Abstract
The protective effect of Lactobacillus rhamnosus 231 (Lr 231) against potent carcinogen N-Methyl-N'-Nitro-N-Nitrosoguanidine (MNNG) in the rat model is studied. Daily feeding with Lr 231 improved the body weight of male Wistar rats compared with control groups. Fecal azoreductase (p < 0.001) and nitroreductase (p < 0.01) enzyme activity decreased significantly in Lr 231 group in comparison with control groups that received only phosphate buffer or MNNG. Oral administration of MNNG led to a significant increase in Glutathione transferase (GST) while Glutathione reductase (GSH) showed decreased activity. Conversely, feeding Lr 231 showed significantly increased GSH and decreased GST activity in comparison to the MNNG group, emphasizing the protection provided by Lr 231 against MNNG. Histopathological analysis of liver, spleen and colon showed decreased signs of inflammation in the Lr 231 group. The present study highlights that inclusion of active Lr 231 in regular diets could be used to prevent MNNG induced colon carcinoma.
Introduction
Colorectal cancer is multifactorial; and epidemiological, etiological and experimental studies indicate that this disease is associated with the accumulation of relevant genetic changes and DNA damage in the target tissue.Citation1 Colon cancer is the second to third most frequent type of cancer in Western countries. Exposure of genotoxic compounds or carcinogens to the colon and rectum are the primary cause for DNA damage in these tissues that lead to cancer. These carcinogens are of exogenous origin or endogenous origin. Heterocyclic amines or polycyclic aromatic hydrocarbons are exogenous, which are formed due to improper handling of food. The N-nitroso-compounds, fecapentaenes and bile acids are known to form endogenously.Citation2 The awareness of this disease has augmented the increased consumption of fruits, vegetables and food rich in total dietary fiber that tend to reduce the risk of development of colon cancer.Citation3,Citation4 Prebiotics, probiotics, synbiotics might be a feasible chemo-preventive measure against colon cancer in humans, and may present a novel therapeutic or preventive option.Citation5,Citation6 A number of studies have recently emphasized the beneficial therapeutic effects of Lactic acid bacteria (LAB) as a probiotic.Citation7,Citation8 LAB and its metabolites exhibit antimutagenic and anticarcinogenic properties in in vitro and in vivo.Citation9-Citation11 Oral supplementation of L. acidophilus in humans reduces activity of fecal bacterial enzymes such as β-glucuronidase, nitroreductase and azoreductase that activate pro-carcinogens into carcinogen; and facilitate in their excretion through feces and urine.Citation12
MNNG (N-Methyl-N′-nitro-N-nitrosoguanidine), a direct-acting potent carcinogen, causes cancer in all laboratory-tested species such as mouse, rat, hamster, rabbit and dog and is preferred as a representative compound for endogenously formed N-nitroso compounds.Citation13 Diet high in meat could induce a 3-fold increase of endogenous N-nitroso compound production in human gut system.Citation14
L. rhamnosus 231 (Lr 231) exhibits in vitro binding of MNNG resulting in biotransformation and detoxification.Citation15 The present study determines the protective effect of Lr 231 against MNNG in rat model.
Results
Feed intake (FI) and body weight (BW) variations with Lr 231 intake
FI and BW were increased in Lr 231 group compared with MNNG-fed and control group (). The MNNG-fed group showed decreased FI and reduced BW, however, it was statistically insignificant.
