Abstract
Intestinal homeostasis results from a complex mutualism between gut microbiota and host cells. Defining the molecular network regulating such mutualism is currently of increasing interest, as its deregulation is reported to lead to increased susceptibility to infections, chronic inflammatory bowel diseases and cancer. Until now, the focus has been on the mechanism, by which the composition of indigenous microbiota shapes the immune system. In a recent study, we have shown that dietary compounds have also the ability to affect innate immune system. This regulation involves aryl hydrocarbon receptor (AhR), a sensor of plant-derived phytochemicals, which mediates the maintenance of Retinoic acid related orphan receptor γ t-expressing innate lymphoid cells (RORγt+ ILC) in the gut and consequently formation of postnatal lymphoid follicles. Thus, AhR represents the first evidence of a molecular link between diet and immunity at intestinal mucosal surfaces.
Introduction
Our knowledge of the intestinal immune system has greatly increased over the last few years. By definition, immune system aims at recognizing and eliminating the “non-self.” However, the role of immune system in the gut seems different. At steady-state, it recognizes diverse dietary and bacterial antigens in order to promote intestinal tissue homeostasis. A growing family of innate lymphocytes has been found to play an important role in maintaining tissue homeostasis in the intestine.Citation1,Citation2 RORγt+ ILC were originally identified as inducers of lymph node and Peyer’s patch formation. These cells are present in large numbers in adult intestinal tissues being an important source of interleukin (IL-)-22.Citation3-Citation7 IL-22 is a cytokine known to participate in epithelial cell homeostasis and in induction of anti-microbial peptide production by epithelial cells. In lamina propria of the small intestine, RORγt+ ILC are required for the formation of lymphoid follicles named crytopatches (CP). These clusters appear early after birth. CP can further recruit B cells and mature into isolated lymphoid follicles (ILF) after colonization of the gut lumen by microbiota.Citation8-Citation10 The role of microbiota in the formation of intestinal lymphoid follicles and in the development of RORγt+ ILC is well studied. Although the ILF development is dependent on the bacterial signals, cryptopatches and RORγt+ ILC are normally present in the absence of the microbiota, which suggests a role for other environmental factors, such as food-derived components. AhR, a sensor of diverse environmental factors, has recently been shown to be important in regulating various processes in the immune system.Citation11
AhR belongs to the basic helix-loop-helix/Per-Arnt-Sim (bHLH/PAS) family of proteins important in adapting multicellular organisms to environment (e.g., hypoxia response, circadian rhythm etc.). AhR is a ligand-inducible transcription factor that mediates cellular responses to low-molecular-weight chemicals by activating transcription of the genes with promoters containing xenobiotic response elements (XRE), AhR binding sites. AhR was originally described as a receptor for dioxin, a toxic compound formed as a by-product of various industrial processes. However, several other ligands have been described to be able to activate AhR. Natural AhR ligands are mainly found in plants, including quercetin in apples, resveratrol in red wine and indole-3-carbinol (I3C) in cruciferous plants such as broccoli and Brussel’s sprouts,Citation11-Citation13 which can be converted into high-affinity AhR ligands in the acidic environment of digestive tract. Numerous studies have shown a key role of AhR in mediating toxicity of low-molecular-weight chemicals. However, the importance of AhR in regulating immune system has recently become obvious.
