Abstract
Occurrence of resistance, especially to clarithromycin, renders the standard triple therapy used to cure Helicobacter pylori infection ineffective. This review presents the bacteriological and pharmacological basis for H. pylori therapy and the current recommendations. The third-line treatment must be based on clarithromycin susceptibility testing. If the bacteria are still susceptible, failure may come from problems of compliance, hyperacidity or high bacterial load which can be overcome. If the bacteria are resistant, different regimens must be considered, including bismuth and non-bismuth-based quadruple therapies (sequential or concomitant), as well as triple therapies where amoxicillin is administered several times a day to obtain an optimal concentration at the gastric mucosal level. The treatments are becoming more and more complex and ecologically unsatisfactory, waiting for new agents or vaccines.
Introduction
The discovery of Helicobacter pylori and of the role of H. pylori infection in the main diseases of the stomach led to the impression that we could rapidly get rid of these diseases by using antimicrobials and eventually a vaccine. In 1993, an effective therapy was finally proposed based on the association of two antibiotics: clarithromycin and amoxicillin or metronidazole, and an antisecretory drug essentially a proton pump inhibitor (PPI).Citation1,Citation2 This regime has been confirmed efficacious in large multicenter trialsCitation3,Citation4 and recommended by the various consensus conferences held around the world.Citation5-Citation7
However, it now appears that the aim was not achieved as simply as that because H. pylori, like other bacteria, can become resistant to antibiotics and we are now faced with numerous failures which make the use of this clarithromycin-based standard triple therapy unacceptable in many parts of the world.
In this review, the rationale for the current strategies of H. pylori treatment as well as the latest guidelines will be presented.
Basis for H. pylori Eradication Treatment
The aim of the treatment is to obtain a mucosal concentration of the antibiotic against H. pylori above the minimal bactericidal concentration (MBC) at the site of the infection, i.e., the gastric mucus, and during a sufficient time lapse to eradicate all of the bacteria present. For practical reasons, the minimal inhibitory concentration (MIC) is used as a surrogate of MBC.
Bacteriological data first indicated the antibiotics with the best potential: macrolides (especially clarithromycin), βlactams (essentially amoxicillin), tetracycline, metronidazole, rifampins (essentially rifabutin) and later fluoroquinolones (especially levofloxacin). The MICs of these drugs against H. pylori are low () (EUCAST: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Consultation/EUCAST_clinical_breakpoints_for_Helicobacter_pylori.pdf) and a mucosal concentration above the MIC can be expected by taking therapeutic doses. Dosages in the gastric mucosa have been performed for some of them and support the postulate. Furthermore, clinical trials have confirmed the experimental data.
Table 1. EUCAST proposed breakpoints for Helicobacter pylori
However, it was also shown that the drugs need to be absorbed and released in the gastric mucosa over long periods. The aminoglycosides, which are not absorbed despite their in vitro activity, are not usable and for the same reason bismuth salts which have an antimicrobial activity cannot be used as the sole drug.
It is important to note that a few drugs do not lead to good clinical results despite their belonging to the antibiotic groups considered active. Such is the case for doxycycline among the tetracyclinesCitation8 and ciprofloxacin among the fluoroquinolones.
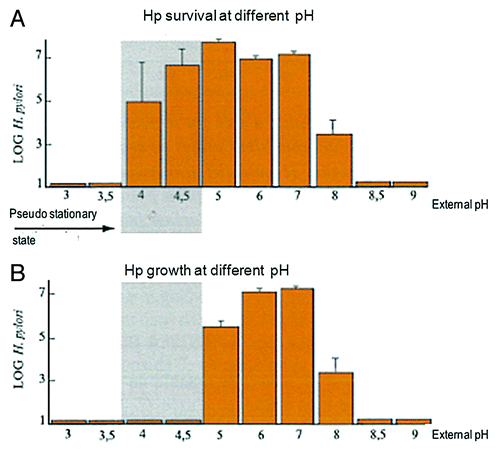
Another point to consider is the acidity of the stomach. There is a pH gradient from 7.2 to 2 in the gastric mucus where H. pylori lives. H. pylori, while not a true acidophile, may still proliferate at pH 5 and survives at pH 4 ().Citation9,Citation10 Most of the antibiotics are not active at low pH, and are only active on dividing bacteria, so it is necessary to add an antisecretory drug to increase the pH of the stomach, and a double-dose of PPI has been the most valuable addition.
Figure 1. Survival (A) and growth (B) of H. pylori according to pH.Citation10
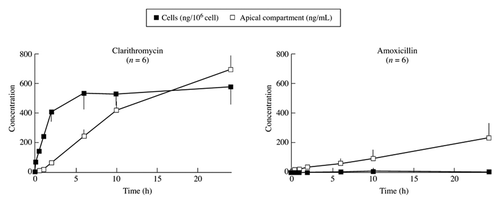
The amoxicillin concentration detectable in gastric juice is in the range of this drug’s MIC against H. pylori. The concomitant PPI treatment, by decreasing the gastric juice volume, will increase its efficacy. In contrast, clarithromycin is able to concentrate in gastric juice compared with blood but a PPI does not increase the gastric transfer fraction. Metronidazole is the drug which concentrates the most in gastric juice. It has been explained by the pH partition hypothesis, i.e., weak bases are trapped in the acidic compartment of the stomach. Metronidazole is indeed predominantly unionized at neutral pH (in blood) but ionizes as the pH decreases (in the gastric mucosa) and then does not diffuse anymore through membranes. Accordingly, the gastric transfer fraction decreases when taking a PPI but is still significant, because the pH remains moderately acidic.Citation11
A sufficient concentration of the antibiotic must be maintained in the mucus over a long period of time. In this respect, the antibiotics which have an intracellular diffusion like clarithromycin have an advantage because the antibiotic can then be gradually released from the epithelial cells in the mucus while for those without intracellular diffusion, e.g., amoxicillin, a permanently high blood concentration is needed to obtain a sufficient concentration of the drug in the mucosa probably via a paracellular pathway ().Citation12,Citation13
Figure 2. Long-term basal-to-apical (serosal-to-mucosal) transport of clarithromycin and amoxicillin across HT29–19A cell monolayers in cell culture inserts (mean ± s.d.).Citation12
All of the data obtained have established that clarithromycin, a macrolide derived from erythromycin, is indeed the basis for H. pylori treatment because of its low MIC, good mucosal diffusion, and limited effect of the acidity. However, it was also shown that a second antibiotic should be added to obtain high and permanent eradication rates, either amoxicillin which also has excellent MICs but limited intracellular diffusion, or metronidazole, a small molecule which concentrates in the mucus as seen before. This standard triple therapy was satisfactory for many years but is now faced with the problem of H. pylori resistance to clarithromycin.
