Abstract
Recently, we developed a novel automated, high throughput screening (HTS) methodology for the anaerobic intestinal parasite Entamoeba histolytica. We validated this HTS platform by screening a chemical library containing US Food and Drug Administration (FDA)-approved drugs and bioactive compounds. We identified an FDA-approved drug, auranofin, as most active against E. histolytica both in vitro and in vivo. Our cell culture and animal studies indicated that thioredoxin reductase, an enzyme involved in reactive oxygen species detoxification, was the target for auranofin in E. histolytica. Here, we discuss the rationale for drug development for three parasites which are major causes of diarrhea worldwide, E. histolytica, Giardia lamblia and Cryptosporidium parvum and extend our current finding of antiparasitic activity of auranofin to Entamoeba cysts, G. lamblia and C. parvum. These studies support the use of HTS assays and reprofiling FDA-approved drugs for new therapy for neglected tropical diseases.
Introduction
E. histolytica, G. lamblia, and Cryptosporidium sp are ancient protozoan parasites that cause amebiasis, giardiasis and cryptosporidiosis, three of the most common diarrheal diseases worldwide. The earliest record of amebiasis is possibly from the Sanskrit document Brigu-samhita, written about 1000 BC, which refers to bloody mucose diarrhea.Citation1,Citation2 Hippocrates (460 to 377 BC) recognized a patient with dysentery and fever, possibly suffering from amebiasis.Citation3 One coprolite of human origin, collected from excavations along the north-central coast of Peru, dated between ca. 2375 and 1525 BC contained Giardia sp cysts, demonstrating that Giardia has been infecting humans since prehistoric times.Citation4 Cryptosporidium sp was identified in Andean mummies with reported dates of 500 to 3,000 y ago.Citation5 Infections with these parasites are major causes of morbidity and mortality in tropical countries. Approximately 500 million people worldwide are infected annually with E. histolytica, resulting in 50 million cases of invasive diseaseCitation6 and about 70,000 annual deaths.Citation7 Giardiasis has an estimated worldwide prevalence of 280 million cases annually. Furthermore, giardial infections contribute substantially to the 2.5 million annual deaths from diarrheal disease.Citation8,Citation9 In Asia, Africa and Latin America, around 500,000 new giardiasis cases are reported each year.Citation8 Cryptosporidium accounts for about 20% and up to 9% of diarrheal episodes in children in developing and developed countries, respectively.Citation10 Infection with Cryptosporidium can be the most chronic and debilitating in immunosuppressed individuals and malnourished children.Citation11 Because of its link with poverty, Giardia and Cryptosporidium were included in the WHO Neglected Diseases Initiative in 2004.Citation12E. histolytica, G. lamblia and C. parvum have been listed by the National Institutes of Health (NIH) as category B priority biodefense pathogens due to low infectious dose and potential for dissemination through compromised food and water supplies in the United States. Despite the prevalence of amebiasis, giardiasis and cryptosporidiosis there are no vaccines or prophylactic drugs. The first-line drugs for invasive amebiasis and giardiasis chemotherapy are nitroimidazoles, with the prototype, metronidazole, being the most common drug used worldwide.Citation13 Newer metronidazole derivatives such as tinidazoleCitation14 and nitazoxanide, a nitrothiazoly-salicylamide derivative,Citation15 have fewer side-effects and are shorter treatment courses. Other drugs, such as furazolidone, albendazole and paromomycin are used for giardiasis to a lesser extent with similar and/or lower success rates. Metronidazole has been shown to be both mutagenic in a microbiological system and carcinogenic to rodentsCitation16 and frequently causes gastrointestinal side effects.Citation17 In vitro E. histolytica trophozoites easily adapt to therapeutically relevant levels of metronidazole.Citation18 In spite of the efficacy of nitroimidazole drugs, treatment failures in giardiasis occur in up to 20% of cases.Citation19 Clinical resistance of G. lamblia to metronidazole is proven and cross resistance occurs to the newer drugs, tinidazole and nitazoxanide,Citation9 so drug resistance is a concern with all commonly used antigiardial drugs.Citation9,Citation19 Nitazoxanide, the only FDA-approved drug for the treatment of cryptosporidiosis, is effective in the treatment of immunocompetent patientsCitation20 and partially effective for immunosuppressed patients.Citation11 Therefore, it is critical to search for more effective drugs to treat amebiasis, giardiasis and cryptosporidiosis.