Generation of Ahr-deficient mice revealed various physiological roles of AhR. Ahr-deficient mice have been made independently by three groups. Either exon1Citation14,Citation15 or exon2Citation16 was deleted. Ahr-deficient mice used in our study are generated in the laboratory of Bradfield.Citation16 All of the different Ahr-deficient mice are unable to induce xenobiotic metabolizing enzymes in responses to dioxin. Moreover, a common phenotype for all of these mice is the liver pathology, such as smaller livers, abnormal vasculature, abnormal hepatic circulation and portal tract fibrosis albeit with variable levels.Citation17,Citation18 Mice generated in the laboratory of Gonzalez also showed immune phenotype, such as reduced numbers of lymphocytes in the spleen and lymph nodes.Citation14 However, this phenotype was not seen in mice generated by other groups. At the time Ahr-deficient mice were generated, they were on a mixed genetic background, possibly causing the controversies in their phenotypes. All the Ahr-deficient mice are nowadays on a pure B6 background. Recently, a broader role of AhR in immune system has been characterized. Most importantly, AhR has been shown to be crucial for the development and function of Th17 cells and T regulatory cells depending on the activating ligand present.Citation19,Citation20
Role of Aryl Hydrocarbon Receptor in the Development of Lymphoid Follicles
AhR has been found to be expressed in RORγt+ ILC.Citation3,Citation21,Citation22 In fact, using Ahr-deficient mice we and others observed that AhR plays an essential role for RORγt+ ILC function and maintenance.Citation22-Citation24 The absence of AhR leads to failure to form intestinal lymphoid clusters, such as CP and ILF.Citation22,Citation23 Moreover, specific deletion of Ahr in RORγt+ ILC resulted in a similar impairment of lymphoid cluster formation in the small intestine, providing the evidence of an intrinsic effect of AhR on RORγt+ ILC (). AhR signaling did not affect the fetal RORγt+ ILC, since the prenatally formed lymph nodes and Peyer’s patches developed normally in the absence of AhR.Citation22-Citation24 RORγt+ ILC were also present in normal numbers in the lymph nodes and in the spleen of the Ahr-deficient mice, indicating that AhR signaling is not needed for the differentiation and maintenance of RORγt+ ILC at non-mucosal sites.Citation22,Citation23
Figure 1. Aryl Hydrocarbon Receptor (AhR) activity in RORγt+ ILC is essential for the first step of lymphoid follicle formation in the gut. Intestinal lymphoid follicles such as cryptopatches (CP) appear early after birth via the recruitment and the expansion of RORγt+ ILC at site. This step is independent of the presence of the indigenous microbiota in the gut. After CP development, invading microbiota drives some of them to further evolve into isolated lymphoid follicles (ILF) via the recruitment of plasma cells. Importantly, RORγt+ ILC expansion seems to be dependent on external factors such as nutrients. RORγt+ ILC, in particular the CD4-negative cell subset, are able to sense variation in phytochemical compound in the diet via the AhR. Activation of AhR induces upregulation of Kit and potentially Notch2 and IL-7R on RORγt+ ILC, supporting their maintenance. In Ahr-deficient mice, CD4- RORγt+ ILC cannot survive and expand leading to a lack of intestinal lymphoid follicle formation.
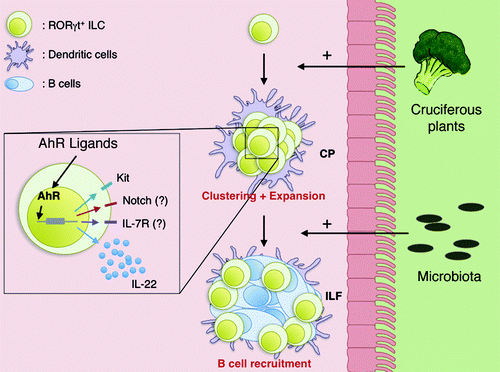
RORγt+ ILC are a heterogeneous population of CD4- and CD4+ RORγt+ ILC.Citation7,Citation25 Fetal RORγt+ ILC are described to be CD4+ 26. In contrast, RORγt+ ILC population in the adult small intestine is mainly CD4- 7, 25. The function of these different populations remains elusive. At birth, CD4- and CD4+ RORγt+ ILC are equally represented.Citation22,Citation25 The absolute numbers of CD4+ RORγt+ ILC remain stable after birth, but CD4- RORγt+ ILC expand strongly over time.Citation22 In newborn Ahr-deficient mice, RORγt+ ILC are present in normal numbers in the small intestine. However, the postnatal expansion of CD4- RORγt+ ILC is highly dependent on AhR activity.Citation22 Thus, these results demonstrate that AhR is not required for the prenatal development of RORγt+ ILC, but rather for the postnatal expansion of CD4- RORγt+ ILC in the intestine.