H. pylori, like all bacteria, is able to become resistant to antibiotics. Fortunately, the genetic basis is not the spread of genetic elements (plasmids, transposons) which can lead to a horizontal transfer as an epidemic in a population of bacteria, but essentially a selection of bacteria with point mutations which occur mostly by chance during the replication process.Citation14
Clarithromycin acts by inhibition of protein synthesis at the ribosomal level. A mutation at certain positions on the 23S rRNA gene modifies the structure of the ribosomes inhibiting clarithromycin binding at the level of the peptidyl transferase loop, and leads to higher MICs than what can be achieved by a therapeutic dose.Citation15,Citation16 There is cross resistance for all of the other macrolides. Clinical trials have shown that the rate of success of the standard triple therapy in many regions is now in the range of 70%, and is much lower when the H. pylori strain is resistant vs. susceptible (20% vs. 90%, respectively).Citation17
The other antibiotic for which resistance is important to consider is levofloxacin. This antibiotic has been proposed to replace clarithromycin in rescue treatments and is indeed efficacious. It inhibits the A subunit of the gyrase encoded by the gyrA gene of H. pylori, an enzyme which unravels DNA to allow DNA replication. The occurrence of a point mutation in a special region of the gyrA gene, the so-called quinolone resistance determining region (QRDR), leads to a modification of the target and an increase in MIC, which cannot be overcome by the concentration of the antibiotic in the mucosa.Citation18,Citation19 There is also cross-resistance for all fluoroquinolones. Clinical trials have shown the efficacy of a PPI-amoxicillin-levofloxacin therapy.Citation20 Later it was shown that the success of this regimen decreases radically in the case of levofloxacin resistant compared with levofloxacin susceptible H. pylori.Citation21
For several drugs eventually used to treat H. pylori infection, resistance is rare. Resistance to rifampins (point mutations on the rpoB gene)Citation22 may be found in patients previously treated with rifampicin especially for tuberculosis.Citation23 Resistance to tetracycline requires the change in a nucleotidic triplet occurring on the 16S rRNA gene which inhibits tetracycline binding to the h1 loop,Citation24 a very rare event indeed, seldomly reported except in Korea and Brazil.Citation25,Citation26 However, in both countries there are also reports indicating a low prevalence of resistance to this antibiotic.Citation27,Citation28 Only a few amoxicillin resistant strains have been found due to mutations in the pbp1 geneCitation29 and it is possible that those strains labeled resistant in some reports but with no further investigation are not truly resistant.
A special mention must be given for metronidazole. Metronidazole is a prodrug, which must be reduced to hydroxylamine inside the bacterial cell by different enzymes to cause DNA breaks.Citation30 This reduction is very much dependent on the redox potential inside the cell which itself depends on the redox potential in the environment, a characteristic which is neither controlled in vivo nor in vitro during testing. Therefore, there is a problem of reproducibility in the MIC measures performed for metronidazole.Citation31 This fact, in addition to the poor correlation observed between the in vitro and the in vivo data, led to consider metronidazole resistance as irrelevant and its testing is not recommended at an individual level in routine practice.Citation32 However, we must recognize that at the population level metronidazole resistance does have some impact which is much less than that of clarithromycin and levofloxacin, and is also dependent on the other drugs used as well as the length of treatment. Tinidazole has the same bacteriological activity as metronidazole on H. pylori.Citation33 However, it has a significantly longer half-life and lower total clearance.Citation34
In addition to the question of H. pylori resistance, it appears to be important to evaluate the consequence of the intervention, i.e., H. pylori eradication, on the general microbiota of the patient but there are no data on this topic yet. Studies performed in Sweden have shown the impact of the standard clarithromycin-based triple therapy on the resistance of some bacteria present in the flora from saliva and intestine. Samples showed an increase in amoxicillin MICs for Streptococcus sp., Staphylococcus sp. and Enterococcus sp. and an increase in clarithromycin MICs also for Enterobacteriaceae and Bacteroides sp., while a suppression of other anaerobic bacteria occurred. Persistence of high level clarithromycin resistant enterococci were found for 3 y in 3 out of 5 patients in a long-term follow-up.Citation35,Citation36
Current Recommendations for Treatment
The prevalence of H. pylori resistance to clarithromycin which is the main risk factor for the success of the standard H. pylori eradication therapy prescribed empirically is highly variable between countries or regions throughout the world. The prevalence depends on the selection pressure of the corresponding group of antibiotics in a given area. In Europe a significant correlation has been shown between the consumption of long acting macrolides in the community, expressed as a defined daily dose per 1,000 inhabitants per day, and the proportion of H. pylori-resistant strains.Citation37
Antibiotic resistance mediated by chromosome mutations is vertically transmitted, to the descendants. Apparently, for macrolides, there is limited biological cost to the bacterium, or compensary mutations can occurCitation38 which explains why they can be maintained even when the selective agent is absent. European surveys performed at 10 y intervals showed that H. pylori resistance increased by approximately 1% per year, from 9.9% in 1998Citation39 to 17.5% in 2008–2009.Citation37 A similar increase in clarithromycin resistance has also been documented in the Far East (Japan, Korea)Citation25,Citation40 while there are few data concerning the US.
Based on these results, the recommendation is to follow a different empiric treatment strategy according to the level of primary clarithromycin resistance observed, the threshold ranging from 15–20% ().Citation32 For regions with low clarithromycin resistance, the recommendation is to continue the use of standard triple therapy as a first-line treatment and, in the case of failure, to choose either a bismuth-based quadruple therapy or a PPI-levofloxacin-amoxicillin therapy as a second-line treatment.
