Abstract
Helicobacter pylori infection is predominantly acquired early in life. The prevalence of the infection in childhood is low in developed countries, whereas in developing countries most children are infected by 10 y of age. In poor resource settings, where malnutrition, parasitic/enteropathogen and H. pylori infection co-exist in young children, H. pylori might have potentially more diverse clinical outcomes. This paper reviews the impact of childhood H. pylori infection in developing countries that should now be the urgent focus of future research. The extra-gastric manifestations in early H. pylori infection in infants in poor resource settings might be a consequence of the infection associated initial hypochlorhydria. The potential role of H. pylori infection on iron deficiency, growth impairment, diarrheal disease, malabsorption and cognitive function is discussed in this review.
Introduction
Helicobacter pylori colonizes the stomach of more than a half of the world’s population and its acquisition occurs predominantly in early childhood, especially in preschool age.Citation1 In developed countries, the prevalence of H. pylori infection is low in childhood whereas in developing countries most children are infected by 10 y of age.Citation1,Citation2 In some settings such as Peru shanty towns over 70% of infants are infected in the first year of lifeCitation3,Citation4 but in other similar settings in South America H. pylori infection occurs between 1–5 y of age.Citation2,Citation4 In poor resource settings where the infection co-exists with other morbidities such as malnutrition and parasitic and enteropathogen infections, H. pylori infection in young children may have potentially more diverse clinical outcomes than in developed countries, and include not only iron deficiency or iron deficiency anemia but growth retardation, diarrheal disease, malabsorption and impaired cognitive function.
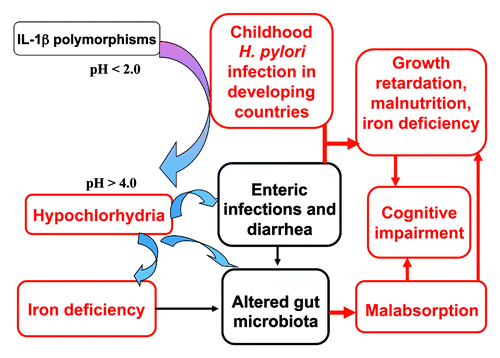
This review focuses on the impact of childhood H. pylori infection in poor resource settings. With rapidly falling levels of H. pylori infection in children in developed countries, childhood H. pylori infection in developing countries, where infection in early childhood remains high, should now be the urgent focus of future research. Importantly, the extra-gastric manifestations of H. pylori infection as a consequence of the initial hypochlorhydria associated with H. pylori infectionCitation5 have been little investigated in developing countries. The potential role of H. pylori on iron deficiency, growth impairment, diarrheal disease, malabsorption and cognitive function is discussed in this review. Although these are all potential extra-gastric manifestations of H. pylori infection, recent evidence points to the importance of hypochlorhydriaCitation6 and proinflammatory IL-1β gene cluster polymorphismsCitation7 in iron deficiency in H. pylori infected children. The consequences of hypochlorhydria in early H. pylori infections in infants in poor resource settings may also be central to increased diarrheal disease, growth impairment, malabsorption and impaired cognitive function (). H. pylori infection in developing countries could be initiating a vicious cycle of events in infants,Citation5 the global impact of which on childhood morbidity may be diverse, and largely a consequence of extra-gastric manifestations of H. pylori infection.
Figure 1. Potential multiple outcomes of childhood H. pylori infection in developing countries. Infection with H. pylori is frequent in children in developing countries.Citation2H. pylori infection is accompanied by a period of hypochlorhydria for several months. Hypochlorhydria in H. pylori infected children has been associated with iron deficiency.Citation6 Polymorphisms in IL-1β gene cluster may control the extent and duration of hypochlorhydria with initial H. pylori infection. Pro-inflammatory polymorphisms in the IL-1β gene cluster in H. pylori infected children are associated with hypoferritinaemia and reduced hemoglobin concentrations.Citation7 The period of hypochlorhydria associated with H. pylori infection in poor resource settings is a potential window for the acquisition of other enteric infections and diarrheal disease.Citation90,Citation91 The clinical consequences and potential synergism between H. pylori infection and diarrheal disease in poor resource settings will promote not only malnutrition and growth impairment, but also cognitive impairment.Citation150 The hypochlorhydria induced by H. pylori infection may also result in alterations in the gut microbiota and contribute to small intestinal permeability changes and malabsorption, thus also impacting on malnutrition and growth impairment.