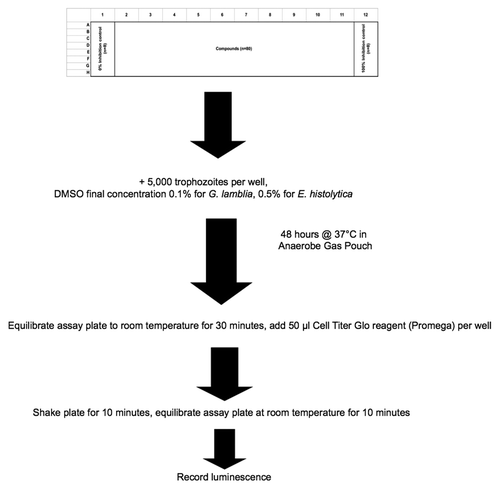
High Throughput Drug Screen: Single Assay for Two Anaerobic Parasites
Traditionally, research on drug development in E. histolytica employed two approaches: (1) identification of molecular targets and (2) measurement of compound efficacy by phenotypic screening in vitro and/or animal models of disease. The latter approach is usually without specific knowledge of the target and/or mechanism of action or for which bioactivity has been characterized in other parasitological or biomedical settings. Both are of proven value but the pace of discovery with these techniques is somewhat slow, relying on discrete, focused compound sets or small number of compounds with known pharmacological actions.Citation21 To accelerate the identification of better drugs, we developed an HTS that used a rapid readout assay and could be conducted in an oxygen-free environment, to mimic the natural habitat of E. histolytica trophozoites. We developed this automated screen for compound libraries for activity against E. histolytica for the first time.Citation22
Like E. histolytica, G. lamblia is an anaerobic parasite and our automated assay developed for E. histolytica can also be used for screening compounds against G. lamblia (). Thus, the use of similar assays for two different parasites can potentially decrease the time required for screening a large number of chemical compounds.
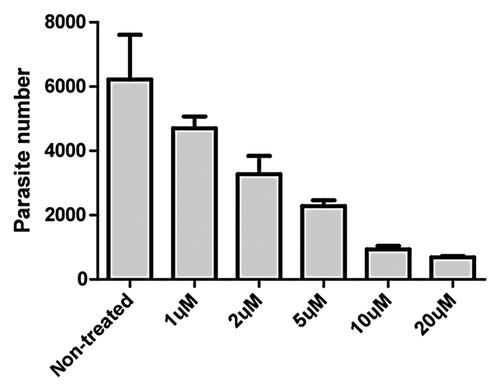
In vitro Compound Efficacy Study for C. parvum
Human colorectal adenocarcinoma cell line (HCT-8) was used for the C. parvum in vitro model of infection.Citation23 We selected this cell line because these cells were from the ileocecal region of the intestines, corresponding to the site of human infection in the ileum. The experiment was performed in a 24-well microtiter plate, and the cells were treated with 1, 2, 5, 10 and 20 μM of compounds for 48 h. In order to quantify compound efficacy against C. parvum, we looked at the ratio of parasite burden between compound-treated wells to the untreated wells. To achieve this, we quantified the amount of parasite DNA per well by real-time PCRCitation24 using C. parvum heat shock protein 70 (Hsp70)-specific primers. In order to associate a threshold cycle number to a concentration of parasites, a standard curve was generated by real-time PCR using DNA from serially diluted C. parvum oocysts ranging from 107 parasites/mL to 101 parasites/mL.
Killing Three Parasites with One Drug
Once we were able to design a high throughput assay for drug screening with E. histolytica, our next step was to screen large compound libraries, including drugs that were already approved for other uses by the FDA. The screening of existing drugs for new purposes has several advantages. First, these drugs have an established safety record that can save significant time in the development process. Second, the combination of an off-patent drug, known clinical safety and possible low production costs can potentially bring down drug pricing to make reprofiled drugs affordable throughout the world. This is particularly important with neglected tropical diseases, which are not a priority for drug companies. The screening of a chemical library of 910 compounds, consisting of 746 approved drugs and 164 bioactive compounds, identified auranofin as the most potent of all against E. histolytica with the half-maximal effective concentration (EC50) 10-fold better in vitro than metronidazole (0.5 vs. 5 μM). Oral auranofin at 1 mg/kg/day and 3 mg/kg/day for 7 d significantly decreased the number of parasites and inflammation in a mouse model of colitis and a hamster model of amebic liver abscess, respectively, while the same dose of metronidazole did not.
