Abstract
Recent evidence indicates that segmented filamentous bacteria (SFB), "Candidatus Arthromitus", play a unique role in different aspects of the maturation of the immune system, including T cell responses.
Introduction
Segmented filamentous bacteria (SFB) are Gram-positive, spore-forming and yet non culturable bacteria commonly considered not pathogenic and host-specific members of gut microbiota in virtually all species of investigated animals: mammals (including man), birds, fishes and insects.
Important experimental studiesCitation1,Citation2 and reviewsCitation3,Citation4 focusing the attention of the scientific community on these bacteria were recently published. These studies showed that the presence of SFB represents a necessary condition for a specific and coordinated induction of T cell activities, particularly in respect to Th17 lineage and regulatory T cell responses. The proposal that, among millions of commensal bacteria living in the gut, only a genus can recapitulate fundamental immune responses draws a scenario equally fascinating than that experienced after the discovery that a bacterial genus, Helicobacter, can live in the acid environment of the stomach and is associated with gastric diseases. The relatively recent discover of Th17 cell lineage and its role in physiology and in the pathogenesis of inflammatory and autoimmune diseases, together with the increasing knowledge concerning the role in both physiology and pathology of regulatory T cells and pattern recognition receptors and cells of innate immunity, makes the picture even more intriguing.
Nowadays, it seems especially important to know the history of this old acquaintance as a necessary condition both to correctly drive future research and to verify if and in what measure an answer to two main questions is available: Why just SFB among millions of intestinal bacteria? How and by means of which bacterial characteristics SFB exercise their fundamental effects on the immune system?
The fact that SFB can modulate the activity of different elements of the immune system is anything but new. These bacteria were identified for the first time more than 160 years ago, as early as 1849, by Joseph LeidyCitation5 in the intestines of myriapods and termites as very long “jointed thread” (Arthromitus). Successively, these bacteria have been described more than 30 years ago in a number of mammalian species.
Since SFB can not be cultured in vitro, they do not have an official taxonomic name: the provisional status Candidatus and the genus name Arthromitus, originally coined by Leidy, have been proposed for this group of bacteria.Citation6 Candidatus Arthromitus can be divided into different species on the basis of host specificity and 16S rRNA sequences and should be referred to as Candidatus Arthromitus muris, Candidatus Arthromitus ratti, etc. This genus contains different species of segmented bacteria, which grow as septate filaments with peculiar morphological characteristics.
Notably, the observation that in most animals SFB appear rather explosively at the time of weaning, then expand to become one of the dominant bacteria, and finally their numbers decrease to become stable in the adult animals is likely correlated with the age-dependent maturation and activity of the immune system.Citation7 This suggests that these bacteria are probably involved in driving the maturation and possibly the differentiation of components of the gut-associated lymphoid tissue. It should also be remembered that a rapid growth of SFB is suggested by the rapid rate of epithelial cell renewal in the small intestine: thus, these microorganisms need to complete their life cycle every two days.
More strikingly, SFB have been already recognized since long time as the single member of the gut microflora able to induce specific immune responses: (1) SFB induce the development of IgA plasma cells in gut lamina propria and natural IgA in gut secretions, being the most potent microbial stimulus for the induction of intestinal IgA-producing cells.Citation8,Citation9 On the other hand, an aberrant and persistent expansion of SFB throughout the small intestine has also been demonstrated in IgA deficent mice;Citation10 (2) SFB selectively induce the expression of the major histocompatibility complex class II molecules on the intestinal epithelial cells;Citation9 (3) SFB specifically induce T cell responses. In fact, SFB have been demonstrated to induce both the cellular elements of intraepithelial lymphocytes (IEL): NK cells and CD8+ T cells. Colonization of mice by SFB induces a significant rise in cytotoxic activity, and these functional changes are associated with an increase in both NK and CD8+ IEL.Citation11 Importantly, IEL have acquired in the last years an increasing relevance both in physiology and in different pathological conditions: IEL are hallmarks of celiac disease, intimately related to the pathogenesis of this condition, and they are considered important biomarkers in irritable bowel syndrome. Finally, it has been recently shown that SFB selectively induce CD4+ T cells that produce IL-17 and IL-22 (Th 17 cell lineage) in the intestinal lamina propria,Citation2 and colonization by these bacteria can even recapitulate the T cell responses induced by the whole mouse microflora simultaneously inducing Th1, Th2, Th17 and T regulatory responses.Citation1 These latter findings, by opening new avenues to understand the relationship between gut microbiota and immunity, have received a great deal of attention by the scientific community.
