Abstract
Up to around a quarter of all infants cry excessively and unsoothably during their first months of life. This phenomenon has been termed “infant colic.” In most cases, physicians are unable to determine the cause of the colicky behavior. In a recent study, and by means of comprehensive and deep analyses of more than 1000 intestinal phylotypes, we found that infants with colic showed lower microbiota diversity and stability than control infants in the first weeks of life. Colic-control differences in the abundance of certain bacteria were also found at 2 weeks. These microbial signatures possibly explain the colic phenotype. In this addendum we discuss other recent publications on the subject and present previously unpublished analyses of our own. We address possible mechanisms behind the links between microbiota and crying, and present future directions that could further help elucidate the hypothesized relations between intestinal microbiota and infant colic.
Introduction
Up to around a quarter of all infants spend a great part of their first months of life crying excessively and not responding to parental soothing attempts. This phenomenon has been termed “infant colic” and is often a source of great worry for parents, even though it mostly resolves by 3 or 4 mo of age. Many parents seek professional help for their colicky infant, and the situation produces exhaustion and considerable strain, frequently preventing parents from functioning optimally both at home and at the workplace. In 5‒10% of the infants with colic, an underlying cause of the crying can be found (e.g., cow milk allergy), but in most cases, physicians are unable to determine the cause of the colicky behavior. There is accumulating evidence that the intestinal microbiota in infants with colic differs from that of healthy controls. In studies that were mostly based on traditional culturing approaches, the stools of colicky infants were found to display reduced diversity in microbiota, lower counts of lactobacilli and higher numbers of gram-negative bacteria. However, these reports described differences in infants already diagnosed with colic and usually of over 6 weeks of age. In a recent study, and by means of comprehensive and deep analyses of more than 1000 intestinal phylotypes in over 200 samples, we found that infants with colic showed lower microbiota diversity and stability than control infants in the first weeks of life. Already as early as at 2 weeks after birth, specific differences in the abundance of certain bacteria were found when fecal samples of colic and control babies were compared. Although the study was aimed to unravel associations, it is tempting to assume that the observed microbial signatures may be, at least partially, causative of many colicky infants’ excessive crying. In this addendum we further discuss our results in the light of other recent publications on the subject, and present previously unpublished analyses of our own. Additionally, we address the possible mechanisms for the observed specific microbial signatures and infant crying, providing support for a theoretical causal model. Finally, we discuss future directions that could further help elucidate the hypothesized relations between intestinal microbiota and infant colic.
Microbial Signatures of Infants with Colic
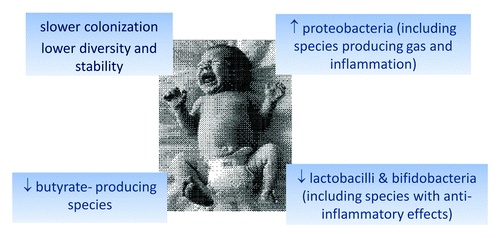
At birth, the intestines of the infant are virtually sterile. Within minutes, the colonization by bacteria begins. The bacteria originate mainly from the mother and the environment, and the colonization is influenced by factors such as prematurity, mode of delivery, sanitary conditions of hospital and home environments, feeding type, antibiotic use, and presence of siblings or pets.Citation1-Citation4 A well-balanced colonization of the neonatal intestine is important for the development of the immune system, such that a late acquisition of intestinal bacteria or a reduced complexity of the microbiota, may delay its maturation.Citation1,Citation5 In addition to affecting the development of the immune system, delays and abnormalities in the colonization process may have other, immediate effects in the young infant. In our study, we compared nine fecal samples of the first 100 d of life from infants that at 6 weeks of age were determined to have colic with those of a control group of infants with low levels of crying behavior.Citation6 DNA was isolated from all samples and analyzed for their global bacterial composition by using the Human Intestinal Tract Chip (HITChip), a phylogenetic microarray platform that allows a comprehensive and deep analysis of over 1000 known intestinal bacteria and has been used to analyze over 5000 intestinal samples (Salojarvi et al., unpublished observations).Citation7 The main results of our study relating to colic babies are summarized in . We observed that fecal microbiota diversity gradually increased after birth in the control group but not in the colic group. In the first postnatal weeks, the diversity of the colic group microbiota was found to be significantly lower than that of the control group. Additionally, the stability of the microbiota of successive samples was significantly lower in the colic infants for the first weeks.