Auranofin was equally active against G. lamblia, both in vitro and in vivo, and the drug showed efficacy at low concentrations against divergent G. lamblia isolates (Tejman-Yarden et al., manuscript submitted). Most importantly, auranofin overrode the metronidazole resistance in Giardia and the EC50 of auranofin for Giardia was not significantly different between metronidazole-sensitive parental isolates and their metronidazole-resistant isogenic derivative lines, indicating that auranofin may be a therapeutic option for giardiasis where metronidazole-resistance is linked to clinical failure.
Auranofin was found efficacious in vitro against C. parvum with EC50 about 2 μM (), which was comparable to nitazoxanide,Citation24 the current drug of choice.
Auranofin is Cysticidal
Although millions of people are infected by E. histolytica each year, most individuals remain asymptomatic but may transmit amebiasis through fecal excretion of infective cysts.Citation25 Therefore, asymptomatic E. histolytica intestinal carriage should also be treated because of its potential for causing invasive disease and public health risks.Citation10,Citation26,Citation27 Because metronidazole is readily and almost completely absorbed from the gastrointestinal tract,Citation28 it lacks cysticidal activity against E. histolytica. Therefore, an additional drug, paromomycin, which is poorly absorbed from the gastrointestinal tract, is recommended to treat patient with intestinal and asymptomatic infections following metronidazole.Citation29 The relapse rate has been reported to be less with tinidazole,Citation14 but its efficacy with cysts has not been evaluated. We evaluated the effects of auranofin on cysts of Entamoeba invadens, a closely related parasite that readily encysts. In vitro generated E. invadens cysts were killed within 72 h with auranofin, but not metronidazole (Rosa Andrade, personal communication).Citation30 This study shows that auranofin has cysticidal activity and may allow for shorter treatment courses.
Background of Auranofin
Auranofin (Ridaura®) was the first oral gold-containing compound, which was approved in 1985 for the treatment of rheumatoid arthritis (RA) in adults and is off patent. It is the only oral formulation of a gold salt available for the treatment of RA for patients who have not responded adequately to one or more non-steroidal anti-inflammatory drugs. Auranofin was not approved for use in children, as it was not effective in the treatment of juvenile RA, but no additional safety issues were found in children.Citation31
The published pharmacokinetic data of auranofin comes from studies in rheumatoid arthritis patients following long-term therapy. Auranofin is rapidly metabolized so no intact drug can be detected, but gold levels have been measured. Following an oral dose, 25% of auranofin is absorbed, 60% is plasma protein bound and 85% excreted in feces.Citation32 Auranofin was approved for the long-term treatment of patients with unresponsive rheumatoid arthritis with courses for a minimum of 6 mo at doses of 3 mg once or twice a day. Steady-state levels were achieved in 8–12 weeks with an elimination half-life of 26 d.Citation32 Auranofin is contraindicated in patients with gold allergy and not recommended during pregnancy or severe hepatic or renal insufficiency. The only reported drug interaction is a single patient with elevated phenytoin blood levels. Since treatment for amebiasis or giardiasis is only 5–10 d, the likelihood of gold toxicity should be extremely small.
Auranofin has received Orphan Drug status for treatment of amebiasis from the FDA. Orphan drugs in the US are meant to develop the products for orphan or rare diseases for which drug development costs are unlikely to be recovered through sale in the United States.Citation33,Citation34 Amebiasis is a rare disease in the US, and its Orphan Drug status may bring several incentives such as tax credits totaling half of development costs, research and development grants, fast-track development and approval, access to Investigational New Drug (IND) Program and pre-approval, waived drug application fees and seven year market exclusivity.Citation33-Citation35 The IND application for use of auranofin for giardiasis has been granted by the FDA.
