Abstract
The Gram-negative, non-sporulating, obligately anaerobic species, Fusobacterium nucleatum, is rapidly gaining notoriety as a pathogen with a surprising number of associated diseases. Recently, we have found that F. nucleatum is a more common resident of the GI tract than originally thought, and thus, through several studies, we have attempted to determine its gut-relevant potential for virulence. We have found that F. nucleatum possesses a number of pathogenic traits with relevance to gut diseases such as inflammatory bowel disease (IBD), however, we have also documented strain-associated differences in virulence. An intriguing picture emerges that paints F. nucleatum as both conferring beneficial as well as detrimental effects on host cells; and we suggest that the ultimate effects of F. nucleatum infection in the gut are a consequence of the microbes with which this species aggregates.
A Heterogeneous Species
F. nucleatum is a Gram negative, non-spore forming, strictly anaerobic species of the Fusobacteriaceae family, which currently consists of 9 genera, including Fusobacterium and Leptotrichia. Within the Fusobacterium genus there are currently 14 species described, several of which (including F. nucleatum) are known pathogens of man and animals.Citation1F. nucleatum is perhaps best known for its role as a component of oral plaque, where by virtue of its adhesive abilities it serves as a bridge organism between early and late colonizers of this biofilm.Citation2
F. nucleatum is a highly heterogeneous species and has been the subject of various schemes attempting to classify strains into subspecies or other groupings.Citation3-Citation6 Currently, five subspecies are recognized; animalis, fusiforme, vincentii, polymorphum and nucleatum, although fusiforme is contested; phylogenetic and other groupings place it within the vincentii clade.Citation3,Citation7 Phenotypically, however, classification of the F. nucleatum species is far from straightforward; antigenic determinants appear to be extremely heterogeneous even within a subspecies and even cell and colony morphology differs widely between genetically similar strains.Citation3,Citation5,Citation8
F. nucleatum as a Pathogen of Man
F. nucleatum is associated with many conditions and diseases. It has been isolated from numerous inflammatory processes, including sinusitis, endocarditis, septic arthritis, tonsillitis and abscesses of the brain, skin and liver.Citation9-Citation12 It has also been found in lung and urinary tract infections and has been recently shown to invade the amniotic membrane during pregnancy, leading to preterm labor and stillbirth.Citation13-Citation15 Most of the research on F. nucleatum has focused on its role as one of the principal pathogens in gingivitis and periodontitis.Citation16 It has been demonstrated that F. nucleatum is invasive and pro-inflammatory in human oral epithelial cells, eliciting secretion of the pro-inflammatory chemokine IL-8.Citation17
F. nucleatum in the Gut
Until recently, F. nucleatum was thought to primarily be a component of the oral microbiota of humans and only an occasional resident of the gut. However, this premise was built on culture-based examination of stool, which usually does not contain high numbers of live, epithelium-associated bacteria. FISH was used to elegantly demonstrate an association between invasive Fusobacterium spp (including F. nucleatum) cells with inflamed appendix tissues, suggesting that the gut could be a hitherto unrecognized niche for this pathogen.Citation18 By virtue of its adhesive and aggregative qualities, we suspected that F. nucleatum may be an overlooked member of the gut microbiota associated specifically with the mucosa and in this respect its invasive and pro-inflammatory characteristics might contribute to inflammatory bowel disease (IBD) etiology. Thus, we initiated a culture-based survey of gut biopsy specimens taken from IBD patients and healthy (colon cancer screen) controls; this strategy had the benefit that recovered isolates could be characterized more deeply than a simple metagenomic survey would allow. From a cohort of 59 patients, we recovered a total of 26 Fusobacterium spp isolates of which 18 were speciated as F. nucleatum. Sub speciation of the isolates showed that the majority of isolates from these gut specimens identified with the animalis clade, which was previously thought to be only a rare inhabitant of humans.Citation8
