation14,Citation168 but no specific causal characteristic has been identified. In addition, the usefulness of this type of model has been questioned since the clinical cases of endocarditis associated with LAB are so rare.Citation105 A rabbit model to evaluate if a probiotic can cause infectious endocarditis has also been used and could be considered as a possible alternative animal model for the evaluation of infectivity.Citation137
Another animal model with potential for assessing infectivity might be derived from the standardized bacterial host resistance model using the intracellular pathogen Listeria monocytogenes as the challenge organism. This approach is commonly applied for the evaluation of potential immunotoxicity of candidate drugs.Citation171 By adapting this test to a candidate probiotic strain (e.g., a Lactobacillus) either through direct injection into the tail vein or into the peritoneal cavity, one might overcome the naturally poor infectivity inherent to the Lactobacillus genus. This poor infectivity might be also overcome by immunosuppressing the animals (e.g., with cyclosporine A)Citation172 prior to using Lactobacillus as the challenge organism. In this bacterial host resistance model, implementing ex vivo evaluations of resistance to intracellular killing by elicited mouse macrophages could be an additional testing option for assessment of potential effects on innate immunity.Citation137 However, none of these models are validated and implementation to assess safety is likely premature.Citation105 One can also envisage pushing this bacterial host resistance model beyond normal physiological limits by using immunosuppressed animals and Lactobacillus as the challenge organism: immunosuppression could be achieved by injections of cyclosporine A (with a dose of 50 mg/kg BW/dayCitation172). In this model, implementing ex vivo evaluations of resistance to intracellular killing by elicited mouse macrophages could be an additional testing option for assessment of potential effects on innate immunity.Citation137 However, these models are not validated and the PROSAFE conclusion considered that implementation of such models for safety issues is premature.Citation105
Reproductive and developmental toxicity.
Reproductive and developmental toxicity testing is a routine part of the evaluation of most products developed for use as food or drugs, but is rarely discussed for probiotics. Recent advances in understanding the role of the microbiota in the development of the digestive tract suggest that manipulation of the microbial population could have intentional or unintentional effects on development when administered to pregnant women or infants, especially before weaning. As more sophisticated strategies for manipulation of human health using microorganisms are developed, especially when strains beyond those found traditionally in dairy fermentations and other sources of routine human inoculation are introduced in large amounts, it becomes more important to develop tools to gauge the broad effects of these new exposures.
Guidelines for reproductive and developmental testing include the Organization of Economic Cooperation and Development (OECD) guidelines that are reflected in nationalCitation173 and international guidance documents.Citation174 These tests are capable of detecting disruptions in digestive function manifested by monitoring generic signs of health status such as low survival or birth weight of pups, slower growth or development of pups or major histological disruptions in specific organs such as the intestinal walls. Reproductive toxicity studies most frequently employ mice, rats and rabbits, although dogs or pigs can be used based on the specific needs of the evaluation.
Guidelines such as those cited above are meant to be flexible and need to be adapted to those questions that are relevant to the probiotic being tested. In addition to the usual endpoints of developmental toxicity testing, it is likely worth investigating whether a probiotic organism becomes a permanent resident of the adult microbiota of the offspring. This would be particularly relevant to strains isolated from other species that might have properties that allow them to displace, for better or for worse, strains ordinarily present during the crucial initial developmental stages following birth. This would require a sensitive, practical and reliable method for probing for the introduced strain and assumes that the animal being used is a reliable model for the target microbial location. Given the specificity of the symbiotic interactions between the microbial strains and the host, it would seem presumptuous to assume equivalent safety of all members of a particular species when present. On the other hand, it may not be necessary to include some of the usual endpoints of reproductive and developmental testing, such as teratology and male reproductive effects, based on theoretical grounds. The proper design and conduct of a reproductive and developmental toxicity evaluation of a probiotic proposed for widespread use as a food or drug is clearly a topic ripe for further discussion.
The pig as a model for assessing probiotic safety: direct evidence for validation.
Animal testing is a key part of evaluating the safety of anything that is intended for administration to humans, but it is most desirable that the animal model be selected with an eye to the ability to predict human response from the animal's response. In the assessment of the safety of chemical substances, it is common to perform comparative pharmacokinetic studies to determine the similarity of the absorption, distribution, metabolism and excretion of the substance in the chosen animal model and in humans. The validity of animal models is no less important with probiotics and prebiotics as with other substances, but the approach differs because the key site of activity is the gastrointestinal tract itself while for most chemicals the real safety issues arise after absorption. Similarity of gastrointestinal morphology, physiology, permeability, microbiome and metabolic processes are paramount in assuring a valid animal model for probiotics and prebiotics. It is important to clearly define host characteristics in different compartments of the gastrointestinal tract as well as systemic responses.Citation175,Citation176
The pig provides unique features as an animal model for such research because of the resemblance to human digestive physiology and associated metabolic processes.Citation177 Pigs eat an omnivorous diet and the developmental period, especially during infancy, is similar to that of humans.Citation178 Emergence of new data using novel molecular technologies to evaluate the intestinal microbiome supports the similarity between representative bacterial orders in the pig and humans.Citation179
In vivo studies using pigs fed B. animalis subsp. lactis Bb-12 starting from birth showed persistence of Bb-12 in the intestinal tract with daily feeding of 1010 CFU of the probiotic without clinically detectable effects on health status. In addition, pigs born to sows fed Bb-12 during the last trimester of gestation and fed Bb-12 from birth were also clinically normal but showed enhanced upregulation of innate immunity markers.Citation180 Effective colonization of gnotobiotic pigs with L. acidophilus NCFM and induction of innate immune markers have also been demonstrated following rotavirus vaccination.Citation181,Citation182 L. rhamnosus GG and B. animalis subsp. lactis Bb-12 have been shown to adhere to pig intestinal mucosa and displace or inhibit pathogenic Salmonella, Clostridium and Escherichia coli in vitro,Citation183 and to ameliorate in vivo the nematode induced inhibition of glucose uptake without inducing any histological changes.Citation180,Citation184 The impact of probiotic use on prevention and treatment of some serious gastrointestinal diseases affecting neonates, including necrotizing enterocolitisCitation185 and colitis,Citation186 have also been modeled in recent studies using young pigs.
It is clear that the pig colon can be colonized with several different probiotic strains currently used by humans without clinical and local tissue abnormalities. The pig is additionally advantageous because many infectious bacterial, viral, parasitic and fungal agents are in common with humans, and autoimmune, allergic and metabolic disease models have been developed. The availability of new immunological reagents,Citation187 molecular tools for the detection of changes in immune responseCitation188 and tracking of changes in the microbiome in the pig gastrointestinal tract,Citation189–Citation191 should facilitate the validation of safety studies.
Key Initiatives in Probiotic Safety
European Food Safety Authority (EFSA).
Various initiatives addressing the safety of probiotics for humans have been undertaken, in particular in the European Community. Detailed reviews and opinions of current practices in safety assessment in the European Union are published.Citation192,Citation193 No formal safety testing guidelines for food-associated microbes are established within the European Union. EFSA was established in 2002 to address the increasingly important and complex scientific and technical issues in relation to food and feed safety (Regulation No. 178/2002). Nevertheless, the exact regulatory model for approval of a novel probiotic microbe has remained ambiguous. The European Union Novel Food regulation (9258/1997 EEC), which encompasses novel foods and food ingredients that have not been used for human consumption to a significant degree within the Community prior to 1997, provides a regulatory framework for probiotics. However, probiotics that have a basis for historical use or are used as additives or processing aids fall outside the scope of the novel food regulation. To date no probiotic strain has been subjected to such a thorough safety evaluation according to the Novel Food regulation. In contrast to probiotics intended for humans, the European Union regulations concerning safety of microbe-based feed additives for animal agriculture follow a very strict procedure.Citation192,Citation194
Qualified presumption of safety (QPS).