How Auranofin Works on a Molecular Level in Entamoeba and Giardia
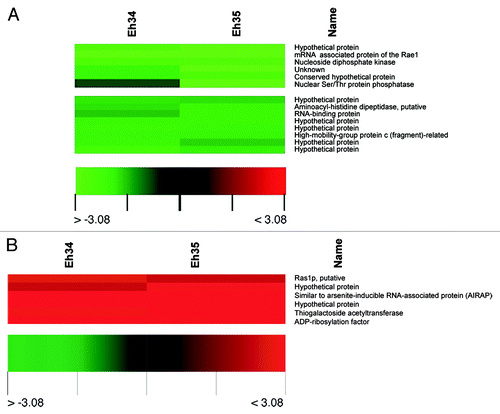
To examine the mechanism of auranofin activity in E. histolytica, transcriptional profiling using E. histolytica oligonucleotide microarrays identified auranofin-induced alterations in critical genes involved in mitosis, nucleotide metabolism, and signal transduction.Citation22 Significant downregulation of the transcript for mRNA associated protein of the Rae1 indicates that auranofin might have role in inhibition of mitotic spindle assemblyCitation36 in E. histolytica (). Nucleoside diphosphate kinases are one of the enzyme families related to cell energy management. These are enzymes involved in energy metabolism transferring high-energy phosphates between nucleosides di- and tri-phosphates, thus maintaining the nucleoside balance in the cell.Citation37 Our microarray data showed decreased expression of nucleoside diphosphate kinase in E. histolytica due to auranofin treatment, leading to decreased nucleotide metabolism (). This is consistent with previous findings where auranofin was evaluated in screening trials as a potential antineoplastic agent and was found to rapidly decrease DNA synthesis.Citation38 Besides transcriptional repression of a few genes, auranofin treatment leads to consistent upregulation of the mRNA levels of a protein similar to arsenite-inducible RNA-associated protein (AIRAP) (). AIRAP is unique among known arsenite-induced genes because its expression is not upregulated in response to other oxidants and is only modestly induced by exposure to other metals, such as zinc.Citation39 The upregulation of the transcript for a gene similar to that encoding AIRAP is not surprising since both arsenite and auranofin are inhibitors of thioredoxin reductase.Citation40-Citation43
Figure 3. Clustered display of data from a portion of E. histolytica microarray, indicating downregulated (A) and upregulated transcripts (B) due to auranofin treatment. Microarray hybridizations (A and B) were performed using RNA from DMSO-treated E. histolytica and 1 µM auranofin-treated E. histolytica. Data from related genes in two separate hybridizations (Eh34 and Eh35) were analyzed by use of Acuity software.Citation22
Auranofin had half-maximal inhibitory concentration (IC50) of 0.4 μM against active, recombinant E. histolytica thioredoxin reductase (EhTrxR). Since E. histolytica does not have a glutathione reductase system, EhTrxR is critical for protecting amebic trophozoites from oxidant attack. When trophozoites were exposed to auranofin, they became more sensitive to killing by H2O2, had increased intracellular reactive oxygen species and the intracellular thioredoxin was predominantly in the oxidized state, whereas the same concentration of metronidazole did not increase intracellular reactive oxygen species.Citation22 Leitsch et al.Citation44 had previously shown that metronidazole forms an adduct with four E. histolytica proteins, including thioredoxin reductase, decreasing the enzymatic activity. These findings suggest that thioredoxin reductase is a valid drug target in E. histolytica, which can be inhibited with auranofin.
Similarly, auranofin inhibited the activity of giardial thioredoxin oxidoreductase (Tejman-Yarden et al. submitted). Like Entamoeba, Giardia trophozoites also lack glutathione and their thioredoxin plays a major role to overcome oxidative stress while living in the small intestinal lumen.Citation45
Future of Auranofin for Amebiasis, Giardiasis and Cryptosporidiosis
Our study led to the development of the first HTS that could be performed in an anaerobic environment.Citation22 We validated this technology by screening a rational chemical library and in the process discovered a new drug lead, auranofin, for the treatment of amebiasis and giardiasis. By bringing a repurposed drug, auranofin, into clinical trials for amebiasis and giardiasis, the cost and development time can be significantly reduced. Since the drug has received the Orphan Drug status and is off patent, there will be future incentives for pharmaceutical companies to develop a drug for an orphan disease. Clinical trials of auranofin for giardiasis and amebiasis are being planned in adults and ultimately will be important in children as malnourished children are more susceptible to amebiasisCitation46 and giardiasis.Citation47,Citation48 If the clinical trials are successful, auranofin could be the first new drug with a defined target for the treatment of amebiasis and giardiasis in over 50 y. The in vitro efficacy of auranofin for Cryptosporidium holds promise for further in vivo testing in animals and subsequently in both immunocompetent and immunocompromised patients.