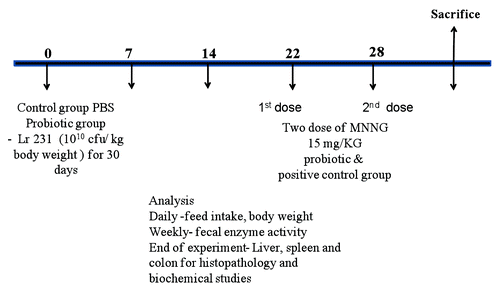
Table 1. Variation in food intake (FI) and body weight (BW) in rat models when treated with only MNNG and Lr 231 group
Fecal azoreductase and fecal nitroreductase assay show variation in the Lr 231 group in comparison to the MNNG-fed group and control groups
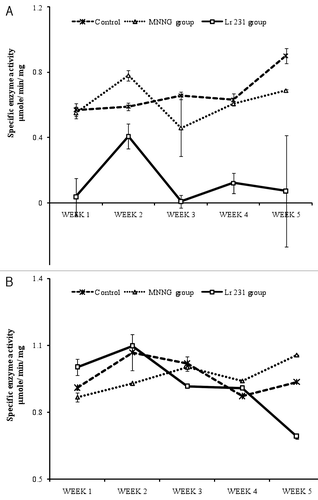
Fecal azoreductase and nitroreductase activities are shown in , respectively. Lr 231 group showed significantly reduced azoreductase (p < 0.001) and nitroreductase activity (p < 0.01) compared with the control groups. The MNNG-fed group showed a marked increase in azoreductase (0.899 μmoles/min/mg and nitroreductase (0.937 μmoles/min/mg) activities on week five in comparsion with azoreductase (0.687 μmoles/min/mg) and nitroreductase (1.057 μmoles/min/mg) of control group.
Figure 1. Effect of Lr 231 on azoreductase and nitroreductase activity in the intestinal microflora of rat models, administered with MNNG on 22nd and 28th day. Azoreductase (A) and nitroreductase (B) activity decreased significantly (p < 0.001 and p < 0.01) in Lr 231 group in comparision to control group. Values are mean ± SE of six different samples.
Antioxidative enzymes assay
Liver, spleen and colon in the MNNG-fed group showed GST activity of 0.14, 0.04 and 0.11 μmole/min/mg, respectively, which was significantly higher than the control group. Feeding Lr 231 decreased GST activity in liver (0.08 μmole/min/mg), spleen (0.01 μmole/min/mg) and colon (0.028 μmole/min/mg). Liver, spleen and colon in the MNNG-fed group showed GSH activity of 6.1, 6.8 and 1.8 μmol/mg, respectively and was significantly lower than the control group having GSH activity as 16.1, 24.6 and 5.1 μmole/min/mg, respectively. Feeding Lr 231 increased GSH activity in liver (11.6 μmole/min/mg), spleen (18.5 μmole/min/mg) and colon (3.3 μmole/min/mg) compared with MNNG group nevertheless it was lesser than control group ().
Table 2. Glutathione transferase and Glutathione reductase activity in rat liver, spleen and colon tissue homogenate at the end of the experiment
Histopathological assay
Histopathology of liver, spleen and colon tissue was performed and severity of the inflammation and change in the shape of cells were evaluated (). Liver and colon sections of the MNNG group showed mild necrosis and inflammation. The Lr 231 group showed reduced inflammation in colon and cells appeared to be normal in liver. Histopathological scoring showed that inflammation in the tissue sections were either reduced or not visible in the Lr 231 group ().
Figure 2. Histopathological sections showing necrosis and inflammation in (1) Liver (2) spleen and (3) colon cells with different groups (a) Control, (b) MNNG-fed and (c) Lr 231.
