Abstract
The bacterial pathogen Helicobacter pylori is predominantly known for its tight association with peptic ulcer disease and gastric cancer. However, recent evidence suggests that chronic infection with H. pylori may also be beneficial to the host by conferring protection against allergies, asthma and inflammatory bowel diseases. The protective effects of H. pylori depend on highly suppressive regulatory T-cells. In this addendum, we summarize results showing that H. pylori infection efficiently re-programs dendritic cells (DCs) toward a tolerance-promoting phenotype; their “tolerogenic” activity requires inflammasome activation and the secretion of interleukin-18. H. pylori-experienced DCs fail to induce T-cell effector functions, but efficiently induce FoxP3 expression in naive T-cells in vitro and in vivo. The experimental depletion of DCs breaks tolerance and results in improved infection control, but also in aggravated T-cell-driven immunopathology. In summary, we propose that H. pylori evades adaptive immune responses by re-programming DCs in favor of tolerance over immunity.
Helicobacter pylori is the etiological agent responsible for almost all cases of severe gastritis and gastric and duodenal ulcers, and chronic infection with H. pylori, generally spanning decades, accounts for the vast majority of gastric cancer cases. The prevalence of these infection-associated conditions has declined in many parts of the Western world as H. pylori infection rates have dropped from > 50% at the beginning of the 20th century to < 10% at its end.Citation1 In the same time frame, the incidence of many immune disorders has increased at an alarming rate.Citation2 Among these are allergic diseases such as hay fever, eczema, and asthma, but also auto-immune diseases (multiple sclerosis, type I diabetes) and chronic inflammatory conditions such as inflammatory bowel disease.Citation2 A series of epidemiological studies describing an inverse association between H. pylori infection and asthma and other allergic and chronic inflammatory disease manifestations, especially in children and young adults, has implied a causal relationship between the two trends.Citation3-Citation9 A possible protective effect of the infection could indeed be substantiated in experimental models of asthma and inflammatory bowel diseases.Citation10,Citation11 In a mouse model of allergen-induced airway hyper-responsiveness and asthma, the protection conferred by H. pylori infection was restricted to mice that had first been exposed to the bacteria during the neonatal period, and was much less pronounced in adult-infected animals.Citation10 Asthma protection was found to be entirely dependent on peripherally induced regulatory T-cells (Tregs), as the depletion of Tregs abrogated protection and the adoptive transfer of highly pure populations of Tregs was sufficient to transfer protection from infected donors to naive recipients.Citation10 Inducible Tregs are believed to be generated in the periphery by tolerogenic dendritic cells (DCs),Citation12 which convert naive T-cells into FoxP3+ Tregs through antigen presentation in the absence of co-stimulatory signals or cytokines.Citation12,Citation13 Additional tolerance-promoting mechanisms utilized by tolerogenic DCs include the production of soluble and membrane-bound factors such as IL-10, TGFβ, retinoic acid, and programmed death ligands.Citation12,Citation13 Tolerogenic properties are generally attributed to semi-mature DCs that have taken up antigen, but have not simultaneously been exposed to ligands of TLRs or NOD-like receptors (NLRs); such DCs are characterized by high levels of MHCII, but low or no expression of co-stimulatory molecules or pro-inflammatory cytokines.Citation12,Citation13
H. pylori Actively Prevents DC Maturation and Re-programs DCs Toward a Tolerogenic Phenotype
Based on our own and others’ findings that H. pylori skews the Treg/Thelper cell 17 (Th17) balance toward regulatory responses via its effects on DCs,Citation14 and that the systemic depletion of DCs improves rather than abrogates immunity to H. pylori infection,Citation15 we focused on investigating the H. pylori/DC interface in more detail.Citation16 We initially relied heavily on bone marrow-derived DCs (BM-DCs), which can be differentiated from bone marrow precursors by treatment with GM-CSF and will subsequently undergo further maturation if treated with strong TLR ligands such as E. coli LPS (a TLR-4 ligand). Upon co-culture with virulent strains of H. pylori, BM-DCs could be observed rapidly phagocytosing the bacteria; to our surprise, we found that the LPS-induced maturation of DCs was almost completely blocked in the H. pylori/DC co-cultures compared with naive DCs.Citation16 DC maturation was assessed in this setting by the flow cytometric quantification of surface CD80, CD86 and CD40 expression, as well as the release of a variety of cytokines, including IL-12, TNF-α and IL-6, into the culture supernatant. Several alternative inducers of DC maturation, i.e., the TLR-2 and -9 ligands Pam3Cys and CpG, mirrored the results we obtained with LPS. The inhibitory effects of the bacteria on DC maturation were completely dependent on direct contact between H. pylori and the DCs, but proved to be independent of the Cag pathogenicity island-encoded type IV secretion system. A mutant lacking a key component of the type IV secretion system, CagE, did not differ from isogenic wild type bacteria in this respect.