Acknowledgments
The authors thank financial support from the NIAID 5U01AI077822 and 5U01AI075527. Rosa Andrade, Noa Tejman-Yarden and Lars Eckmann are acknowledged for sharing unpublished data.
References
- Vaidya AB, Ray DK. Amoebiasis: the tropical scourge. Science Today (India) 1982 pp. 21-26.
- Cox FE. History of human parasitology. Clin Microbiol Rev 2002; 15:595 - 612; http://dx.doi.org/10.1128/CMR.15.4.595-612.2002; PMID: 12364371
- Tanyuksel M, Petri WA Jr.. Laboratory diagnosis of amebiasis. Clin Microbiol Rev 2003; 16:713 - 29; http://dx.doi.org/10.1128/CMR.16.4.713-729.2003; PMID: 14557296
- Ortega YR, Bonavia D. Cryptosporidium, Giardia, and Cyclospora in ancient Peruvians. J Parasitol 2003; 89:635 - 6; http://dx.doi.org/10.1645/GE-3083RN; PMID: 12880276
- Allison MJ, Bergman T, Gerszten E. Further studies on fecal parasites in antiquity. Am J Clin Pathol 1999; 112:605 - 9; PMID: 10549246
- WHO/PAHO/UNESCO report.. WHO/PAHO/UNESCO report. A consultation with experts on amoebiasis. Mexico City, Mexico 28-29 January, 1997. Epidemiol Bull 1997; 18:13 - 4; PMID: 9197085
- World Health Organization. The World Health Report 1998: life in the 21st century: a vision for all. Geneva: World Health Organization. 1998.
- Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev 2001; 14:447 - 75; http://dx.doi.org/10.1128/CMR.14.3.447-475.2001; PMID: 11432808
- Wright JM, Dunn LA, Upcroft P, Upcroft JA. Efficacy of antigiardial drugs. Expert Opin Drug Saf 2003; 2:529 - 41; http://dx.doi.org/10.1517/14740338.2.6.529; PMID: 14585063
- Fletcher SM, Stark D, Harkness J, Ellis J. Enteric protozoa in the developed world: a public health perspective. Clin Microbiol Rev 2012; 25:420 - 49; http://dx.doi.org/10.1128/CMR.05038-11; PMID: 22763633
- Cabada MM, White AC Jr.. Treatment of cryptosporidiosis: do we know what we think we know?. Curr Opin Infect Dis 2010; 23:494 - 9; http://dx.doi.org/10.1097/QCO.0b013e32833de052; PMID: 20689422
- Savioli L, Smith H, Thompson A. Giardia and Cryptosporidium join the ‘Neglected Diseases Initiative’. Trends Parasitol 2006; 22:203 - 8; http://dx.doi.org/10.1016/j.pt.2006.02.015; PMID: 16545611
- Freeman CD, Klutman NE, Lamp KC. Metronidazole. A therapeutic review and update. Drugs 1997; 54:679 - 708; http://dx.doi.org/10.2165/00003495-199754050-00003; PMID: 9360057
- Fung HB, Doan TL. Tinidazole: a nitroimidazole antiprotozoal agent. Clin Ther 2005; 27:1859 - 84; http://dx.doi.org/10.1016/j.clinthera.2005.12.012; PMID: 16507373
- Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Giardia intestinalis and Entamoeba histolytica or E. dispar: a randomized, double-blind, placebo-controlled study of nitazoxanide. J Infect Dis 2001; 184:381 - 4; http://dx.doi.org/10.1086/322038; PMID: 11443569
- Bendesky A, Menéndez D, Ostrosky-Wegman P. Is metronidazole carcinogenic?. Mutat Res 2002; 511:133 - 44; http://dx.doi.org/10.1016/S1383-5742(02)00007-8; PMID: 12052431
- Sweetman SC. Martindale: The Complete Drug Reference, 33rd ed. Pharmaceutical Press, London, 2002:594.
