Abstract
The health benefits of a high fiber diet (HFD) result in part from the action of metabolic end products made by gut commensals on the host epithelium. Butyrate is one such beneficial metabolite; however, butyrate paradoxically enhances the capacity of Escherichia coli-produced Shiga toxin type 2 (Stx2) to kill tissue culture cells. We recently showed that mice fed an HFD exhibited increased butyrate in gut contents and had an altered intestinal microbiota with reduced numbers of Escherichia species. Furthermore, mice fed an HFD and infected with Stx-producing E. coli (STEC) were colonized to a higher degree, lost more weight and succumbed to infection at greater rates compared with STEC-infected low fiber diet animals. The HFD animals showed higher levels of the Stx receptor globotriaocylceramide (Gb3) in both the gut and kidneys. We speculate that an HFD that leads to increased intestinal butyrate and Gb3 in the intestines and kidneys may explain the higher rate of the hemolytic uremic syndrome in females over males.
Introduction
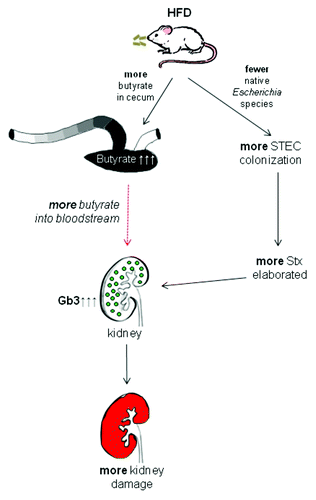
Shiga toxin (Stx)-producing E. coli (STEC) are food- and water-borne pathogens that cause bloody diarrhea. The hemolytic uremic syndrome (HUS) occurs as a sequela of STEC infection in 4–30% of the cases.Citation1-Citation4 Possible host-related influences on which patients will develop the HUS are not defined apart from younger ageCitation5 and female gender.Citation6 However, we recently showed that when the food regimen is altered in mice to a high fiber diet (HFD), there was an increase in butyrate in the gut, and the animals became more susceptible to infection by an E. coli O157:H7 strain that produces Stx2.Citation7 We further demonstrated that mice on an HFD not only had elevated levels of butyrate in the gut but also had enhanced expression of the Stx receptor, Gb3, in or on the mouse gut epithelia and kidney. We theorize that the enhanced virulence of Stx2+ O157:H7 in mice fed an HFD results from two factors. First, we hypothesize that high fiber diets increase local and systemic levels of butyrate and that these elevated butyrate concentrations lead to more Gb3-expressing colonic and renal tubular epithelial cells, respectively. The enrichment of Gb3 on cells in the gut results in more Stx2 binding to these enterocytes and more transfer of Stx2 into the blood stream. This increased toxemia, in turn, leads to more Stx2 that is available to bind to the renal tubules now enriched in Gb3. More tubular necrosis then ensues that ultimately causes more morbidity and mortality in HFD-fed mice than in low fiber diet (LFD)-fed animals. Second, an HFD reduces the population of commensal Escherichia species in the gut;Citation7 therefore, we speculate that a niche is made available for the incoming STEC and facilitates the increased colonization by Stx2+ O157:H7 that we observed in the HFD mice. A model of the two-pronged proposed mechanism of the enhanced virulence of STEC is shown in .
Figure 1. Model of diet-based enhanced susceptibility to E. coli O157:H7 infection. An HFD leads to an increase in gut butyrate (black), most concentrated in the cecum and falling in concentration (gray shading) toward the rectum. Butyrate enhances Gb3 levels in the gut and kidney (green) and thus results in enhanced sensitivity to Stx and more kidney damage (red); a concomitant decrease in the competitive resident Escherichia species in the intestinal tract due to the HFD allows for increased colonization by O157:H7 and thus more Stx production in the intestine.