Importantly, SFB also provide host protection against pathogens. This can be realized both by competing for the same attachment areas and by promoting an effective immune response against pathogens; the latter can also be due to the proposed induction of Th17 cells, IL-22 and the epithelial production of a bactericidal C-type lectin.Citation12 To date, SFB have shown in experimental studies to induce resistance to Listeria monocitogenes,Citation13 enteropathogenic Escherichia coli, Salmonella enteritidisCitation14,Citation15 and Citrobacter rodentium.Citation2
Would it be possible that SFB induce all these activities if a peculiar and intimate relationship between these bacteria and selected intestinal epithelial cells does not exist?
Although some studies focusing on SFB morphology have been published,Citation16,Citation17 how and why SFB specifically interact with the immune system has not been attentively evaluated. In particular, we believe that as long as cultural methods for these bacteria will not be available, more attention should be given to the morphological patterns of contact between SFB and the intestinal epithelium.Citation18 This may allow to better understand the reasons for the impressive and specific inductions of immune responses by these peculiar “commensal” bacteria.
We have attentively evaluated transmission electron microscopy pictures of the mouse terminal ileum (male Swiss mice 2–3 months old) to investigate the SFB life-cycle and their modes of contact with the epithelium.
Results
SFB presented both a developmental and a vegetative form. The developmental form is characterized by the presence of intrasegmental bodies: during the first stages of their growth the bacteria can be shorter but already contain intrasegmental bodies (), then they become long filaments and each filament is made up of segments, and each segment contains one or two intrasegmental bodies ( and C). These segments have the ultrastructure of a prokaryotic cell and show a homogeneous cytoplasm and nuclear areas. The intrasegmental bodies also have a prokaryotic cell structure with cell-wall, cytoplasm and nuclear areas distinct from that of the surrounding cell, some of these cells appear to be dividing, and probably when the intrasegmental bodies are two the cell division has just happened. These cells do not have the ultrastructure of bacteria endospores. When the filaments have completed their growth the segmental bodies disappear and the long filaments appear in their vegetative form (). Only this vegetative form of SFB is mature and ready to anchor the epithelial cells with the holdfast segment. The cytoskeletal structures of this specialized segment remain relaxed when the vegetative forms of SFB are seen floating in the lumen (), whereas they assume a contracted aspect when SFB are seen anchored to the epithelial cells ().
It is important to underline that the bacterial form containing intrasegmental bodies has never been seen and reported attached to enterocytes. For this reason we are confident with our proposal of distinguish: (1) a bacterial form containing intrasegmental bodies always seen floating in the lumen and never found attached to the epithelial cells. This form is probably deputed to a rapid growth of the filaments and, under certain conditions, to the development of spore-like bodies; (2) a bacterial form without intrasegmental bodies and deputed to the attachment to the epithelial cells. We named, according to recent suggestions by other authors,Citation19 these two well distinct SFB forms as developmental and vegetative, respectively.
Even more notably, transmission electron microscopy shows that SFB present peculiar modalities of contact with intestinal epithelial cells. They preferentially adhere to follicle-associated epithelium (enterocytes and specialized M cells) of terminal ileal Peyer's patches (PP). PP are the principal sites for the sampling of antigens in the gut by their unique ability to take up antigens via endocytosis or phagocytosis and then deliver them via transcytosis to antigen presenting cells or directly to lymphocytes. The direct attachment of a vegetative form of SFB to epithelial cells first causes an invagination of the plasma membrane, displacement of microvilli confined to the site of attachment while the rest of the microvillous border remains usually intact, and promotes the formation of a thickened and electrondense underlying zone in the epithelial cells identified as an accumulation of actin filaments at the attachment site (). This accumulation of polymerizedactin filamentsCitation20 indicates a specific host reaction and implies a cell response similar to that induced by pathogens such as enteropathogenic Escherichia coliCitation21 and Salmonella.Citation22 After their attachment, SFB seem to release their segments while only the holdfast segments penetrate more and more deeply in the epithelial cells (–D). Finally, restoration of the overhanging plasma membrane and microvillous border are seen ( and F). In addition, a true phagocytosis of SFB by epithelial cells has been previously demonstrated.Citation17,Citation23 Therefore, all the mechanisms of endocytosis, phagocytosis and transcytosis, characterizing the follicular epithelium, seem to be involved in the interaction between SFB and epithelial host cells. This determines the conditions for a massive presentation of bacterial antigens, possibly evoking impressive immune responses.