AhR Function in RORγt+ ILC Homeostasis
AhR is known to regulate the expression of numerous genes, including potential candidate genes required for the development and/or maintenance of RORγt+ ILC (). Our data show that Ahr-deficient RORγt+ ILC proliferate less and express lower levels of receptor tyrosine kinase, Kit.Citation22 Kit and its ligand, stem cell factor (SCF), have been shown to be important for the maintenance of intestinal RORγt+ ILC.Citation27 Interestingly, addition of synthetic AhR ligands in in vitro cultures induced upregulation of Kit expression by RORγt+ ILC.Citation22 In fact, we could show that AhR interacts directly with the two canonical XRE-elements found in the Kit promoter and induces its transcription. Qiu et al. reported that the IL-7 signaling is impaired in the absence of AhR and might cause poor maintenance of RORγt+ ILC.Citation24 They showed that expression of IL-7 was reduced in the colon epithelium of Ahr-deficient mice. However, the decrease of epithelial derived IL-7 is unlikely to affect the phenotype seen in Ahr-deficient mice, as the effect of AhR was observed to be cell-intrinsic.Citation22,Citation23 Additionally, IL-7R expression on RORγt+ ILC was found to be decreased in the absence of AhR.Citation24 It is known that the numbers of RORγt+ ILC in the intestine are strongly reduced in the absence of IL-7R signaling and that IL-7 is needed for the stabilization of the RORγt expression in RORγt+ ILC.Citation7,Citation28 Therefore, the reduced expression of IL-7R on RORγt+ ILC together with the decreased IL-7 expression in the epithelium of Ahr-deficient mice might partially be the cause of the poor maintenance of RORγt+ ILC. However, no significant reduction of IL-7R expression by RORγt+ ILC was observed in two other studies.Citation22,Citation23 In addition, increased apoptosis of RORγt+ ILC and in line with this, decreased expression of antiapoptotic genes such as Bcl2 and Bcl2l1 by RORγt+ ILC were associated with the lack of AhR.Citation24
In intestinal tissues, a subset of RORγt+ ILC expressing NK-cell receptors (NKR) was also observed to be dependent on AhR signaling for its maintenance.Citation23 This subset of cells was described to differentiate from NKR- RORγt+ ILCCitation7 and produce high levels of IL-22.Citation3-Citation6 AhR was suggested to regulate the pool size of NKR+ RORγt+ ILC via Notch pathway.Citation23 Notch genes have been identified as AhR target genes.Citation29,Citation30 Consistently, Notch and a target gene of Notch signaling, Hes-1, were found to be upregulated in AhR expressing human NKR+ RORγt+ ILC when compared with the conventional NK cells which do not express AhR.Citation3 Interestingly, Notch2 has been shown to be required for differentiation of RORγt+ ILC from the precursors in vitro.Citation31 Indeed, mice lacking Notch expression in the hematopoietic compartment had an impaired development of NKR+ RORγt+ ILC.Citation23 It remains to be determined whether Notch signaling-driven maturation of NKR+ RORγt+ ILC is cell-intrinsic and regulated by AhR signaling.
In addition to the altered gene expression in Ahr-deficient mice, lack of AhR may interfere with the colonization of microbiota in the intestine, affecting in turn the development and/or maintenance of RORγt+ ILC. In fact, Ahr-deficient mice were observed to have an increased bacterial load in the small intestine but not in the colon.Citation32 This higher bacterial load was associated with an increase in the phylum Bacteroidetes. Other phyla, such as Firmicutes, Actinomyces or Proteobacteria were not altered in Ahr-deficient mice. Another study could not observe any difference in bacterial burden when comparing Ahr-proficient to Ahr-deficient mice.Citation24 By now, all Ahr-deficient mice are on a pure C57BL/6 genetic background. Thus, these controversial results could not be explained by a mixed genetic background but rather by a different experimental setup. Indeed, the first study investigated the amount and composition of bacteria in a tissue (i.e., small intestine or colon), whereas the second study analyzed the amount of bacteria in fecal samples. Moreover, the housing of mice in different animal facilities associated with a different rodent diet may cause variations in the microbiota. Nevertheless, altered microbiota in the absence of AhR are unlikely to cause the phenotype seen in Ahr-deficient mice. It is known that commensal microflora is not needed for the development of RORγt+ ILC, as in the germ free mice cryptopatches and RORγt+ ILC are normally present.Citation8,Citation10
AhR Sensing of Dietary Compounds Regulates RORγt+ ILC
Transcriptional activity of AhR requires an interaction with one of its ligands (). The first possible source of AhR ligands after birth is the mother milk. To our current knowledge, there is no data available regarding the influence of mother milk on the expansion of RORγt+ ILC in the gut. Milk itself is not known to naturally contain any AhR ligands. However, milk is a potential source of synthetic AhR ligands derived from the environment and natural AhR ligands derived from the maternal diet.