To further assess the functional consequences of the H. pylori/DC interaction, we adapted an experimental system that allows for the quantitative assessment of DC tolerogenicity. To this end, BM-DCs are cultured for three days with naive CD4+CD25- T-cells at a 1:2 ratio in the presence of anti-CD3 antibody, IL-2 and TGF-β. Tolerogenic DCs will be able to induce CD25 and FoxP3 expression in co-cultured T-cells as assessed by flow cytometry, whereas inflammatory or immunogenic DCs will not. In line with the semi-mature status of H. pylori-experienced DCs, and the well-documented inherent tolerogenic activity of semi-mature DCs,Citation12 we observed that DC populations that had been infected with H. pylori were much better inducers of FoxP3/CD25 in co-cultured T-cells than their naive counterparts. Interestingly, this was not only true in the BM-DC system, but could be verified with immunomagnetically isolated CD11c+ DCs from mesenteric lymph nodes (MLN) of naive donors, which we cultivated and infected with H. pylori ex vivo. H. pylori-experienced BM-DCs or MLN-DCs were further incapable of priming antigen-specific T-effector responses (for example to ovalbumin), demonstrating that they had indeed acquired tolerogenic rather than immunogenic properties upon exposure to H. pylori.
Perhaps the most unexpected observation associated with this investigation came from examining the tolerogenicity of MLN-DCs that we immunomagnetically isolated from infected animals. Without any additional exposure to H. pylori ex vivo, these MLN-DCs exhibited astonishing tolerogenic capacity, inducing FoxP3/CD25 in up to 50% of co-cultured T-cells, i.e., 3–5 times more than DCs isolated in the same manner from uninfected controls.Citation16 DCs isolated from mice that had been infected at an early age were more tolerogenic than DCs from adult-infected animals. The combined results suggested that H. pylori actively targets DCs in vitro and in vivo and re-programs them toward acquiring tolerogenic rather than immunogenic functions.
H. pylori Activates the Inflammasome and Caspase-1 to Induce Proteolytic Processing of IL-1β and IL-18
Most gram-negative pathogens express not only TLR ligands such as the ones described above, but are also sensed by an additional, cytoplasmic set of pattern recognition receptors (PRRs), the Nod-like receptors (NLRs). Activation of NLRs can either result in the transcriptional activation of innate and adaptive immune response genes through the transcription factor NF-κB as in the case of the Nod-like receptors 1 and 2Citation17 or lead to the assembly of multi-protein complexes called inflammasomes, which activate the cysteine protease caspase-1.Citation18 NLRs in the latter category include NLRC4, the cytoplasmic ligand for bacterial flagellinsCitation19,Citation20 and NALP3, which detects urate crystals, ATP, bacterial pore-forming toxins and particulate matter such as asbestos and silica.Citation20,Citation21 The ultimate outcomes of inflammasome/caspase-1 activation are a special caspase-1-dependent form of apoptosis termed pyroptosis and/or the processing and secretion of the cytokines IL-1β and IL-18.Citation19,Citation21
There has been a long-standing interest in the Helicobacter field in IL-1β, which is known to strongly promote gastritis and to trigger the preneoplastic changes characteristic of chronically infected individuals. IL-1β levels are induced in the gastric mucosa of symptomatic H. pylori-infected individualsCitation22 and polymorphisms associated with increased steady-state levels of IL-1β predispose carriers to gastric cancer.Citation23 Indeed, the stomach-specific expression of human IL-1β is sufficient to induce gastric inflammation and gastric cancer in transgenic mice.Citation24 In contrast, surprisingly little was known until recently regarding the activities of the other caspase-1 substrate, IL-18, and the inflammasome itself in the pathogenesis of H. pylori infection. H. pylori peptidoglycan is detected by the NLR Nod1 in a type IV secretion system-dependent mannerCitation25 and triggers the activation of the NF-κB and ISGF3 signaling pathways.Citation25,Citation26 Other NLR ligands have not been identified, and inflammasome-activating NLRs have to date not been shown to be involved in Helicobacter sensing.