- Wassmann C, Hellberg A, Tannich E, Bruchhaus I. Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J Biol Chem 1999; 274:26051 - 6; http://dx.doi.org/10.1074/jbc.274.37.26051; PMID: 10473552
- Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev 2001; 14:150 - 64; http://dx.doi.org/10.1128/CMR.14.1.150-164.2001; PMID: 11148007
- Rossignol JF, Ayoub A, Ayers MS. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of Nitazoxanide. J Infect Dis 2001; 184:103 - 6; http://dx.doi.org/10.1086/321008; PMID: 11398117
- Abdulla MH, Ruelas DS, Wolff B, Snedecor J, Lim KC, Xu F, et al. Drug discovery for schistosomiasis: hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PLoS Negl Trop Dis 2009; 3:e478; http://dx.doi.org/10.1371/journal.pntd.0000478; PMID: 19597541
- Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nat Med 2012; 18:956 - 60; http://dx.doi.org/10.1038/nm.2758; PMID: 22610278
- von Oettingen J, Nath-Chowdhury M, Ward BJ, Rodloff AC, Arrowood MJ, Ndao M. High-yield amplification of Cryptosporidium parvum in interferon gamma receptor knockout mice. Parasitology 2008; 135:1151 - 6; http://dx.doi.org/10.1017/S0031182008004757; PMID: 18667105
- Cai X, Woods KM, Upton SJ, Zhu G. Application of quantitative real-time reverse transcription-PCR in assessing drug efficacy against the intracellular pathogen Cryptosporidium parvum in vitro. Antimicrob Agents Chemother 2005; 49:4437 - 42; http://dx.doi.org/10.1128/AAC.49.11.4437-4442.2005; PMID: 16251280
- Stauffer W, Ravdin JI. Entamoeba histolytica: an update. Curr Opin Infect Dis 2003; 16:479 - 85; http://dx.doi.org/10.1097/00001432-200310000-00016; PMID: 14502002
- Stanley SL Jr.. Amoebiasis. Lancet 2003; 361:1025 - 34; http://dx.doi.org/10.1016/S0140-6736(03)12830-9; PMID: 12660071
- Pritt BS, Clark CG. Amebiasis. Mayo Clin Proc 2008; 83:1154 - 9, quiz 1159-60; http://dx.doi.org/10.4065/83.10.1154; PMID: 18828976
- Reynolds JEF. Martindale: The Extra Pharmacopoeia, 30th ed. Pharmaceutical Press, London, 1993:516-21.
- Haque R, Huston CD, Hughes M, Houpt E, Petri WA Jr.. Amebiasis. N Engl J Med 2003; 348:1565 - 73; http://dx.doi.org/10.1056/NEJMra022710; PMID: 12700377
- Buckner FS, Waters NC, Avery VM. Recent highlights in anti-protozoan drug development and resistance research. Int J Parasitol: Drugs and Drug Res 2012; In press http://dx.doi.org/10.1016/j.ijpddr.2012.05.002
- Giannini EH, Brewer EJ Jr., Kuzmina N, Shaikov A, Wallin B, The USA Pediatric Rheumatology Collaborative Study Group. The USSR Cooperative Children’s Study Group. Auranofin in the treatment of juvenile rheumatoid arthritis. Results of the USA-USSR double-blind, placebo-controlled trial. Arthritis Rheum 1990; 33:466 - 76; http://dx.doi.org/10.1002/art.1780330402; PMID: 2183804
- Gottlieb NL. Pharmacology of auranofin: overview and update. Scand J Rheumatol Suppl 1986; 63:19 - 28; PMID: 3110942
- Griggs RC, Batshaw M, Dunkle M, Gopal-Srivastava R, Kaye E, Krischer J, et al, Rare Diseases Clinical Research Network. Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab 2009; 96:20 - 6; http://dx.doi.org/10.1016/j.ymgme.2008.10.003; PMID: 19013090
- Wellman-Labadie O, Zhou Y. The US Orphan Drug Act: rare disease research stimulator or commercial opportunity?. Health Policy 2010; 95:216 - 28; http://dx.doi.org/10.1016/j.healthpol.2009.12.001; PMID: 20036435