Since no consensus document existed at the time, the European Union Scientific Committee on Animal Nutrition published a position paper in 2002 for safety assessment and regulatory aspects of microorganisms in feed and food applications. In this document, the Scientific Committee on Animal Nutrition proposed an approach to safety known as “Qualified Presumption of Safety”.Citation195 The scientific committee to EFSA advised that QPS was a suitable system for a common European approach to safety of bacterial dietary supplements. EFSA is currently finalizing the 2008 review of QPS guidelines. Details of this system are available from EFSACitation196 and a general scheme is presented in . Establishing QPS involves four steps: (1) definition of the taxonomy of the microbe; (2) collection of sufficient information providing the basis for QPS status, including any scientific literature, history of use, industrial applications, ecological data and human intervention data; (3) exclusion of pathogenicity; and (4) definition of the end use. If there are no safety concerns for a certain taxonomic group, or if any safety concerns have been allayed (qualification), then QPS status may be granted. Subsequently, a microbial strain categorically assigned to a QPS group would be exempt from further safety assessment, other than satisfying any qualifications specified. Microbes that are not considered suitable for QPS would remain subject to a full safety assessment. Members of the lactic acid bacteria, which include some species of probiotic bacteria, will be among the first groups to be evaluated. The introduction of this system appears to be favorably received so far and is considered by some more flexible than the GRAS system used in the United States taking into account new emerging safety risks such as acquisition of antibiotic resistance and virulence determinants.Citation193,Citation194
PROSAFE.
A European Union-funded project “Biosafety Evaluation of Probiotic Lactic Acid Bacteria for Human Consumption” (PROSAFE) had the objective to propose recommendations towards an evidence-based safety assessment of probiotics for human use. Conclusions from this project were presented at a workshop in 2006,Citation105,Citation168 and these recommendations were:
Molecular methods, such as ribosomal DNA sequencing, conducted by an expert laboratory, should be used to taxonomically classify LAB.Citation106 Moreover, the probiotic strain should be deposited in a public culture collection, albeit with restricted access.
Microbes not belonging to the wild-type distributions of resistance levels to relevant antimicrobials should not be developed as future products for consumption by humans or animals in the food chain without proper risk assessment.Citation123
Use of Enterococcus probiotics or other LAB that harbor known and confirmed virulence genes should be avoided. The presence of other properties including bile acid deconjugation, adhesion to cell lines and extracellular matrix proteins were insufficiently associated with virulence, and along with survival of probiotics in in vitro colon models, were considered irrelevant for biosafety assessment.
In addition to following European guidelines for in vivo safety assessment, human colonization should be studied in a randomized, placebo-controlled, double-blind design but details of such a study were not specified.
The potential use of animal models to measure safety was very controversial. However, the rat model of experimental endocarditis was suggested as the most reliable animal model.Citation168,Citation169
FAO/WHO.
As part of the 2002 effort to establish guidelines for the use of probiotics in foods, a group of experts described a general approach to characterization of a probiotic.Citation103 Criteria considered useful for establishing safety by this group overlapped with the PROSAFE conclusions listed above, and included the following. Note that although the document advises certain assessments, guidance in how to assess and how to interpret results from such assessments are generally absent from this document.
Established history of safe use in traditional products
Absence of a significant risk with regard to transferable antibiotic resistance
Absence of a significant risk with regard to virulence properties
Assessment of certain metabolic activities, such as D-lactate production, bile salt deconjugation
Testing for toxin production if the strain under evaluation belongs to a species that is a known mammalian toxin producer
Determination of hemolytic activity if the strain under evaluation belongs to a species with known hemolytic potential
Assessment of side-effects during human studies
Epidemiological surveillance of adverse incidents in consumers (post-market)
ACE-ART.
The subject of probiotics serving as potential reservoirs of transferable antibiotic resistance genes was addressed in an European Uniion project “Assessment and Critical Evaluation of Antibiotic Resistance Transferability in the Food Chain” (ACE-ART). This project focused on non-pathogenic LAB as well as bifidobacteria and provided recommendations for assessment and interpretation of antibiotic resistance in probiotics for human use.Citation127
Health Canada: Natural health products regulations of the Canadian food and drugs act.
Health Canada has been active in addressing safety, efficacy and quality issues related to probiotics,Citation197,Citation198 and are currently developing guidance for industry focused on “Recommendations for the Evidence Requirements for Efficacy, Safety and Quality of Natural Health Products containing Probiotics.” Probiotics used in food are covered under the Food Directorate,Citation198 whereas probiotics (excluding genetically modified strains), are considered “natural health products” under the Natural Health Products Regulations.
The approach used by Health Canada for safety, efficacy and quality of probiotics is based on recommendations of the Joint FAO/WHO working group for the evaluation of probiotics in foodCitation103 and recommendations published by EFSA. In addition, unless applicants can demonstrate otherwise, efficacy, safety and quality will be considered based on the understanding that both advantageous and deleterious probiotic effects on human health are strain-specific.
Safety dossiers should minimally provide, for all strains in the formulation, evidence of safe historical use of the species, a strain-specific literature review or in vitro characterization for each strain of adverse metabolic activities (for example, D-lactate production, bile salt deconjugation, bacteriocins, enzymatic activities), identity information at both the genotypic and phenotypic level, strain-specific characterization of antibiotic resistance and justification by both literature review and assay that antibiotic resistance is not transferable. Alternatively, a comprehensive justification for omission of this testing would be required for each strain that was not tested.
United States.
In the United States, the term “probiotic” is not legally defined at the national level. The Food and Drug Administration approach is to regulate probiotics using the regulatory structure pertinent for the intended use of the probiotic. Possible regulatory categories would include food, food ingredient, dietary supplement or drug. Each category has specific standards regarding efficacy, allowable claims, safety and good manufacturing practices. SandersCitation199 provides some perspective of United States regulatory issues pertinent to probiotics. Recently, the National Institutes of Health and the United States Food and Drug Administration commissioned an evidence-based review of probiotic safety through the Agency for Health Care Quality and Research. The purpose of the project is to catalogue what is known about the safety of probiotics (Lactobacillus, Bifidobacterium, Saccharomyces, Streptococcus, Enterococcus and Bacillus) used in research to reduce the risk of, prevent, or treat disease. The literature review will assess the quality and completeness of the available information, and the degree of confidence to interpret this information. The project will provide information relevant to practitioners, researchers and regulators for assessing the value and safety of probiotic administration to reduce risk, or prevent or treat disease, as well as to identify priorities or needs for future research. The preliminary results of this review reveal that few publications describe research designs that systematically probe the safety (as opposed to the efficacy) of the probiotic. This review may begin the process of developing a consensus on the safety endpoints that are appropriate to clinical trials.
Others.
In Europe, there have been several national initiatives regarding safety of probiotics. The Netherlands National Institute of Public Health and Environment (Rijksinstituut voor Volksgezondheid en Milieu; RIVM) published a report with a scheme to consider as a basis to evaluate the safety of newly marketed probiotics strains or products.Citation200
The Norwegian Scientific Committee for Food Safety evaluated the safety of the use of a specific probiotic (L. rhamnosus GG) to be used in infants and children 4–36 months of age.Citation73 The committee concluded that in the absence of convincing data that L. rhamnosus GG can prevent disease in this age group, and considering that the long-term effects on immune function are unknown, that there was insufficient evidence to substantiate safety for this use. In addition, a subcommittee of this same organization in 2009 conducted a benefit/risk assessment for the use of probiotics for patients in hospitals. They considered: (1) bacterial translocation from the gut and infectious disease; (2) virulence factors including toxicity; (3) metabolic functions including host storage, platelet aggregation, deconjugation of bile acids and degradation of mucin; and (4) resistance to antimicrobials. They concluded that due to reported adverse effects, probiotic bacteria should not be consumed by critically ill individuals.
The International Life Sciences Institute (ILSI) Europe has proposed a new activity on functional safety of probiotics in particular to address the differences between probiotics used for foods compared to bio-therapeutic agents to cure disease.Citation201
In India, the government announced its intent to formulate guidelines for regulation of probiotic foods which would be in place by January 2010. A task force set up by the Indian Council of Medical Research is responsible for formulating the guidelines that in addition to product safety, would address quality and reliability.