Figure 3. Therapies recommended for Helicobacter pylori eradication. Maastricht-4 Consensus Report.Citation32
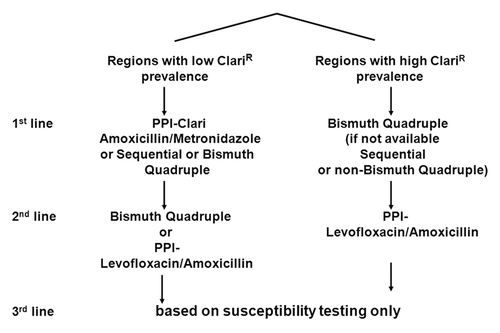
To the contrary, for regions with a high clarithromycin resistance, the first option is a bismuth-based quadruple therapy. If bismuth is not available, a non-bismuth quadruple therapy could be used, preferably as a sequential therapy. This sequential therapy is comprised of 5 d of PPI-amoxicillin followed by 5 d of PPI-clarithromycin-metronidazole.Citation41 Resistance to clarithromycin only slightly affects the outcome of the treatment, the rationale being that the PPI-amoxicillin course already eliminates most of the bacteria, decreasing the chance of encountering a mutation since the rate of mutation for clarithromycin is in the range of (10−9).42It has also been proposed to give the 4 drugs concomitantly. However, in the case of clarithromycin resistance, only the 2 other antibiotics are effective. In the case of failure of this first-line therapy, a PPI-levofloxacin-amoxicillin combination can be used. A limit of the empiric strategy is the use of levofloxacin for which resistance is emerging in many places, again due to the widespread use of this family of drugs in the community.Citation37
These recommendations are an extension of the previous guidelines published in EuropeCitation43 and USA in 2007.Citation44
What to Do When Empiric Treatment Fails Twice?
According to the current recommendations,Citation32 when an empiric treatment fails twice, it is necessary to prescribe a tailored treatment, i.e., the antibiotic susceptibility of H. pylori should be tested in order to prescribe the most adapted regimen, and tools are available for this purpose.Citation45-Citation47
All bacteriology laboratories are indeed able to culture H. pylori. The pre-analytic phase is crucial, i.e., the obtention of gastric biopsies which must be taken from both the antrum and the fundus before taking biopsies for pathology, followed by their introduction into a transport medium maintained at 4°C, because temperature, desiccation and contact with air are harmful for the bacteria. Recommendations for culture and susceptibility testing either by disk diffusion or E-test have been published.Citation14 The minimal testing is for clarithromycin and levofloxacin, and possibly other antibiotics depending on the area concerned.
This traditional approach is demanding in the lab and a delay of several days is necessary to obtain the results. Molecular methods using mainly PCR have been developed to detect the point mutations responsible for clarithromycin resistance. Several PCR formats exist. An interesting one is the real-time PCR using the fluorescence resonance energy transfer principle (FRET-RT-PCR). This FRET-RT-PCR allows first a detection of H. pylori if an amplification occurs and second the possibility to perform a melting curve analysis of the amplicons once obtained. The presence of a mutation is responsible for a DNA mismatch and consequently a lower melting temperature, so it is easy to detect in comparison to the wild type.Citation48 There are commercial kits available for this purpose which can also be used on faeces. A non-PCR method, a fluorescence in situ hybridization (FISH) technique, can also be performed on histological preparations; it detects H. pylori with one probe and clarithromycin resistance with another probe.Citation49
Given the high number of mutations which can lead to levofloxacin resistance, another approach has been developed: a multiplex PCR followed by DNA hybridization on a strip.Citation50 This commercially available method can detect mutations responsible for both levofloxacin and clarithromycin resistance.
What if the strain is still susceptible to clarithromycin?
Clarithromycin resistance occurs in approximately two thirds of the cases of failure with standard triple therapy so susceptibility is not unusual, still present in one third of the cases. On the other hand, in the case of a clarithromycin susceptible strain before treatment, a 100% eradication rate is seldomly obtained due to other potential causes which, while not frequent, can be responsible for failure of the standard triple therapy. However, triple therapy can still be used after correcting the following problems.
The first is compliance and for this we have to rely on the patients' claims which are difficult to verify. A study using special containers (Medication event monitoring System) showed that there were more than 10% of poor compliers (i.e., taking less than 85% of the total amount of drugs) leading to much lower eradication rates.Citation51 H. pylori treatments are relatively complex compared with others, so it is important to convince the patient to follow the prescription and to inform him/her of potential adverse events which are common and should not lead to stopping the treatment. There is room for improvement in this field, especially with general practitioners who are not often faced with this type of patient and who need to be taught by the specialists.
Another possible reason is that we are dealing with an acid hypersecretor patient for whom the quantity of PPI prescribed is not sufficient to obtain an adequate pH for the antibiotics to be active. Independently of gastrinoma, such patients have been described in the past.Citation52
There is also the possibility of patients who are rapid PPI metabolizers. PPI are metabolized in the liver by the cytochrome P450 isoenzyme CYP2C19 for which polymorphisms do exist and patients can be separated into rapid, intermediate and low metabolizers. Rapid metabolizers may have a pH one point lower than the low metabolizers, when taking the same dose of PPI.Citation53 The proportion of H. pylori eradication using standard triple therapy was only 64.7% for the rapid metabolizers vs. 79.4% and 100% for the intermediate and low metabolizers, respectively, indicating the need for a higher dose of PPI in a recent study.Citation54 The frequency of rapid metabolizers in the Western populations remains however to be determined.
A higher bacterial load, determined by the DOB value of the semi-quantitative test, i.e., the urea breath test, has also been shown to be a cause of more frequent failures.Citation55
The CagA negative status is also a potential risk factor for failure, possibly because these strains may not replicate as quickly as the CagA positive.Citation56 Furthermore, this may explain the constant finding of superior eradication rates in patients with peptic ulcer disease vs. those with non-ulcer dyspepsia.Citation57
Under such circumstances, only increasing dosages and the duration of treatment can be done.
What if the strain is resistant to clarithromycin?
This situation is indeed the most frequent and there are several possibilities to be reviewed for each given patient, none of which is a panacea.
Bismuth-based quadruple therapy
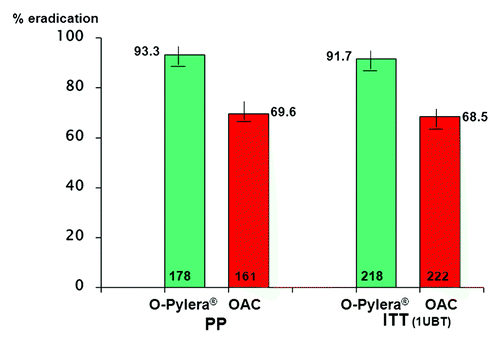
The patient has most likely already received this regime but it must be noted that there is now a commercially available 3-in-1 capsule (Pylera®), prescribed with a PPI for 10 d. This galenic form favors compliance and the 10 d treatment duration can be superior to the 7 d which the patient may have received.Citation58 This regimen has shown excellent results independently of the metronidazole resistant status of the strain ().