Significantly distinct microbial signatures were found in the colic group at 2 or 4 weeks of age, while early indications for the observed aberrations were already detectable in the first week of life. Proteobacterial DNA was increased more than 2-fold in colic as compared with control infants, especially specific groups of bacteria related to Escherichia, Klebsiella, Serratia, Vibrio, Yersinia, and Pseudomonas. Notably, Escherichia and Klebsiella spp. are known for their gas-producing properties as well as the potential production of inflammatory lipopolysaccharides (LPS). Contrarily, the DNA levels of bacteria belonging to the Bacteroidetes and Firmicutes were reduced, the latter including some canonical groups known to produce butyrate. We have evidence that the latter group of strict anaerobes is present in early life and includes bacteria notably related to Eubacterium halli (unpublished observations). These members of the Clostridium cluster XIVa group are capable of producing butyrate from acetate and lactate.Citation8 While butyrate is known for its anti-inflammatory, enterocyte-fuelling and pain-reducing effects in the adult intestine,Citation9 lactate and acetate do not have this positive connotation although these acids are produced in ample amounts by the abundant bifidobacteria. The decreased DNA levels of Bacteroides in colic babies are of specific interest, as recently these were found to be reduced as well in infants suffering from atopic eczema.Citation10,Citation11 Finally, bifidobacteria and lactobacilli DNA was reduced in infants with colic. Detailed analysis of our data indicated that the reduced level of lactobacilli notably related to relatives of L. plantarum that also includes L. rhamnosus.Citation7 In line with this, we observed relatively high levels of L. rhamnosus in healthy babies of the studied cohort based on deep metagenomic analysis (De Been et al., unpublished observations). In conclusion, our present results confirm and extend those of earlier studies in older infants, which mostly employed traditional culturing methods and focused on specific bacteria.Citation12-Citation16
The microbial differences between the colic and control groups described above were only explained by crying but not by other factors, such as infant sex, mode and place of delivery, breast-feeding, birth weight, and attendance of center-based childcare. Finally, the differences between the colic and control groups disappeared by 3–4 mo of age, indicating that the colic microbial signatures may be only temporary and not indicative of a permanently altered intestinal microbiota. This contrasts with the studies on atopic eczema where differences persist for over a year of age.Citation10,Citation11
Since the publication of our colic study, two papers have appeared describing the results of randomized double-blind placebo-controlled probiotic trials in colicky infants in two European countries, Italy and Czech Republic.Citation17,Citation18 Both studies used designs with Lactobacillus reuteri as a probiotic, administering the same dose (108 colony-forming units) for the same 21-d period. The mean ages of the infants upon entering the study were 30 d (Italy study, n = 29) and 35 d (Czech Republic study, n = 80). Importantly, in both studies the crying decreased by 2-fold or more, significantly more often in the group of infants receiving probiotics than in those receiving placebo. The study in the Czech Republic reported these significant differences in crying behavior already 7 d after starting the treatment.Citation18 The study by Roos et al.Citation17 reanalyzed an earlier L. reuteri trial by addressing the intestinal microbiota of the colicky infants by medium depth pyrosequencing. No effect of the probiotic on the global composition of the intestinal microbiota was found, but when the responders to the probiotic intervention were analyzed they had a higher level of Bacteroides spp. abundance than the non-responders. The results from these independent but comparable studies point in the direction of a causal relation between intestinal microbiota and excessive crying. Modification of the infant’s microbiota by means of an oral probiotic treatment apparently directly affects the crying behavioral phenotype.
Another recent study on the links between microbiota and colic reported a highly increased odds ratio for the presence of Helicobacter pylori in feces of colicky infants as compared with controls [odds ratio, 15.3 (95% CI, 17.9‒29.8)].Citation19 The infants were between 2 weeks and 4 mo of age, and of the 55 infants with colic, 45 (81.8%) tested positive for H.pylori, while of the 30 healthy controls, 7 (23.3%) tested positive for H. pylori. When revisiting this species in our earlier study, we found on average a 2-fold increased level of Helicobacter-related bacteria in colic babies as compared with the healthy control babies at 2 weeks (p = 0.01).