Review of Standards for Safety for Foods, Supplements and Drugs
The degree of risk that is regarded as acceptable can vary depending on the standard being used to evaluate safety. There is no such thing as “zero risk”; in any risk assessment some element of uncertainty remains. Regulatory definitions or limits recognize these concepts. For example, risk standards in the United States differ for conventional foods “reasonable certainty of no harm” [Congressional Record, H.R. Report No. 2284, 85th Congress 1958]) and for ingredients in dietary supplements (“reasonably likely to be safe” [United States Federal Food Drug and Cosmetic Act 21 U.S.C. 350b] and lack of “unreasonable risk of illness or injury under the… conditions of use” [United States Federal Food Drug and Cosmetic Act 21 U.S.C. 342(f)]. Closely related to the concept of risk is the balancing concept of benefit. There is widespread, perhaps universal, agreement that some level of anticipated benefit is an inherent part of any risk evaluation. It would be difficult to find support for the position that even a tiny deliberately imposed risk (for example, that resulting from voluntary addition of a substance or microorganism to a food) is acceptable if there is absolutely no benefit.
Application of different standards for evaluating safety can result in different conclusions. This is exemplified by the difference in the conclusion reached regarding the use of L. rhamnosus GG (LGG) in infant formula by the Norwegian Scientific Committee for Food SafetyCitation73 on the one hand, and the non-governmental GRAS Expert Panel that submitted an analysis to the FDA on the other. The Norwegian committee found that “the data available are not sufficient to support the suggested beneficial effects, or the safety, of LGG in infant formula…” The GRAS panel concluded that this intended use of L. rhamnosus GG was safe, and, further, that this safety was generally recognized in the scientific community. The FDA did not question this conclusionCitation202 of the GRAS panel, despite the fact that the VKM opinion had been released a year earlier.
The apparent contradiction is explained by several differences in the ways the VKM and the GRAS panel evaluated safety. Importantly, the VKM was charged with evaluating benefit, and then evaluating safety in light of the strength or weakness of the evidence for benefit. The GRAS panel conducted in the United States assessed only safety. Of perhaps greater importance is that the GRAS panel used the “reasonable certainty” standard, while the VKM determined that “our lack of knowledge [of long-term effects from manipulating the immune function of neonates] necessitates the application of precautionary principles.” The VKM based its conclusion on both actual data (such as case reports of translocation) and theoretical possibilities (such as a possible long-term effect on the colonizing microbiota). The GRAS panel was equally aware of these concerns, but determined that they were sufficiently rare or unlikely as to fall under the level of negligible risk permitted by the “reasonable certainty” standard, and the FDA accepted this conclusion. This case study exemplifies the importance of including in any risk assessment a clear statement of the safety standard employed.
Conclusions
The assessment of safety of probiotics comprises consideration of a variety of factors:
Record isolation history and taxonomic classification of the candidate probiotic
Manufacturing controls that eliminate contamination (including cross-contamination between batches) of the probiotic with microbes or other substances.
Absence of association of the probiotic with infectivity or toxicity, assessed at the strain level.
Absence of transferable antibiotic resistance genes
Physiological status of the consuming population. Special consideration must be made for use in vulnerable populations, including newborn infants and the critically ill.
Dose administered
Method of administration (oral or otherwise)
Absences of allergenic material (for example, dairy proteins) for products targeted for allergic populations.
Considering all of these factors, it is paramount that it is recognized that a general conclusion that “probiotics are safe” cannot be broadly made, and such an assessment is specific to the many conditions indicated above. To the extent that commercial probiotics are marketed as foods or dietary supplements, they need to be determined to be safe for the general population. For drug uses (the prevention, treatment, cure or mitigation of disease), the safety assessment must balance risk with benefit. Furthermore, the pre-market assessment of safety should never be presumed, but must entail a considered assessment of the many factors outlined here. Careful and comprehensive post-marketing surveillance is essential to monitor the occurrence of any unintended consequences, especially for use in vulnerable populations.
Footnote
As part of the 6th meeting of the International Scientific Association for Probiotics and Prebiotics held in London, Ontario, Canada in November 2008, a workshop was convened on the topic of safety of probiotics. The workshop included representatives from industry, academia and government. The focus of this discussion was on immunological, microbiological and toxicological issues that must be considered when reviewing safety of use, with special consideration given to emerging concerns and appropriateness of currently available model systems. The area of genetically recombinant microbes was not included. This paper reflects the discussion and compiles the conclusions that were reached.
Workshop composition. Chair: Mary Ellen Sanders (Dairy & Food Culture Technologies), Co-chair: James Heimbach (JHEIMBACH LLC), Participants: Louis Akkermans (University Medical Center—Utrecht), Martin Antonsson (Probi AB), Simon Cutting (University of London, Surrey), Linda Duffy (National Center for Complementary and Alternative Medicine, Bethesda, MD), Cara Fiore (United States Food and Drug Administration, Center for Biologics Evaluation Research), Bénédicte Flambard (Chr. Hansens), Dirk Haller (ZIEL - Research Centre for Nutrition and Food Science), Cathy Hammerman (Shaare Zedek Medical Center), Geert Huys (Ghent University), Mary-Anne Kent (Health Canada), Artem Khlebnikov (Danone Research), Dan Levy (United States Food and Drug Administration, Center for Food Safety and Applied Nutrition), David Mack (Children's Hospital of eastern Ontario), Peggy Martini (Kraft Foods), Lorenzo Morelli (Catholic University—Piacenza), Phoukham Phothirath (Nestlé Research Center), Gloria Solano-Aguilar (United States Department of Agriculture), Michelle Sumeray (Wyeth Consumer Healthcare), Rob Unal (Yakult USA), Luuk van Duijn (Winclove), Elaine Vaughan (Unilever R&D).
Abbreviations
| AAD | = | antibiotic-associated diarrhea |
| CFU | = | colony forming units |
| EFSA | = | European Food Safety Authority |
| FDA | = | United States Food and Drug Administration |
| GRAS | = | generally recognized as safe |
| LAB | = | lactic acid bacteria |
| MIC | = | minimal inhibitory concentration |
| NEC | = | necrotizing enterocolitis |
| PCR | = | polymerase chain reaction |
| PFGE | = | pulsed field gel electrophoresis |
| QPS | = | qualified presumption of safety |
Figures and Tables
Figure 1 A general scheme for the assessment of suitability for QPS status of microorganisms.Citation196 Reprinted with permission.