H. pylori and Iron Deficiency
Iron deficiency (ID), the most common nutritional disorder in the world, affects 20–50% of people globally,Citation8-Citation11 iron deficiency anemia (IDA) being the final stage in the spectrum of a persistent negative iron balance. In developing countries, anemia represents a major public health problem and approximately 50 percent of all cases are due to ID.Citation9,Citation11 Factors including low iron intake, low dietary iron bioavailability and gastrointestinal parasite infections contribute to the high frequency of ID/IDA in developing countries.Citation11,Citation12 In childhood/adolescence the increased requirement for iron due to growth, expansion of red cell mass and menstrual blood loss in adolescent females favors the development of ID/IDA.Citation11,Citation13,Citation14 In childhood, iron deficiency has been associated with deficits of immune, cognitive and motor function.Citation11,Citation15,Citation16 Clinically advanced IDA is associated with reduced growth, increased susceptibility to infectious diseases and increased mortality.Citation10,Citation17
The first descriptions of an association between IDA and H. pylori infection in children were case reports published in 1991 by Blecker et al.Citation17 of a 15-y-old girl with a chronic active hemorrhagic gastritis associated with H. pylori infection and in 1993 by Dufour et al.Citation19 of a 7-y-old boy with H. pylori chronic antral gastritis without hemorrhage. These two patients had IDA that was refractory to iron therapy in the boy.Citation19 Eradication of H. pylori without iron supplementation resulted in reversion of the anemia in both cases. Since then, other case reports and series of cases have demonstrated an improvement in iron parameters after eradication of H. pylori in children.Citation20-Citation22
Epidemiological studies
Several observational epidemiological studies in children demonstrating an association between H. pylori infection and lower serum ferritin concentrations and/or increased prevalence of iron deficiencyCitation23-Citation28 have reinforced the association between H. pylori infection and impaired iron status. Studies conducted in developedCitation23-Citation27 and developingCitation28 countries showed lower serum ferritin concentrationsCitation23,Citation24,Citation26,Citation27 and/or higher proportion of ID or IDACitation25-Citation28 in H. pylori-positive than in H. pylori-negative children.
Of note, in a large cohort of 937 pubescent children in South Korea,Citation23a higher H. pylori-positive serology rate in those with either hypoferritinemia or ID/IDA was largely restricted to girls, which might be explained by lower iron storage in females. In 1040 children and adolescentsCitation29 and in nearly 700 school-aged Alaskan children,Citation25 where H. pylori status was evaluated by ELISA or 13C urea breath test (13C-UBT), an increased risk for low serum ferritin concentrationsCitation29 or increased prevalence of ID in children older than 8 y of ageCitation25 was observed in H. pylori infected than in non-infected children. Cardenas et al.Citation26 by evaluating 1,771 children and adolescents among 7,462 subjects from the 1999–2000 USA National Health and Nutrition Examination Survey (NHANES) showed that positive serology for H. pylori was associated with decreased levels of serum ferritin and hemoglobin and increased risk for IDA. The authors estimated that 32.3% of the cases of IDA and 13.6% of ID in the United States might be related to H. pylori infection. In poor resource settings the contribution of H. pylori to ID may well be greater.