Figure 4.H. pylori eradication with Pylera®-omeprazole (10d) vs. standard clarithromycin-based triple therapy (7d) European Multicentric Study.Citation58
Sequential or concomitant non-bismuth quadruple therapies
Clarithromycin resistance has a limited impact on the sequential treatment so, theoretically, it could be used.Citation41,Citation59However, recent trials from Asia were not all as promising as the previous ones.
The so-called concomitant therapy has the advantage to favor compliance. It was successful in several trials and especially on clarithromycin resistant strains in a Spanish trial.Citation60 A review of this therapy involving 2,070 patients from 19 studies obtained a mean eradication rate of 88%.Citation61
There are also some attempts to combine the 2 approaches, sequential and concomitant, by prescribing a 14-d sequential regimen but continuing amoxicillin during the entire period, the so-called hybrid therapy.Citation62
The sequential and concomitant regimens were compared with the standard triple therapy in a large trial performed in six Latin American countries. Surprisingly, the best results were obtained with the standard triple therapy (82.2% eradication intention to treat) surpassing concomitant therapy by 8.6% and sequential therapy by 5.6%. These treatments were all given empirically, so results of clarithromycin susceptibility are not available.Citation63
PPI-amoxicillin-levofloxacin for 10 d
If the patient already received this treatment, levofloxacin resistance is almost always present after treatment failure. Otherwise it is an option, and even better following testing. This regimen has mainly been used as a second-line treatment. A large study performed in Spain led to a 73.8% eradication rate,Citation64 as well as 78.1% in Taiwan.Citation65 A metaanalysis even showed this regimen to be superior to bismuth-based quadruple therapies.Citation66
Beside levofloxacin, moxifloxacin has also been used successfully,Citation67 but it is now recommended to limit the use of this drug in several countries due to various adverse events. There are promising data from Japan especially with sitafloxacin, a fluoroquinolone with higher efficacy especially in case of resistance associated mutationsCitation68 and active at acidic pH. In a large multicenter trial in Japan, in patients receiving a third-line therapy in PPI-amoxicillin-sitafloxacin led to a 70% eradication rate ITT.Citation69 Another fluoroquinolone, gemifloxacin, appears to have the same property to overcome quinolone resistance.Citation70
PPI-amoxicillin-metronidazole for 14 d
This old regimen can lead to good eradication rates. It is frequently used in Japan as a second-line treatment. Data from 1,373 patients from 15 sites in the Tokyo Metropolitan area during 5 y were compared. There was no significant trend for decrease efficacy with an overall eradication rate of 92%.Citation71 It can also be improved by increasing the number of times amoxicillin is taken because, as mentioned before, it is important to maintain a high blood concentration of amoxicillin to maintain a gastric mucus concentration higher than the MIC.
Furuta et al. compared a PPI amoxicillin-metronidazole therapy with amoxicillin administered bid (750 mg), tid (500 mg) and qid (500 mg). They obtained an eradication rate of 82.1%, 96%, and 100%, respectively.Citation72
PPI-amoxicillin-rifabutin
Rifabutin has not the best safety profile. Rifabutin is toxic to the blood cell lineage leading to neutropenia and thrombocytopenia and furthermore, it is better to reserve it for mycobacterial infections. This regimen should be considered only if everything else has failed.
A PPI-amoxicillin-rifabutin therapy, used as a rescue therapy after 3 failures in 2 studies, led to a 50% eradication rate only.Citation73,Citation74
PPI-amoxicillin
There is also a comeback of this dual therapy from the early days but with some adaptations, because as mentioned above, it is important to maintain a high blood concentration during the nycthemeral cycle. Furuta et al. succeeded by administering the drug 4 times a day instead of twice a day.Citation75
Other antibiotics are also used but not available in many countries such as furazolidone, which can be used in bismuth-based quadruple therapy instead of metronidazole, but has side effects, especially in case of high dosage, rifaximin, a non-absorbed antibiotic of the rifampin group, nitazoxanide, a drug related to metronidazole. Currently there is a trend to mix different drugs in order to get high eradication rates.
The interest in adding an adjuvant therapy, such as probiotics is also questioned. Certain probiotics, such as Loctobacillus reuteri, have a potent anti-H. pylori activity in vitro but none were able to eradicate H. pylori in animal models. It is accepted that the benefit obtained with probiotics is essentially by their capacity to prevent side effects, especially diarrhea. The metaanalyses performed have shown an increase in eradication of about 5–10%.Citation76,Citation77
All these treatments are not usable in all patients. The problem of βlactam allergy in some patients restricts the possibilities. In this respect, replacing amoxicillin by tetracycline may be worth considering. A PPI-tetracycline-metronidazole has shown to be effective.Citation78 In children fluoroquinolones and tetracyclines cannot be used and therefore limit considerably the possibilities. As first line treatment, either PPI-amoxicillin-imidazole or PPI-amoxicillin-clarithromycin or bismuth salts-amoxicillin-imidazole or sequential therapy is recommended. Susceptibility testing is also recommended for clarithromycin before using this antibiotic in areas of known high resistance. After failure, a tailored treatment is recommended as well as increasing dose and/or duration of therapy.Citation79 In obese patients the eradication rate appears to be inferior to the normal populationCitation80 possibly because of an increased distribution volume.