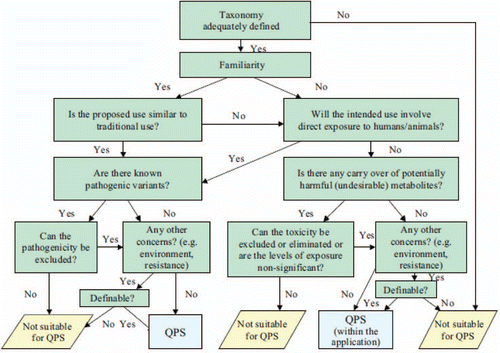
Table 1 Effects of probiotic treatment in 7 randomized controlled trials in surgical patients with a high risk of post operative bacterial infections
References
- Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria 2001; 2009 http://www.who.int/foodsafety/publications/fs_management/en/probiotics.pdf
- Cannon JP, Lee TA, Bolanos JT, Danziger LH. Pathogenic relevance of Lactobacillus: a retrospective review of over 200 cases. Eur J Clin Microbiol Infect Dis 2005; 24:125 - 126
- Land MH, Rouster-Stevens K, Woods CR, Cannon ML, Cnota J, Shetty AK. Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005; 115:178 - 181
- Salminen MK, Rautelin H, Tynkkynen S, Poussa T, Saxelin M, Valtonen V, et al. Lactobacillus bacteremia, species identification and antimicrobial susceptibility of 85 blood isolates. Clin Infect Dis 2006; 42:35 - 44
- Bernardeau M, Guguen M, Vernoux JP. Beneficial lactobacilli in food and feed: long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol Rev 2006; 30:487 - 513
- Besselink MG, van Santvoort HC, Buskens E, Boermeester MA, van Goor H, Timmerman HM, et al. Probiotic prophylaxis in predicted severe acute pancreatitis: a randomised, double-blind, placebo-controlled trial. Lancet 2008; 371:651 - 659
- Hennequin C, Kauffmann-Lacroix C, Jobert A, Viard JP, Ricour C, Jacquemin JL, et al. Possible role of catheters in Saccharomyces boulardii fungemia. Eur J Clin Microbiol Infect Dis 2000; 19:16 - 20
- Rautio M, Jousimies-Somer H, Kauma H, Pietarinen I, Saxelin M, Tynkkynen S, et al. Liver abscess due to a Lactobacillus rhamnosus strain indistinguishable from L. rhamnosus strain GG. Clin Infect Dis 1999; 28:1159 - 1160
- Mackay AD, Taylor MB, Kibbler CC, Hamilton-Miller JM. Lactobacillus endocarditis caused by a probiotic organism. Clin Microbiol Infect 1999; 5:290 - 292
- Spinosa MR, Braccini T, Ricca E, De Felice M, Morelli L, Pozzi G, et al. On the fate of ingested Bacillus spores. Res Microbiol 2000; 151:361 - 368
- Oggioni MR, Pozzi G, Valensin PE, Galieni P, Bigazzi C. Recurrent septicemia in an immunocompromised patient due to probiotic strains of Bacillus subtilis. J Clin Microbiol 1998; 36:325 - 326
- Kunz AN, Noel JM, Fairchok MP. Two cases of Lactobacillus bacteremia during probiotic treatment of short gut syndrome. J Pediatr Gastroenterol Nutr 2004; 38:457 - 458
- Boyle RJ, Robins-Browne RM, Tang ML. Probiotic use in clinical practice: what are the risks?. Am J Clin Nutr 2006; 83:1256 - 1264
- Vesterlund S, Vankerckhoven V, Saxelin M, Goossens H, Salminen S, Ouwehand AC. Safety assessment of Lactobacillus strains: presence of putative risk factors in faecal, blood and probiotic isolates. Int J Food Microbiol 2007; 116:325 - 331
- Borriello SP, Hammes WP, Holzapfel W, Marteau P, Schrezenmeir J, Vaara M, et al. Safety of probiotics that contain lactobacilli or bifidobacteria. Clin Infect Dis 2003; 36:775 - 780
- Salminen MK, Tynkkynen S, Rautelin H, Saxelin M, Vaara M, Ruutu P, et al. Lactobacillus bacteremia during a rapid increase in probiotic use of Lactobacillus rhamnosus GG in Finland. Clin Infect Dis 2002; 35:1155 - 1160
- Bernardeau M, Vernoux JP, Henri-Dubernet S, Gueguen M. Safety assessment of dairy microorganisms: the Lactobacillus genus. Int J Food Microbiol 2008; 126:278 - 285
- Snydman DR. The safety of probiotics. Clin Infect Dis 2008; 46:104 - 111
- O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep 2006; 7:688 - 693
- Gill HS, Rutherfurd KJ, Cross ML. Dietary probiotic supplementation enhances natural killer cell activity in the elderly: an investigation of age-related immunological changes. J Clin Immunol 2001; 21:264 - 271
- Hoffmann M, Rath E, Hölzlwimmer G, Quintanilla-Martinez L, Loach D, et al. Lactobacillus reuteri 100-23 transiently activates intestinal epithelial cells of mice that have a complex microbiota during early stages of colonization. J Nutr 2008; 138:1684 - 1691
- Mater DD, Langella P, Corthier G, Flores MJ. A probiotic Lactobacillus strain can acquire vancomycin resistance during digestive transit in mice. J Mol Microbiol Biotechnol 2008; 14:123 - 127
- Kruis W, Fric P, Pokrotnieks J, Lukás M, Fixa B, Kascák M, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004; 53:1617 - 1623
- Gionchetti P, Rizzello F, Morselli C, Poggioli G, Tambasco R, Calabrese C, et al. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum 2007; 50:2075 - 2082
- Kalliomaki M, Salminen S, Poussa T, Arvilommi H, Isolauri E. Probiotics and prevention of atopic disease: 4-year follow-up of a randomised placebo-controlled trial. Lancet 2003; 361:1869 - 1871
- Di Giacinto C, Marinaro M, Sanchez M, Strober W, Boirivant M. Probiotics ameliorate recurrent Th1-mediated murine colitis by inducing IL-10 and IL-10-dependent TGFβ-bearing regulatory cells. J Immunol 2005; 174:3237 - 3246
- Clark JA, Coopersmith CM. Intestinal crosstalk: a new paradigm for understanding the gut as the “motor” of critical illness. Shock 2007; 28:384 - 393
- Alverdy JC, Laughlin RS, Wu L. Influence of the critically ill state on host-pathogen interactions within the intestine: gut-derived sepsis redefined. Crit Care Med 2003; 31:598 - 607
- Liévin V, Peiffer I, Hudault S, Rochat F, Brassart D, Neeser JR, et al. Bifidobacterium strains from resident infant human gastrointestinal microflora exert antimicrobial activity. Gut 2000; 47:646 - 652
- Resta-Lenert S, Barrett KE. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 2003; 52:988 - 997
- Sherman PM, Johnson-Henry KC, Yeung HP, Ngo PS, Goulet J, Tompkins TA. Probiotics reduce enterohemorrhagic Escherichia coli O157:H7- and enteropathogenic E. coli O127:H6-induced changes in polarized T84 epithelial cell monolayers by reducing bacterial adhesion and cytoskeletal rearrangements. Infect Immun 2005; 73:5183 - 5188
- Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 2002; 277:50959 - 50965
- Yan F, Cao H, Cover TL, Whitehead R, Washington MK, Polk DB. Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 2007; 132:562 - 575
- Petrof EO, Kojima K, Ropeleski MJ, Musch MW, Tao Y, De Simone C, et al. Probiotics inhibit nuclear factor-κB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 2004; 127:1474 - 1487
- Czerucka D, Dahan S, Mograbi B, Rossi B, Rampal P. Saccharomyces boulardii preserves the barrier function and modulates the signal transduction pathway induced in enteropathogenic Escherichia coli-infected T84 cells. Infect Immun 2000; 68:5998 - 6004
- Hart AL, Lammers K, Brigidi P, Vitali B, Rizzello F, Gionchetti P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut 2004; 53:1602 - 1609
- Galdeano CM, de Moreno de LeBlanc A, Vinderola G, Bonet ME, Perdigon G. Proposed model: mechanisms of immunomodulation induced by probiotic bacteria. Clin Vaccine Immunol 2007; 14:485 - 492
- Sugawara G, Nagino M, Nishio H, Ebata T, Takagi K, Asahara T, et al. Perioperative synbiotic treatment to prevent postoperative infectious complications in biliary cancer surgery: a randomized controlled trial. Ann Surg 2006; 244:706 - 714
- Gollin G, Marks C, Marks WH. Intestinal fatty acid binding protein in serum and urine reflects early ischemic injury to the small bowel. Surgery 1993; 113:545 - 551
- Besselink MG, van Santvoort HC, Renooij W, de Smet MB, Boermeester MA, Fischer K, et al. Intestinal Barrier Dysfunction in a Randomized Trial of a Specific Probiotic Composition in Acute Pancreatitis. Ann Surg 2009; 250:712 - 719