In a cross-sectional study evaluating symptomatic South-American children undergoing upper gastrointestinal endoscopy, H. pylori infection was also a significant predictor of low serum ferritin and hemoglobin concentrations.Citation30 Negative correlations between mean cell volume (MCV) and mean cell hemoglobin (MCH) values and the degree of antral and corpus inflammation were also observed in the Brazilian and Chilean children.Citation30 An association between H. pylori infection and reduced serum ferritin levels was also found in children from Turkey undergoing endoscopy.Citation31
Other studies in children have failed to demonstrate associations between H. pylori infection and iron/anemia parameters.Citation32-Citation35 Explanations include differences in study design, inclusion/exclusion criteria, number and age of the evaluated children, H. pylori diagnosis criteria and adjustment for confounding factors. For instance, very few studies have investigated children undergoing endoscopy for upper gastrointestinal symptoms,Citation30,Citation31,Citation36 a strategy which permits better evaluation of H. pylori status and the exclusion of common causes of iron deficiency such as gastrointestinal bleeding, peptic ulcer disease, extensive erosions and celiac disease. Exclusion of female adolescents with heavy menstrual blood loss,Citation30 an important determinant of iron status in young women, and intestinal parasitic infectionsCitation6,Citation30 that lead to blood loss have not been adopted by all authors. Exclusion of subjects with enteric parasitic infection is vital in studies on H. pylori infection and iron deficiency in developing countries. The choice of diagnostic methods for H. pylori infection may also contribute to disagreement among the studies. Indirect methods such as serology have a low accuracy rate for the diagnosis of H. pylori in young children.Citation37
Interventional clinical trials
The strongest evidence for a cause-and-effect comes from interventional trials in children from developedCitation38-Citation40 and developing countriesCitation41-Citation44 or from low socioeconomic populations in a developed countryCitation45 that demonstrated an improvement of the anemia after H. pylori eradication. A series of therapeutic trials in children without iron replacement in South Korea demonstrated improvement of hemoglobin and/or ferritin levels after H. pylori eradication.Citation38-Citation40 In a controlled, household-randomized trial in rural Alaska, Fagan et al.,Citation42 by evaluating 176 native school-age children, demonstrated higher ferritin concentrations in children who remained H. pylori negative at 40 mo after H. pylori eradication therapy than in children who were re-infected with H. pylori, or who were untreated for H. pylori. Eradication of H. pylori in 110 Hispanic children of low socio-economic level aged 3 to 10 y without ID was accompanied by 3-fold increased serum ferritin baseline values.Citation45 However, Sarker et al.Citation46 did not observe differences in the hemoglobin concentrations and iron parameters between Bangladeshi children with IDA or ID in whom H. pylori was eradicated and children who remained H. pylori-positive in the three months-follow-up.
Two meta analyses studies including 16 and 8 randomized controlled trialsCitation47,Citation48 conducted in Asian children and adults, and one trial in Alaska,Citation47 compared the effect of oral iron supplementation alone, or in combination with H. pylori eradication therapy, on hematological parameters in patients with iron deficiency anemia. The meta analyses indicated eradication of H. pylori with oral iron significantly increased hemoglobin, serum ferritinCitation47,Citation48 and serum ironCitation47 from baseline compared with oral iron therapy alone. The meta analyses confirm that oral iron alone has no beneficial effect on serum iron, hemoglobin and serum ferritin in the absence of H. pylori eradication. This indicates that gastric H. pylori infection is perturbing the normal physiological mechanisms of iron absorption.
An important question is whether eradication of H. pylori infection to improve iron status in children in developing countries could have adverse effects. Recent important studies in Tanzanian children indicate that the iron status influences Plasmodium falciparum malaria risk.Citation49 Iron deficiency was associated with significantly reduced parasitemia and severity of malaria and malaria-associated mortality.Citation49 In areas of high malaria risk H. pylori may have a beneficial role protecting from severe parasitaemia. H. pylori eradication in such areas for iron deficiency should only be undertaken in associated with effective malaria control.
Mechanisms of ID/IDA due to H. pylori infection
The mechanisms by which H. pylori infection might cause ID/IDA have not been well defined. Increased blood loss due to H. pylori-induced gastric lesions, deficient iron absorption due to decreased gastric acidity/gastric juice ascorbic acid concentrations and iron uptake by H. pylori have been proposed.