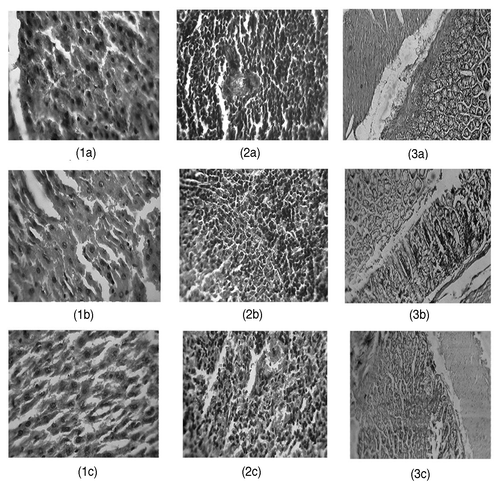
Table 3. Histopathological scoring and changes observed in the tissue sections of liver, spleen and colon
Discussion
Colon cancer and other forms of cancer are diet-dependent and dose-dependent.Citation3,Citation16 Meat and dietary fat act as the primary risk factors. Cancer induction is a complex multi-step process caused by the accumulation of mutagens and induction of the mutation of tumor suppressor genes.Citation3 Lactobacilli can influence several mechanisms possibly linked to carcinogenesis such as preventing mutations, binding mutagens, inhibiting bacterial enzymes that coverts procarcinogens to carcinogens in the colon, decreasing pH in the colon lumen or activating the immune system.Citation9
In this study we report the protective role of Lr 231 against potent mutagen MNNG in the male Wister rat model. Potential probiotic human strain Lr 231 was isolated from healthy human feces. It possesses antimicrobial activity against several human pathogens and food spoilage organisms and it is partially attributed to low molecular weight glycopeptide.Citation17 Lr 231 exhibits in vitro binding and antimutagenic activity against potent mutagens AO, MNNG and MeIQx.Citation17
Lr 231 had positive influence on the rats’ appetite, even though they were fed with MNNG on day 22 and 28. In control and Lr 231 group, increase in body weight and feed intake showed a similar fashion. In MNNG-fed group body weight increased and feed intake was comparatively lesser than other groups. Even though, the feed intake rate was lesser in Lr 231 group after third week, the body weight did not change indicating no significant adverse effects of Lr 231 on feed intake and body weight.
Azoreductase and nitroreductase are potential mediators of colon carcinogenesis. These enzymes are involved in the conversion of pro-carcinogens to carcinogens that increases risk of colon cancer.Citation18,Citation19 Gut microflora consisting of both harmful and beneficial bacteria differ in their enzyme activity. Reduction in fecal azoreducatase and nitroreducatase activity in Lr 231 group in comparison to control and MNNG group indicates that continuous feeding of Lr 231 for four weeks protected rats against MNNG (). This could be due to the low fecal enzyme activity of lactobacilli involved in the conversion of promutagens to mutagens and carcinogens.Citation20 Nitroreductase activity remained similar in the MNNG group and control group while in Lr 231 group, it reduced significantly at the end of the experiment (). Lr 231 exhibit in vitro antibacterial activity against pathogenic bacteria.Citation17 This property of Lr 231 might positively influence the gastrointestinal microflora, thereby, reducing their fecal enzymatic activity thereby protecting against MNNG. Rats fed with L. acidophilus showed reduced β-glucuronidase, nitroreductase and azoreductase activity.Citation19 In another study, nitroreductase activity decreased by 30–40% in LAB treated groups in comparison with azoxymethane alone in rat model.Citation21
Antioxidant plays an important role in preventing the cells from oxidative damage. GSTs are important against oxidative stress and GSH are important cellular antioxidants. Lr 231 group showed a similar trend for GST and GSH activity, as that of control, whereas MNNG-fed groups had high GST and low GSH activity (). This improved GST and GSH activity in Lr 231 groups indicate the protective effect of Lr 231 against MNNG. Enzyme GST helps in sequestering carcinogens.Citation22 They catalyze the conjugation of glutathione with mutagens containing electrophilic centers. It is important to deal with active electrophiles since they can react with macromolecules controlling cell growth such as DNA, RNA and proteins. Thus, GST plays an important role in detoxifying strong electrophiles having toxic, mutagenic and carcinogenic properties. Lr 231 group showed marked decrease in specific enzyme activity of GST compared with MNNG-fed group. GST activity was high in liver compared with spleen and colon indicating elevated detoxification activity in this organs.Citation22 The activity of GSH is used as indicator for oxidative stress and it is higher when ratio of GSSG to GSH is increased.Citation23 This ratio in Lr 231 group was lesser compared with the MNNG group showing lesser oxidative stress implying that feeding of Lr 231 have a protective effect against MNNG. GSH activity was higher in Lr 231 fed group compared with MNNG probably because Lr 231 bound to mutagen and made it less available. Lr 231 exhibits in vivo antioxidative activity and thereby preventing against oxidative epithelial damage caused by MNNG.