- Lutgendorff F, Nijmeijer RM, Sandström PA, Trulsson LM, Magnusson KE, Timmerman HM, et al. Probiotics prevent intestinal barrier dysfunction in acute pancreatitis in rats via induction of ileal mucosal glutathione biosynthesis. PLoS ONE 2009; 4:4512
- Lutgendorff F, Trulsson LM, van Minnen LP, Rijkers GT, Timmerman HM, Franzén LE, et al. Probiotics enhance pancreatic glutathione biosynthesis and reduce oxidative stress in experimental acute pancreatitis. Am J Physiol Gastrointest Liver Physiol 2008; 295:1111 - 1121
- Ammori BJ. Role of the gut in the course of severe acute pancreatitis. Pancreas 2003; 26:122 - 129
- Siriwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, et al. Randomised, double blind, placebo controlled trial of intravenous antioxidant (n-acetylcysteine, selenium, vitamin C) therapy in severe acute pancreatitis. Gut 2007; 56:1439 - 1444
- Voltan S, Martines D, Elli M, Brun P, Longo S, Porzionato A, et al. Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-γ in the intestinal mucosa. Gastroenterology 2008; 135:1216 - 1227
- Abdelrahman M, Sivarajah A, Thiemermann C. Beneficial effects of PPAR-γ ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc Res 2005; 65:772 - 781
- The use of probiotics for patients in hospitals. A benefit and risk assessment. Opinion of the Steering Committee of the Norwegian Scientific Committee for Food Safety 2009; 2009 http://www.vkm.no/dav/009488e0b8.pdf
- Doherty G, Bennett G, Patil S, Cheifetz A, Moss AC. Interventions for prevention of post-operative recurrence of Crohn's disease. Cochrane Database Syst Rev 2009; 6873
- Rolfe VE, Fortun PJ, Hawkey CJ, Bath-Hextall F. Probiotics for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2006; 4826
- Butterworth AD, Thomas AG, Akobeng AK. Probiotics for induction of remission in Crohn's disease. Cochrane Database Syst Rev 2008; 6634
- Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double blind, placebo-controlled trial. Gastroenterology 2000; 119:305 - 309
- Mimura T, Rizzello F, Helwig U, Poggioli G, Schreiber S, Talbot IC, et al. Once daily high dose probiotic therapy (VSL#3) for maintaining remission in recurrent or refractory pouchitis. Gut 2004; 53:108 - 114
- Shen B, Brzezinski A, Fazio VW, Remzi FH, Achkar JP, Bennett AE, et al. Maintenance therapy with a probiotic in antibiotic-dependent pouchitis: experience in clinical practice. Aliment Pharmacol Ther 2005; 22:721 - 728
- Mallon P, McKay D, Kirk S, Gardiner K. Probiotics for induction of remission in ulcerative colitis. Cochrane Database Syst Rev 2007; 5573
- Tsuda Y, Yoshimatsu Y, Aoki H, Nakamura K, Irie M, Fukuda K, et al. Clinical effectiveness of probiotics therapy (BIO-THREE) in patients with ulcerative colitis refractory to conventional therapy. Scand J Gastroenterol 2007; 42:1306 - 1311
- Huynh HQ, deBruyn J, Guan L, Diaz H, Li M, Girgis S, et al. Probiotic preparation VSL#3 induces remission in children with mild to moderate acute ulcerative colitis: a pilot study. Inflamm Bowel Dis 2009; 15:760 - 768
- Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol 2009; 104:437 - 443
- Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, et al. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol 2009; 7:1202 - 1209
- Farina C, Arosio M, Mangia M, Moioli F. Lactobacillus casei subsp. rhamnosus sepsis in a patient with ulcerative colitis. J Clin Gastroenterol 2001; 33:251 - 252
- Palmer C, Bik EM, Digiulio DB, Relman DA, Brown PO. Development of the Human Infant Intestinal Microbiota. PLoS Biol 2007; 5:177
- Gewolb IH, Schwalbe RS, Taciak VL, Harrison TS, Panigrahi P. Stool microflora in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed 1999; 80:167 - 173
- Fanaro S, Chierici R, Guerrini P, Vigi V. Intestinal microflora in early infancy: composition and development. Acta Paediatr Suppl 2003; 91:48 - 55
- Meng D, Newburg DS, Young C, Baker A, Tonkonogy SL, Sartor RB, et al. Bacterial symbionts induce a FUT2-dependent fucosylated niche on colonic epithelium via ERK and JNK signaling. Am J Physiol Gastrointest Liver Physiol 2007; 293:780 - 787
- Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut differentiation. Dev Biol 2006; 297:374 - 386
- Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol 2007; 119:192 - 198
- Saavedra JM, Abi-Hanna A, Moore N, Yolken RH. Long-term consumption of infant formulas containing live probiotic bacteria: tolerance and safety. Am J Clin Nutr 2004; 79:261 - 267
- Saxelin M, Chuang NH, Chassy B, Rautelin H, Mäkelä PH, Salminen S, et al. Lactobacilli and bacteremia in Southern Finland, 1989–1992. Clin Infect Dis 1996; 22:564 - 566
- Hata D, Yoshida A, Ohkubo H, Mochizuki Y, Hosoki Y, Tanaka R, et al. Meningitis caused by Bifidobacterium in an infant. Pediatr Infect Dis J 1988; 7:669 - 671
- Barton LL, Rider ED, Coen RW. Bacteremic infection with Pediococcus: vancomycin-resistant opportunist. Pediatrics 2001; 107:775 - 776
- Honeycutt TC, El Khashab M, Wardrop RM 3rd, McNeal-Trice K, Honeycutt AL, Christy CG, et al. Probiotic administration and the incidence of nosocomial infection in pediatric intensive care: a randomized placebo-controlled trial. Pediatr Crit Care Med 2007; 8:452 - 458
- Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics 2005; 115:5 - 9
- Vendt N, Grünberg H, Tuure T, Malminiemi O, Wuolijoki E, Tillmann V, et al. Growth during the first 6 months of life in infants using formula enriched with Lactobacillus rhamnosus GG: double-blind, randomized trial. J Hum Nutr Diet 2006; 19:51 - 58
- Risk assessment on use of Lactobacillus rhamnosus (LGG) as an ingredient in infant formula and baby foods (II) 2007; 2009 http://www.vkm.no/dav/63bb45d3eb.pdf
- Abrahamsson TR, Jakobsson T, Böttcher MF, Fredrikson M, Jenmalm MC, Björkstén B, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2007; 119:1174 - 1180
- Chouraqui JP, Grathwohl D, Labaune JM, Hascoet JM, de Montgolfier I, Leclaire M, et al. Assessment of the safety, tolerance and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial. Am J Clin Nutr 2008; 87:1365 - 1373
- Gibson RA, Barclay D, Marshall H, Moulin J, Maire JC, Makrides M. Safety of supplementing infant formula with long-chain polyunsaturated fatty acids and Bifidobacterium lactis in term infants: a randomised controlled trial. Br J Nutr 2009; 101:1706 - 1713
- Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet 2001; 357:1076 - 1079
- Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, et al. Long-term safety and impact on infection rates of postnatal probiotic and prebiotic (synbiotic) treatment: randomized, double-blind, placebo-controlled trial. Pediatrics 2008; 122:8 - 12
- Puccio G, Cajozzo C, Meli F, Rochat F, Grathwohl D, Steenhout P. Clinical evaluation of a new starter formula for infants containing live Bifidobacterium longum BL999 and prebiotics. Nutrition 2007; 23:1 - 8
- Vlieger AM, Robroch A, van Buuren S, Kiers J, Rijkers G, Benninga MA, et al. Tolerance and safety of Lactobacillus paracasei ssp. paracasei in combination with Bifidobacterium animalis ssp. lactis in a prebiotic-containing infant formula: a randomised controlled trial. Br J Nutr 2009; 102:869 - 875
- JW D, K W, PN B, et al. Safety aspects of probiotic bacterial strains Lactobacillus rhamnosus HN001 and Bifidobacterium animalis ssp. lactis HN019 in human infants aged 0 to 2 years. Int Dairy J 2009; 19:149 - 154
- Wickens K, Black PN, Stanley TV, Mitchell E, Fitzharris P, Tannock GW, et al. A differential effect of 2 probiotics in the prevention of eczema and atopy: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol 2008; 122:788 - 794