We have demonstrated recently that caspase-1 is activated upon H. pylori infection in cultured BM-DCs, as well as in vivo in experimentally infected animals. Cells with active caspase-1 can be detected using a fluorescently labeled caspase-1 substrate in the mesenteric lymph nodes of infected mice, and IL-1β and IL-18 are abundantly produced in the infected gastric mucosa.Citation27 Mice lacking either caspase-1, IL-18 or the IL-1 receptor due to gene-specific deletions exhibit striking and very robust phenotypes, and reveal dual functions for caspase-1 in H. pylori infection that are mediated by IL-1 and IL-18 signaling, respectively. Whereas caspase-1-mediated IL-1β processing and IL1R signaling are absolutely required for the generation of Helicobacter-specific Th1 and Th17 responses, for the efficient control of the infection and for the induction of Helicobacter-associated gastric immunopathology (manifesting as chronic gastritis, atrophy, epithelial hyperplasia and intestinal metaplasia), active IL-18 appears to counteract the effects of IL-1β.Citation27 The processing and secretion of IL-18 restricts pathogenic Th17 responses; therefore, IL-18−/− animals mount more pronounced Helicobacter-specific Th17 responses, exhibit more pathology, and control the infection more effectively.Citation27 A mouse strain lacking caspase-1 phenocopies the defects of both cytokine (receptor)-deficient mice.Citation27 The results revealed for the first time that the two caspase-1 substrates have largely opposing functions in a bacterial infection model, and reinforce the notion that IL-18 exhibits predominantly anti-inflammatory/regulatory properties in the gut. Our data are in line with results from experimental models of inflammatory bowel disease, which have documented an increased sensitivity of IL-18−/− mice relative to wild type mice of the same strain background.Citation28,Citation29
DC-Intrinsic IL-18 Expression is Required for the Tolerogenicity of H. pylori-Experienced DCs In Vitro and In Vivo
As outlined above, the exposure of BM-DCs or MLN-derived DCs to H. pylori generates cells with predominantly tolerogenic properties, i.e., with the capacity to induce FoxP3/CD25 expression in co-cultured naive T-cells.Citation16 Based on the infection-associated phenotypes of the caspase-1−/− and IL-18−/− strains, we speculated that DC-derived IL-18 might skew antigen-activated T-cells away from Th17 polarization and toward T-regulatory functions. To test this idea, we compared the tolerogenicity of wild type and IL-18−/− BM-DCs after exposure to H. pylori and found that IL-18−/− DCs indeed fail to induce FoxP3/CD25 expression in T-cells.Citation16 Along the same line of evidence, T-cells lacking the IL-18 receptor were incapable of converting to FoxP3+CD25+ Tregs upon co-culture with (wild type) H. pylori-experienced DCs. The combined results suggest that IL-18 signaling is critical for Treg differentiation, at least in vitro. Interestingly, we made similar observations in in vivo models of H. pylori infection: infected mice lacking either IL-18 or its receptor exhibited significantly lower numbers of Tregs in the draining mesenteric lymph nodes than infected wild type mice.Citation16 Furthermore, MLN-DCs from infected IL-18−/− or IL-18R−/− animals did not exhibit the tolerogenic properties characteristic of MLN-DCs of infected wild type animals when examined in the T-cell co-culture assay ex vivo.Citation16 Most compellingly, immunomagnetically isolated CD4+CD25+ T cells from the MLN of infected wild type, but not from infected IL-18−/− animals were capable of conferring asthma protection to naive allergen-exposed recipients, suggesting that IL-18 signaling is required in vivo for the generation of fully suppressive FoxP3+ Tregs.