Histopathological sections of liver, spleen and colon show the severity of MNNG as a potent carcinogen. MNNG is the prime cause of colon carcinoma in many animals including rats.Citation13 It is evident in the current study that even at low doses, MNNG causes inflammation in the tissue sections. In MNNG group, no perivascular infiltration is observed indicating that necrosis and inflammation was solely induced by MNNG. In Lr 231 group this inflammation was reduced ().
The protective behavior of lactobacilli is dependent on its survival rate through the intestinal tract, multiplication and exerting probiotic effect in the colon. This confers the additional advantage of Lr 231 as probiotics or food adjuncts in preventing cancer. Previous studies reported that in vitro binding of MNNG of Lr 231 led to biotransformation and subsequent detoxification.Citation15 Protection received by Lr 231 could be mainly due to MNNG binding and detoxification, reduced fecal enzymes and antioxidative activity.
This work briefly describes the probiotic human strain Lr 231 possesses protective effect against potent colon carcinogen, MNNG. Feeding Lr 231 improved body weight in rats. Reduced fecal azoreductase and nitroreductase activity in Lr 231 group is an indirect evidence for the protective role of Lr 231 against MNNG. Histopathological study of liver, spleen and colon and reduced oxidative stress emphasize the protective effect of Lr 231. In vivo studies specify the significant role of Lr 231 against MNNG induced carcinoma; and suggests that addition of these in the diet will facilitate prevention against cancer. Additionally, determination of immunomodulatory properties of Lr 231 that help in preventing inflammatory lesion development by mutagen is required. Such studies will give us a better understanding and more in-depth knowledge concerning the total deactivating capacities of these important beneficial bacteria. Detail studies to determine mode of action of Lr 231 against MNNG is in progress.
Materials and Methods
Chemicals
MNNG, Sigma-Aldrich, St. Louis, Mo, USA was dissolved in di-methyl sulphoxide (DMSO). DMSO, 1-cholo-2, 4-dinitrobenzene (CDNB) and 5′-dithiobis-2-nitrobenzoic acid (DTNB) were purchased from SRL, India. Glutathione, MRS broth, M-nitro benzoic acid and EDTA were purchased from Himedia, India.
Bacterial strain
Potential probiotic human strain Lr 231 was isolated from feces of healthy human subjects.Citation17 Stock cultures for Lr 231 were preserved in 10% skimmed milk at 4°C until further used for the experiment. Actively growing Lr 231 (2ml) were inoculated in MRS broth (100ml) and incubated at 37°C for 24 h. The cells were harvested by centrifugation at 5000 rpm for 15 min at 4°C and washed twice with PBS (pH 7, 0.1 M) and re-suspended in phosphate buffer (pH 7, 0.1 M) with optical density of 1.0 at 600 nm. The viability of the culture was determined by serial dilution method.
Study animals
Male Wistar rats (6–9 week old, 160–270 g) were maintained in an air conditioned room (22 ± 2°C, RH 55 ± 5%) approved by Institutional Animal Ethics Committee (CPCSEA, SU/DPS/IAEC/9013) and exposed to equal hours of light and dark cycle, fed with water and normal chow ad libitum.
Experimental design
Experimental design adopted for present study is shown in . Animals were divided into three groups (n = 6). Positive and negative controls received PBS daily. Lr 231 group were fed intragastrically with fresh Lr 231 (1010 cfu/ kg body weight). On 21st and 28th day, MNNG (15 mg/ kg) was given intragastrically to positive control and Lr 231 groups, respectivelyCitation24 and daily food intake and body weights were measured. Fresh fecal samples were collected weekly to determine the fecal azoreductase and nitroreductase enzyme activities. On 30th day, rats were sacrificed using chloroform as anesthetic agent; liver, spleen and colon were removed and processed for biochemical and histological studies.