- Stolley H, Droese W. Lactic acid in milk formula, the influence on absorption of nutrients and the influence on the metabolism in young babies. Acta Paediatr Scand 1971; 60:367 - 368
- Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol 2002; 68:219 - 226
- Schultz M, Gottl C, Young RJ, Iwen P, Vanderhoof JA. Administration of oral probiotic bacteria to pregnant women causes temporary infantile colonization. J Pediatr Gastroenterol Nutr 2004; 38:293 - 297
- Gueimonde M, Kalliomaki M, Isolauri E, Salminen S. Probiotic intervention in neonates—will permanent colonization ensue?. J Pediatr Gastroenterol Nutr 2006; 42:604 - 606
- Strachan DP. Hay fever, hygiene and household size. Bmj 1989; 299:1259 - 1260
- Kalliomaki M, Salminen S, Poussa T, Isolauri E. Probiotics during the first 7 years of life: a cumulative risk reduction of eczema in a randomized, placebo-controlled trial. J Allergy Clin Immunol 2007; 119:1019 - 1021
- Kopp MV, Hennemuth I, Heinzmann A, Urbanek R. Randomized, double-blind, placebo-controlled trial of probiotics for primary prevention: no clinical effects of Lactobacillus GG supplementation. Pediatrics 2008; 121:850 - 856
- Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol 2007; 119:184 - 191
- Prescott SL, Wiltschut J, Taylor A, Westcott L, Jung W, Currie H, et al. Early markers of allergic disease in a primary prevention study using probiotics: 2.5-year follow-up phase. Allergy 2008; 63:1481 - 1490
- Sakata S, Tonooka T, Ishizeki S, Takada M, Sakamoto M, Fukuyama M, et al. Culture-independent analysis of fecal microbiota in infants, with special reference to Bifidobacterium species. FEMS Microbiol Lett 2005; 243:417 - 423
- Bin-Nun A, Bromiker R, Wilschanski M, Kaplan M, Rudensky B, Caplan M, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight neonates. J Pediatr 2005; 147:192 - 196
- Lin HC, Hsu CH, Chen HL, Chung MY, Hsu JF, Lien RI, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008; 122:693 - 700
- Lin HC, Su BH, Chen AC, Lin TW, Tsai CH, Yeh TF, et al. Oral probiotics reduce the incidence and severity of necrotizing enterocolitis in very low birth weight infants. Pediatrics 2005; 115:1 - 4
- Deshpande G, Rao S, Patole S. Probiotics for prevention of necrotising enterocolitis in preterm neonates with very low birthweight: a systematic review of randomised controlled trials. Lancet 2007; 369:1614 - 1620
- Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. A prospective double-blind study. Biol Neonate 2002; 82:103 - 108
- Stappenbeck TS, Hooper LV, Gordon JI. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc Natl Acad Sci USA 2002; 99:15451 - 15455
- Wagner RD. Effects of microbiota on GI health: gnotobiotic research. Adv Exp Med Biol 2008; 635:41 - 56
- Hormannsperger G, Haller D. Molecular crosstalk of probiotic bacteria with the intestinal immune system: Clinical relevance in the context of inflammatory bowel disease. Int J Med Microbiol 2009;
- Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol 2009; 6:306 - 314
- Sonnenburg JL, Chen CT, Gordon JI. Genomic and metabolic studies of the impact of probiotics on a model gut symbiont and host. PLoS Biol 2006; 4:413
- Joint FAO/WHO working group report on drafting guidelines for the evaluation of probiotics in food 2002; 2009 ftp://ftp.fao.org/es/esn/food/wgreport2.pdf
- Saarela M, Mogensen G, Fonden R, Matto J, Mattila-Sandholm T. Probiotic bacteria: safety, functional and technological properties. J Biotechnol 2000; 84:197 - 215
- Vankerckhoven V, Huys G, Vancanneyt M, et al. Biosafety assessment of probiotics used for human consumption: recommendations from the EU-PROSAFE project. Trend Food Sci Tech 2008; 19:102 - 114
- Huys G, Vancanneyt M, D'Haene K, Vankerckhoven V, Goossens H, Swings J. Accuracy of species identity of commercial bacterial cultures intended for probiotic or nutritional use. Res Microbiol 2006; 157:803 - 810
- Masco L, Huys G, De Brandt E, Temmerman R, Swings J. Culture-dependent and culture-independent qualitative analysis of probiotic products claimed to contain bifidobacteria. Int J Food Microbiol 2005; 102:221 - 230
- Temmerman R, Scheirlinck I, Huys G, Swings J. Culture-independent analysis of probiotic products by denaturing gradient gel electrophoresis. Appl Environ Microbiol 2003; 69:220 - 226
- Green DH, Wakeley PR, Page A, Barnes A, Baccigalupi L, Ricca E, et al. Characterization of two Bacillus probiotics. Appl Environ Microbiol 1999; 65:4288 - 4291
- Duc le H, Hong HA, Barbosa TM, Henriques AO, Cutting SM. Characterization of Bacillus probiotics available for human use. Appl Environ Microbiol 2004; 70:2161 - 2171
- Mohania D, Nagpal R, Kumar M, Bhardwaj A, Yadav M, Jain S, et al. Molecular approaches for identification and characterization of lactic acid bacteria. J Dig Dis 2008; 9:190 - 198
- Collado MC, Moreno Y, Cobo JM, Hernandez M. Microbiological evaluation and molecular characterization of bifidobacteria strains in commercial fermented milks. Eur Food Res Technol 2006; 222:112 - 117
- Coudeyras S, Marchandin H, Fajon C, Forestier C. Taxonomic and strain-specific identification of the probiotic strain Lactobacillus rhamnosus 35 within the Lactobacillus casei group. Appl Environ Microbiol 2008; 74:2679 - 2689
- Massi M, Vitali B, Federici F, Matteuzzi D, Brigidi P. Identification method based on PCR combined with automated ribotyping for tracking probiotic Lactobacillus strains colonizing the human gut and vagina. J Appl Microbiol 2004; 96:777 - 786
- Naser SM, Dawyndt P, Hoste B, Gevers D, Vandemeulebroecke K, Cleenwerck I, et al. Identification of lactobacilli by pheS and rpoA gene sequence analyses. Int J Syst Evol Microbiol 2007; 57:2777 - 2789
- Ventura M, van Sinderen D, Fitzgerald GF, Zink R. Insights into the tazonomy genetics and physiology of bifidobacteria. Antonia van Leeuwenhoek 2004; 86:205 - 223
- Rooney AP, Price NP, Ehrhardt C, Swezey JL, Bannan JD. Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. inaquosorum subsp. nov. Int J Syst Evol Microbiol 2009; 59:2429 - 2436
- Lee YK, Ho PS, Low CS, Arvilommi H, Salminen S. Permanent colonization by Lactobacillus casei is hindered by the low rate of cell division in mouse gut. Appl Environ Microbiol 2004; 70:670 - 674
- Alander M, Satokari R, Korpela R, Saxelin M, Vilpponen-Salmela T, Mattila-Sandholm T, et al. Persistence of colonization of human colonic mucosa by a probiotic strain, Lactobacillus rhamnosus GG, after oral consumption. Appl Environ Microbiol 1999; 65:351 - 354
- Klarin B, Johansson ML, Molin G, Larsson A, Jeppsson B. Adhesion of the probiotic bacterium Lactobacillus plantarum 299v onto the gut mucosa in critically ill patients: a randomised open trial. Crit Care 2005; 9:285 - 293
- Sorokulova I. Preclinical testing in the development of probiotics: a regulatory perspective with Bacillus strains as an example. Clin Infect Dis 2008; 46:92 - 95
- Klare I, Konstabel C, Müller-Bertling S, Reissbrodt R, Huys G, Vancanneyt M, et al. Evaluation of new broth media for microdilution antibiotic susceptibility testing of Lactobacilli, Pediococci, Lactococci and Bifidobacteria. Appl Environ Microbiol 2005; 71:8982 - 8986
- Klare I, Konstabel C, Werner G, Huys G, Vankerckhoven V, Kahlmeter G, Hildebrandt B, et al. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use. J Antimicrob Chemother 2007; 59:900 - 912
- Kastner S, Perreten V, Bleuler H, Hugenschmidt G, Lacroix C, Meile L. Antibiotic susceptibility patterns and resistance genes of starter cultures and probiotic bacteria used in food. Syst Appl Microbiol 2006; 29:145 - 155