Intriguingly, we found that F. nucleatum was recovered more frequently from patients with Crohn disease than from healthy individuals (50% of the total F. nucleatum isolates were from diseased patients whereas 17.6% of the isolates were from healthy controls). All of the disease-associated F. nucleatum isolates were recovered from Crohn disease patients, however the study cohort contained only a few patients with ulcerative colitis (n = 4) so it is difficult to draw conclusions about a possible association of F. nucleatum with this latter form of IBD. We postulated that the IBD-associated strains might have pathogenic tendencies and we assayed strains from both IBD and control patients for invasion capabilities in a Caco-2 cell-line model. A clear pattern emerged from these studies correlating invasion with IBD status of the host from which the strain was recovered; the most invasive strains were invariably those that were isolated from patients with Crohn disease and active inflammation at the time of biopsy recovery, whereas strains isolated from healthy controls were minimally invasive at best. That F. nucleatum strains differ in their pathogenic tendencies has been demonstrated before,Citation19 although the reasons behind these differences are unclear. A total of 11 of the F. nucleatum isolates that we recovered during the course of our study have been subjected to genome sequencing through the Human Microbiome ProjectCitation20 and the resulting genome sequences (found at www.broadinstitute.org/annotation/genome/fusobacterium_group/GenomesIndex.html) are currently undergoing comparative analysis in the hope that invasion-associated loci might be identified.
Link Between Mouth and Gut in IBD
The association of F. nucleatum with IBD is intriguing in light of this pathogen’s well-characterized role in oral inflammatory diseases, given that there exists a potential link between IBD and periodontal disease (PD). The pathogenesis of PD and IBD share some key similarities. Both PD and IBD display hypersensitivity against commensal bacteria and much like in IBD, antibodies against various bacterial plaque components are present in the serum of patients with PD.Citation21 Additionally, extra intestinal manifestations of IBD include oral ulcers and mucogingivitis and those with active vs. inactive CD have an increase in the number of plaque retention sites, gingival bleeding and oral mucosal inflammation.Citation22-Citation24 It is also interesting to note that F. nucleatum comprises a greater percentage of the oral flora in children and young adults with PD than adults with PD or healthy individuals, particularly since it is often in the teenage years that IBD patients first begin to develop signs and symptoms and are diagnosed.Citation25 Additionally, CD patients with oral CD are significantly more likely to have perianal disease compared with CD patients without oral involvement.Citation22 These observations might indicate that the presence of certain F. nucleatum strains contribute to both the intestinal and oral manifestations of IBD and thus the presence of such strains in the gut may be predictive of disease phenotype.
F. nucleatum Effects on Host Cell Physiology
Since some strains of F. nucleatum appear to be invasive and the microorganism is associated with several diseases for which inflammation is a hallmark, the question arises as to the consequences of host-pathogen interactions on host cells. In our laboratories, we have focused on the effects of F. nucleatum colonization and invasion on MUC2 mucin production, as mucin forms a central component of the innate mucosal barrier, disruption of which can lead to exposure of underlying epithelial cells to microbial antigenic determinants which could initiate an inflammatory response. We infected a high-mucin-producing human colonic cell line with several isolates derived from IBD patients and healthy controls and observed that F. nucleatum isolates which displayed a strong invasion phenotype also promoted a dose-dependent increase in mucin secretion, whereas a less invasive isolate from healthy tissues had no significant effect in this assay.