- Cheung RY, Cohen JC, Illingworth P. Orphan drug policies: implications for the United States, Canada, and developing countries. Health Law J 2004; 12:183 - 200; PMID: 16539081
- Blower MD, Nachury M, Heald R, Weis KA. A Rae1-containing ribonucleoprotein complex is required for mitotic spindle assembly. Cell 2005; 121:223 - 34; http://dx.doi.org/10.1016/j.cell.2005.02.016; PMID: 15851029
- Parks RE Jr., Brown PR, Cheng YC, Agarwal KC, Kong CM, Agarwal RP, et al. Purine metabolism in primitive erythrocytes. Comp Biochem Physiol B 1973; 45:355 - 64; http://dx.doi.org/10.1016/0305-0491(73)90070-9; PMID: 4351428
- Simon TM, Kunishima DH, Vibert GJ, Lorber A. Screening trial with the coordinated gold compound auranofin using mouse lymphocyte leukemia P388. Cancer Res 1981; 41:94 - 7; PMID: 6778607
- Sok J, Calfon M, Lu J, Lichtlen P, Clark SG, Ron D. Arsenite-inducible RNA-associated protein (AIRAP) protects cells from arsenite toxicity. Cell Stress Chaperones 2001; 6:6 - 15; http://dx.doi.org/10.1379/1466-1268(2001)006<0006:AIRAPA>2.0.CO;2; PMID: 11525245
- Rigobello MP, Scutari G, Boscolo R, Bindoli A. Induction of mitochondrial permeability transition by auranofin, a gold(I)-phosphine derivative. Br J Pharmacol 2002; 136:1162 - 8; http://dx.doi.org/10.1038/sj.bjp.0704823; PMID: 12163349
- Omata Y, Folan M, Shaw M, Messer RL, Lockwood PE, Hobbs D, et al. Sublethal concentrations of diverse gold compounds inhibit mammalian cytosolic thioredoxin reductase (TrxR1). Toxicol In Vitro 2006; 20:882 - 90; http://dx.doi.org/10.1016/j.tiv.2006.01.012; PMID: 16510263
- Lu J, Chew EH, Holmgren A. Targeting thioredoxin reductase is a basis for cancer therapy by arsenic trioxide. Proc Natl Acad Sci U S A 2007; 104:12288 - 93; http://dx.doi.org/10.1073/pnas.0701549104; PMID: 17640917
- Angelucci F, Sayed AA, Williams DL, Boumis G, Brunori M, Dimastrogiovanni D, et al. Inhibition of Schistosoma mansoni thioredoxin-glutathione reductase by auranofin: structural and kinetic aspects. J Biol Chem 2009; 284:28977 - 85; http://dx.doi.org/10.1074/jbc.M109.020701; PMID: 19710012
- Leitsch D, Kolarich D, Wilson IB, Altmann F, Duchêne M. Nitroimidazole action in Entamoeba histolytica: a central role for thioredoxin reductase. PLoS Biol 2007; 5:e211; http://dx.doi.org/10.1371/journal.pbio.0050211; PMID: 17676992
- Brown DM, Upcroft JA, Edwards MR, Upcroft P. Anaerobic bacterial metabolism in the ancient eukaryote Giardia duodenalis. Int J Parasitol 1998; 28:149 - 64; http://dx.doi.org/10.1016/S0020-7519(97)00172-0; PMID: 9504342
- Mondal D, Haque R, Sack RB, Kirkpatrick BD, Petri WA Jr.. Attribution of malnutrition to cause-specific diarrheal illness: evidence from a prospective study of preschool children in Mirpur, Dhaka, Bangladesh. Am J Trop Med Hyg 2009; 80:824 - 6; PMID: 19407131
- Busatti HG, Santos JF, Gomes MA. The old and new therapeutic approaches to the treatment of giardiasis: where are we?. Biologics 2009; 3:273 - 87; PMID: 19707415
- Cacciò SM, Ryan U. Molecular epidemiology of giardiasis. Mol Biochem Parasitol 2008; 160:75 - 80; http://dx.doi.org/10.1016/j.molbiopara.2008.04.006; PMID: 18501440