Finally, a recently published study by Gelfand et al.Citation20 reports that 2-mo-old infants of mothers with a history of migraine are 2.6 times as likely to have colic than infants whose mother do not suffer from migraine. The authors propose that colic may be an early life precursor of migraine, as migraine has a strong genetic underpinning. However, the fact that the excessive crying that characterizes colic disappears at 3‒4 mo of age, places questions about the crying representing an early life manifestation of migraine. An alternative, and perhaps even provocative, explanation may lie in a possible relation between maternal migraine and intestinal microbiota. Both explanations may be related, perhaps in an indirect way, such as through maternal diet. To our knowledge, there are no studies relating adult migraine to intestinal microbiota. However, a report addressing 12-y-old children suffering from migraine by endoscopic techniques found evidence of inflammatory lesion in 29 out of 31 children.Citation21 The authors speculate that recurrent abdominal pain may be implicated in the etiology of migraine. In this line, it is possible to hypothesize that abnormalities in the microbiota of the maternal gastrointestinal tract may underlie both the maternal migraine as well as the infant colicky phenotype.
Possible Mechanisms Linking Intestinal Microbiota and Infant Crying
Remarkably, the differences between colic and control microbiota that we found in our study were all in the first month of life, before the colic peak of 6 weeks.Citation6 Based on these results, we therefore propose that early increased levels of pathogenic bacteria and reductions of lactobacilli, bifidobacteria or butyrate-producing bacteria produce intestinal pain and inflammation in the infant, and that this in turn causes excessive crying. However, it is not possible to determine causality in our study, and we cannot discard the possibility that other, unknown factors are behind both the microbial signatures and the crying. In any case, we can consider the observed patterns () as early warning signals. Remarkably, these patterns share some features with those recently observed in adults with type 2 diabetes.Citation22
It is appealing to take a closer look at the process of early colonization, as this process in many cases may hold the answer over the origin of the colic and possibly of other phenotypes. As stated before, infants are born with virtually sterile intestines,Citation23 and in our work we observed a low bacterial load in the meconium samples that were analyzed,Citation6 The subsequent colonization by microbiota commences almost immediately, and proceeds at a rapid pace, with the infant intestinal tract becoming the habitat of dozens of bacterial species after only a few days. presents a model of the factors affecting an infant’s microbial colonization of the intestinal tract in the first weeks of postnatal life.
Figure 2. Major factors affecting a full-term infant’s microbial colonization of the gut in the first weeks of postnatal life.
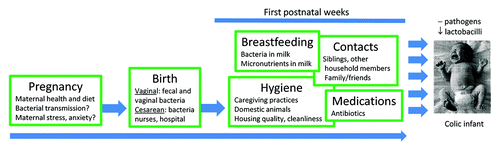
Abnormalities in any of these factors, together with fortuitous encounters with pathogens may result in the colic phenotype. It is yet unknown whether bacteria may reach the fetal intestine during pregnancy, although there are speculations on early colonization processes taking place through regular fetal swallowing of amniotic fluid.Citation24 However, there are other indications that maternal state during pregnancy may influence the neonate’s intestinal colonization. Bailey et al.Citation25 found that prenatal stress reduced the number of bifidobacteria and lactobacilli in offspring of prenatally stressed rhesus monkeys (especially those stressed in late pregnancy). These authors also found a trend for stressed offspring to have more Shigella flexneri than non-stressed offspring. Whether human infants’ intestinal colonization is similarly influenced by maternal (psychological) stress and anxiety during pregnancy remains to be determined, and we are currently exploring this area by investigating the effects of late pregnancy maternal stress on the infant’s colonization process.