Conclusion
Our possibilities are currently limited by the lack of new drugs. All attempts tend to get the best results from old antibiotics by combining them in different ways, and so treatment success is obtained at an ecological cost for the human microbiome. There is however, room for improvement in general practice by following current guidelines including follow-up control by performing a urea breath test after 4 to 6 weeks. It is also important to keep in mind that in some cases immediate eradication is not mandatory, and can be delayed, hoping for better drugs in the future.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Bazzoli F, Zagari R, Fossi S, et al. Efficacy and tolerability of a short-term low-dose triple therapy for eradication of Helicobacter pylori.. Am J Gastroenterol 1993; 104:40A
- Lamouliatte HC, Cayla R, Megraud F, Zerbib F, Stablo M, Bouchard S, et al. Amoxicillin-clarithromycin-omeprazole: the best therapy for Helicobacter pylori infection. Acta Gastroenterol Belg 1993; 56:A140
- Lind T, Veldhuyzen van Zanten S, Unge P, Spiller R, Bayerdörffer E, O’Morain C, Bardhan KD, Bradette M, Chiba N, Wrangstadh M, et al. Eradication of Helicobacter pylori using one-week triple therapies combining omeprazole with two antimicrobials: the MACH I Study. Helicobacter 1996; 1:138 - 44; http://dx.doi.org/10.1111/j.1523-5378.1996.tb00027.x; PMID: 9398894
- Lind T, Mégraud F, Unge P, Bayerdörffer E, O’morain C, Spiller R, Veldhuyzen Van Zanten S, Bardhan KD, Hellblom M, Wrangstadh M, et al. The MACH2 study: role of omeprazole in eradication of Helicobacter pylori with 1-week triple therapies. Gastroenterology 1999; 116:248 - 53; http://dx.doi.org/10.1016/S0016-5085(99)70119-8; PMID: 9922303
- European Helicobacter Pylori Study Group. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. Gut 1997; 41:8 - 13; http://dx.doi.org/10.1136/gut.41.1.8; PMID: 9274464
- Lam SK, Talley NJ. Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol 1998; 13:1 - 12; http://dx.doi.org/10.1111/j.1440-1746.1998.tb00537.x; PMID: 9737564
- Coelho LG, León-Barúa R, Quigley EM. Latin-American Consensus Conference on Helicobacter pylori infection. Latin-American National Gastroenterological Societies affiliated with the Inter-American Association of Gastroenterology (AIGE). Am J Gastroenterol 2000; 95:2688 - 91; http://dx.doi.org/10.1016/S0002-9270(00)01967-5; PMID: 11051336
- Borody TJ, George LL, Brandl S, Andrews P, Lenne J, Moore-Jones D, Devine M, Walton M. Helicobacter pylori eradication with doxycycline-metronidazole-bismuth subcitrate triple therapy. Scand J Gastroenterol 1992; 27:281 - 4; http://dx.doi.org/10.3109/00365529209000075; PMID: 1589705
- Meyer-Rosberg K, Scott DR, Rex D, Melchers K, Sachs G. The effect of environmental pH on the proton motive force of Helicobacter pylori.. Gastroenterology 1996; 111:886 - 900; http://dx.doi.org/10.1016/S0016-5085(96)70056-2; PMID: 8831583
- Sachs G, Meyer-Rosberg K, Hong C, Melchers K, Scott D.. Biologie de Helicobacter pylori dans l'estomac. La lettre de l'Infectiologue 1997; 12:16 - 20
- Goddard AF, Jessa MJ, Barrett DA, Shaw PN, Idström JP, Cederberg C, Spiller RC. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology 1996; 111:358 - 67; http://dx.doi.org/10.1053/gast.1996.v111.pm8690200; PMID: 8690200
- Matysiak-Budnik T, Heyman M, Candalh C, Lethuaire D, Mégraud F. In vitro transfer of clarithromycin and amoxicillin across the epithelial barrier: effect of Helicobacter pylori.. J Antimicrob Chemother 2002; 50:865 - 72; http://dx.doi.org/10.1093/jac/dkf219; PMID: 12461005
- Nakamura M, Spiller RC, Barrett DA, Wibawa JI, Kumagai N, Tsuchimoto K, Tanaka T. Gastric juice, gastric tissue and blood antibiotic concentrations following omeprazole, amoxicillin and clarithromycin triple therapy. Helicobacter 2003; 8:294 - 9; http://dx.doi.org/10.1046/j.1523-5378.2003.00156.x; PMID: 12950601
- Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 2007; 20:280 - 322; http://dx.doi.org/10.1128/CMR.00033-06; PMID: 17428887
- Versalovic J, Shortridge D, Kibler K, Griffy MV, Beyer J, Flamm RK, Tanaka SK, Graham DY, Go MF. Mutations in 23S rRNA are associated with clarithromycin resistance in Helicobacter pylori.. Antimicrob Agents Chemother 1996; 40:477 - 80; PMID: 8834903
- Occhialini A, Urdaci M, Doucet-Populaire F, Bébéar CM, Lamouliatte H, Mégraud F. Macrolide resistance in Helicobacter pylori: rapid detection of point mutations and assays of macrolide binding to ribosomes. Antimicrob Agents Chemother 1997; 41:2724 - 8; PMID: 9420046
- Mégraud F. H pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 2004; 53:1374 - 84; http://dx.doi.org/10.1136/gut.2003.022111; PMID: 15306603
- Moore RA, Beckthold B, Wong S, Kureishi A, Bryan LE. Nucleotide sequence of the gyrA gene and characterization of ciprofloxacin-resistant mutants of Helicobacter pylori.. Antimicrob Agents Chemother 1995; 39:107 - 11; http://dx.doi.org/10.1128/AAC.39.1.107; PMID: 7695290
- Tankovic J, Lascols C, Sculo Q, Petit JC, Soussy CJ. Single and double mutations in gyrA but not in gyrB are associated with low- and high-level fluoroquinolone resistance in Helicobacter pylori.. Antimicrob Agents Chemother 2003; 47:3942 - 4; http://dx.doi.org/10.1128/AAC.47.12.3942-3944.2003; PMID: 14638505
- Cammarota G, Cianci R, Cannizzaro O, Cuoco L, Pirozzi G, Gasbarrini A, Armuzzi A, Zocco MA, Santarelli L, Arancio F, et al. Efficacy of two one-week rabeprazole/levofloxacin-based triple therapies for Helicobacter pylori infection. Aliment Pharmacol Ther 2000; 14:1339 - 43; http://dx.doi.org/10.1046/j.1365-2036.2000.00846.x; PMID: 11012480