Interestingly, while defective for Treg conversion, IL-18−/− DCs are actually better inducers of Th17 polarization in naive T-cells than wild type DCs.Citation16 This finding explains why caspase1−/− and IL-18−/− mice launch stronger anti-Helicobacter Th17 responses and are able to spontaneously control an experimental H. pylori infection better than wild type animals.Citation27 Th17 cells and Tregs represent developmentally related lineages, which both rely on TGF-βCitation30 and retain a high level of plasticity even when fully differentiated; in particular, Tregs can convert to Th17 cells under the influence of IL-1β and IL-6 in vitro and in vivo.Citation31-Citation33 We speculate that the relative abundance of IL-18 (driving Treg differentiation) and IL-1β (promoting Th1 and Th17 differentiation) at the DC/T-cell interface may tip the balance toward predominant Treg or Th1/Th17 responses, respectively, with additional determinants being the (semi-) mature status of the H. pylori-experienced DC and the availability of co-stimulatory signals (). It is interesting to note in this context that IL-18 and IL-1β are regulated very differently at the transcriptional level; whereas IL-18 is constitutively expressed and stored in pre-formed granules, to be released upon inflammasome/caspase activation and proteolytic processing, IL-1β needs to be activated at the transcriptional level before the pre-form can be processed and released. The transcriptional activation of the IL1B gene occurs through an NF-κB-dependent process that is activated by TLR or NLR ligation at the cell surface or in the cytoplasm. According to our working hypothesis (), exposure to H. pylori, which lacks many relevant TLR ligands and/or has evolved to avoid TLR recognition,Citation34-Citation36 triggers relatively little IL-1β production whereas IL-18 is abundantly expressed and secreted. The consequence of this relative abundance of IL-18 over IL-1β is the predominant induction of Tregs. If IL-1β is transcriptionally induced due to exogenous addition of bioactive E. coli LPS in vitro (which binds to TLR4) or certain adjuvants in vivo (such as the mycobacterial cord factor we have used extensively),Citation15 IL-1β production is increased, the IL-18/IL-1β balance shifts toward IL1β and Th1/Th17 responses are preferentially induced.
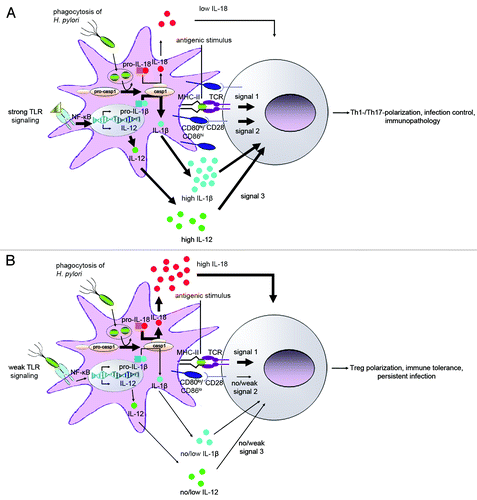
Figure 1. Schematic representation of the DC/T-cell interface under immunogenic and tolerogenic conditions. (A) DC exposure to H. pylori in inflamed tissues, e.g., upon challenge of mice that have previously been immunized with an H. pylori-specific vaccine, results in DCs with predominantly immunogenic properties. Such immunogenic DCs are characterized by high expression of co-stimulatory molecules (CD80, CD86), abundant secretion of IL-12 and IL-1β and little if any production of IL-18. Immunogenic DCs provide three signals to T-cells: the antigenic stimulus, co-stimulatory signals and T-effector cell-inducing cytokines. The ultimate outcome is Th1/Th17 polarization, efficient control of H. pylori infection and severe T-cell-driven immunopathology. (B) Under tolerance-promoting conditions, e.g., exposure to H. pylori during the neonatal period, the uptake of H. pylori renders DCs tolerogenic; tolerogenic DCs express little or no CD80/CD86, IL-12 and IL-1β, but instead produce large amounts of IL-18 upon inflammasome and caspase-1 activation. Tolerogenic DCs provide a strong antigenic stimulus to T-cells in the absence of signals 2 and 3, and preferentially induce Treg differentiation. Strong Treg and weak T-effector responses favor immune tolerance and persistent infection and cross-protect against allergen-specific T-cell responses and allergic disease manifestations.