Discussion
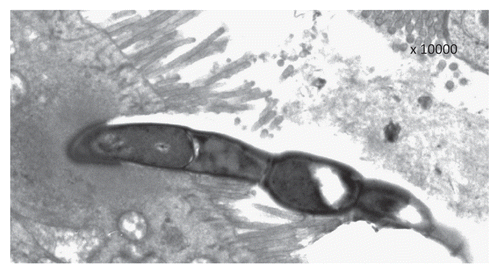
Based on the observation that single holdfast segments and full SFB forms not containing intrasegmental bodies are often seen attached to enterocytes, and that some segments are more rarely found attached to the holdfast, previous ultrastructural studiesCitation24,Citation25 suggested a SFB life cycle different from that proposed in the present investigation. In particular, it has been suggested that holdfast segments attach to the enterocytes in the terminal ileum and this attachment represents the signal promoting filament elongation. However, it is worthy of mention that none of the published ultrastructural pictures can demonstrate the proposed pattern of life cycle; therefore the proposal by Ferguson and Birch-AndersenCitation24 should be considered a speculative hypothesis. On the contrary, we can substantiate our point of view not only based on TEM pictures showing a detachment of the rest of the segmented bacteria from the attached holdfast segment, but also by showing a progressive penetration of the holdfast segments within the epithelial cells. This fact is of paramount importance because it represents the basis of bacterial endocytosis, transcytosis and phagocytosis, that has been already demonstrated by other authors.Citation17–Citation23 This permits an impressive presentation of bacterial antigens to antigen presenting cells and, in the case of the M cells, directly to the lymphocytes contained in the lymphoid packets characteristic of this specialized cells. Notably, in the rare cases in which few segments remain attached to the holdfast segment, that represent the principal morphological findings supporting the above reported hypothesis, degenerative changes of the residual segments and enlargements of the intrasegmental junctions are often evident (). This induces us to strongly suppose a final detachment of all the segments from the holdfast also in these cases, permitting its penetration into the epithelial cells. These aspects probably represent the morphological basis to explain the unique role played by these bacteria in both maturation and activation of the different components of the immune system.
Although SFB do not cause an apparent inflammatory reaction in the lamina propria of colonized areasCitation26 and are commonly considered not pathogenic bacteria, three lines of evidence suggest the possibility that “different levels of pathogenicity” should be considered: (1) T lymphocytes in the intestinal mucosa are normally in a more activated state than similar cells in peripheral lymph nodes and the spleen; the commensal SFB on their own seem capable of activating both humoral and cellular mucosal immune system in a non-pathogenic manner;Citation13 (2) SFB together with a defined cocktail of specific pathogen-free bacteria were effective in triggering intestinal inflammation in a mouse model of inflammatory bowel disease;Citation27 (3) SFB are suspected to play a role in the aetio-pathogenesis of rainbow trout gastroenteritis.Citation19
Moreover, if manipulation of processes mediated by SFB may provide new therapeutic opportunities,Citation2 it has to be remembered that both antibiotics and probiotics have proved to be active against SFB in mice. In fact, penicillin apparently eliminates SFB from the mouse terminal ileum and a recolonization of the ileum is observed some weeks after the antibiotic treatment is stopped.Citation28 The high increment in the number of SFB found in ileum samples of immunosuppressed mice was restored to normal values when animals received a strain of Lactobacillus plantarum.Citation29 Importantly, recent data obtained with two complimentary genetic mouse models, demonstrated that Paneth cell alfa-defensins play a crucial role in regulating microbial ecology. In particular these antimicrobial peptides induce striking losses of SFB and fewer IL-17-producing T cells in the small intestinal lamina propria; this observation provides evidence for a key role of alfa-defensins in regulating intestinal microbial ecology in general and SFB homeostasis in particular.Citation30
We believe that, lacking the possibility to grow these organisms in culture and also independently from this fact, much more attention should be given to the morphological aspects related to SFB. In this experimental ultrastructural study we have identified two distinct morphological forms of SFB with separate functions in their life cycle. We also attempted a first characterization of the patterns of contact between SFB and the specialized follicular epithelium, showing that surely exist sufficient morphological bases to explain the peculiar relationships between these organisms and the immune system.
Ileal biopsies are commonly performed in patients and SFB is now ready to be made known to gastroenterologists: the possible role of these intriguing bacteria in the physiopathology of human diseases, starting off with Inflammatory Bowel Diseases, should be investigated in retrospective and prospective studies, and obviously further attempts to grow SFB in cultural media should be carried out. We are pretty convinced that a large number of studies involving human patients will be published in the next few years on this topic that recently received a great deal of attention.Citation1,Citation2,Citation31 These studies will certainly increase our knowledge on the relationship between the gut microbiota and the host immune responses, and hopefully will provide new strategies for the treatment of gastrointestinal pathologies including chronic inflammatory and autoimmune diseases.