We observed variable kinetics of mucin secretion as a result of infection by different strains which corresponded to the kinetics of invasion for these strains and since neither heat-killed cells nor bacterium-free culture supernatants had any significant effect on mucin secretion, we conclude that mucin secretion seen in response to F. nucleatum association is governed in some way by invasion. Further experiments in vivo using a rat ligated colonic loop model confirmed our in vitro findings and allowed histological visualization of robust mucus secretion from goblet cells with formation of mucus plugs in the lumen, in loops inoculated with the more invasive F. nucleatum strains. We also examined the effects of F. nucleatum strain infection on host cell gene expression both in vitro (LS 174T cells) and in vivo (rat colonic cells), targeting MUC1, MUC2 and MUC3 as markers of mucin gene expression, genes for TFF-3 and RELMß as markers of goblet cell mediators, as well as genes for pro-inflammatory cytokines TNFα and IL1β. We observed significant correlations between infection with highly invasive isolates and concomitant upregulation of MUC2 and TNFα gene expression, as well as variable ability among these invasive isolates to upregulate MUC1 gene expression. In contrast, none of the tested F. nucleatum strains elicited an IL-1β gene expression response in vivo, (although a marginal response was seen in vitro) and to our surprise, RELM-β and TFF expression were also unaffected by F. nucleatum infection, suggesting that MUC2 expression could be decoupled from these key mucin gene regulators. From these studies we concluded that a subset of F. nucleatum strains, which display a more invasive phenotype for colonic epithelial cells, have the potential to interfere with a critical component of the host cell mucosal barrier. Although the consequences of this disturbance have not yet been studied, it is possible that such perturbation could be a key factor in a subsequent inflammatory response.
In the oral niche, studies of F. nucleatum interactions with host cells have led to a confusing picture. On the one hand, F. nucleatum is regarded as a commensal organism, with attributes including stimulation (priming) of elements of the innate immune response, such as human β-defensins, allowing for appropriate immune surveillance of a mucosal surface that is intimately associated with potential pathogens.Citation26,Citation27 Additionally, cell wall extracts of F. nucleatum strongly upregulate host protease inhibitors, which can both act to dampen down the tissue damage caused by neutrophil protease release during oral infections, as well as potentially interfere with the virulence-associated protease activities of various oral pathogens such as Porphyromonas gingivalis, Treponema denticola and Tannerella forsythensis (reviewed by Signat et al.Citation28). On the other hand, F. nucleatum was found to enhance the adherence as well as invasion of P. gingivalis to human oral cells.Citation29,Citation30 F. nucleatum is also well known in its capacity to stimulate secretion of IL-8 as well as several other pro-inflammatory cytokines,Citation17,Citation31 to induce cell death in human lymphocytesCitation32 and to also promote release of matrix metalloproteinases, MMP-2, MMP-9 and MMP-13.Citation27 Promotion of MMP-9 release was found to occur through bacterial binding of the complement regulatory protein CD46,Citation33 a protein that serves as an important receptor for several notorious pathogens, including Streptococcus pyogenes and pathogenic Neisseria sp.Citation34 Thus, with such disparate roles, it is unclear to what extent F. nucleatum colonization (which is ubiquitous in the oral setting) influences pathogenesis in periodontal disease.
F. nucleatum Invasion and Subsequent Fate
F. nucleatum’s ability to invade many types of host cells suggests pathogenic intentions in line with many other invasive bacterial species. The process of invasion is remarkable from the point of view that F. nucleatum cells are long and thin, yet we and others have observed host cell entry via a zipper mechanism whereby cells enter pole firstCitation8,Citation17; this in turn suggests that the bacterial adhesins involved are differentially displayed on the bacterial surface. A novel adhesin, FadA, has been associated with both attachment and invasion of host cells and is unusual in that it appears to exist in two separate forms that together mediate functional oligomerization and host cell attachment and invasion.Citation35
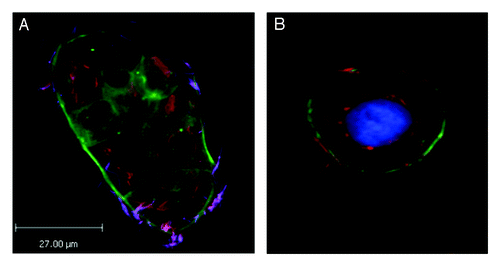