Fiber, Butyrate and Gb3
The amount of butyrate an individual produces in the gut is determined by diet and gut microbiota. Adding fiber to a low fiber diet increases fecal butyrate and the concentrations of other short-chain fatty acids (SCFA).Citation8,Citation9 The specific fiber source plays a considerable role, however, in the amount of butyrate produced. What is popularly known as “dietary fiber” is the non-starch polysaccharide (NSP), or non α-glucan polysaccharide.Citation10 NSP includes plant material such as pectins, guar and cellulose.Citation10 Fiber in the human diet is typically NSP.Citation11 Another fiber type is resistant starch (RS). RS is a type of starch that resists digestion in the small bowel and is thus available as a substrate for fermentation in the large bowel. Examples of RS include corn, peas, beans, cracked grains, potatoes and bananas.Citation10 In general, the amount of butyrate produced in the gut for a given fiber type is governed by the fermentability of a given fiber substrate and the quantity of the fiber present in the gut. For example, pigs fed white rice, which contains low fiber content (RS type) have a distal colonic butyrate pool of 0.06mol, compared with 0.47mol in pigs fed brown rice, which has high fiber content (RS type).Citation12 In the latter example, most of the white rice was digested in the stomach and small intestines, while the brown rice was more resistant to digestion and provided fermentable material to the colon for production of SCFA such as butyrate.
Butyrate has a profound effect on cell morphology and function and acts as a primary energy source for colonic enterocytes.Citation13,Citation14 In fiber-rich diets, crypt deepening occurs, and crypt duplication can take place, events that increase the number of crypts per unit length and total crypt depth in response to diet.Citation15 Hence, butyrate levels alter the mucosa, although the possible effect of butyrate-mediated anatomical and physiological changes in the gut on STEC colonization is unknown. However, butyrate and other SCFA are most concentrated on the right side of the colon and levels fall progressively toward the distal colon.Citation10 Of note, STEC-induced pathology occurs in that same region (the ascending and transverse colon).Citation16 Indeed, in STEC infections, the cecum and right colon are described as markedly abnormal, exhibiting edema, erosion, hemorrhage and surface ulceration, whereas the descending colon typically has mild or no changes.Citation17 We speculate that the reason for the apparent co-localization of the highest levels of intestinal butyrate and STEC-evoked pathology is due to butyrate-mediated increased levels of Gb3 expression in the same region. Despite the profound effect of butyrate on Gb3 expression, additional effects of the butyrate cannot be ruled out. Butyrate and other SCFA produced in the large bowel are rapidly absorbed and pass into the portal vein,Citation18,Citation19 encounter the liver and then circulate through the body to the kidney. The kidney naturally expresses high levels of Gb3, and we found that an HFD can increase those levels further.
Disparity in Cases of HUS between Different Groups
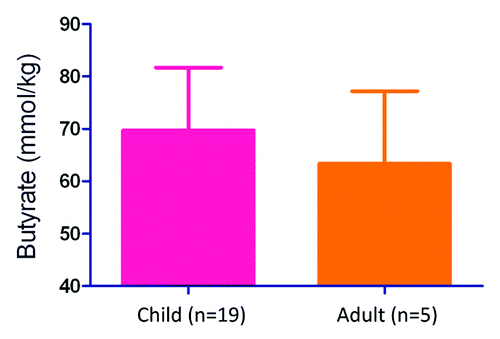
A disparity exists between those who develop the HUS following STEC infection and those who do not, i.e., children < 10 y old are 10 times more likely on average to acquire that serious sequela.Citation5 While there is some evidence of age-related changes in intestinal bacterial populations,Citation20 we are not aware of any studies that assess general butyrate content as a function of age. We therefore asked whether an inherent difference in the capacity to produce butyrate exists between children and adults by assessing the butyrate content in stool from those groups. The amount of butyrate measured in stool is likely an underestimate of the overall colonic concentration of that fatty acid because greater than 95% of gut butyrate is absorbed systemically by the time digesta reaches the anus.Citation10 We found that although children exhibit a slight trend toward more abundant butyrate, there was no significant difference in butyrate content in the stool of children compared with adults in this study, .
Figure 2. Butyrate content in the stool of uninfected children and adults. Butyrate content in the stool of children <10 y old is not statistically different from adults >~25 y old. Butyrate was extracted from frozen stool samples and measured by acidic extraction followed by gas chromatography/mass spectroscopy as described previously.Citation7 Error bars represent standard error of the mean.