Material and Methods
Fifteen male Swiss mice (2–3 months old; weight 25–30 g) were used; they were housed in 425 × 266 × 155 mm cages under standard conditions. Immediately after the mice were sacrificed, all the terminal ileal samples were analyzed by using Transmission Electron Microscopy (TEM). The specimens were fixed in 2.5% glutharaldeyde in 0.1 phosphate buffer pH 7.4. Then they were cut into small pieces and post-fixed in the same buffer. They were dehydrated in acetone and embedded in Araldite Durcupan ACM (Fluka). The semi-thin sections obtained with a Reichet Ultracut S ultramicrotome were stained with a 1% toluidine blue in an aqueous sodium borate solution and examined under light microscopy. Ultrathin sections of selected areas were contrasted with uranyl-acetate and lead-citrate by a Reichert Ultrostainer (Leica) and observed with a Zeiss EM900 electron microscope.
Abbreviations
| IEL | = | intraepithelial lymphocytes |
| PP | = | Peyer's patches |
| SFB | = | segmented filamentous bacteria |
Figures and Tables
Figure 1 The life cycle of SFB seems to start by a rapid growth of these segmented organisms in their developmental form: short SFB composed by few segments containing intrasegmental bodies (A) grow quickly increasing the number of their segments and each segment contains one or two intrasegmental bodies (B and C). When SFB complete their growth the intrasegmental bodies disappear and the organisms present specialized ends for the attachment to the epithelial cells (D); these specialized holdfast segments present relaxed cytoskeletal structures when the vegetative forms are seen floating in the lumen (E), but they become contracted when the holdfast segments are engaged in the “bite” to the epithelial cells (F).
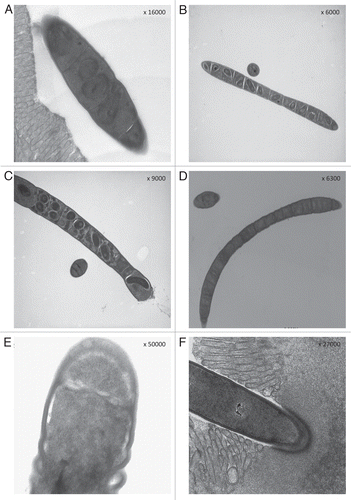
Figure 2 After the initial “bite” to the epithelial cells (A), the holdfast segments move away from the remaining part of the organisms (B and C), and only the holdfast segments penetrate more and more deeply in the epithelial cells while microvillar borders and cytoplasmic membranes seem to come back to an apparent integrity (D–F).
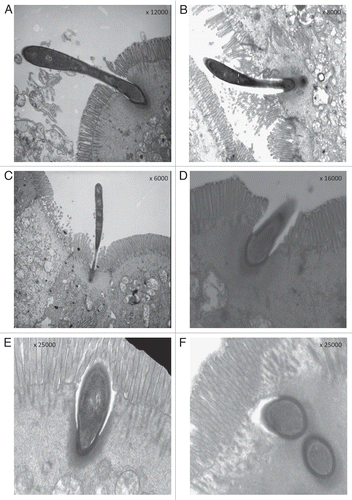
Figure 3 Some residual segments are seen attached to the holdfast segment anchored to the surface of an epithelial cell presenting the typical microvillar aspect of a M cell, but the degenerative changes of these segments associated with the enlargement of the intrasegmental junction with the holdfast strongly suggest that the complete detachment of the holdfast segment from the rest of the filament is imminent.