Once inside the host cell, F. nucleatum resides at least for the short-term in a membrane-bound vacuole and appears to proliferate. Invasion and subsequent survival in HaKaT (human skin) keratinocytes by Fusobacterium spp. including F. nucleatum was dependent on the host cell actin cytoskeleton but not microtubules.Citation36 Other studies have shown a dependence on the actin cytoskeleton for bacterial invasion for human gingival epithelial cells.Citation17 Viable bacterial cells can be recovered from infected host cells, including colonic cell lines, after 24 h or moreCitation36 (and unpublished observations), which is remarkable considering that the bacteria are obligate anaerobes; it is likely that the bacteria upregulate genes involved in aero tolerance in response to certain cues taken from the infective process.Citation37 Bacterial survival within host cells may be dependent on the cell line and/or F. nucleatum strain studied as some studiesCitation38 were unable to recover viable F. nucleatum from cultured human gingival epithelial cells beyond 12 h. In our studies using immunofluorescence microscopy and Caco-2 cells we found that the highly invasive F. nucleatum strains achieved maximal invasion by 4 h post infection and are located close to the sites of invasion at the periphery of the host cells. By 24 h post-infection, most of the bacteria are found localized in the peri-nuclear region (), although the reasons for this are unclear and require further investigation. Host cells that are infected with minimally invasive strains (isolated from healthy individuals) do not traffic bacteria to the peri-nuclear region and bacterial cells cannot be easily detected in host cells by 24 h, suggesting that they have been degraded via the normal endocytosis pathway. Intriguingly, invasion by F. nucleatum does not seem to have any cytopathic effects on host cells of all types thus far studied over the time period of observation (up to 48 h) (see refs Citation36 and Citation38; unpublished observations).
Figure 1. Immunofluorescence micrographs showing images of Caco-2 cells infected with F. nucleatum strain EAVG_002 for 24 h, showing localization of bacteria in the perinuclear region. Strain EAVG_002 is an example of a highly invasive strain isolated from an inflamed intestinal biopsy taken from an IBD patient and has been extensively characterized.Citation3,Citation8,Citation47 (A) This merged image shows differentially stained bacteria (see ref. Citation8 for differential staining method used) allowing clear delineation between bacteria that have invaded and are internalized within the imaged group of host cells and bacteria that remain outside of these host cells; actin is labeled with Phalloidin 488 (green), internalized bacteria are orange (Cy3) and bacteria external to the cells appear purple (Cy3 + Alexa 350). (B) Merged image showing detail of a representative host cell from sample taken from same experimental set as for (A) except that for labeling purposes this time differential staining was not done and instead samples were stained with DAPI (blue) and Phalloidin 488 (green) to reveal the host cell nuclei and actin cytoskeleton respectively and all bacteria were stained orange using Cy3.
F. nucleatum Overrepresentation in Colon Cancer
The role of infectious agents in cancer etiology is increasingly recognized with an estimated 15% of the world’s burden of cancer attributable to known bacterial and viral pathogens.Citation39 Recently, two independent studies using metagenomics to probe the prevalence of potential infectious agents associated with colorectal cancer have been performed; total DNA and RNA were extracted from colorectal cancer tumors and matched normal tissues and subjected to high-throughput sequencing following which sequence reads from the human genome were removed. Using this strategy and with the increasing availability of microbial genome catalogs, the remaining sequencing reads could be identified. In both studies, a marked over-representation of F. nucleatum sequences in tumors relative to control specimens was seen and confirmed by quantitative PCR in further matched tumor pairs.Citation40,Citation41 In one study, F. nucleatum presence was confirmed in situ in tumor tissue,Citation41 whereas in the other study, a F. nucleatum strain was recovered from a frozen biopsy sample and subsequently found to be invasive in Caco-2 cellsCitation40; together both studies indicate that live, invasive F. nucleatum cells are associated with colorectal cancer. Whether this association is involved in colon carcinoma pathology, or simply the result of F. nucleatum exploitation of an ecological niche created as a result of the cancer tumor microenvironment, remains to be tested.