Erosive gastritis
Occult blood loss due to chronic erosive/hemorrhagic gastritis was one of the first supposed mechanisms of ID in H. pylori infection.Citation18 However, it does not appear to be the most relevant factor because neither gastric bleeding lesions are frequently found in H. pylori-positive children undergoing endoscopy,Citation19,Citation20,Citation38,Citation40 nor is the faecal occult blood test positive in the patients.Citation19,Citation40
Deficient iron absorption and reduced gastric juice ascorbic acid
Another proposed mechanism promoting ID in H. pylori infection involves deficient iron absorption due to decreased gastric acidity.Citation5,Citation50 Gastric acid is essential for iron absorption as it reduces ferric iron to the more soluble and absorbable ferrous iron form.Citation50 It has been demonstrated that H. pylori infected children have significantly lower basal and stimulated acid output than uninfected children and that eradication of H. pylori infection improves gastric acid output.Citation51 In addition, in Chilean children infected with H. pylori an association between hypochlorhydria and low serum iron and transferrin was observed.Citation6
Initial H. pylori infection is accompanied by a period of hypochlorhydria of variable duration.Citation52-Citation54 This could be linked to increased concentrations of IL-1β and TNF-α, potent inhibitors of gastric acid secretion by parietal cells,Citation55 in the gastric mucosa of H. pylori infected adults and children.Citation56-Citation58 Similar perturbations in gastric acid secretion occur in animals following infection with gastric Helicobacter species.Citation59,Citation60 In the study of Takashima et al.Citation60 in gerbils, treatment with recombinant IL-1 receptor antagonist reversed the hypochlorhydria induced by H. pylori infection implicating the IL-1B gene cluster in the hypochlorhydric response to H. pylori. Clinically the duration of hypochlorhydria is variable but experimental human infection studies indicate hypochlorhydria can extend for several months.Citation61 Data from infants in Gambia, using a non-invasive urinary test of gastric acid secretion,Citation62 similarly indicate H. pylori infected infants have extended periods of hypochlorhydria.Citation63
As H. pylori infection is mainly acquired in childhood,Citation1 it is plausible that infected children with hypochlorhydria may be at increased risk of developing ID/IDA. In fact, a recent study evaluating a cohort of symptomatic Brazilian children undergoing upper gastrointestinal endoscopyCitation7 demonstrated that the concentration of gastric IL-1β was inversely associated with blood ferritin and hemoglobin concentrations, suggesting that in the early phase of H. pylori infection, high gastric secretion of IL-1β inhibits acid secretion and impairs the absorption of iron. IL1β could also participate in the impairment of iron absorption by upregulating hepcidin as demonstrated in vivo.Citation64,Citation65 However, association between the serum concentrations of hepcidin and H. pylori infection has not been observed.Citation66
In long-term chronic H. pylori infection in adults, hypochlorhydria resulting from development of gastric atrophy with loss of parietal cells is considered an important mechanism of iron deficiency.Citation67,Citation68 Gastric corpus atrophy in H. pylori infected adults is more frequently observed in carriers of the IL1 gene cluster polymorphisms.Citation57 In Brazilian children it was demonstrated that hemoglobin and hematocrit values are lower in carriers of IL1RN polymorphic alleles than in children with the wild genotype.Citation7 High gastric secretion of IL-1β in the former group might cause more severe hypochlorhydria in the acute phase of H. pylori infection that is mainly acquired in early childhood.
Decreased gastric juice ascorbic acid concentrations have also been considered to contribute to deficient iron absorption in H. pylori-infected patients. Ascorbic acid maintains iron stability by chelating ferric iron as well as participating in the maintenance of iron in the more absorbable ferrous form.Citation50 Baysoy et al.Citation69 demonstrated decreased gastric juice ascorbic acid concentrations in H. pylori positive children, especially in those infected with cagA-positive strains. However, there were no differences in ascorbic acid concentrations between H. pylori infected children with, and without, IDA. However, an association between low gastric juice ascorbic acid and IDA in adults infected by H. pylori has been demonstrated.Citation67
Finally, H. pylori-positive childrenCitation69 and adultsCitation70 with IDA have more frequently a pattern of gastritis with corpus involvement that is accompanied by decreased gastric acid secretion that could impair iron absorption. Baysoy et al.Citation69 observed lower serum iron concentrations in infected children with pangastritis than in those with antral gastritis.