Table 1. Representative AhR ligands
A significant source of AhR ligands are the plant-derived compounds of diets (). Indeed, AhR dietary ligands were observed to be important for the postnatal expansion of RORγt+ ILC and for the development of intestinal lymphoid follicles.Citation22 Synthetic mouse diet devoid of plant products lacks AhR ligands. Mice fed with a synthetic diet had lower levels of Kit expression in RORγt+ ILC, impaired postnatal expansion of RORγt+ ILC and delayed formation of the intestinal lymphoid follicles. Importantly, all these parameters could be rescued with addition of one AhR ligand to the synthetic diet. However, the lack of AhR ligands in the diet could only delay the formation of intestinal lymphoid organs. This finding indicates that at later time points other sources of AhR ligands (e.g., bacterial origin) can compensate the lack of dietary AhR ligands. Bacteria-derived ligands are unlikely to be the only maintenance regulators of RORγt+ ILC, as in germ free mice the numbers of RORγt+ ILC are not changed and cryptopatches develop normally.Citation8,Citation10 However, bacteria-derived ligands might together with the diet-derived ligands contribute to the maintenance of RORγt+ ILC. Intriguingly, AhR dietary ligands have also been shown to regulate the maintenance of γδ T cells in epithelium of the intestine.Citation32 This study additionally showed that the absence of γδ T cells due to the lack of AhR resulted in an increased amount of bacteria in the small intestine, an elevated activation of immune system and an increased susceptibility to DSS induced epithelial damage.Citation32 Together, these studies demonstrate the importance of diet in regulating homeostasis of intestinal immune system and consequently in affecting the progression of intestinal diseases.
In addition to dietary AhR ligands, endogenous AhR agonists may be important in the maintenance of RORγt+ ILC. Arachidonic acid metabolites, heme metabolites and tryptophan metabolites belong to endogenous AhR ligands.Citation13 However, these compounds rarely reach the concentration needed for activation of AhR. Yet, it is likely that endogenous AhR ligands contribute to the maintenance of RORγt+ ILC. In the future, the function of endogenous AhR ligands will be an important issue to analyze in more detail.
The Role of AhR in Intestinal Inflammation
The importance of RORγt+ ILC in Citrobacter (C.) rodentium infection is well established.Citation33 C. rodentium belongs to an attaching and effacing (A/E) family of bacteria and is the only A/E pathogen that infects mice. Therefore, C. rodentium has become an important mouse model to study human pathogens, such as enteropathogenic E. coli (EPEC) and enterohemorrhagic E. coli (EHEC).Citation34,Citation35 IL-22 is necessary for the early phase of host defense against C. rodentium infection.Citation36 The increased IL-23 levels during C. rodentium infection result in an enhanced IL-22 production mainly by RORγt+ ILC. Consequently, enhanced IL-22 production leads to an increased expression of antimicrobial peptides of regenerating islet-derived 3 (Reg3) family.Citation33,Citation36
AhR activation is known to regulate IL-22 production by Th17 cells.Citation19 Consistently, RORγt+ ILC deficient for AhR produce less IL-22.Citation22-Citation24 Ahr-deficient mice suffered from severe inflammation after C. rodentium infection due to a diminished IL-22 production by RORγt+ ILC and due to reduced numbers of IL-22 producing cells. Qiu et al. demonstrated that AhR controls directly IL-22 expression.Citation24 Il22 locus contains several XRE-elements for AhR binding, which are clustered with ROR-responsive elements (RORE). Qiu et al. showed by using Chromatin IP (ChIP) assays with T cell line that RORγt interacts directly with Il22 locus. When constitutively active AhR was expressed alone, no binding to Il22 locus was detected. However, when AhR and RORγt were expressed together, AhR interacted with the IL-22 promoter. This finding indicates that RORγt facilitates binding of AhR to Il22 locus. In fact, AhR and RORγt were shown to physically interact with each other. In addition, coexpression of AhR and RORγt induced synergistically an upregulation of IL-22 transcription.