F. nucleatum: Friend or Foe?
F. nucleatum is a very interesting bacterial species that shows associations with a growing number of human diseases, but yet can also be isolated from healthy subjects. As indicated above, there are many examples of F. nucleatum’s incongruent behavior during interactions with host cells. Specifically in the gut, even the consequences of the basic physiology of the microorganism can be read in two ways: F. nucleatum produces considerable amounts of both butyrate and hydrogen sulfide, the former being a recognized energy source for colonocytes with known anti-inflammatory effectsCitation42 and the latter being the highly toxic end product of cystein metabolism, which can act to inhibit effective use of butyrate by colonocytes.Citation43 So, should F. nucleatum be considered a pathogen or not? Our studies show that all F. nucleatum strains are not equal in their virulence potential and the genetic basis for this is currently a focus for our research. However, this may not be the whole story. Many of the studies done to examine the invasive and pro-inflammatory properties of F. nucleatum are performed as single species infection assays with tissue culture cells, however F. nucleatum is naturally co-aggregative and would likely exist in the human gut microbiota as a feature of a larger microbial grouping.
In the oral niche, F. nucleatum displays a degree of synergism with other bacterial species. F. nucleatum co-aggregates with nearly all bacterial species involved in oral plaque formation, including Streptococcus gordonii, Veillonella parvula, Prevotella intermedia and Porphyromonas gingivalis among others and thus acts as a ‘bridge organism’ between early and late colonizers in plaque.Citation2 Co-aggregation between S. gordonii and Actinomyces naeslundii, which are both components of dental plaque, results in increased expression of genes involved in arginine biosynthesis and pyruvate metabolism in S. gordonii, which may provide a survival advantage in nutrient limiting environments.Citation44 Thus, co-aggregation may be an important tool used by commensal bacteria with pathogenic tendencies. The ability to adhere to other bacterial species could also enable gene transfer. Thus, if this synergism also exists in the gut, it is possible that some F. nucleatum strains may have acquired genes through horizontal transfer, conferring increased virulence potential. These strains could prove to be more prevalent in IBD patients or more pathogenic in genetically susceptible hosts.
It has been demonstrated that F. nucleatum, by virtue of its impressive adhesive properties, can bind to and transport otherwise non-invasive bacterial species into host cells, acting as a shuttle in this respect (see ref. Citation45; unpublished observations). If this is the case, then the involvement of F. nucleatum in disease could be a function of the microbes to which it adheres (perhaps coupled with its apparent ability to disturb the protective mucus layer of the host epithelium), rather than a direct result of its own virulence determinants. Indeed, associations of F. nucleatum with the oral commensal, Streptococcus cristatus, attenuate virulence of the former in both KB and OKF6/TERT-2 cell models of infection.Citation46 Whatever the case, the growing number of gastrointestinal diseases with which F. nucleatum seems to be associated can only mean that this enigmatic microbe is worthy of further study in the context of the gut.
Acknowledgments