In Vivo Evidence from Humans and Experimentally Infected Mice Suggests an Essential Role for Tregs and DCs in H. pylori-Specific Immune Tolerance, Persistence and Asthma Protection
We have reported previously that the systemic depletion of DCs by administration of sublethal doses of diphtheria toxin (DT) to cd11c-DT receptor-transgenic mice enhances immune control over H. pylori in an experimental infection model, and also strongly improves vaccine-induced protective immunity afforded by H. pylori-specific vaccination.Citation15 This at first glance paradoxical effect now makes a lot of sense in light of our more recent finding that DCs are a potentially critical target of H. pylori immunomodulation. Indeed, we obtained similar results upon DC depletion in a neonatal infection model of H. pylori, for which the mice are experimentally infected during a time when their immune system is immature and inherently prone to develop tolerance rather than immunity to commensals, infectious agents and other foreign antigens.Citation37 Neonatally infected mice fail to generate H. pylori-specific T-effector cell responses and cannot control the infection, but are protected against gastritis and gastric immunopathology.Citation37 Their tolerant status is retained for many months if not years, and possibly for life if the infection is left untreated.Citation37 The immune tolerance of neonatally infected mice depends on Tregs; systemic Treg depletion rapidly breaks tolerance and leads to clearance of the infection accompanied by severely accelerated immunopathology.Citation37 We show now that the depletion of DCs has similar, albeit not quite as drastic, effects on immune tolerance as the depletion of Tregs.Citation16 Neonatally infected mice depleted of DCs control their infection more effectively, generate stronger Th1 and Th17 responses and develop more gastritis.Citation16 The phenotype of (neonatally) infected mice is reminiscent of reports in humans that have consistently found gastric H. pylori-specific T-regulatory responses to be the dominant T-cell responses in asymptomatic children, and also in asymptomatic adult H. pylori carriers relative to duodenal ulcer patients.Citation38,Citation39 We have now also begun to examine DC responses in human H. pylori carriers. Extensive quantification and immunophenotyping of DCs infiltrating the (H. pylori-infected) gastric mucosa revealed that (1) DCs are actively recruited to the infected relative to the uninfected gastric mucosa and that (2) gastric DCs in infected individuals retain a semi-mature MHChiCD80loCD83loCD86lo status.Citation16 These results indeed suggest that gastric DCs that have been in direct contact with H. pylori may phenotypically represent the human cell counterpart of the tolerogenic DCs that are characteristic of (neonatally) infected mice.
As mentioned earlier, H. pylori infection appears to protect against asthma and other allergic and chronic inflammatory disease manifestations in humansCitation3-Citation9 and prevents the airway hyper-responsiveness, lung inflammation, eosinophilia and goblet cell metaplasia that are characteristic of experimentally induced allergic asthma in mice.Citation10 Asthma protection is clearly dependent on Tregs, and can even be transferred to naive recipients via Tregs alone.Citation10 The protective effects of the infection are most evident in neonatally infected mice that are tolerant to the bacteria; similarly, the inverse correlation between H. pylori infection in humans with allergies and asthma was strongest in children and young adults and was particularly pronounced with respect to early-onset asthma.Citation4,Citation5,Citation9 Whether tolerogenic DCs play a role in asthma protection can only be inferred, but not definitively proven at this point, as the depletion of DCs abrogates allergic asthma in our experimental model.Citation40 We have documented earlier that semi-mature DCs accumulate in the lungs of protected mice, and that the high ratio of semi-mature to mature DCs is a strong indicator of protection.Citation10 Identifying the specific DC subsets that establish and maintain H. pylori-specific immune tolerance in the lungs and in the gastro-intestinal tract, and determining the mechanisms and molecular players that mediate asthma protection will remain a formidable challenge for future work.
Acknowledgments
Research in the laboratory of A.M. is supported by grants from the Cancer Leagues of Switzerland and the Canton of Zurich, the Swiss National Science Foundation, the Gebert Rüf Foundation and the University Research Priority Program in Systems Biology.