Fecal azoreductase and nitroreductase assay
Fecal samples collected from each group were prepared in 5% phosphate buffer (pH 6.8). Homogenized samples were centrifuged (5,000 rpm × 15 min, 4°C) and filtered using 0.45 μm millipore filter; and were used for azoreductase and nitroreductase assay.Citation25
Azoreductase assay
Fecal samples (3 ml) were homogenized in 0.1 ml of amaranth (7.75 mM), warmed (37°C) and incubated at 37°C for 1 h. An aliquot (0.3 ml) made up to 3 ml in 1 mM sodium azide solution, was centrifuged (12,000 rpm × 15 min, 4°C) using (Remi) and absorbance (A521) of supernatant was measured using UV-Visible spectrophotometer (Shimadzu). Phosphate buffer (0.1M) was used as blank. Concentrations of the residual substrate were calculated using standard curve of amaranth, and specific enzyme activity was determined.
Nitroreductase assay
Fecal sample diluted (1:5) in 1.5 mM M-nitro benzoic acid (pH 6.8) were incubated anaerobically at 37°C for 1 h, using vacuum desiccator (Polylab) containing sodium thiosulphate as oxygen scavenger and anhydrous calcium sulfate was used as desiccant. Enzyme activity was arrested using 1.2 N HCl, centrifuged (5,000 rpm, 15 min, 4°C) and absorbance (A266) of supernatant was measured. Phosphate buffer was used as blank.
Histopathology
Liver, spleen and colon were washed in 10% buffered formalin and fixed in Bouin’s fixative. Tissues were then subjected to serial dilutions of alcohol (10–100%) and alcohol-xylene mix; and thereafter, were embedded in paraffin blocks and sectioned for histopathological study. Eosin yellow was used for staining and the sections were photographed. Histopathological sections were given scores according to the degree of inflammation by a blinded pathologist.Citation26
Antioxidative enzymes activity
Liver, spleen and colon were washed and homogenized (10%) using chilled normal saline and evaluated for glutathione transferase and glutathione reductase activity. All analysis was performed at 4°C.
Glutathione transferase activity (GST)
GSH transferase activity was measured spectrophotometrically.Citation27 The reaction mixture (3 ml) containing 1 ml phosphate buffer (0.1 M, pH 7), 0.1 ml of 1-cholro-2, 4-dinitrobenzene (CDNB) (30 mM) and 1.7 ml distilled water were pre-incubated (37°C, 5 min). The reaction was initiated by adding 0.1 ml of tissue homogenate and 0.1 ml glutathione (5mM) as substrate. Absorbance was determined at 340 nm at 0 and 5 min. CDNB was omitted from the control tube. The reaction mixture without enzyme was used as blank. The activity of GST is expressed as μmoles of GSH-CDNB conjugate formed/min/mg protein using extinction coefficient of 9.6/mM/cm.
Glutathione reductase activity
Reaction mixture containing 200 mM phosphate buffer (pH 7) and 2 mM EDTA (pH 9), were homogenized with tissue and reaction was initiated by the adding 10 mM DTNB in phosphate buffer (pH 7). The sulphydryl groups available in glutathione formed colored complex with DTNB that was measured instantaneously using colorimeter at 412 nm. The extinction coefficient of 1.36 × 10−7/mM/cm was used for calculation and results were expressed as μmol of GSH/ min/ mg of protein.Citation28,Citation29
Protein estimation
Total protein in fecal sample and homogenized liver, spleen and colon was determined.Citation30
Statistical analysis
All the results obtained were statistically analyzed and were expressed as means ± SD (n as indicated). Comparisons were done with the statistical software package (SPSS 7.0). Differences in treatments were evaluated by one-way ANOVA followed by a Student’s t-test; and the differences were considered significant.