During delivery, infants encounter the first major source of microbes: the maternal fecal and vaginal communities. The quality of the maternal vaginal and intestinal microbiota will therefore play a major role in the initial phase of colonization, and perhaps even have long-term consequences for infant development and health.Citation26 In healthy pregnant women as compared with non-pregnant control women, the diversity and richness of the vaginal microbiome are reduced, with dominance of Lactobacillus species.Citation27 However, women suffering from unbalances or (subclinical) infections in their vaginal or intestinal microbiota will most probably transmit these to their infants, constituting a non-optimal basis for the further development of the intestinal microbiota. In infants delivered by cesarean section, the natural colonization process is disrupted, and these neonates will acquire their intestinal microbiota from the environment (e.g., ward staff, other infants and children, and family and friends) and from the maternal skin.Citation28 Studies show that these infants are characterized by low bacterial richness and diversity, lower bifidobacteria, lower numbers of Bacteroides fragilis, and higher numbers of Clostridium difficile.Citation29,Citation30 According to a recent review, the delivery mode has an important role in the numbers and diversity of both lactobacilli and bifidobacteria, and these effects may persist for some time, affecting the infant’s subsequent health and development.Citation31
Feeding has a crucial impact on the infant’s intestinal microbiota, as the composition of the early diet will guide the colonization process.Citation31,Citation32 Bifidobacteria are more dominant, and often more diverse, in the intestine of breastfed infants as compared with formula fed infants. These differences may be due to the unique composition of maternal milk, but also to the direct presence of bacteria in breast milk. In addition to containing appropriate nutrients for the growing infant, maternal milk is a source of oligosaccharides that selectively promote the growth of Bifidobacterium in the infant intestine,Citation33 and of lactoferrin (antimicrobial agent), and lysozyme (enzyme capable of digesting bacterial cell walls).Citation31 Human milk energy content may depend on the sex of the infant: a recent study found that mothers of male infants produced milk with a 25% greater energy content than mothers of female infants.Citation34 These sex-biased differences in quality of milk may in turn affect bacterial colonization of the intestines. With respect to bacteria, human breast milk contains over 360 prokaryotic genera,Citation35 and may be an important direct source of lactobacilli and bifidobacteria,Citation36 as well as of other bacteria for the infant.Citation37-Citation39 These findings are relatively new, and many questions about the role of maternal milk remain. For example, is the colonization process influenced by the frequency and duration of each breastfeeding episode? Cultures around the world vary greatly in their frequency of feeding, from at least twice an hour (e.g., Kung San)Citation40 to Western patterns of once every 3 or 4 h. Whether and how these differences affect an infant’s colonization process is as yet unknown. Also, how does the maternal peripartum psychological state affect the (bacterial) composition of her breast milk? There is a high prevalence of perinatal mental disorders, most commonly depression and anxiety, with rates of 10–13% in high-income countries, and of 16–20% in low- and lower-middle-income countries.Citation41 These mental disorders are closely related to the physiological stress system and to diet. Again, how these states are related to breast milk composition is a matter of further research.
Hygienic properties of the early environment, such as housing characteristics, and cleanliness, will most probably play a role in the colonization process. The presence of companion animals may influence hygienic conditions of the house, while at the same time playing an indirect role in transmission of bacteria. Dog-owning adults namely share more skin-like microbiota with their own dogs than with other dogs, and having a dog also increases the shared skin microbiota in cohabiting adults.Citation4 Parental hygienic and caregiving practices will constitute a direct link between the environment and the bacteria reaching their infant. A recent example of this parental influence comes from a Swedish study on pacifier cleaning practices.Citation42 The researchers studied two methods for cleaning a pacifier during an infant’s first 6 mo of life: boiling vs. parental sucking of the pacifier, and found that the latter was related to a reduced risk for allergy development and an altered oral microbiota in the child. The authors speculate that microbes transferred to the infant via the parent’s saliva may stimulate the infant’s immune system by influencing the development of the oral/pharyngeal microbiota, but also by being swallowed and influencing the development of the intestinal microbiota. However, the oral microbiota was only characterized by profiling and hence one may also envisage that the sucking of the pacifier by the parents is associated with other hygienic conditions leading to altered microbiota in oral and possibly intestinal microbiota.
The structure of the family an infant is born into is likely to influence the colonization of the intestinal tract. Family members share more of their skin, oral, and intestinal microbiota than individuals from different households, with stronger effects of cohabitation on skin microbiota.Citation4 Also, 1-mo-old infants without siblings tend to have lower counts of bifidobacteria in the intestinal tract than infants with older siblings.Citation30 First-born infants are also more rapidly colonized by Clostridium species and by Enterobacteriaceae other than E. coli.Citation43 Fortuitous contacts of the infant with further family and friends could influence the establishment of the intestinal microbiota, but to date little is known about these environmental influences on the colonization process.
In the first postnatal weeks, infectious illnesses together with the use of antibiotics can have major effects on the development of the infant’s intestinal microbiota, producing large shifts in taxonomic groups, and altering overall diversity.Citation32 For example, oral antibiotics taken in the first month of life result in a decreased count of fecal bifidobacteria and B. fragilis-group species, and increased Enterococcus.Citation30,Citation44
Finally, and for simplicity reasons not included in , a first factor that may naturally play a role in the microbial colonization is the infant’s genetic and epigenetic make-up that may affect the physical and physiological state of the intestinal tract, in turn making it more or less attractive for different bacterial species.