- Perna F, Zullo A, Ricci C, Hassan C, Morini S, Vaira D. Levofloxacin-based triple therapy for Helicobacter pylori re-treatment: role of bacterial resistance. Dig Liver Dis 2007; 39:1001 - 5; http://dx.doi.org/10.1016/j.dld.2007.06.016; PMID: 17889627
- Heep M, Beck D, Bayerdörffer E, Lehn N. Rifampin and rifabutin resistance mechanism in Helicobacter pylori.. Antimicrob Agents Chemother 1999; 43:1497 - 9; PMID: 10348780
- Suzuki S, Suzuki H, Nishizawa T, Kaneko F, Ootani S, Muraoka H, Saito Y, Kobayashi I, Hibi T. Past rifampicin dosing determines rifabutin resistance of Helicobacter pylori.. Digestion 2009; 79:1 - 4; http://dx.doi.org/10.1159/000191204; PMID: 19142036
- Trieber CA, Taylor DE. Mutations in the 16S rRNA genes of Helicobacter pylori mediate resistance to tetracycline. J Bacteriol 2002; 184:2131 - 40; http://dx.doi.org/10.1128/JB.184.8.2131-2140.2002; PMID: 11914344
- Lee JW, Kim N, Kim JM, Nam RH, Chang H, Kim JY, Shin CM, Park YS, Lee DH, Jung HC. Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter 2013; 18:206 - 14; http://dx.doi.org/10.1111/hel.12031; PMID: 23241101
- Ribeiro ML, Gerrits MM, Benvengo YH, Berning M, Godoy AP, Kuipers EJ, Mendonça S, van Vliet AH, Pedrazzoli J Jr., Kusters JG. Detection of high-level tetracycline resistance in clinical isolates of Helicobacter pylori using PCR-RFLP. FEMS Immunol Med Microbiol 2004; 40:57 - 61; http://dx.doi.org/10.1016/S0928-8244(03)00277-3; PMID: 14734187
- Chung JW, Lee GH, Jeong JY, Lee SM, Jung JH, Choi KD, Song HJ, Jung HY, Kim JH. Resistance of Helicobacter pylori strains to antibiotics in Korea with a focus on fluoroquinolone resistance. J Gastroenterol Hepatol 2012; 27:493 - 7; http://dx.doi.org/10.1111/j.1440-1746.2011.06874.x; PMID: 21793912
- Suzuki RB, Almeida CM, Sperança MA. Absence of Helicobacter pylori high tetracycline resistant 16S rDNA AGA926-928TTC genotype in gastric biopsy specimens from dyspeptic patients of a city in the interior of São Paulo, Brazil. BMC Gastroenterol 2012; 12:49; http://dx.doi.org/10.1186/1471-230X-12-49; PMID: 22594560
- Gerrits MM, Schuijffel D, van Zwet AA, Kuipers EJ, Vandenbroucke-Grauls CM, Kusters JG. Alterations in penicillin-binding protein 1A confer resistance to beta-lactam antibiotics in Helicobacter pylori.. Antimicrob Agents Chemother 2002; 46:2229 - 33; http://dx.doi.org/10.1128/AAC.46.7.2229-2233.2002; PMID: 12069978
- Mendz GL, Mégraud F. Is the molecular basis of metronidazole resistance in microaerophilic organisms understood?. Trends Microbiol 2002; 10:370 - 5; http://dx.doi.org/10.1016/S0966-842X(02)02405-8; PMID: 12160635
- Glupczynski Y, Broutet N, Cantagrel A, Andersen LP, Alarcon T, López-Brea M, Mégraud F. Comparison of the E test and agar dilution method for antimicrobial suceptibility testing of Helicobacter pylori.. Eur J Clin Microbiol Infect Dis 2002; 21:549 - 52; http://dx.doi.org/10.1007/s10096-002-0757-6; PMID: 12172749
- Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al, European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012; 61:646 - 64; http://dx.doi.org/10.1136/gutjnl-2012-302084; PMID: 22491499
- Novo Y, Gavinet AM, Megraud F, Bebear C. Susceptibility of Campylobacter pylori to nitroimidazole compounds in vitro. P 165-7 In: Megraud F, Lamouliatte H (Eds) Gastroduodenal pathology and Campylobacter pylori. Excerpta Medica ICS847, 1989. Amsterdam.
- Mattila J, Männistö PT, Mäntylä R, Nykänen S, Lamminsivu U. Comparative pharmacokinetics of metronidazole and tinidazole as influenced by administration route. Antimicrob Agents Chemother 1983; 23:721 - 5; http://dx.doi.org/10.1128/AAC.23.5.721; PMID: 6870221
- Adamsson I, Nord CE, Lundquist P, Sjöstedt S, Edlund C. Comparative effects of omeprazole, amoxycillin plus metronidazole versus omeprazole, clarithromycin plus metronidazole on the oral, gastric and intestinal microflora in Helicobacter pylori-infected patients. J Antimicrob Chemother 1999; 44:629 - 40; http://dx.doi.org/10.1093/jac/44.5.629; PMID: 10552979
- Sjölund M, Wreiber K, Andersson DI, Blaser MJ, Engstrand L. Long-term persistence of resistant Enterococcus species after antibiotics to eradicate Helicobacter pylori.. Ann Intern Med 2003; 139:483 - 7; http://dx.doi.org/10.7326/0003-4819-139-6-200309160-00011; PMID: 13679325
- Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, Andersen LP, Goossens H, Glupczynski Y, Study Group participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013; 62:34 - 42; http://dx.doi.org/10.1136/gutjnl-2012-302254; PMID: 22580412
- Björkholm B, Sjölund M, Falk PG, Berg OG, Engstrand L, Andersson DI. Mutation frequency and biological cost of antibiotic resistance in Helicobacter pylori.. Proc Natl Acad Sci U S A 2001; 98:14607 - 12; http://dx.doi.org/10.1073/pnas.241517298; PMID: 11717398
- Glupczynski Y, Mégraud F, Lopez-Brea M, Andersen LP. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori.. Eur J Clin Microbiol Infect Dis 2001; 20:820 - 3; http://dx.doi.org/10.1007/s100960100611; PMID: 11783701
- Kobayashi I, Murakami K, Kato M, Kato S, Azuma T, Takahashi S, Uemura N, Katsuyama T, Fukuda Y, Haruma K, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol 2007; 45:4006 - 10; http://dx.doi.org/10.1128/JCM.00740-07; PMID: 17942652
- Zullo A, Vaira D, Vakil N, Hassan C, Gatta L, Ricci C, De Francesco V, Menegatti M, Tampieri A, Perna F, et al. High eradication rates of Helicobacter pylori with a new sequential treatment. Aliment Pharmacol Ther 2003; 17:719 - 26; http://dx.doi.org/10.1046/j.1365-2036.2003.01461.x; PMID: 12641522