Acknowledgments
The authors gratefully acknowledge Dr. H.V. Oza, Head and Professor of Department of Pathology, Dr. Grishma Jobanputra, P.D.O. Medical College, Rajkot and Dr. Arpita P. Rathod, Consultant pathologist and microbiologist at B.T. Savani Kidney Hospital, Rajkot, for their sincere support and invaluable assistance in facilitating the histopathological studies. Sincere regards to Dr. Ramadasan Kuttan, Amala Cancer Research Center, Kerala, India for providing MNNG, and Dr. Kanthi Kiran Kondepudi, Labmedicin, Klinisk Mikrobiologi, Division of Bacteriology, Lund University for the critical corrections of the MS.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Fearon ER, Vogelstein BA. Genetic model for colorectal tumorigenesis. Cell 1990; 61:759 - 67; http://dx.doi.org/10.1016/0092-8674(90)90186-I; PMID: 2188735
- de Kok TM, van Maanen JM. Evaluation of fecal mutagenicity and colorectal cancer risk. Mutat Res 2000; 463:53 - 101; http://dx.doi.org/10.1016/S1383-5742(00)00003-X; PMID: 10838209
- Ferguson LR, Philpott M, Karunasinghe N. Dietary cancer and prevention using antimutagens. Toxicology 2004; 198:147 - 59; http://dx.doi.org/10.1016/j.tox.2004.01.035; PMID: 15138038
- Rafter J, Bennett M, Caderni G, Clune Y, Hughes R, Karlsson PC, et al. Dietary synbiotics reduce cancer risk factors in polypectomized and colon cancer patients. Am J Clin Nutr 2007; 85:488 - 96; PMID: 17284748
- Rauch M, Lynch SV. Probiotics manipulation of the gastrointestinal microbiota. Gut Microbes 2010; 1:335 - 8; http://dx.doi.org/10.4161/gmic.1.5.13169; PMID: 21327043
- De Preter V, Hamer HM, Windey K, Verbeke K. The impact of pre- and/or probiotics on human colonic metabolism: Does it affect human health?. Mol Nutr Food Res 2011; 55:46 - 57; http://dx.doi.org/10.1002/mnfr.201000451; PMID: 21207512
- Ljungh Ǻ, Wadström T. Lactic acid bacteria as probiotics. Curr Issues Intest Microbiol 2006; 7:73 - 89; PMID: 16875422
- O'Flaherty S, Saulnier DM, Pot B, Versalovic J. How can probiotics and prebiotics impact mucosal immunity?. Gut Microbes 2010; 1:293 - 300; http://dx.doi.org/10.4161/gmic.1.5.12924; PMID: 21327037
- Commane D, Hughes R, Shortt C, Rowland I. The potential mechanisms involved in the anti-carcinogenic action of probiotics. Mutat Res 2005; 591:276 - 89; http://dx.doi.org/10.1016/j.mrfmmm.2005.02.027; PMID: 16095630
- Liong MT. Roles of probiotics and prebiotics in colon cancer prevention: Postulated mechanisms and in-vivo evidence. Int J Mol Sci 2008; 9:854 - 63; http://dx.doi.org/10.3390/ijms9050854; PMID: 19325789
- Vorobjeva LI, Abilev SK. Antimutagenic properties of bacteria. [review] Appl Biochem Microbiol 2002; 38:115 - 27; http://dx.doi.org/10.1023/A:1014338712108
- Lidbeck A, Nord CE, Gustafson JA, Rafter J. Effect of Lactobacillus acidophilus supplements on mutagen excretion in feces and urine in humans Lactobacilli, anticarcinogenic activities and human intestinal microflora. Eur J Cancer Prev 1992; 1:341 - 53; http://dx.doi.org/10.1097/00008469-199208000-00002; PMID: 1463986
- IARC Monographs. 1974; 4:183-95
- Bingham SA, Pignatelli B, Pollock JR, Ellul A, Malaveille C, Gross G, et al. Does increased endogenous formation of N-nitroso compounds in the human colon explain the association between red meat and colon cancer?. Carcinogenesis 1996; 17:515 - 23; http://dx.doi.org/10.1093/carcin/17.3.515; PMID: 8631138