Despite having strongly decreased numbers of RORγt+ ILC, Ahr-deficient mice have a tendency to develop RORγt+ ILC–less B cell follicles in the colon.Citation22 Similar follicles have been described in the colon of Rorc(γt)−/− mice lacking all RORγt+ ILC.Citation37 Formation of these B cell follicles was dependent on the lymphotoxin α1β2 expression on B cells and was enhanced during inflammation.Citation37 Interestingly, RORγt+ ILC–less clusters were shown to contain inappropriately activated B cells and to promote colitis. In addition to the lack of protective IL-22 due to the failing postnatal expansion of RORγt+ ILC, the presence of inflammation-promoting B cell clusters in Ahr-deficient mice might enhance the severity of C. rodentium infection-induced inflammation.Citation22-Citation24 Interestingly, older Ahr-deficient mice are prone to develop anal prolapse, indicating the existence of an intestinal inflammation (E.A.K. and C.V., unpublished data). It is attractive to speculate on the role of increased numbers of the RORγt+ ILC–less B cell follicles and the lack of protective IL-22 in Ahr-deficient mice in promoting the development of this spontaneous intestinal inflammation.
Intriguingly, recent data suggest that AhR is also involved in human intestinal inflammations. However, the results from studies with Crohn’s disease (CD) patients are contradictory. In one study, the expression of AhR was found to be increased in mucosal tissues of CD patients.Citation38 In contrast, another study showed that the cells from lamina propria of CD patients had a decreased expression of AhR in comparison to healthy controls.Citation39 The reason for such discrepancies might be a different methodology used for AhR expression assessment. Additionally, different environmental and lifestyle factors, such as exposure to environmental toxins and cigarette smoking, may influence the results. Supplementary studies are needed to better comprehend the role of AhR in inflammatory bowel disease. However, it has been already shown that low consumption of fruits and vegetables in children is associated with CD. These data indicate that diet with high amount of AhR ligands has an effect on intestinal inflammations also in humans.Citation40
Future work will reveal whether AhR signaling in humans has a similar role as in mice. It remains to be investigated whether AhR signaling in humans transmits the beneficial effects of vegetable rich diets and promotes the RORγt+ ILC mediated intestinal homeostasis. Nevertheless, understanding the role of AhR and the environmental factors in the regulation of mucosal intestinal homeostasis will provide helpful knowledge to better characterize the mechanisms behind intestinal inflammations.
Acknowledgments
We thank A. Diefenbach and the members of the Diefenbach laboratory for support and valuable discussions and J. Kiss for comments on the manuscript. Work in the Diefenbach laboratory is supported by the Deutsche Forschungsgemeinschaft (SGBM, GRK1104, SFB620/A14 to E.A.K.) and Centrum für Chronische Immundefizienz (to C.V.).