- Wang G, Wilson TJ, Jiang Q, Taylor DE. Spontaneous mutations that confer antibiotic resistance in Helicobacter pylori.. Antimicrob Agents Chemother 2001; 45:727 - 33; http://dx.doi.org/10.1128/AAC.45.3.727-733.2001; PMID: 11181351
- Malfertheiner P, Megraud F, O’Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56:772 - 81; http://dx.doi.org/10.1136/gut.2006.101634; PMID: 17170018
- Chey WD, Wong BC, Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102:1808 - 25; http://dx.doi.org/10.1111/j.1572-0241.2007.01393.x; PMID: 17608775
- Kawai T, Yamagishi T, Yagi K, Kataoka M, Kawakami K, Sofuni A, Itoi T, Sakai Y, Moriyasu F, Osaka Y, et al. Tailored eradication therapy based on fecal Helicobacter pylori clarithromycin sensitivities. J Gastroenterol Hepatol 2008; 23:Suppl 2 S171 - 4; http://dx.doi.org/10.1111/j.1440-1746.2008.05408.x; PMID: 19120893
- Liou JM, Chen CC, Chang CY, Chen MJ, Fang YJ, Lee JY, Chen CC, Hsu SJ, Hsu YC, Tseng CH, et al, Taiwan Helicobacter Consortium. Efficacy of genotypic resistance-guided sequential therapy in the third-line treatment of refractory Helicobacter pylori infection: a multicentre clinical trial. J Antimicrob Chemother 2013; 68:450 - 6; http://dx.doi.org/10.1093/jac/dks407; PMID: 23099849
- Fiorini G, Vakil N, Zullo A, Saracino IM, Castelli V, Ricci C, Zaccaro C, Gatta L, Vaira D. Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2013; 11:507 - 10; http://dx.doi.org/10.1016/j.cgh.2012.12.007; PMID: 23267869
- Oleastro M, Ménard A, Santos A, Lamouliatte H, Monteiro L, Barthélémy P, Mégraud F. Real-time PCR assay for rapid and accurate detection of point mutations conferring resistance to clarithromycin in Helicobacter pylori.. J Clin Microbiol 2003; 41:397 - 402; http://dx.doi.org/10.1128/JCM.41.1.397-402.2003; PMID: 12517879
- Rüssmann H, Adler K, Haas R, Gebert B, Koletzko S, Heesemann J. Rapid and accurate determination of genotypic clarithromycin resistance in cultured Helicobacter pylori by fluorescent in situ hybridization. J Clin Microbiol 2001; 39:4142 - 4; http://dx.doi.org/10.1128/JCM.39.11.4142-4144.2001; PMID: 11682543
- Cambau E, Allerheiligen V, Coulon C, Corbel C, Lascols C, Deforges L, Soussy CJ, Delchier JC, Megraud F. Evaluation of a new test, genotype HelicoDR, for molecular detection of antibiotic resistance in Helicobacter pylori.. J Clin Microbiol 2009; 47:3600 - 7; http://dx.doi.org/10.1128/JCM.00744-09; PMID: 19759218
- Wermeille J, Cunningham M, Dederding JP, Girard L, Baumann R, Zelger G, Buri P, Metry JM, Sitavanc R, Gallaz L, et al. Failure of Helicobacter pylori eradication: is poor compliance the main cause?. Gastroenterol Clin Biol 2002; 26:216 - 9; PMID: 11981460
- Hirschowitz BI, Haber MM. Helicobacter pylori effects on gastritis, gastrin and enterochromaffin-like cells in Zollinger-Ellison syndrome and non-Zollinger-Ellison syndrome acid hypersecretors treated long-term with lansoprazole. Aliment Pharmacol Ther 2001; 15:87 - 103; http://dx.doi.org/10.1046/j.1365-2036.2001.00876.x; PMID: 11136282
- Furuta T, Shirai N, Xiao F, Ohashi K, Ishizaki T. Effect of high-dose lansoprazole on intragastic pH in subjects who are homozygous extensive metabolizers of cytochrome P4502C19. Clin Pharmacol Ther 2001; 70:484 - 92; http://dx.doi.org/10.1067/mcp.2001.119721; PMID: 11719736
- Demir K, Ormeci A, Emrence Z, Gokturk S, Baran B, Mutlauay Soyer O, et al. Effect of genotypic differences in CYP2C19 and on cure rates for Helicobacter pylori infection by triple therapy with different proton pump inhibitors. DDW 2013; 2013:a1915
- Perri F, Clemente R, Festa V, Quitadamo M, Conoscitore P, Niro G, Ghoos Y, Rutgeerts P, Andriulli A. Relationship between the results of pre-treatment urea breath test and efficacy of eradication of Helicobacter pylori infection. Ital J Gastroenterol Hepatol 1998; 30:146 - 50; PMID: 9675647
- Broutet N, Marais A, Lamouliatte H, de Mascarel A, Samoyeau R, Salamon R, Mégraud F. cagA Status and eradication treatment outcome of anti-Helicobacter pylori triple therapies in patients with nonulcer dyspepsia. J Clin Microbiol 2001; 39:1319 - 22; http://dx.doi.org/10.1128/JCM.39.4.1319-1322.2001; PMID: 11283049
- Broutet N, Tchamgoué S, Pereira E, Lamouliatte H, Salamon R, Mégraud F. Risk factors for failure of Helicobacter pylori therapy--results of an individual data analysis of 2751 patients. Aliment Pharmacol Ther 2003; 17:99 - 109; http://dx.doi.org/10.1046/j.1365-2036.2003.01396.x; PMID: 12492738
- Malfertheiner P, Bazzoli F, Delchier JC, Celiñski K, Giguère M, Rivière M, Mégraud F, Pylera Study Group. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet 2011; 377:905 - 13; http://dx.doi.org/10.1016/S0140-6736(11)60020-2; PMID: 21345487
- Gisbert JP, Calvet X, O’Connor A, Mégraud F, O’Morain CA. Sequential therapy for Helicobacter pylori eradication: a critical review. J Clin Gastroenterol 2010; 44:313 - 25; PMID: 20054285
- Molina-Infante J, Pazos-Pacheco C, Vinagre-Rodriguez G, Perez-Gallardo B, Dueñas-Sadornil C, Hernandez-Alonso M, Gonzalez-Garcia G, Mateos-Rodriguez JM, Fernandez-Bermejo M, Gisbert JP. Nonbismuth quadruple (concomitant) therapy: empirical and tailored efficacy versus standard triple therapy for clarithromycin-susceptible Helicobacter pylori and versus sequential therapy for clarithromycin-resistant strains. Helicobacter 2012; 17:269 - 76; http://dx.doi.org/10.1111/j.1523-5378.2012.00947.x; PMID: 22759326
- Gisbert JP, Calvet X. Update on non-bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori.. Clin Exp Gastroenterol 2012; 5:23 - 34; http://dx.doi.org/10.2147/CEG.S25419; PMID: 22457599