Another example of host-related differences found during STEC outbreaks is that in two recent large, produce-associated outbreaks, women were more likely to develop the infection than children or men.Citation3,Citation21 Could the disparity in STEC cases for these two outbreaks simply be attributed to the healthier eating habits of women compared with men?Citation22 Maybe so for the disparity in the number of STEC infections between females and males, but, for the HUS, women do appear to be generally more susceptible to that sequela.Citation6 Further, in one of the produce examples—the sprout-linked German outbreak due to an unusual Stx2+ enteroaggregative E. coli—30% of the STEC cases in women resulted in the HUS, whereas just 15% of the infected men developed the HUS.Citation23 So, if healthier eating habits alone cannot explain the difference in the rate of the HUS between females and males where else can we look for answers? A study by Lampe, et al. suggests that there may be gender differences in colonic function. In that study, despite equal fiber intakes by men and women, mean fecal transit times were consistently faster and fecal weights greater for men than women on all diets.Citation24 This longer transit time resulted in enhanced digestibility in women and seemed to be primarily due to greater digestion of wheat bran and vegetable fiber diets.Citation24 The increased digestion in women would lead to more gut butyrate as would the diet rich in fiber. Therefore, the reason females are more likely to develop the HUS than males may be due to their higher concentrations of intestinal butyrate that subsequently lead to increased Gb3 levels and enhanced susceptibility to Stx.
Finally, a Note of Caution about Oral Rehydration Therapy
No specific therapy for STEC infections exists. Treatments for STEC patients are symptom-based, and the use of antibiotics or anti-motility agents are generally contraindicated.Citation25 However, intravenous volume expansion may reduce the likelihood of anuria and the HUS.Citation26,Citation27 In contrast, for diarrhea due to cholera, oral rehydration (ORS) therapies can shorten the duration and volume of diarrhea. The addition of guar gum or amylase-resistant starch to ORS has in some studies reduced persistent diarrhea in children or reduced the duration and severity of cholera or non-cholera diarrhea.Citation28-Citation30 The hypothesis for why the addition of carbohydrates such as guar gum or amylase-resistant starch to ORS may reduce diarrheal symptoms is that those carbohydrates are fermented to SCFA in the colon, the SCFA are then absorbed, and as a consequence, net fluid absorption is increased and the volume and duration of diarrhea is decreased.Citation31 We caution however, that the use of starch- or guar gum-supplemented ORS for the treatment of non-cholera diarrhea mediated by STEC might be deleterious for the patient as the predicted increase in SFCA in the colon could lead to increased levels of Gb3 in the colon and the kidney and, consequently, could increase the susceptibility of the patient to the effects of the Stxs.
| Abbreviations: | ||
| STEC | = | Shiga toxin-producing E. coli |
| HFD | = | high fiber diet |
| HUS | = | hemolytic uremic syndrome |
| Stx | = | Shiga toxin |
| Gb3 | = | globotriaosylceramide |
| SCFA | = | short-chain fatty acids |
| NSP | = | non-starch polysaccharide |
| RS | = | resistant starch |
| EAEC | = | enteroaggregative E. coli |
Acknowledgments
We thank Dr Phil Tarr (University of Washington, St. Louis, MO) for providing pediatric stool samples; Dr Louise Teel (Uniformed Services University, Bethesda, MD) for providing adult stool samples; and Kenneth Gable for assistance with GC/MS analysis. This work was supported by NIH grant R37 AI020148 to ADO.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Mead PS, Slutsker L, Dietz V, McCaig LF, Bresee JS, Shapiro C, Griffin PM, Tauxe RV. Food-related illness and death in the United States. Emerg Infect Dis 1999; 5:607 - 25; http://dx.doi.org/10.3201/eid0505.990502; PMID: 10511517
- Rowe PC, Orrbine E, Lior H, Wells GA, McLaine PN. A prospective study of exposure to verotoxin-producing Escherichia coli among Canadian children with haemolytic uraemic syndrome. The CPKDRC co-investigators. Epidemiol Infect 1993; 110:1 - 7; http://dx.doi.org/10.1017/S0950268800050615; PMID: 8432313
- California Food Emergency Response Team California Department of Health Services, U.S. Food and Drug Administration. Investigation of an Escherichia coli O157:H7 outbreak associated with Dole pre-packaged spinach. California Department of Health Services, 2007.