Iron-uptake by H. pylori
H. pylori has a high affinity uptake system to scavenge iron from the host with many proteins, such as FeoB, FrpB, iron-repressible outer membrane proteins (IROMPs), Pfr and NapA involved in the transport/storage of the metal,Citation71-Citation76 and the Fur protein, encoded by the fur gene, involved in ferric uptake regulation.Citation77 The sources of iron that H. pylori utilizes during colonization are not clear, but uptake of ferrous iron by FeoBCitation75,Citation76 and heme uptake by heme/hemoglobin-binding proteins such as FrpB2Citation76,Citation78 and IROMPsCitation72,Citation79 have been demonstrated.
Iron uptake from host transferrinCitation76,Citation80 and lactoferrinCitation73 have also been described. In the process of iron acquisition from transferrin, the virulence factors, VacA and CagA, are involved in the relocation of the transferrin/transferrin receptor from the basolateral to the apical surface of the gastric epithelial cell where the iron can be utilized by H. pylori.Citation81 Importantly, experimental studies indicate that only CagA-positive H. pylori strains are able to colonize iron deficient gerbils.Citation81 This raises the intriguing question of whether the high prevalence of cag pathogenicity island (cag PAI) positive H. pylori strains in many developing countries is a consequence of high levels of iron deficiency in the respective populations.
It seems that some H. pylori strains have enhanced iron-uptake activity as demonstrated by Yokota et al.Citation82 who compared strains isolated from patients with IDA to those from patients without IDA. The authors postulated that the high ability of H. pylori to uptake iron might be a causative factor for iron-deficiency anemia. Corroborating this finding, the expression of IROMPs under iron restricted conditions is higher in H. pylori strains isolated from patients with IDA than from those without IDA.Citation79
The proteomic profiles of H. pylori strains isolated from patients with IDA seems to be different from those isolated from patients without IDA,Citation83 suggesting that polymorphisms in genes that encode proteins involved in H. pylori iron acquisition might influence the development of ID in the host. The Thr70-type polymorphism in napA that encodes a bacterioferritin-like protein was associated with enhanced Fe iron uptake and occurred more frequently in strains isolated from Japanese patients with, than in those without, iron-deficiency.Citation84 Other polymorphisms in the napA gene have also been more frequently observed in strains isolated from IDA Korean adolescents.Citation85 Polymorphisms in the H. pylori feoB geneCitation86 that encodes FeoB, a high-affinity cytoplasmic membrane Fe+2 transporter protein, were reported to associate with IDA in Korean children, but not in adult Japanese patients. Polymorphisms in other Helicobacter genes such as pfrCitation87 that encodes a prokaryotic ferritin and fur,Citation84 a transcription repressor of iron acquisition systems, were not significantly associated with IDA.
High lactoferrin concentrations, which decreased after eradication of H. pylori, were observed in the gastric mucosa of H. pylori positive Korean adolescents with IDA compared with those without anemia, or in non-infected adolescents with IDA.Citation88 This points to lactoferrin sequestration in the gastric mucosa as another potential mechanism of iron deficiency associated with H. pylori infection.
H. pylori and Diarrheal Disease
The importance of gastric acid in preventing enteric infections is well established.Citation89,Citation90 Although some bacterial pathogens are less susceptible to gastric acid,Citation91 hypochlorhydria increases the risk of several infections including cholera,Citation92 typhoid and non-typhoidal salmonellosisCitation93 and enteric viruses.Citation94 In developed countries pharmacological induced hypochlorhydria is a recognized risk factor for Clostridium difficile-associated diarrhea.Citation95
In developing countries where enteric infections are endemic, the hypochlorhydria associated with initial H. pylori infection in infants and children, which can last several months, may be a critical window for acquisition of other enteropathogens and diarrheal disease (). Few studies have examined the impact of initial H. pylori infection in infants and children on diarrheal disease. Early studies in Gambian infants identified an association between H. pylori seropositivity and chronic diarrhea.Citation96 In Lima, Peru H. pylori seroconverting infants and children had increased diarrhea days and diarrhea episodes in the year after H. pylori infection than uninfected children.Citation97 Interestingly, the effects of H. pylori on diarrheal disease were greatest in the two months after infection,Citation97 the period when post-infection hypochlorhydria would be most evident.