- Sardarian H, Fakheri H, Hosseini V, Taghvaei T, Maleki I, Mokhtare M. Comparison of hybrid and sequential therapies for Helicobacter pylori eradication in Iran: a prospective randomized trial. Helicobacter 2013; 18:129 - 34; http://dx.doi.org/10.1111/hel.12017; PMID: 23121338
- Greenberg ER, Anderson GL, Morgan DR, Torres J, Chey WD, Bravo LE, Dominguez RL, Ferreccio C, Herrero R, Lazcano-Ponce EC, et al. 14-day triple, 5-day concomitant, and 10-day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet 2011; 378:507 - 14; http://dx.doi.org/10.1016/S0140-6736(11)60825-8; PMID: 21777974
- Gisbert JP, Pérez-Aisa A, Bermejo F, Castro-Fernández M, Almela P, Barrio J, Cosme A, Modolell I, Bory F, Fernández-Bermejo M, et al, H. pylori Study Group of the Asociación Española de Gastroenterología (Spanish Gastroenterology Association). Second-line therapy with levofloxacin after failure of treatment to eradicate helicobacter pylori infection: time trends in a Spanish Multicenter Study of 1000 patients. J Clin Gastroenterol 2013; 47:130 - 5; http://dx.doi.org/10.1097/MCG.0b013e318254ebdd; PMID: 22647827
- Chuah SK, Hsu PI, Chang KC, Chiu YC, Wu KL, Chou YP, Hu ML, Tai WC, Chiu KW, Chiou SS, et al. Randomized comparison of two non-bismuth-containing second-line rescue therapies for Helicobacter pylori.. Helicobacter 2012; 17:216 - 23; http://dx.doi.org/10.1111/j.1523-5378.2012.00937.x; PMID: 22515360
- Di Caro S, Fini L, Daoud Y, Grizzi F, Gasbarrini A, De Lorenzo A, Di Renzo L, McCartney S, Bloom S. Levofloxacin/amoxicillin-based schemes vs quadruple therapy for Helicobacter pylori eradication in second-line. World J Gastroenterol 2012; 18:5669 - 78; http://dx.doi.org/10.3748/wjg.v18.i40.5669; PMID: 23155306
- Wu C, Chen X, Liu J, Li MY, Zhang ZQ, Wang ZQ. Moxifloxacin-containing triple therapy versus bismuth-containing quadruple therapy for second-line treatment of Helicobacter pylori infection: a meta-analysis. Helicobacter 2011; 16:131 - 8; http://dx.doi.org/10.1111/j.1523-5378.2011.00826.x; PMID: 21435091
- Murakami K, Okimoto T, Kodama M, Tanahashi J, Fujioka T, Ikeda F, Muraoka H, Takigawa M, Saika T, Hasegawa M, et al. Sitafloxacin activity against Helicobacter pylori isolates, including those with gyrA mutations. Antimicrob Agents Chemother 2009; 53:3097 - 9; http://dx.doi.org/10.1128/AAC.01552-08; PMID: 19380599
- Murakami K, Furuta T, Ando T, Nakajima T, Inui Y, Oshima T, Tomita T, Mabe K, Sasaki M, Suganuma T, et al, For the Japan GAST Study Group.. Multi-center randomized controlled study to establish the standard third-line regimen for Helicobacter pylori eradication in Japan. [Epub ahead of print] J Gastroenterol 2013; http://dx.doi.org/10.1007/s00535-012-0731-8; PMID: 23307042
- Chang WL, Kao CY, Wu CT, Huang AH, Wu JJ, Yang HB, Cheng HC, Sheu BS. Gemifloxacin can partially overcome quinolone resistance of H. pylori with gyrA mutation in Taiwan. Helicobacter 2012; 17:210 - 5; http://dx.doi.org/10.1111/j.1523-5378.2012.00935.x; PMID: 22515359
- Asaoka D, Nagahara A, Matsuhisa T, Takahashi SI, Tokunaga K, Kawai T, Kawakami K, Suzuki H, Suzuki M, Nishizawa T, et al. Trends of second-line eradication therapy for Helicobacter pylori in Japan: A multicenter study in the Tokyo metropolitan area. Helicobacter 2013; PMID: 23773231
- Furuta T, Sugimoto M, Yamade M, Uotani T, Sahara S, Ichikawa H, et al. Effects of different dosing schedules of amoxicillin on the eradication rates of Helicobacter pylori by triple therapy with a proton pump inhibitor, amoxicillin, and either clarithromycin or metronidazole. DDW 2013; 2013:a245
- Gisbert JP, Castro-Fernandez M, Perez-Aisa A, Cosme A, Molina-Infante J, Rodrigo L, Modolell I, Cabriada JL, Gisbert JL, Lamas E, et al. Fourth-line rescue therapy with rifabutin in patients with three Helicobacter pylori eradication failures. Aliment Pharmacol Ther 2012; 35:941 - 7; http://dx.doi.org/10.1111/j.1365-2036.2012.05053.x; PMID: 22372560
- D’Elios MM, Silvestri E, Emmi G, Barnini T, Prisco D. Helicobacter pylori: usefulness of an empirical fourth-line rifabutin-based regimen. Expert Rev Gastroenterol Hepatol 2012; 6:437 - 9; http://dx.doi.org/10.1586/egh.12.32; PMID: 22928895
- Furuta T, Sugimoto M, Kodaira C, Nishino M, Yamade M, Uotani T, Ikuma M, Shirai N. The dual therapy with 4 times daily dosing of rabeprazole and amoxicillin as the 3rd rescue regimen for eradication of H. pylori.. Hepatogastroenterology 2010; 57:1314 - 9; PMID: 21410079
- Tong JL, Ran ZH, Shen J, Zhang CX, Xiao SD. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2007; 25:155 - 68; http://dx.doi.org/10.1111/j.1365-2036.2006.03179.x; PMID: 17229240
- Szajewska H, Horvath A, Piwowarczyk A. Meta-analysis: the effects of Saccharomyces boulardii supplementation on Helicobacter pylori eradication rates and side effects during treatment. Aliment Pharmacol Ther 2010; 32:1069 - 79; http://dx.doi.org/10.1111/j.1365-2036.2010.04457.x; PMID: 21039671
- Realdi G, Dore MP, Piana A, Atzei A, Carta M, Cugia L, Manca A, Are BM, Massarelli G, Mura I, et al. Pretreatment antibiotic resistance in Helicobacter pylori infection: results of three randomized controlled studies. Helicobacter 1999; 4:106 - 12; http://dx.doi.org/10.1046/j.1523-5378.1999.99002.x; PMID: 10382124
- Koletzko S, Jones NL, Goodman KJ, Gold B, Rowland M, Cadranel S, Chong S, Colletti RB, Casswall T, Elitsur Y, et al, H pylori Working Groups of ESPGHAN and NASPGHAN. Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr 2011; 53:230 - 43; PMID: 21558964
- Abdullahi M, Annibale B, Capoccia D, Tari R, Lahner E, Osborn J, Leonetti F, Severi C. The eradication of Helicobacter pylori is affected by body mass index (BMI). Obes Surg 2008; 18:1450 - 4; http://dx.doi.org/10.1007/s11695-008-9477-z; PMID: 18443890