- Masco L, Van Hoorde K, De Brandt E, Swings J, Huys G. Antimicrobial susceptibility of Bifidobacterium strains from humans, animals and probiotic products. J Antimicrob Chemother 2006; 58:85 - 94
- Huys G, D'Haene K, Swings J. Genetic basis of tetracycline and minocycline resistance in potentially probiotic Lactobacillus plantarum strain CCUG 43738. Antimicrob Agents Chemother 2006; 50:1550 - 1551
- van Hoek AHAM, Margolles A, Domig KJ, et al. Molecular assessment of erythromycin and tetracycline resistance genes in lactic acid bacteria and bifidobacteria and their relation to the phenotypic resistance. International Journal of Probiotics and Prebiotics 2008; 3:271 - 280
- Update of the criteria used in the assessment of bacterial resistance to antibiotics of human or veterinary importance 2008; 2009 http://www.efsa.europa.eu/cs/BlobServer/Guidance_of_Panel/feedap_op_ej732_tg_antimicrobial_resistance_en.pdf?ssbinary=true
- Jorgensen J. Methods for Antimicrobial Dilution and Disk Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria; Approved Guideline, M45-A Clinical and Laboratory Standards Institute 2006;
- Agency Additional Correspondence Letter GRAS Notice No. GRN 000049 2005; 2009 http://www.fda.gov/Food/FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/GRASListings/ucm154391.htm
- Vancanneyt M, Huys G, Lefebvre K, Vankerckhoven V, Goossens H, Swings J. Intraspecific genotypic characterization of Lactobacillus rhamnosus strains intended for probiotic use and isolates of human origin. Appl Environ Microbiol 2006; 72:5376 - 5383
- van de Guchte M, Penaud S, Grimaldi C, Barbe V, Bryson K, Nicolas P, et al. The complete genome sequence of Lactobacillus bulgaricus reveals extensive and ongoing reductive evolution. Proc Natl Acad Sci USA 2006; 103:9274 - 9279
- Olah A, Belagyi T, Issekutz A, Gamal ME, Bengmark S. Randomized clinical trial of specific lactobacillus and fibre supplement to early enteral nutrition in patients with acute pancreatitis. Br J Surg 2002; 89:1103 - 1107
- Rayes N, Seehofer D, Hansen S, Boucsein K, Müller AR, Serke S, et al. Early enteral supply of lactobacillus and fiber versus selective bowel decontamination: a controlled trial in liver transplant recipients. Transplantation 2002; 74:123 - 127
- Kirjavainen PV, Salminen SJ, Isolauri E. Probiotic bacteria in the management of atopic disease: underscoring the importance of viability. J Pediatr Gastroenterol Nutr 2003; 36:223 - 227
- Sharieff W, Bhutta Z, Schauer C, Tomlinson G, Zlotkin S. Micronutrients (including zinc) reduce diarrhoea in children: the Pakistan Sprinkles Diarrhoea Study. Arch Dis Child 2006; 91:573 - 579
- Asahara T, Takahashi M, Nomoto K, Takayama H, Onoue M, Morotomi M, et al. Assessment of safety of lactobacillus strains based on resistance to host innate defense mechanisms. Clin Diagn Lab Immunol 2003; 10:169 - 173
- Franz CM, Muscholl-Silberhorn AB, Yousif NM, Vancanneyt M, Swings J, Holzapfel WH. Incidence of virulence factors and antibiotic resistance among Enterococci isolated from food. Appl Environ Microbiol 2001; 67:4385 - 4389
- Eaton TJ, Gasson MJ. Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates. Appl Environ Microbiol 2001; 67:1628 - 1635
- Ge J, Catt DM, Gregory RL. Streptococcus mutans surface α-enolase binds salivary mucin MG2 and human plasminogen. Infect Immun 2004; 72:6748 - 6752
- Mack DR. D(−)-lactic acid-producing probiotics, D(−)-lactic acidosis and infants. Can J Gastroenterol 2004; 18:671 - 675
- Hove H, Mortensen PB. Colonic lactate metabolism and D-lactic acidosis. Dig Dis Sci 1995; 40:320 - 330
- Walter J. Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol 2008; 74:4985 - 4996
- Bongaerts G, Bakkeren J, Severijnen R, Sperl W, Willems H, Naber T, et al. Lactobacilli and acidosis in children with short small bowel. J Pediatr Gastroenterol Nutr 2000; 30:288 - 293
- Connolly E, Abrahamsson T, Bjorksten B. Safety of D(−)-lactic acid producing bacteria in the human infant. J Pediatr Gastroenterol Nutr 2005; 41:489 - 492
- Uribarri J, Oh MS, Carroll HJ. D-lactic acidosis. A review of clinical presentation, biochemical features and pathophysiologic mechanisms. Medicine 1998; 77:73 - 82
- Hove H. Lactate and short chain fatty acid production in the human colon: implications for D-lactic acidosis, short-bowel syndrome, antibiotic-associated diarrhoea, colonic cancer and inflammatory bowel disease. Dan Med Bull 1998; 45:15 - 33
- Munakata S, Arakawa C, Kohira R, Fujita Y, Fuchigami T, Mugishima H. A case of D-lactic acid encephalopathy associated with use of probiotics. Brain Dev 2009;
- Uchida H, Yamamoto H, Kisaki Y, Fujino J, Ishimaru Y, Ikeda H. D-lactic acidosis in short-bowel syndrome managed with antibiotics and probiotics. J Pediatr Surg 2004; 39:634 - 636
- MMWR, Leads from the MMWR. Scombroid fish poisoning—New Mexico, 1987. Jama 1988; 260:1217
- Straub BW, Kicherer M, Schilcher SM, Hammes WP. The formation of biogenic amines by fermentation organisms. Z Lebensm Unters Forsch 1995; 201:79 - 82
- Pessione E, Mazzoli R, Giuffrida MG, Lamberti C, Garcia-Moruno E, Barello C, et al. A proteomic approach to studying biogenic amine producing lactic acid bacteria. Proteomics 2005; 5:687 - 698
- Hoa NT, Baccigalupi L, Huxham A, Smertenko A, Van PH, Ammendola S, et al. Characterization of Bacillus species used for oral bacteriotherapy and bacterioprophylaxis of gastrointestinal disorders. Appl Environ Microbiol 2000; 66:5241 - 5247
- Coeuret V, Gueguen M, Vernoux JP. Numbers and strains of lactobacilli in some probiotic products. Int J Food Microbiol 2004; 97:147 - 156
- Milazzo I, Speciale A, Musumeci R, Fazio D, Blandino G. Identification and antibiotic susceptibility of bacterial isolates from probiotic products available in Italy. New Microbiol 2006; 29:281 - 291
- Moneret-Vautrin DA, Morisset M, Cordebar V, Codreanu F, Kanny G. Probiotics may be unsafe in infants allergic to cow's milk. Allergy 2006; 61:507 - 508
- Lee TT, Morisset M, Astier C, Moneret-Vautrin DA, Cordebar V, Beaudouin E, et al. Contamination of probiotic preparations with milk allergens can cause anaphylaxis in children with cow's milk allergy. J Allergy Clin Immunol 2007; 119:746 - 747
- Momose H, Igarashi M, Era T, Fukuda Y, Yamada M, Ogasa K. Toxicological studies on Bifidobacterium longum BB536. Appl Pharmacol 1979; 17:881 - 887
- Ishibashi N, Yamazaki S. Probiotics and safety. Am J Clin Nutr 2001; 73:465 - 470
- Lara-Villoslada F, Sierra S, Diaz-Ropero MP, Olivares M, Xaus J. Safety assessment of the human milk-isolated probiotic Lactobacillus salivarius CECT5713. J Dairy Sci 2007; 90:3583 - 3589
- Salminen MK, Tynkkynen S, Rautelin H, Poussa T, Saxelin M, Ristola M, et al. The efficacy and safety of probiotic Lactobacillus rhamnosus GG on prolonged, noninfectious diarrhea in HIV Patients on antiretroviral therapy: a randomized, placebo-controlled, crossover study. HIV Clin Trials 2004; 5:183 - 191
- Gil L, Siems W, Mazurek B, Gross J, Schroeder P, Voss P, et al. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res 2006; 40:495 - 505
- Nielsen K, Duncan J. Animal Brucellosis 1990; Boca Raton, FL CRC Press, Inc
- Konikoff MR, Denson LA. Role of fecal calprotectin as a biomarker of intestinal inflammation in inflammatory bowel disease. Inflamm Bowel Dis 2006; 12:524 - 534
- Patti JM, Bremell T, Krajewska-Pietrasik D, Abdelnour A, Tarkowski A, Rydén C, et al. The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis. Infect Immun 1994; 62:152 - 161