In summary, many factors affecting the early colonization of the infant intestinal tract have been identified. As such these factors may have a smaller or larger influence in the origins of unbalances in microbiota, and hence be implicated in the development of the colic phenotype. However, several gaps in the knowledge about the colonization process remain. Specifically information about the factors that play a determining role in the development of the colic signatures is lacking. Further prospective research carrying out intensive sampling designs, interventions and other cause-effect studies should help shed light on the mechanisms that underlie colic, and promote therapies that can prevent this disruptive condition.
Challenges and Future Directions
Although the development of early fecal tests to detect microbial signatures predictive of future colic would seem an attractive advance in preventive medicine, it does not appear to be a feasible endeavor for the near future. The laboratory analyses used to detect the characteristic microbial signatures of infants with colic are presently too complex, time consuming and expensive for application as a diagnostic screening instrument. A more reasonable and pragmatic approach appears to lie in the realm of maternal and infant ingestion of pre- and pro-biotics to reinforce the chances of the infant developing a diverse, and normal intestinal microbiota. Modulating maternal intestinal microbiota during pregnancy and lactation could directly diminish the infant’s chances of developing colic. Moreover, given the importance of the intestinal microbiota for the maturation and development of the intestinal tract, the immune system, and perhaps even the brain, these effects may extend far beyond those of the first couple of months, having a direct effect on the infant’s future health. For example, recent studies indicate how interactions between the early intestinal microbiome and the environment may affect metabolic programming already from infancy, leading in some cases to childhood obesity.Citation45
With respect to infant colic, the two studies described above,Citation17,Citation18 together with an earlier studyCitation46 present promising results as they indicate that administration of L. reuteri to colicky infants reduced their crying. A next step is to investigate whether the excessive crying can be prevented by giving these or other lactobacilli to the infant from birth (i.e., before the colic is apparent), and even to the mother toward the end of the pregnancy and during the breastfeeding period. Such treatments might in many cases help prevent the excessive crying altogether, at the same time giving the infant a boost of beneficial bacteria for the intestinal colonization. Problems that remain to be solved, however, are that the “ideal” composition of the infant intestinal microbiota is yet to be determined, as well as the identity of the core microbes that will lead to the most favorable health outcomes.Citation31 In the same line, researchers still face the challenge of determining the best (preventive) pre- and probiotics. A recent study by Aloisio et al.Citation47 investigated the features of 46 strains of Bifidobacterium and identified four promising strains for functioning as probiotics for the treatment of infant colic. The authors plan to test the selected strains in a validation clinical trial. Endeavors of this type are necessary to further identify the best strains for probiotic treatments. Similarly, the optimal dose, frequency, and timing of administration for the infants and pregnant women are some aspects that need to be evaluated.Citation48 For example, there are indications that administration of a single dose of probiotics to low birth weight infants may have long-term effects on the microbiota,Citation49 raising the question about the necessity for a pattern of daily administration.
Acknowledgments
We are very grateful for the continuous efforts of the families who kindly participate in the BIBO study. We also thank all the research assistants, Master students and PhD students, for their assistance with data collection. This research was supported by the Netherlands Organization for Scientific Research (NWO) by a personal Vidi Grant to CW (grant number 452–04–320) and an unrestricted Spinoza Award and the ERC Advanced Grant 250172 MicrobesInside to WMV.