The study was supported by a grant from the Canadian Institute for Health Research (CIHR)(K.C) and a Grant in Aid of Research from the Crohn’s and Colitis Foundation of Canada (E.A.-V.). J.S was funded by a CIHR doctoral scholarship award.
References
- Citron DM. Update on the taxonomy and clinical aspects of the genus Fusobacterium.. Clin Infect Dis 2002; 35:S22 - 7; http://dx.doi.org/10.1086/341916; PMID: 12173104
- Kolenbrander PE, Palmer RJ Jr., Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontol 2000 2006; 42:47 - 79; http://dx.doi.org/10.1111/j.1600-0757.2006.00187.x; PMID: 16930306
- Strauss J, White A, Ambrose C, McDonald J, Allen-Vercoe E. Phenotypic and genotypic analyses of clinical Fusobacterium nucleatum and Fusobacterium periodonticum isolates from the human gut. Anaerobe 2008; 14:301 - 9; http://dx.doi.org/10.1016/j.anaerobe.2008.12.003; PMID: 19114111
- Suchett-Kaye G, Decoret D, Barsotti O. Clonal analysis by ribotyping of Fusobacterium nucleatum isolates obtained from healthy young adults with optimal plaque control. J Periodontal Res 1998; 33:179 - 86; http://dx.doi.org/10.1111/j.1600-0765.1998.tb02189.x; PMID: 9689613
- Thurnheer T, Guggenheim B, Gruica B, Gmür R. Infinite serovar and ribotype heterogeneity among oral Fusobacterium nucleatum strains?. Anaerobe 1999; 5:79 - 92; http://dx.doi.org/10.1006/anae.1999.0188
- Morris ML, Andrews RH, Rogers AH. Investigations of the taxonomy and systematics of Fusobacterium nucleatum using allozyme electrophoresis. Int J Syst Bacteriol 1997; 47:103 - 10; http://dx.doi.org/10.1099/00207713-47-1-103; PMID: 8995811
- Gmür R, Munson MA, Wade WG. Genotypic and phenotypic characterization of fusobacteria from Chinese and European patients with inflammatory periodontal diseases. Syst Appl Microbiol 2006; 29:120 - 30; http://dx.doi.org/10.1016/j.syapm.2005.07.011; PMID: 16464693
- Strauss J, Kaplan GG, Beck PL, Rioux K, Panaccione R, Devinney R, et al. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis 2011; 17:1971 - 8; http://dx.doi.org/10.1002/ibd.21606; PMID: 21830275
- Shammas NW, Murphy GW, Eichelberger J, Klee D, Schwartz R, Bachman W. Infective endocarditis due to Fusobacterium nucleatum: case report and review of the literature. Clin Cardiol 1993; 16:72 - 5; http://dx.doi.org/10.1002/clc.4960160116; PMID: 8416766
- Brook I, de Leyva F. Microbiology of tonsillar surfaces in infectious mononucleosis. Arch Pediatr Adolesc Med 1994; 148:171 - 3; PMID: 8118535
- Roberts GL. Fusobacterial infections: an underestimated threat. Br J Biomed Sci 2000; 57:156 - 62; PMID: 10912293
- Marina M, Strong CA, Civen R, Molitoris E, Finegold SM. Bacteriology of anaerobic pleuropulmonary infections: preliminary report. Clin Infect Dis 1993; 16:Suppl 4 S256 - 62; http://dx.doi.org/10.1093/clinids/16.Supplement_4.S256; PMID: 8324128
- Cahill RJ, Tan S, Dougan G, O'gaora P, Pickard D, Kennea N, et al. Universal DNA primers amplify bacterial DNA from human fetal membranes and link Fusobacterium nucleatum with prolonged preterm membrane rupture. Mol Hum Reprod 2005; In press http://dx.doi.org/10.1093/molehr/gah234; PMID: 16254004
- Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. BJOG 2002; 109:527 - 33; http://dx.doi.org/10.1111/j.1471-0528.2002.01349.x; PMID: 12066942
- Han YW, Redline RW, Li M, Yin L, Hill GB, McCormick TS. Fusobacterium nucleatum induces premature and term stillbirths in pregnant mice: implication of oral bacteria in preterm birth. Infect Immun 2004; 72:2272 - 9; http://dx.doi.org/10.1128/IAI.72.4.2272-2279.2004; PMID: 15039352
- Moore WE, Moore LV. The bacteria of periodontal diseases. Periodontol 2000 1994; 5:66 - 77; http://dx.doi.org/10.1111/j.1600-0757.1994.tb00019.x; PMID: 9673163