- Beckerman KP, Rogers HW, Corbett JA, Schreiber RD, McDaniel ML, Unanue ER. Release of nitric oxide during the T cell-independent pathway of macrophage activation. Its role in resistance to Listeria monocytogenes. J Immunol 1993; 150:888 - 895
- Gras V, Bouffandeau B, Montravers PH, Lalau JD. Effect of metformin on survival rate in experimental sepsis. Diabetes Metab 2006; 32:147 - 150
- Vankerckhoven V, Moreillon P, Piu S, Giddey M, Huys G, Vancanneyt M, Goossens H, et al. Infectivity of Lactobacillus rhamnosus and Lactobacillus paracasei isolates in a rat model of experimental endocarditis. J Med Microbiol 2007; 56:1017 - 1024
- Moreillon P, Que YA. Infective endocarditis. Lancet 2004; 363:139 - 149
- Moreillon P, Entenza JM, Francioli P, McDevitt D, Foster TJ, François P, et al. Role of Staphylococcus aureus coagulase and clumping factor in pathogenesis of experimental endocarditis. Infect Immun 1995; 63:4738 - 4743
- Cohen MD. Bacterial host resistance models in the evaluation of immunotoxicity. Methods 2007; 41:20 - 30
- Vessie EL, Hirsch GM, Lee TD. Aortic allograft vasculopathy is mediated by CD8(+) T cells in Cyclosporin A immunosuppressed mice. Transpl Immunol 2005; 15:35 - 44
- Toxicological Principles for the Safety Assessment of Food Ingredients. Chapter IC.C.9.a: Guideline for Reproduction Studies 2007; 2009 http://www.fda.gov/Food/GuidanceComplianceRegulatoryInformation/GuidanceDocuments/FoodIngredientsandPackaging/Redbook/default.htm
- International Conference On Harmonisation Of Technical Requirements For Registration Of Pharmaceuticals For Human Use. Detection Of Toxicity To Reproduction For Medicinal Products & Toxicity To Male Fertility. Harmonised Tripartite Guideline S5(R2) 2005; 2009 http://www.ich.org/LOB/media/MEDIA498.pdf
- Claud EC, Walker WA. Bacterial colonization, probiotics and necrotizing enterocolitis. J Clin Gastroenterol 2008; 42:46 - 52
- Walker WA. Mechanisms of action of probiotics. Clin Infect Dis 2008; 46:87 - 91
- Patterson JK, Lei XG, Miller DD. The pig as an experimental model for elucidating the mechanisms governing dietary influence on mineral absorption. Exp Biol Med 2008; 233:651 - 654
- Garthoff LH, Henderson GR, Sager AO, Sobotka TJ, O'Dell R, Thorpe CW, et al. The Autosow raised miniature swine as a model for assessing the effects of dietary soy trypsin inhibitor. Food Chem Toxicol 2002; 40:487 - 500
- Pang X, Hua X, Yang Q, Ding D, Che C, Cui L, et al. Inter-species transplantation of gut microbiota from human to pigs. Isme J 2007; 1:156 - 162
- Solano-Aguilar G, Dawson H, Restrepo M, Andrews K, Vinyard B, Urban JF Jr. Detection of Bifidobacterium animalis subsp. lactis (Bb12) in the intestine after feeding of sows and their piglets. Appl Environ Microbiol 2008; 74:6338 - 6347
- Zhang W, Azevedo MS, Wen K, Gonzalez A, Saif LJ, Li G, et al. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine 2008; 26:3655 - 3661
- Wen L, Ley RE, Volchkov PY, Stranges PB, Avanesyan L, Stonebraker AC, et al. Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 2008; 455:1109 - 1113
- Collado MC, Grzeskowiak L, Salminen S. Probiotic strains and their combination inhibit in vitro adhesion of pathogens to pig intestinal mucosa. Curr Microbiol 2007; 55:260 - 265
- Solano-Aguilar G, Shea-Donohue T, Madden K, Dawson H, Ledbetter T, Urban JF Jr. Gasbarre LC. The effect of human derived probiotic bacteria on the intestinal function of pigs. AAVP Symposium: New approaches in the study of animal parasites. Vet Parasitol 2004; 125:147 - 161
- Siggers RH, Siggers J, Boye M, Thymann T, Mølbak L, Leser T, et al. Early administration of probiotics alters bacterial colonization and limits diet-induced gut dysfunction and severity of necrotizing enterocolitis in preterm pigs. J Nutr 2008; 138:1437 - 1444
- Harding SV, Fraser KG, Wykes LJ. Probiotics stimulate liver and plasma protein synthesis in piglets with dextran sulfate-induced colitis and macronutrient restriction. J Nutr 2008; 138:2129 - 2135
- Piriou-Guzylack L, Salmon H. Membrane markers of the immune cells in swine: an update. Vet Res 2008; 39:54
- Porcine Immunology and Nutrition (PIN) database 2009 http://www.ars.usda.gov/Services/docs.htm?docid=6065
- Chowdhury SR, King DE, Willing BP, Band MR, Beever JE, Lane AB, et al. Transcriptome profiling of the small intestinal epithelium in germfree versus conventional piglets. BMC Genomics 2007; 8:215
- Dethlefsen L, Huse S, Sogin ML, Relman DA. The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 2008; 6:280
- Dowd SE, Sun Y, Wolcott RD, Domingo A, Carroll JA. Bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) for microbiome studies: bacterial diversity in the ileum of newly weaned Salmonella-infected pigs. Foodborne Pathog Dis 2008; 5:459 - 472
- Von Wright A. Regulating the safety of probiotics—the European approach. Curr Pharm Des 2005; 11:17 - 23
- Wassenaar TM, Klein G. Safety aspects and implications of regulation of probiotic bacteria in food and food supplements. J Food Prot 2008; 71:1734 - 1741
- Opinion of the Scientific Committee on Animal Nutrition on the criteria for assessing the safety of micro-organisms resistant to antibiotics of human clinical and veterinary importance 2002; 2009 http://ec.europa.eu/food/fs/sc/scan/out108_en.pdf
- Introduction of a Qualified Presumption of Safety (QPS) approach for assessment of selected microorganisms referred to EFSA—Opinion of the Scientific Committee 2007 2009 http://www.efsa.europa.eu/EFSA/efsa_locale-1178620753812_1178667590178.htm
- On a generic approach to the safety assessment of micro-organisms used in feed/food and feed/food production 2003; 2009 http://ec.europa.eu/food/fs/sc/scf/out178_en.pdf
- Probiotics 2009; 2009 http://www.hc-sc.gc.ca/dhp-mps/prodnatur/applications/licen-prod/monograph/mono_probioti-eng.php
- Guidance Document—The Use of Probiotic Microorganisms in Food 2009; 2009 http://www.hc-sc.gc.ca/fn-an/legislation/guide-ld/probiotics_guidance-orientation_probiotiques-eng.php
- Sanders M. How do we know when something called ”probiotic” is really a probiotic? A guideline for consumers and healthcare professionals. Functional Food Rev 2009; 1:3 - 12
- Immunomodulation by probiotics: efficacy and safety evaluation 2005; 2009 http://www.rivm.nl/bibliotheek/rapporten/340320003.html
- Probiotics Task Force 2009; 2009 http://www.ilsi.org/Europe/Pages/TF_Probiotics.aspx
- Agency response letter: GRAS Notice No. GRN 000231 2008; 2009 http://www.fda.gov/Food/FoodIngredientsPackaging/GenerallyRecognizedasSafeGRAS/GRASListings/ucm153907.htm
- Rayes N, Hansen S, Seehofer D, Müller AR, Serke S, Bengmark S, et al. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition 2002; 18:609 - 615
- Rayes N, Seehofer D, Theruvath T, Schiller RA, Langrehr JM, Jonas S, et al. Supply of pre- and probiotics reduces bacterial infection rates after liver transplantation—a randomized, double-blind trial. Am J Transplant 2005; 5:125 - 130
- Kanazawa H, Nagino M, Kamiya S, Komatsu S, Mayumi T, Takagi K, et al. Synbiotics reduce postoperative infectious complications: a randomized controlled trial in biliary cancer patients undergoing hepatectomy. Langenbecks Arch Surg 2005; 390:104 - 113
- Rayes N, Seehofer D, Theruvath T, Mogl M, Langrehr JM, Nüssler NC, et al. Effect of enteral nutrition and synbiotics on bacterial infection rates after pylorus-preserving pancreatoduodenectomy: a randomized, double-blind trial. Ann Surg 2007; 246:36 - 41
- Nomura T, Tsuchiya Y, Nashimoto A, Yabusaki H, Takii Y, Nakagawa S, et al. Probiotics reduce infectious complications after pancreaticoduodenectomy. Hepatogastroenterology 2007; 54:661 - 663