- Johnson RP, Clarke R, Wilson JB, Read SC, Rahn K, Renwick S, et al. Growing concerns and recent outbreaks involving non-O157:H7 serotypes of verotoxigenic Escherichia coli.. J Food Prot 1996; 59:1112 - 22
- Barton Behravesh C, Jones TF, Vugia DJ, Long C, Marcus R, Smith K, Thomas S, Zansky S, Fullerton KE, Henao OL, et al, FoodNet Working Group. Deaths associated with bacterial pathogens transmitted commonly through food: foodborne diseases active surveillance network (FoodNet), 1996-2005. J Infect Dis 2011; 204:263 - 7; http://dx.doi.org/10.1093/infdis/jir263; PMID: 21673037
- Gould LH, Demma L, Jones TF, Hurd S, Vugia DJ, Smith K, Shiferaw B, Segler S, Palmer A, Zansky S, et al. Hemolytic uremic syndrome and death in persons with Escherichia coli O157:H7 infection, foodborne diseases active surveillance network sites, 2000-2006. Clin Infect Dis 2009; 49:1480 - 5; http://dx.doi.org/10.1086/644621; PMID: 19827953
- Zumbrun SD, Melton-Celsa AR, Smith MA, Gilbreath JJ, Merrell DS, O’Brien AD. Dietary choice affects Shiga toxin-producing Escherichia coli (STEC) O157:H7 colonization and disease. Proc Natl Acad Sci U S A 2013; 110:E2126 - 33; http://dx.doi.org/10.1073/pnas.1222014110; PMID: 23690602
- Fleming SE, Rodriguez MA. Influence of dietary fiber on fecal excretion of volatile fatty acids by human adults. J Nutr 1983; 113:1613 - 25; PMID: 6308193
- Metcalfe-Gibson A, Ing TS, Kuiper JJ, Richards P, Ward EE, Wrong OM. In vivo dialysis of faeces as a method of stool analysis. II. The influence of diet. Clin Sci 1967; 33:89 - 100; PMID: 6059306
- Cummings JH, Macfarlane GT. The control and consequences of bacterial fermentation in the human colon. J Appl Bacteriol 1991; 70:443 - 59; http://dx.doi.org/10.1111/j.1365-2672.1991.tb02739.x; PMID: 1938669
- Southgate DA. Food components that act as dietary fiber. In: Spiller G, ed. CRC Handbook of Dietary Fiber in Human Nutrition, Third Edition. Boca Raton, FL: CRC Press LLC, 2001.
- Marsano Y. Complex Carbohydrates and Lipids in Rice Products: Effects on Large Bowel Volatile Fatty Acids and Plasma Cholesterol in Animals. Bedford Park: Flinders University of South Australia, 1995.
- Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut 1994; 35:Suppl S35 - 8; http://dx.doi.org/10.1136/gut.35.1_Suppl.S35; PMID: 8125387
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 2008; 27:104 - 19; http://dx.doi.org/10.1111/j.1365-2036.2007.03562.x; PMID: 17973645
- Perrin P, Pierre F, Patry Y, Champ M, Berreur M, Pradal G, Bornet F, Meflah K, Menanteau J. Only fibres promoting a stable butyrate producing colonic ecosystem decrease the rate of aberrant crypt foci in rats. Gut 2001; 48:53 - 61; http://dx.doi.org/10.1136/gut.48.1.53; PMID: 11115823
- Riley LW, Remis RS, Helgerson SD, McGee HB, Wells JG, Davis BR, Hebert RJ, Olcott ES, Johnson LM, Hargrett NT, et al. Hemorrhagic colitis associated with a rare Escherichia coli serotype. N Engl J Med 1983; 308:681 - 5; http://dx.doi.org/10.1056/NEJM198303243081203; PMID: 6338386
- Petras RE, Frankel W. Large Intestine (colon). In: Weidner N CR, Suster S, Weiss LM, ed. Modern Surgical Pathology. Philadelphia: Saunders, 2009.