References
- Sanos SL, Vonarbourg C, Mortha A, Diefenbach A. Control of epithelial cell function by interleukin-22-producing RORγt+ innate lymphoid cells. Immunology 2011; 132:453 - 65; http://dx.doi.org/10.1111/j.1365-2567.2011.03410.x; PMID: 21391996
- Spits H, Cupedo T. Innate lymphoid cells: emerging insights in development, lineage relationships, and function. Annu Rev Immunol 2012; 30:647 - 75; http://dx.doi.org/10.1146/annurev-immunol-020711-075053; PMID: 22224763
- Cella M, Fuchs A, Vermi W, Facchetti F, Otero K, Lennerz JK, et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 2009; 457:722 - 5; http://dx.doi.org/10.1038/nature07537; PMID: 18978771
- Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, et al. Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat Immunol 2009; 10:75 - 82; http://dx.doi.org/10.1038/ni.1681; PMID: 19029904
- Sanos SL, Bui VL, Mortha A, Oberle K, Heners C, Johner C, et al. RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat Immunol 2009; 10:83 - 91; http://dx.doi.org/10.1038/ni.1684; PMID: 19029903
- Satoh-Takayama N, Vosshenrich CA, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 2008; 29:958 - 70; http://dx.doi.org/10.1016/j.immuni.2008.11.001; PMID: 19084435
- Vonarbourg C, Mortha A, Bui VL, Hernandez PP, Kiss EA, Hoyler T, et al. Regulated expression of nuclear receptor RORγt confers distinct functional fates to NK cell receptor-expressing RORγt(+) innate lymphocytes. Immunity 2010; 33:736 - 51; http://dx.doi.org/10.1016/j.immuni.2010.10.017; PMID: 21093318
- Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, et al. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med 1996; 184:1449 - 59; http://dx.doi.org/10.1084/jem.184.4.1449; PMID: 8879216
- Eberl G, Littman DR. Thymic origin of intestinal alphabeta T cells revealed by fate mapping of RORgammat+ cells. Science 2004; 305:248 - 51; http://dx.doi.org/10.1126/science.1096472; PMID: 15247480
- Pabst O, Herbrand H, Friedrichsen M, Velaga S, Dorsch M, Berhardt G, et al. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol 2006; 177:6824 - 32; PMID: 17082596
- Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol 2009; 30:447 - 54; http://dx.doi.org/10.1016/j.it.2009.06.005; PMID: 19699679
- Stevens EA, Mezrich JD, Bradfield CA. The aryl hydrocarbon receptor: a perspective on potential roles in the immune system. Immunology 2009; 127:299 - 311; http://dx.doi.org/10.1111/j.1365-2567.2009.03054.x; PMID: 19538249
- Nguyen LP, Bradfield CA. The search for endogenous activators of the aryl hydrocarbon receptor. Chem Res Toxicol 2008; 21:102 - 16; http://dx.doi.org/10.1021/tx7001965; PMID: 18076143
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, et al. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science 1995; 268:722 - 6; http://dx.doi.org/10.1126/science.7732381; PMID: 7732381
- Mimura J, Yamashita K, Nakamura K, Morita M, Takagi TN, Nakao K, et al. Loss of teratogenic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the Ah (dioxin) receptor. Genes Cells 1997; 2:645 - 54; http://dx.doi.org/10.1046/j.1365-2443.1997.1490345.x; PMID: 9427285
- Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A 1996; 93:6731 - 6; http://dx.doi.org/10.1073/pnas.93.13.6731; PMID: 8692887
- Lahvis GP, Bradfield CA. Ahr null alleles: distinctive or different?. Biochem Pharmacol 1998; 56:781 - 7; http://dx.doi.org/10.1016/S0006-2952(98)00134-8; PMID: 9774139
- Esser C. The immune phenotype of AhR null mouse mutants: not a simple mirror of xenobiotic receptor over-activation. Biochem Pharmacol 2009; 77:597 - 607; http://dx.doi.org/10.1016/j.bcp.2008.10.002; PMID: 18983984
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008; 453:106 - 9; http://dx.doi.org/10.1038/nature06881; PMID: 18362914
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008; 453:65 - 71; http://dx.doi.org/10.1038/nature06880; PMID: 18362915
- Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity 2010; 32:803 - 14; http://dx.doi.org/10.1016/j.immuni.2010.06.007; PMID: 20620944
- Kiss EA, Vonarbourg C, Kopfmann S, Hobeika E, Finke D, Esser C, et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 2011; 334:1561 - 5; http://dx.doi.org/10.1126/science.1214914; PMID: 22033518