The risk of co-infection with specific enteropathogenic infections in H. pylori positive children and/or adults has also been investigated in developing countries. In Peru H. pylori positivity has been associated with increased Vibrio cholerae infection in young children under 10 y of ageCitation98 and adults with chronic atrophic gastritis,Citation99 the latter study emphasing the important protective role of gastric acidity. In contrast, studies in Bangladeshis involving both children and adults have found no increase in cholera in H. pylori infected subjects;Citation100 however, evaluation of gastric pathology was not undertaken and the period of acute H. pylori infection was not specifically evaluated. H. pylori infection has also been associated with increased Shigella infection in young childrenCitation101 and Salmonella typhi in adults.Citation102
In contrast to the studies in PeruCitation97 and Gambia,Citation96 a study in Thai children did not observe an association between H. pylori infection and increased diarrheal disease.Citation103 Furthermore, studies in adults in two developed countries, USA and Germany, found a negative association between H. pylori infection and gastroenteritis.Citation104,Citation105 The conflicting results between developed and developing countries is unsurprising given the contrasting exposure to enteric pathogens and differing availability of clean water and sanitation. Further studies are required in infants and children to assess the role of H. pylori in childhood diarrheal disease in developing countries.
H. pylori and Growth Retardation
There is considerable variation in the results of studies evaluating the effect of H. pylori infection on growth in children. Cross-sectional studies in different countries points to the presenceCitation24,Citation106-Citation113 or absenceCitation114-Citation121 of association. There are other studies demonstrating associations only in the presence of iron-deficiency or dyspeptic symptoms.Citation122,Citation123 However, almost all longitudinal studies analyzing growth velocity, considered a more sensitive indicator of health status than weight and height,Citation124 point to association.Citation124-Citation128 Recently, new evidence on the role of H. pylori infection on growth emerged from the studies of Mera et al.Citation129 and Yang et al.Citation130 who demonstrated a beneficial effect of eradication of H. pylori on the growth of children from Colombia and China, respectively. However, only one studyCitation127 has examined childhood growth in the first two years of life, when the greatest impact of infection on growth is expected to occur and no birth-cohort studies have been conducted until recently.
Most of the cross-sectional studies evaluating differences in the height-for-age, weight-for-age, or the frequency of short stature, according to H. pylori status are from developing countries. In most studies, no adjustment for genetic factors and socioeconomic status, which are known to influence childhood growth, was done. Association between H. pylori infection and short stature was observed in prepubescent girls from a low income community in northeast Brazil,Citation110 in Egyptian childrenCitation111 and in children from low resource setting in Mexico.Citation112 Results of studies from Turkey are controversial: a negative effect of H. pylori infection on growthCitation31,Citation108,Citation113 and no association,Citation117,Citation119 or associations only in children with recurrent abdominal painCitation123 were observed. Absence of an association between height and H. pylori infection were described in Alaska native children;Citation118 in African refugee children from Australia;Citation120 in dyspeptic children from IranCitation121 and in children from Guatemala.Citation116
In developed countries, studies from Italy,Citation106 Germany,Citation107 and JapanCitation109 also point to negative effects of H. pylori infection on growth in childhood. However, no difference in the prevalence of H. pylori infection could be detected in Italian children with short statureCitation114,Citation115 as well as no difference in the weight and height could be demonstrated in children from UK after adjusting for socioeconomic factors.Citation131
Longitudinal cohort studies evaluating children from ColombiaCitation124,Citation126,Citation132 and EcuadorCitation128 demonstrate reduction on the velocity of growth associated with acquisition of H. pylori infection as diagnosed by urea breath test and stool antigen test. Of note, the associations remained significant after controlling for confounding variables linked to socio-economic status.
Bravo et al.Citation126 demonstrated that the deceleration of growth velocity occurs one to two months after acquisition of the infection, independently of the age of the children, and the final effect of H. pylori infection on the growth velocity was 0.5 cm/year. Mera et al.Citation132 observed that the reduction on growth velocity was significant for up to 6 mo after acquisition of H. pylori and after 8 mo the cumulative difference between H. pylori-positive and -negative children was 0.25 cm. In the study of Goodman et al.Citation124 H. pylori-positive children grew an average of 0.022 cm/month slower than the non-infected children. Egorov et al.Citation128 observed a reduction in the linear growth velocity of 9.7 mm/year in children with new H. pylori infection compared with uninfected children and 6.4 mm/year compared with children with chronic H. pylori infection.