- Ambalam P, Dave JM, Nair BM, Vyas BR. In vitro mutagen binding and antimutagenic activity of human Lactobacillus rhamnosus 231. Anaerobe 2011; 17:217 - 22; http://dx.doi.org/10.1016/j.anaerobe.2011.07.001; PMID: 21777684
- Levi F, La Vecchia C, Lucchini F, Te VC, Franceschi S. Mortality from Hodgkin's disease and other lymphomas in Europe, 1960-1990. Oncology 1995; 52:93 - 6; http://dx.doi.org/10.1159/000227437; PMID: 7854782
- Ambalam PS, Prajapati JB, Dave JM, Nair BM, Ljungh Å, Vyas BRM. Isolation and characterization of antimicrobial proteins produced by a potential probiotic strain of human Lactobacillus rhamnosus 231 and its effect on selected human pathogens and food spoilage organisms. Microb Ecol Health Dis 2009; 21:211 - 20; http://dx.doi.org/10.3109/08910600903429052
- Gorbach SL, Goldin BR. The intestinal microflora and the colon cancer connection. Rev Infect Dis 1990; 12:S252 - 61; http://dx.doi.org/10.1093/clinids/12.Supplement_2.S252; PMID: 2154844
- Goldin BR, Gorbach SL. Alteration of the intestinal microflora by diet, oral antibiotics and Lactobacillus: decreased production of free amines from aromatic nitro compounds, azo dyes and glucoronides. J Natl Cancer Inst 1984; 73:689 - 95; PMID: 6433097
- Pool-Zobel BL, Bertram B, Knoll M, Lambertz R, Neudecker C, Schillinger U, et al. Antigenotoxic properties of lactic acid bacteria in vivo in the gastrointestinal tract of rats. Nutr Cancer 1993; 20:271 - 81; http://dx.doi.org/10.1080/01635589309514295; PMID: 8108276
- Lee SM, Lee WK. Effect of lactic acid bacteria on intestinal microbial enzyme activity and composition in rats treated with azoxymethane. J Microbiol 2001; 39:154 - 61
- Sherratt JP, Hayes JD. Glutathione S-transferase. In: Ioannides C, ed. Anderson D, Waters MD, Timothy CM, Series eds. Enzyme Systems that Metabolize Drugs and Other Xenobiotics. New York: John Wiley & Sons, Ltd, 2002: 319-352.
- Kumaresan KR, Springhorn SS, Lacks SA. Lethal and mutagenic actions of N-methyl-N’-nitro-N-nitrosoguanidine potentiated by oxidized glutathione, a seemingly harmless substance in the cellular environment. J Bacteriol 1995; 177:3641 - 6; PMID: 7601826
- Wollowski I, Ji ST, Bakalinsky AT, Neudecker C, Pool-Zobel BL. Bacteria used for the production of yogurt inactivate carcinogen and prevent DNA damage in the colon of rats. J Nutr 1999; 129:77 - 82; PMID: 9915879
- Carman RJ, Woodburn MA. Azoreductase and nitroreductase in human feces: Comparison of methods to measure specific activities in the absence of oxygen. Microb Ecol Health Dis 1999; 11:208 - 10; http://dx.doi.org/10.1080/08910609908540829
- Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22:696 - 9; http://dx.doi.org/10.1016/0168-8278(95)80226-6; PMID: 7560864
- Habig WH, Pabst MJ, Jakoby WB. Glutathione-S-transferase: the first enzymatic step in mercapturic acid formation. J Biol Chem 1974; 249:7130 - 9; PMID: 4436300
- Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys 1959; 82:70 - 7; http://dx.doi.org/10.1016/0003-9861(59)90090-6; PMID: 13650640
- Smith IK, Vierheller TL, Thorne CA. Assay of glutathione reductase in crude tissue homogenates using 5, 5′-dithiobis (2-nitrobenzoic acid). Anal Biochem 1988; 175:408 - 13; http://dx.doi.org/10.1016/0003-2697(88)90564-7; PMID: 3239770
- Lowry OH, Rosebrough NF, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem 1951; 193:265 - 75; PMID: 14907713