- Han YW, Shi W, Huang GT, Kinder Haake S, Park NH, Kuramitsu H, et al. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun 2000; 68:3140 - 6; http://dx.doi.org/10.1128/IAI.68.6.3140-3146.2000; PMID: 10816455
- Swidsinski A, Dorffel Y, Loening-Baucke V, Theissig F, Ruckert JC, Ismail M, et al. Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum.. Gut 2011; 60:34 - 40; http://dx.doi.org/10.1136/gut.2009.191320; PMID: 19926616
- Dabija-Wolter G, Cimpan MR, Costea DE, Johannessen AC, Sornes S, Neppelberg E, et al. Fusobacterium nucleatum enters normal human oral fibroblasts in vitro. J Periodontol 2009; 80:1174 - 83; http://dx.doi.org/10.1902/jop.2009.090051; PMID: 19563299
- Peterson J, Garges S, Giovanni M, McInnes P, Wang L, Schloss JA, et al. The NIH Human Microbiome Project. Genome Res 2009; 19:2317 - 23; http://dx.doi.org/10.1101/gr.096651.109; PMID: 19819907
- Brandtzaeg P. Inflammatory bowel disease: clinics and pathology. Do inflammatory bowel disease and periodontal disease have similar immunopathogeneses?. Acta Odontol Scand 2001; 59:235 - 43; http://dx.doi.org/10.1080/00016350152509265; PMID: 11570527
- Harty S, Fleming P, Rowland M, Crushell E, McDermott M, Drumm B, et al. A prospective study of the oral manifestations of Crohn's disease. Clin Gastroenterol Hepatol 2005; 3:886 - 91; http://dx.doi.org/10.1016/S1542-3565(05)00424-6; PMID: 16234026
- Halme L, Meurman JH, Laine P, von Smitten K, Syrjanen S, Lindqvist C, et al. Oral findings in patients with active or inactive Crohn's disease. Oral Surg Oral Med Oral Pathol 1993; 76:175 - 81; http://dx.doi.org/10.1016/0030-4220(93)90200-N; PMID: 8361727
- Van Dyke TE, Dowell VR Jr., Offenbacher S, Snyder W, Hersh T. Potential role of microorganisms isolated from periodontal lesions in the pathogenesis of inflammatory bowel disease. Infect Immun 1986; 53:671 - 7; PMID: 3462153
- Moore WE, Holdeman LV, Cato EP, Smibert RM, Burmeister JA, Palcanis KG, et al. Comparative bacteriology of juvenile periodontitis. Infect Immun 1985; 48:507 - 19; PMID: 3988344
- Gupta S, Ghosh SK, Scott ME, Bainbridge B, Jiang B, Lamont RJ, et al. Fusobacterium nucleatum-associated beta-defensin inducer (FAD-I): identification, isolation and functional evaluation. J Biol Chem 2010; 285:36523 - 31; http://dx.doi.org/10.1074/jbc.M110.133140; PMID: 20847052
- Gursoy UK, Kononen E, Uitto VJ. Stimulation of epithelial cell matrix metalloproteinase (MMP-2, -9, -13) and interleukin-8 secretion by fusobacteria. Oral Microbiol Immunol 2008; 23:432 - 4; http://dx.doi.org/10.1111/j.1399-302X.2008.00453.x; PMID: 18793368
- Signat B, Roques C, Poulet P, Duffaut D. Fusobacterium nucleatum in Periodontal Health and Disease. Curr Issues Mol Biol 2011; 13:25 - 36; PMID: 21220789
- Saito A, Inagaki S, Kimizuka R, Okuda K, Hosaka Y, Nakagawa T, et al. Fusobacterium nucleatum enhances invasion of human gingival epithelial and aortic endothelial cells by Porphyromonas gingivalis.. FEMS Immunol Med Microbiol 2008; 54:349 - 55; http://dx.doi.org/10.1111/j.1574-695X.2008.00481.x; PMID: 19049647
- Metzger Z, Blasbalg J, Dotan M, Tsesis I, Weiss EI. Characterization of coaggregation of Fusobacterium nucleatum PK1594 with six Porphyromonas gingivalis strains. J Endod 2009; 35:50 - 4; http://dx.doi.org/10.1016/j.joen.2008.09.016; PMID: 19084124
- Stathopoulou PG, Benakanakere MR, Galicia JC, Kinane DF. Epithelial cell pro-inflammatory cytokine response differs across dental plaque bacterial species. J Clin Periodontol 2010; 37:24 - 9; http://dx.doi.org/10.1111/j.1600-051X.2009.01505.x; PMID: 20096064