Reduction in growth velocity associated with acquisition of the infection, evaluated by urea breath test and controlled for socioeconomic and demographic variables, in two cohorts of children from Gambia was transiently observed in children who acquired H. pylori infection early, at 3/6 mo of age, but reduced growth velocity was no longer detected when the children were 5 to 8 y old.Citation127 In developed countries, Patel et al.,Citation125 by evaluating 554 Scottish children, demonstrated that the growth of H. pylori-positive girls after four years follow-up was significantly lower than that of the uninfected girls.
Recently, two interventional studiesCitation129,Citation130 demonstrated that eradication of Helicobacter pylori increases childhood growth. In Colombia, the authors showed that although no difference in the height between H. pylori-positive and -negative children could be detected at baseline, in the 3.7 y follow-up, treated but not untreated children were 2.98 cm taller, after adjusting for demographic and socioeconomic variables.Citation129 In another study, Yang et al.Citation130 found that Taiwanese children with successful eradication of the bacterium had higher height and weight than the non-infected ones.
The mechanism by which H. pylori causes growth impairment in children remains to be investigated. Whether growth failure is a direct effect of the H. pylori-induced inflammation or a consequence of indirect effects such as infection-induced anorexia, H. pylori-associated intestinal permeability changes/malabsorption or diarrheal disease is unclear. The likelihood is both direct and indirect effects will contribute to growth impairment. Experimental studies on inflammation induced animal models of colitis indicate IL-6, which suppresses insulin-like growth factor (IGF-1), mediates growth failure.Citation133 Further studies are required to determine whether IL6 -174 G/C promoter polymorphisms associated with growth failure in children with Crohn diseaseCitation133 impact on growth impairment in infants/children with H. pylori infection.
Does H. pylori Infection Contribute to Malabsorption?
Mucosal small intestinal enteropathy and associated chronic permeability changes in infants in developing countries are considered a major factor in growth retardation.Citation134 The precise mechanisms of small intestinal mucosal damage contributing to malabsorption and its association with diarrhea and growth retardation has long been debated and subject of recent comprehensive reviews.Citation135,Citation136 To date no specific pathogens have been associated with enteropathy and malabsorption. The hypochlorhydria associated with initial H. pylori infections in infants in developing countries may result in establishment of small intestinal bacterial flora and lead, via microbial disturbances of intestinal barrier function, to changes in small intestinal permeability (). Whether such changes in intestinal microbiota resolve following restoration of normal acid secretion in H. pylori infected infants is unknown. While several recent studies have investigated changes in the gut microbiota in the first few years of life,Citation135 the changes in gut microbiota associated with H. pylori infection in poor resource settings is unknown.
Recent experimental studies in H. pylori infected mice have shown, using ex vivo techniques to measure jejunal intestinal permeability to 51Cr-EDTA and 14C-mannitol, that small intestinal permeability is significantly increased in H. pylori infected mice compared with uninfected controls.Citation137 Interestingly, eradication of infection in mice did not significantly improve permeability at two months post-eradication. Clinical studies indicate H. pylori infection in adults also increases intestinal permeability compared with H. pylori negative controls.Citation138
The extent and duration of hypochlorhydria in infants and children on initial H. pylori infection may vary with the cag PAI status of the colonizing H. pylori strain as well as polymorphisms in the IL-1β gene cluster.Citation6 In both animal modelsCitation139 and symptomatic adultsCitation140,Citation141 cag PAI strains are more likely to colonize the corpus mucosa with associated inflammation. In developed countries both increases, and changes in composition, of gastric non-H. pylori microbiota occur in H. pylori infected adults who have reduced acid secretion as a consequence of corpus atrophy, or following pharmacological treatment with proton pump inhibitors.Citation142 Similar changes in both gastric and small intestinal microbiota during the hypochlorhydric phase following initial H. pylori infection in infants in poor resource settings are entirely possible, but remain to be investigated.