- Dawson AM, Holdsworth CD, Webb J. Absorption of Short Chain Fatty Acids in Man. Proc Soc Exp Biol Med 1964; 117:97 - 100; http://dx.doi.org/10.3181/00379727-117-29505; PMID: 14219969
- Ruppin H, Bar-Meir S, Soergel KH, Wood CM, Schmitt MG Jr.. Absorption of short-chain fatty acids by the colon. Gastroenterology 1980; 78:1500 - 7; PMID: 6768637
- Hopkins MJ, Sharp R, Macfarlane GT. Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut 2001; 48:198 - 205; http://dx.doi.org/10.1136/gut.48.2.198; PMID: 11156640
- Frank C, Werber D, Cramer JP, Askar M, Faber M, an der Heiden M, Bernard H, Fruth A, Prager R, Spode A, et al, HUS Investigation Team. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N Engl J Med 2011; 365:1771 - 80; http://dx.doi.org/10.1056/NEJMoa1106483; PMID: 21696328
- Martin S. Surprise! Women eat healthier than men. CMAJ 2002; 167:913; PMID: 12406966
- Koch-Institute R. Final presentation and evaluation of the epidemiological findings in the EHEC O104:H4 outbreak, Germany 2011. Berlin 2011, 2011.
- Lampe JW, Fredstrom SB, Slavin JL, Potter JD. Sex differences in colonic function: a randomised trial. Gut 1993; 34:531 - 6; http://dx.doi.org/10.1136/gut.34.4.531; PMID: 8387940
- Thielman NM, Guerrant RL. Clinical practice. Acute infectious diarrhea. N Engl J Med 2004; 350:38 - 47; http://dx.doi.org/10.1056/NEJMcp031534; PMID: 14702426
- Ake JA, Jelacic S, Ciol MA, Watkins SL, Murray KF, Christie DL, Klein EJ, Tarr PI. Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics 2005; 115:e673 - 80; http://dx.doi.org/10.1542/peds.2004-2236; PMID: 15930195
- Hickey CA, Beattie TJ, Cowieson J, Miyashita Y, Strife CF, Frem JC, Peterson JM, Butani L, Jones DP, Havens PL, et al. Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch Pediatr Adolesc Med 2011; 165:884 - 9; http://dx.doi.org/10.1001/archpediatrics.2011.152; PMID: 21784993
- Alam NH, Meier R, Sarker SA, Bardhan PK, Schneider H, Gyr N. Partially hydrolysed guar gum supplemented comminuted chicken diet in persistent diarrhoea: a randomised controlled trial. Arch Dis Child 2005; 90:195 - 9; http://dx.doi.org/10.1136/adc.2003.040089; PMID: 15665181
- Raghupathy P, Ramakrishna BS, Oommen SP, Ahmed MS, Priyaa G, Dziura J, Young GP, Binder HJ. Amylase-resistant starch as adjunct to oral rehydration therapy in children with diarrhea. J Pediatr Gastroenterol Nutr 2006; 42:362 - 8; http://dx.doi.org/10.1097/01.mpg.0000214163.83316.41; PMID: 16641573
- Alam NH, Meier R, Schneider H, Sarker SA, Bardhan PK, Mahalanabis D, Fuchs GJ, Gyr N. Partially hydrolyzed guar gum-supplemented oral rehydration solution in the treatment of acute diarrhea in children. J Pediatr Gastroenterol Nutr 2000; 31:503 - 7; http://dx.doi.org/10.1097/00005176-200011000-00010; PMID: 11144434
- Binder HJ. Role of colonic short-chain fatty acid transport in diarrhea. Annu Rev Physiol 2010; 72:297 - 313; http://dx.doi.org/10.1146/annurev-physiol-021909-135817; PMID: 20148677