- Kaplan CW, Ma X, Paranjpe A, Jewett A, Lux R, Kinder-Haake S, et al. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect Immun 2010; 78:4773 - 8; http://dx.doi.org/10.1128/IAI.00567-10; PMID: 20823215
- Mahtout H, Chandad F, Rojo JM, Grenier D. Fusobacterium nucleatum binding to complement regulatory protein CD46 modulates the expression and secretion of cytokines and matrix metalloproteinases by oral epithelial cells. J Periodontol 2011; 82:311 - 9; http://dx.doi.org/10.1902/jop.2010.100458; PMID: 20843232
- Cattaneo R. Four viruses, two bacteria and one receptor: membrane cofactor protein (CD46) as pathogens' magnet. J Virol 2004; 78:4385 - 8; http://dx.doi.org/10.1128/JVI.78.9.4385-4388.2004; PMID: 15078919
- Xu M, Yamada M, Li M, Liu H, Chen SG, Han YW. FadA from Fusobacterium nucleatum utilizes both secreted and nonsecreted forms for functional oligomerization for attachment and invasion of host cells. J Biol Chem 2007; 282:25000 - 9; http://dx.doi.org/10.1074/jbc.M611567200; PMID: 17588948
- Gursoy UK, Kononen E, Uitto VJ. Intracellular replication of fusobacteria requires new actin filament formation of epithelial cells. APMIS 2008; 116:1063 - 70; http://dx.doi.org/10.1111/j.1600-0463.2008.00868.x; PMID: 19133009
- Silva VL, Diniz CG, Cara DC, Santos SG, Nicoli JR, Carvalho MA, et al. Enhanced pathogenicity of Fusobacterium nucleatum adapted to oxidative stress. Microb Pathog 2005; 39:131 - 8; http://dx.doi.org/10.1016/j.micpath.2005.07.002; PMID: 16125361
- Ji S, Shin JE, Kim YC, Choi Y. Intracellular degradation of Fusobacterium nucleatum in human gingival epithelial cells. Mol Cells 2010; 30:519 - 26; http://dx.doi.org/10.1007/s10059-010-0142-8; PMID: 21057979
- Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118:3030 - 44; http://dx.doi.org/10.1002/ijc.21731; PMID: 16404738
- Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2011; http://dx.doi.org/10.1101/gr.126516.111; PMID: 22009989
- Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 2011; http://dx.doi.org/10.1101/gr.126573.111; PMID: 22009990
- Pryde SE, Duncan SH, Hold GL, Stewart CS, Flint HJ. The microbiology of butyrate formation in the human colon. FEMS Microbiol Lett 2002; 217:133 - 9; http://dx.doi.org/10.1111/j.1574-6968.2002.tb11467.x; PMID: 12480096
- Roediger WE, Duncan A, Kapaniris O, Millard S. Reducing sulfur compounds of the colon impair colonocyte nutrition: implications for ulcerative colitis. Gastroenterology 1993; 104:802 - 9; PMID: 8440437
- Jakubovics NS, Gill SR, Iobst SE, Vickerman MM, Kolenbrander PE. Regulation of gene expression in a mixed-genus community: stabilized arginine biosynthesis in Streptococcus gordonii by coaggregation with Actinomyces naeslundii.. J Bacteriol 2008; 190:3646 - 57; http://dx.doi.org/10.1128/JB.00088-08; PMID: 18359813
- Edwards AM, Grossman TJ, Rudney JD. Fusobacterium nucleatum transports noninvasive Streptococcus cristatus into human epithelial cells. Infect Immun 2006; 74:654 - 62; http://dx.doi.org/10.1128/IAI.74.1.654-662.2006; PMID: 16369022
- Zhang G, Rudney JD. Streptococcus cristatus attenuates Fusobacterium nucleatum-induced cytokine expression by influencing pathways converging on nuclear factor-kappaB. Mol Oral Microbiol 2011; 26:150 - 63; http://dx.doi.org/10.1111/j.2041-1014.2010.00600.x; PMID: 21375705
- Dharmani P, Strauss J, Ambrose C, Allen-Vercoe E, Chadee K. Fusobacterium nucleatum infection of colonic cells stimulates MUC2 mucin and tumor necrosis factor alpha. Infect Immun 2011; 79:2597 - 607; http://dx.doi.org/10.1128/IAI.05118-11; PMID: 21536792