- Lee JS, Cella M, McDonald KG, Garlanda C, Kennedy GD, Nukaya M, et al. AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat Immunol 2011; http://dx.doi.org/10.1038/ni.2187; PMID: 22101730
- Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, et al. The Aryl Hydrocarbon Receptor Regulates Gut Immunity through Modulation of Innate Lymphoid Cells. Immunity 2011; PMID: 22177117
- Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, et al. Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science 2010; 330:665 - 9; http://dx.doi.org/10.1126/science.1194597; PMID: 20929731
- Mebius RE, Rennert P, Weissman IL. Developing lymph nodes collect CD4+CD3- LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 1997; 7:493 - 504; http://dx.doi.org/10.1016/S1074-7613(00)80371-4; PMID: 9354470
- Chappaz S, Gärtner C, Rodewald HR, Finke D. Kit ligand and Il7 differentially regulate Peyer’s patch and lymph node development. J Immunol 2010; 185:3514 - 9; http://dx.doi.org/10.4049/jimmunol.1000665; PMID: 20709954
- Satoh-Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich CA, et al. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med 2010; 207:273 - 80; http://dx.doi.org/10.1084/jem.20092029; PMID: 20142427
- Boverhof DR, Burgoon LD, Tashiro C, Sharratt B, Chittim B, Harkema JR, et al. Comparative toxicogenomic analysis of the hepatotoxic effects of TCDD in Sprague Dawley rats and C57BL/6 mice. Toxicol Sci 2006; 94:398 - 416; http://dx.doi.org/10.1093/toxsci/kfl100; PMID: 16960034
- Dere E, Lo R, Celius T, Matthews J, Zacharewski TR. Integration of genome-wide computation DRE search, AhR ChIP-chip and gene expression analyses of TCDD-elicited responses in the mouse liver. BMC Genomics 2011; 12:365; http://dx.doi.org/10.1186/1471-2164-12-365; PMID: 21762485
- Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, et al. Notch signaling is necessary for adult, but not fetal, development of RORγt(+) innate lymphoid cells. Nat Immunol 2011; 12:949 - 58; http://dx.doi.org/10.1038/ni.2105; PMID: 21909092
- Li Y, Innocentin S, Withers DR, Roberts NA, Gallagher AR, Grigorieva EF, et al. Exogenous stimuli maintain intraepithelial lymphocytes via aryl hydrocarbon receptor activation. Cell 2011; 147:629 - 40; http://dx.doi.org/10.1016/j.cell.2011.09.025; PMID: 21999944
- Sonnenberg GF, Monticelli LA, Elloso MM, Fouser LA, Artis D. CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 2011; 34:122 - 34; http://dx.doi.org/10.1016/j.immuni.2010.12.009; PMID: 21194981
- Schauer DB, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun 1993; 61:2486 - 92; PMID: 8500884
- Schauer DB, Falkow S. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect Immun 1993; 61:4654 - 61; PMID: 8406863
- Zheng Y, Valdez PA, Danilenko DM, Hu Y, Sa SM, Gong Q, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med 2008; 14:282 - 9; http://dx.doi.org/10.1038/nm1720; PMID: 18264109
- Lochner M, Ohnmacht C, Presley L, Bruhns P, Si-Tahar M, Sawa S, et al. Microbiota-induced tertiary lymphoid tissues aggravate inflammatory disease in the absence of RORgamma t and LTi cells. J Exp Med 2011; 208:125 - 34; http://dx.doi.org/10.1084/jem.20100052; PMID: 21173107
- Arsenescu R, Arsenescu V, Zhong J, Nasser M, Melinte R, Dingle RW, et al. Role of the xenobiotic receptor in inflammatory bowel disease. Inflamm Bowel Dis 2011; 17:1149 - 62; http://dx.doi.org/10.1002/ibd.21463; PMID: 20878756
- Monteleone I, Rizzo A, Sarra M, Sica G, Sileri P, Biancone L, et al. Aryl hydrocarbon receptor-induced signals up-regulate IL-22 production and inhibit inflammation in the gastrointestinal tract. Gastroenterology 2011; 141:237 - 48, 248, e1; http://dx.doi.org/10.1053/j.gastro.2011.04.007; PMID: 21600206
- D’Souza S, Levy E, Mack D, Israel D, Lambrette P, Ghadirian P, et al. Dietary patterns and risk for Crohn’s disease in children. Inflamm Bowel Dis 2008; 14:367 - 73; http://dx.doi.org/10.1002/ibd.20333; PMID: 18092347
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol 2003; 43:309 - 34; http://dx.doi.org/10.1146/annurev.pharmtox.43.100901.135828; PMID: 12540743