References
- Blaser MJ, Falkow S. What are the consequences of the disappearing human microbiota?. Nat Rev Microbiol 2009; 7:887 - 94; http://dx.doi.org/10.1038/nrmicro2245; PMID: 19898491
- Bach JF. The effect of infections on susceptibility to autoimmune and allergic diseases. N Engl J Med 2002; 347:911 - 20; http://dx.doi.org/10.1056/NEJMra020100; PMID: 12239261
- Blaser MJ, Chen Y, Reibman J. Does Helicobacter pylori protect against asthma and allergy?. Gut 2008; 57:561 - 7; http://dx.doi.org/10.1136/gut.2007.133462; PMID: 18194986
- Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med 2007; 167:821 - 7; http://dx.doi.org/10.1001/archinte.167.8.821; PMID: 17452546
- Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis 2008; 198:553 - 60; http://dx.doi.org/10.1086/590158; PMID: 18598192
- Reibman J, Marmor M, Filner J, Fernandez-Beros ME, Rogers L, Perez-Perez GI, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One 2008; 3:e4060; http://dx.doi.org/10.1371/journal.pone.0004060; PMID: 19112508
- Luther J, Dave M, Higgins PD, Kao JY. Association between Helicobacter pylori infection and inflammatory bowel disease: a meta-analysis and systematic review of the literature. Inflamm Bowel Dis 2010; 16:1077 - 84; PMID: 19760778
- Sładek M, Jedynak-Wasowicz U, Wedrychowicz A, Kowalska-Duplaga K, Pieczarkowski S, Fyderek K. [The low prevalence of Helicobacter pylori gastritis in newly diagnosed inflammatory bowel disease children and adolescent]. Przegl Lek 2007; 64:Suppl 3 65 - 7; PMID: 18431918
- Amberbir A, Medhin G, Erku W, Alem A, Simms R, Robinson K, et al. Effects of Helicobacter pylori, geohelminth infection and selected commensal bacteria on the risk of allergic disease and sensitization in 3-year-old Ethiopian children. Clin Exp Allergy 2011; 41:1422 - 30; http://dx.doi.org/10.1111/j.1365-2222.2011.03831.x; PMID: 21831135
- Arnold IC, Dehzad N, Reuter S, Martin H, Becher B, Taube C, et al. Helicobacter pylori infection prevents allergic asthma in mouse models through the induction of regulatory T cells. J Clin Invest 2011; 121:3088 - 93; http://dx.doi.org/10.1172/JCI45041; PMID: 21737881
- Higgins PD, Johnson LA, Luther J, Zhang M, Sauder KL, Blanco LP, et al. Prior Helicobacter pylori infection ameliorates Salmonella typhimurium-induced colitis: Mucosal crosstalk between stomach and distal intestine. Inflamm Bowel Dis 2010; PMID: 21560200
- Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol 2010; 108:111 - 65; http://dx.doi.org/10.1016/B978-0-12-380995-7.00004-5; PMID: 21056730
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol 2005; 6:1219 - 27; http://dx.doi.org/10.1038/ni1265; PMID: 16244650
- Kao JY, Zhang M, Miller MJ, Mills JC, Wang B, Liu M, et al. Helicobacter pylori immune escape is mediated by dendritic cell-induced Treg skewing and Th17 suppression in mice. Gastroenterology 2010; 138:1046 - 54; http://dx.doi.org/10.1053/j.gastro.2009.11.043; PMID: 19931266
- Hitzler I, Oertli M, Becher B, Agger EM, Müller A. Dendritic cells prevent rather than promote immunity conferred by a helicobacter vaccine using a mycobacterial adjuvant. Gastroenterology 2011; 141:186 - 96, 196, e1; http://dx.doi.org/10.1053/j.gastro.2011.04.009; PMID: 21569773
- Oertli M, Sundquist M, Hitzler I, Engler DB, Arnold IC, Reuter S, et al. DC-derived IL-18 drives Treg differentiation, murine Helicobacter pylori-specific immune tolerance, and asthma protection. J Clin Invest 2012; 122:1082 - 96; http://dx.doi.org/10.1172/JCI61029; PMID: 22307326
- Kim YG, Park JH, Shaw MH, Franchi L, Inohara N, Núñez G. The cytosolic sensors Nod1 and Nod2 are critical for bacterial recognition and host defense after exposure to Toll-like receptor ligands. Immunity 2008; 28:246 - 57; http://dx.doi.org/10.1016/j.immuni.2007.12.012; PMID: 18261938
- Broz P, Monack DM. Molecular mechanisms of inflammasome activation during microbial infections. Immunol Rev 2011; 243:174 - 90; http://dx.doi.org/10.1111/j.1600-065X.2011.01041.x; PMID: 21884176
- Mariathasan S, Monack DM. Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 2007; 7:31 - 40; http://dx.doi.org/10.1038/nri1997; PMID: 17186029
- Brodsky IE, Monack D. NLR-mediated control of inflammasome assembly in the host response against bacterial pathogens. Semin Immunol 2009; 21:199 - 207; http://dx.doi.org/10.1016/j.smim.2009.05.007; PMID: 19539499
- Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009; 10:241 - 7; http://dx.doi.org/10.1038/ni.1703; PMID: 19221555
- Yamaoka Y, Kita M, Kodama T, Sawai N, Kashima K, Imanishi J. Induction of various cytokines and development of severe mucosal inflammation by cagA gene positive Helicobacter pylori strains. Gut 1997; 41:442 - 51; http://dx.doi.org/10.1136/gut.41.4.442; PMID: 9391240
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000; 404:398 - 402; http://dx.doi.org/10.1038/35006081; PMID: 10746728
- Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 2008; 14:408 - 19; http://dx.doi.org/10.1016/j.ccr.2008.10.011; PMID: 18977329
- Viala J, Chaput C, Boneca IG, Cardona A, Girardin SE, Moran AP, et al. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol 2004; 5:1166 - 74; http://dx.doi.org/10.1038/ni1131; PMID: 15489856
- Watanabe T, Asano N, Fichtner-Feigl S, Gorelick PL, Tsuji Y, Matsumoto Y, et al. NOD1 contributes to mouse host defense against Helicobacter pylori via induction of type I IFN and activation of the ISGF3 signaling pathway. J Clin Invest 2010; 120:1645 - 62; http://dx.doi.org/10.1172/JCI39481; PMID: 20389019
- Hitzler I, Sayi A, Kohler E, Engler DB, Koch KN, Hardt WD, et al. Caspase-1 has both proinflammatory and regulatory properties in Helicobacter infections, which are differentially mediated by its substrates IL-1β and IL-18. J Immunol 2012; 188:3594 - 602; http://dx.doi.org/10.4049/jimmunol.1103212; PMID: 22403439
- Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti T-D. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity 2010; 32:379 - 91; http://dx.doi.org/10.1016/j.immuni.2010.03.003; PMID: 20303296
- Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KSB, McIntire CR, LeBlanc PM, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity 2010; 32:367 - 78; http://dx.doi.org/10.1016/j.immuni.2010.02.012; PMID: 20226691
- Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 2006; 24:179 - 89; http://dx.doi.org/10.1016/j.immuni.2006.01.001; PMID: 16473830
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 2008; 29:44 - 56; http://dx.doi.org/10.1016/j.immuni.2008.05.007; PMID: 18585065
- Deknuydt F, Bioley G, Valmori D, Ayyoub M. IL-1beta and IL-2 convert human Treg into T(H)17 cells. Clin Immunol 2009; 131:298 - 307; http://dx.doi.org/10.1016/j.clim.2008.12.008; PMID: 19211307
- Li L, Kim J, Boussiotis VA. IL-1β-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J Immunol 2010; 185:4148 - 53; http://dx.doi.org/10.4049/jimmunol.1001536; PMID: 20817874
- Andersen-Nissen E, Smith KD, Strobe KL, Barrett SL, Cookson BT, Logan SM, et al. Evasion of Toll-like receptor 5 by flagellated bacteria. Proc Natl Acad Sci U S A 2005; 102:9247 - 52; http://dx.doi.org/10.1073/pnas.0502040102; PMID: 15956202
- Gewirtz AT, Yu Y, Krishna US, Israel DA, Lyons SL, Peek RM Jr.. Helicobacter pylori flagellin evades toll-like receptor 5-mediated innate immunity. J Infect Dis 2004; 189:1914 - 20; http://dx.doi.org/10.1086/386289; PMID: 15122529
- Moran AP, Lindner B, Walsh EJ. Structural characterization of the lipid A component of Helicobacter pylori rough- and smooth-form lipopolysaccharides. J Bacteriol 1997; 179:6453 - 63; PMID: 9335296
- Arnold IC, Lee JY, Amieva MR, Roers A, Flavell RA, Sparwasser T, et al. Tolerance rather than immunity protects from Helicobacter pylori-induced gastric preneoplasia. Gastroenterology 2011; 140:199 - 209; http://dx.doi.org/10.1053/j.gastro.2010.06.047; PMID: 20600031
- Harris PR, Wright SW, Serrano C, Riera F, Duarte I, Torres J, et al. Helicobacter pylori gastritis in children is associated with a regulatory T-cell response. Gastroenterology 2008; 134:491 - 9; http://dx.doi.org/10.1053/j.gastro.2007.11.006; PMID: 18242215
- Robinson K, Kenefeck R, Pidgeon EL, Shakib S, Patel S, Polson RJ, et al. Helicobacter pylori-induced peptic ulcer disease is associated with inadequate regulatory T cell responses. Gut 2008; 57:1375 - 85; http://dx.doi.org/10.1136/gut.2007.137539; PMID: 18467372
- van Rijt LS, Jung S, Kleinjan A, Vos N, Willart M, Duez C, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med 2005; 201:981 - 91; http://dx.doi.org/10.1084/jem.20042311; PMID: 15781587