Submitted
06/30/2013
Revised
07/24/2013
Accepted
08/04/2013
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Adlerberth I, Wold AE. Establishment of the gut microbiota in Western infants. Acta Paediatr 2009; 98:229 - 38; http://dx.doi.org/10.1111/j.1651-2227.2008.01060.x; PMID: 19143664
- Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol 2002; 68:219 - 26; http://dx.doi.org/10.1128/AEM.68.1.219-226.2002; PMID: 11772630
- Koenig JE, Spor A, Scalfone N, Fricker AD, Stombaugh J, Knight R, Angenent LT, Ley RE. Succession of microbial consortia in the developing infant gut microbiome. Proc Natl Acad Sci U S A 2011; 108:Suppl 1 4578 - 85; http://dx.doi.org/10.1073/pnas.1000081107; PMID: 20668239
- Song SJ, Lauber C, Costello EK, Lozupone CA, Humphrey G, Berg-Lyons D, Caporaso JG, Knights D, Clemente JC, Nakielny S, et al. Cohabiting family members share microbiota with one another and with their dogs. Elife 2013; 2:e00458; http://dx.doi.org/10.7554/eLife.00458; PMID: 23599893
- Langhendries JP. [Early bacterial colonisation of the intestine: why it matters?]. Arch Pediatr 2006; 13:1526 - 34; http://dx.doi.org/10.1016/j.arcped.2006.09.018; PMID: 17079124
- de Weerth C, Fuentes S, Puylaert P, de Vos WM. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics 2013; 131:e550 - 8; http://dx.doi.org/10.1542/peds.2012-1449; PMID: 23319531
- Rajilić-Stojanović M, Heilig HG, Molenaar D, Kajander K, Surakka A, Smidt H, de Vos WM. Development and application of the human intestinal tract chip, a phylogenetic microarray: analysis of universally conserved phylotypes in the abundant microbiota of young and elderly adults. Environ Microbiol 2009; 11:1736 - 51; http://dx.doi.org/10.1111/j.1462-2920.2009.01900.x; PMID: 19508560
- Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol 2012; 9:577 - 89; http://dx.doi.org/10.1038/nrgastro.2012.156; PMID: 22945443
- Vanhoutvin SA, Troost FJ, Kilkens TO, Lindsey PJ, Hamer HM, Jonkers DM, Venema K, Brummer RJ. The effects of butyrate enemas on visceral perception in healthy volunteers. Neurogastroenterol Motil 2009; 21:952 - e76; http://dx.doi.org/10.1111/j.1365-2982.2009.01324.x; PMID: 19460106
- Abrahamsson TR, Jakobsson HE, Andersson AF, Björkstén B, Engstrand L, Jenmalm MC. Low diversity of the gut microbiota in infants with atopic eczema. J Allergy Clin Immunol 2012; 129:434 - 40, e1-2; http://dx.doi.org/10.1016/j.jaci.2011.10.025; PMID: 22153774
- Nylund L, Satokari R, Nikkilä J, Rajilić-Stojanović M, Kalliomäki M, Isolauri E, Salminen S, de Vos WM. Microarray analysis reveals marked intestinal microbiota aberrancy in infants having eczema compared to healthy children in at-risk for atopic disease. BMC Microbiol 2013; 13:12; http://dx.doi.org/10.1186/1471-2180-13-12; PMID: 23339708
- Savino F, Cordisco L, Tarasco V, Calabrese R, Palumeri E, Matteuzzi D. Molecular identification of coliform bacteria from colicky breastfed infants. Acta Paediatr 2009; 98:1582 - 8; http://dx.doi.org/10.1111/j.1651-2227.2009.01419.x; PMID: 19604166
- Savino F, Cresi F, Pautasso S, Palumeri E, Tullio V, Roana J, Silvestro L, Oggero R. Intestinal microflora in breastfed colicky and non-colicky infants. Acta Paediatr 2004; 93:825 - 9; http://dx.doi.org/10.1111/j.1651-2227.2004.tb03025.x; PMID: 15244234
- Rhoads JM, Fatheree NY, Norori J, Liu Y, Lucke JF, Tyson JE, Ferris MJ. Altered fecal microflora and increased fecal calprotectin in infants with colic. J Pediatr 2009; 155:823 - 8, e1; http://dx.doi.org/10.1016/j.jpeds.2009.05.012; PMID: 19628216
- Lehtonen L, Korvenranta H, Eerola E. Intestinal microflora in colicky and noncolicky infants: bacterial cultures and gas-liquid chromatography. J Pediatr Gastroenterol Nutr 1994; 19:310 - 4; http://dx.doi.org/10.1097/00005176-199410000-00009; PMID: 7815263