References
- Gaboriau-Routhiau V, Rakotobe S, Lecuyer E, Mulder I, Lan A, Bridonneau C, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity 2009; 31:677 - 689
- Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 2009; 139:485 - 498
- Tanoue T, Umesaki Y, Honda K. Immune responses to gut microbiota-cammensals and pathogens. Gut Microbes 2010; 1:1 - 10
- Ivanov II, Littman DR. Segmented filamentous bacteria take the stage. Mucosal Immunol 2010; 3:209 - 212
- Leidy J. On the existence of entophyta in healthy animals, as a natural condition. Proc Acad Natl Sci Phila 1849; 4:225 - 233
- Snel J, Heinen PP, Blok HJ, Carman RJ, Duncan AJ, Allen PC, et al. Comparison of 16S rRNA sequences of segmented filamentous bacteria isolated from mice, rats and chickens and proposal of “Candidatus Arthromitus.”. Int J Syst Bacteriol 1995; 45:780 - 782
- Snel J, Hermsen CC, Smits HJ, Bos NA, Eling WM, Cebra JJ, et al. Interactions between gut-associated lymphoid tissue and colonization levels of indigenous, segmented, filamentous bacteria in the small intestine of mice. Can J Microbiol 1998; 44:1177 - 1182
- Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun 1999; 67:1992 - 2000
- Umesaki Y, Setoyama H, Matsumoto S, Imaoka A, Itoh K. Differential roles of segmented filamentous bacteria and clostridia in development of the intestinal immune system. Infect Immun 1999; 67:3504 - 3511
- Suzuki K, Meek B, Doi Y, Muramatsu M, Chiba T, Honjo T, et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci USA 2004; 101:1981 - 1986
- Cebra JJ, Periwal SB, Lee G, Lee F, Shroff KE. Development and maintenance of the gut-associated lymphoid tissue (GALT): the roles of enteric bacteria and viruses. Dev Immunol 1998; 6:13 - 18
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 2008; 14:282 - 289
- Cebra JJ. Influences of microbiota on intestinal immune system development. Am J Clin Nutr 1999; 69:1046 - 1051
- Garland CD, Lee A, Dickson MR. Segmented filamentous bacteria in the rodent small intestine: their colonization of growing animals and possible role in the host resistence to Salmonella. Microb Ecol 1982; 8:181 - 190
- Heczko U, Abe A, Finlay BB. Segmented filamentous bacteria prevent colonization of enteropathogenic Escherichia coli O103 in rabbits. J Infect Dis 2000; 181:1027 - 1033
- Koopman JP, Stadhouders AM, Kennis HM, De Boer H. The attachment of filamentous segmented microorganisms to the distal ileum wall of the mouse: a scanning and transmission electron microscopy study. Lab Anim 1987; 21:48 - 52
- Yamauchi KE, Snel J. Transmission electron microscopic demonstration of phagocytosis and intracellular processing of segmented filamentous bacteria by intestinal epithelial cells of the chick ileum. Infect Immun 2000; 68:6496 - 6504
- Caselli M. Trasmission electron microscopy of segmented filamentous bacteria 2009; Available at: http://www.cell.com/comments/S0092-8674(09)01248-3
- Del-Pozo J, Crumlish M, Turnbull JF, Ferguson HW. Histopathology and ultrastructure of segmented filamentous bacteria-associated rainbow trout gastroenteritis. Vet Pathol 2010; 47:220 - 230
- Jepson MA, Clark MA, Simmons NL, Hirst BH. Actin accumulation at sites of attachment of indigenous apathogenic segmented filamentous bacteria to mouse ileal epithelial cells. Infect Immun 1993; 61:4001 - 4004
- Finlay BB, Rosenshine I, Donnenberg MS, Kaper JB. Cytoskeletal composition of attaching and effacing lesions associated with enteropathogenic Escherichia coli adherence to HeLa cells. Infect Immun 1992; 60:2541 - 2543
- Finlay BB, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying salmonella entry into epithelial cells. J Cell Sci 1991; 99:283 - 296
- Reimann HA. Microbic phagocytosis by enteric epithelial cells. Jama 1965; 192:1130 - 1132
- Ferguson DJP, Birch-Andersen A. Electron microscopy of a filamentous, segmented bacterium attached to the small intestine of mice from a laboratory animal colony in Denmark. Acta Pathol Microbiol Scand B 1979; 87:247 - 252
- Angert ER. Alternatives to binary fission in bacteria. Nat Rev Microbiol 2005; 3:214 - 224
- Yamauchi K, Isshiki Y, Zhou ZX, Nakahiro Y. Scanning and transmission electron microscopic observations of bacteria adhering to ileal epithelial cells in growing broiler and White Leghorn chickens. Br Poult Sci 1990; 31:129 - 137
- Stepankova R, Powrie F, Kofronova O, Kozakova H, Hudcovic T, Hrncir T, et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm Bowel Dis 2007; 13:1202 - 1211
- Davis CP, Savage DC. Effect of penicillin on the succession, attachment and morphology of segmented, filamentous microbes in the murine small bowel. Infect Immun 1976; 13:180 - 188
- Fuentes S, Egert M, Jimenez-Valera M, Monteoliva-Sanchez M, Ruiz-Bravo A, Smidt H. A strain of Lactobacillus plantarum affects segmented filamentous bacteria in the intestine of immunosuppressed mice. FEMS Microbiol Ecol 2008; 63:65 - 72
- Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 2010; 11:76 - 83
- Ivanov II, Littman DR. Segmented filamentous bacteria take the stage. Mucosal Immunol 2010; 3:209 - 212