- Pärtty A, Kalliomäki M, Endo A, Salminen S, Isolauri E. Compositional development of Bifidobacterium and Lactobacillus microbiota is linked with crying and fussing in early infancy. PLoS One 2012; 7:e32495; http://dx.doi.org/10.1371/journal.pone.0032495; PMID: 22403665
- Roos S, Dicksved J, Tarasco V, Locatelli E, Ricceri F, Grandin U, Savino F. 454 pyrosequencing analysis on faecal samples from a randomized DBPC trial of colicky infants treated with Lactobacillus reuteri DSM 17938. PLoS One 2013; 8:e56710; http://dx.doi.org/10.1371/journal.pone.0056710; PMID: 23468874
- Szajewska H, Gyrczuk E, Horvath A. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: a randomized, double-blind, placebo-controlled trial. J Pediatr 2013; 162:257 - 62; http://dx.doi.org/10.1016/j.jpeds.2012.08.004; PMID: 22981952
- Ali AM. Helicobacter pylori and infantile colic. Arch Pediatr Adolesc Med 2012; 166:648 - 50; http://dx.doi.org/10.1001/archpediatrics.2011.1241; PMID: 22751879
- Gelfand AA, Thomas KC, Goadsby PJ. Before the headache: infant colic as an early life expression of migraine. Neurology 2012; 79:1392 - 6; http://dx.doi.org/10.1212/WNL.0b013e31826c1b7b; PMID: 22972642
- Mavromichalis I, Zaramboukas T, Giala MM. Migraine of gastrointestinal origin. Eur J Pediatr 1995; 154:406 - 10; http://dx.doi.org/10.1007/BF02072116; PMID: 7641777
- de Vos WM, Nieuwdorp M. Genomics: A gut prediction. Nature 2013; 498:48 - 9; http://dx.doi.org/10.1038/nature12251; PMID: 23719383
- Jiménez E, Marín ML, Martín R, Odriozola JM, Olivares M, Xaus J, Fernández L, Rodríguez JM. Is meconium from healthy newborns actually sterile?. Res Microbiol 2008; 159:187 - 93; http://dx.doi.org/10.1016/j.resmic.2007.12.007; PMID: 18281199
- Mshvildadze M, Neu J. The infant intestinal microbiome: friend or foe?. Early Hum Dev 2010; 86:Suppl 1 67 - 71; http://dx.doi.org/10.1016/j.earlhumdev.2010.01.018; PMID: 20116944
- Bailey MT, Lubach GR, Coe CL. Prenatal stress alters bacterial colonization of the gut in infant monkeys. J Pediatr Gastroenterol Nutr 2004; 38:414 - 21; http://dx.doi.org/10.1097/00005176-200404000-00009; PMID: 15085020
- Collado MC, Cernada M, Baüerl C, Vento M, Pérez-Martínez G. Microbial ecology and host-microbiota interactions during early life stages. Gut Microbes 2012; 3:352 - 65; http://dx.doi.org/10.4161/gmic.21215; PMID: 22743759
- Aagaard K, Riehle K, Ma J, Segata N, Mistretta TA, Coarfa C, Raza S, Rosenbaum S, Van den Veyver I, Milosavljevic A, et al. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One 2012; 7:e36466; http://dx.doi.org/10.1371/journal.pone.0036466; PMID: 22719832
- Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A 2010; 107:11971 - 5; http://dx.doi.org/10.1073/pnas.1002601107; PMID: 20566857
- Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, Sears MR, Becker AB, Scott JA, Kozyrskyj AL, CHILD Study Investigators. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 2013; 185:385 - 94; http://dx.doi.org/10.1503/cmaj.121189; PMID: 23401405
- Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006; 118:511 - 21; http://dx.doi.org/10.1542/peds.2005-2824; PMID: 16882802
- Fouhy F, Ross RP, Fitzgerald GF, Stanton C, Cotter PD. Composition of the early intestinal microbiota: knowledge, knowledge gaps and the use of high-throughput sequencing to address these gaps. Gut Microbes 2012; 3:203 - 20; http://dx.doi.org/10.4161/gmic.20169; PMID: 22572829
- Madan JC, Farzan SF, Hibberd PL, Karagas MR. Normal neonatal microbiome variation in relation to environmental factors, infection and allergy. Curr Opin Pediatr 2012; 24:753 - 9; http://dx.doi.org/10.1097/MOP.0b013e32835a1ac8; PMID: 23111681
- Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci U S A 2011; 108:Suppl 1 4653 - 8; http://dx.doi.org/10.1073/pnas.1000083107; PMID: 20679197
- Powe CE, Knott CD, Conklin-Brittain N. Infant sex predicts breast milk energy content. Am J Hum Biol 2010; 22:50 - 4; http://dx.doi.org/10.1002/ajhb.20941; PMID: 19533619
- Ward TL, Hosid S, Ioshikhes I, Altosaar I. Human milk metagenome: a functional capacity analysis. BMC Microbiol 2013; 13:116; http://dx.doi.org/10.1186/1471-2180-13-116; PMID: 23705844
- Martín R, Jiménez E, Heilig H, Fernández L, Marín ML, Zoetendal EG, Rodríguez JM. Isolation of bifidobacteria from breast milk and assessment of the bifidobacterial population by PCR-denaturing gradient gel electrophoresis and quantitative real-time PCR. Appl Environ Microbiol 2009; 75:965 - 9; http://dx.doi.org/10.1128/AEM.02063-08; PMID: 19088308
- Solís G, de Los Reyes-Gavilan CG, Fernández N, Margolles A, Gueimonde M. Establishment and development of lactic acid bacteria and bifidobacteria microbiota in breast-milk and the infant gut. Anaerobe 2010; 16:307 - 10; http://dx.doi.org/10.1016/j.anaerobe.2010.02.004; PMID: 20176122
- Martín R, Heilig GH, Zoetendal EG, Smidt H, Rodríguez JM. Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J Appl Microbiol 2007; 103:2638 - 44; http://dx.doi.org/10.1111/j.1365-2672.2007.03497.x; PMID: 18045446
- Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, Rodríguez JM. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res 2013; 69:1 - 10; http://dx.doi.org/10.1016/j.phrs.2012.09.001; PMID: 22974824
- Konner M. Hunter-gatherer infancy and childhood: The! Kung and others. In: Hewlett BS, Lamb ME, eds. Hunter-gatherer childhoods: Evolutionary, developmental and cultural perpectives. New Brunswick: Transaction Publishers, 2005:34-35.
- Fisher J, Cabral de Mello M, Patel V, Rahman A, Tran T, Holton S, Holmes W. Prevalence and determinants of common perinatal mental disorders in women in low- and lower-middle-income countries: a systematic review. Bull World Health Organ 2012; 90:139G - 49G; http://dx.doi.org/10.2471/BLT.11.091850; PMID: 22423165
- Hesselmar B, Sjöberg F, Saalman R, Aberg N, Adlerberth I, Wold AE. Pacifier cleaning practices and risk of allergy development. Pediatrics 2013; 131:e1829 - 37; http://dx.doi.org/10.1542/peds.2012-3345; PMID: 23650304
- Adlerberth I, Strachan DP, Matricardi PM, Ahrné S, Orfei L, Aberg N, Perkin MR, Tripodi S, Hesselmar B, Saalman R, et al. Gut microbiota and development of atopic eczema in 3 European birth cohorts. J Allergy Clin Immunol 2007; 120:343 - 50; http://dx.doi.org/10.1016/j.jaci.2007.05.018; PMID: 17604093
- Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Tsubouchi M, Kiyohara C, Shirakawa T, Sonomoto K, Nakayama J. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol 2009; 56:80 - 7; http://dx.doi.org/10.1111/j.1574-695X.2009.00553.x; PMID: 19385995
- Luoto R, Kalliomäki M, Laitinen K, Delzenne NM, Cani PD, Salminen S, Isolauri E. Initial dietary and microbiological environments deviate in normal-weight compared to overweight children at 10 years of age. J Pediatr Gastroenterol Nutr 2011; 52:90 - 5; http://dx.doi.org/10.1097/MPG.0b013e3181f3457f; PMID: 21150648
- Savino F, Cordisco L, Tarasco V, Palumeri E, Calabrese R, Oggero R, Roos S, Matteuzzi D. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics 2010; 126:e526 - 33; http://dx.doi.org/10.1542/peds.2010-0433; PMID: 20713478
- Aloisio I, Santini C, Biavati B, Dinelli G, Cencič A, Chingwaru W, Mogna L, Di Gioia D. Characterization of Bifidobacterium spp. strains for the treatment of enteric disorders in newborns. Appl Microbiol Biotechnol 2012; 96:1561 - 76; http://dx.doi.org/10.1007/s00253-012-4138-5; PMID: 22588500
- Marques TM, Wall R, Ross RP, Fitzgerald GF, Ryan CA, Stanton C. Programming infant gut microbiota: influence of dietary and environmental factors. Curr Opin Biotechnol 2010; 21:149 - 56; http://dx.doi.org/10.1016/j.copbio.2010.03.020; PMID: 20434324
- McGee R, O’Connor PM, Russell D, Dempsey EM, Ryan AC, Ross PR, Stanton C. Prolonged faecal excretion following a single dose of probiotic in low birth weight infants. Acta Paediatr 2010; 99:1587 - 8; http://dx.doi.org/10.1111/j.1651-2227.2010.01878.x; PMID: 20491702