Recent exciting studies in Malawian children have identified using discordant monozigous twin pairs for kwashiorkor that the metabolism of gut microbiota and local diet are key to malnutrition.Citation143 Gnotobiotic mice given stool samples obtained from children with kwashiorkor became malnourished on Malawian diet but changed gut microbiota and gained weight on ready-to-use therapeutic food (RUTF).Citation143 Furthermore, a recent double blind placebo controlled study in Malawian infants aged 6 to 59 mo with kwashiorkor highlighted the importance of gut microbiota in malnutrition. Antibiotic therapy, together with RUTF, significantly reduced mortality and increased weight gain in children with malnutrition being superior to RUTF and placebo.Citation144 These important recent studies suggest antibiotic manipulation of the gut microbiota, which contributes to malnutrition, has occurred. This raises the important question of whether antibiotics are targeting specific gut pathogens which contribute to malnutrition.Citation145 While normal H. pylori eradication therapy requires two antibiotics and acid suppressant proton pump inhibitors,Citation146 in H. pylori infected infants and young children with hypochlorhydria, antibiotic treatment alone would be sufficient for H. pylori eradication. At high pH the gastric mucous barrier is decreased increasing the gastric permeability of antibiotics.Citation147 This raises the intriguing question of whether reduced mortality and weight gain following antibiotic treatment in children with malabsorption in the study of Trehan et al.Citation144 was a consequence of H. pylori eradication, restoration of normal acid secretion and loss of small intestinal microbiota mediating permeability changes. In H. pylori positive pre-school children in Bangladeshi suppressed basal and stimulated gastric acid output is restored after successful eradication of H. pylori.Citation51 The restoration of gastric acid secretion after eradication of H. pylori infection may result in changes to gut microbiota relevant to reduced malabsorption. Future studies should aim to address whether antibiotic treatment in children with severe malabsorption in poor resource settings is changing their H. pylori status. Recent studies have validated non-invasive diagnostic 13C urea breath tests and stool antigen assays for detection of H. pylori infection in infants in developing countries.Citation4 This provides the necessary validated tools for non-invasively determining H. pylori status in infants in developing countries and addressing the impact of this infection on globally important potential extra-gastric manifestations of H. pylori infection in infants in developing countries.
Does H. pylori Infection in Children Impair Cognitive Function?
Gastrointestinal parasitic infections and early diarrheal diseaseCitation148-Citation150 in infancy in developing countries have been reported to impact negatively on later cognitive function in childhood and school performance in developing countries. Similarly, it has long been established that iron deficiency in infancy is associated with later impairment of cognitive function.Citation151,Citation152 The potential role of H. pylori infection in infancy on cognitive function has not been investigated in developing countries. Given the association of H. pylori infection with iron deficiency, as well as frequent other enteric infections and diarrheal disease in poor resource settings, investigations on direct or indirect effects of H. pylori infection on cognitive impairment would be challenging and require large longitudinal birth cohorts to be examined for cognitive function at school age.
Muhsen et al.Citation153 have recently assessed the cognitive function of school age children in the Israeli Arab population, who have high levels of H. pylori infection although living in a developed country. Children from three villages of differing socioeconomic status were studied. H. pylori infection in children in the high socioeconomic village was independently associated with impaired cognitive function at early school age assessed by both full-scale Intelligence Quotient (IQ) score and also reduced non-verbal IQ and verbal IQ scores.Citation153 In the low socioeconomic village an association between H. pylori infection and cognitive impairment was not observed, probably due to high levels of H. pylori infection. Further studies are required to evaluate the association of H. pylori infection with cognitive impairment particularly in developing countries. Recent longitudinal birth cohorts of infants in South America of defined H. pylori status, and with known diarrheal episodes and growth over the first two years of lifeCitation4 will be valuable populations in which to analyze the contribution of H. pylori infection to cognitive function in the children at school age. The association of H. pylori with impaired cognitive function is not confined to developing country populations as H. pylori seropositivity has recently been linked with poor cognition in adults in the United States.Citation154
Conclusions
The extra-gastric manifestations of H. pylori infection in children in developing countries are likely to be substantially greater than in developed countries and thus constitute a major public health problem. Childhood H. pylori infection in poor resource settings has been largely under investigated and further studies are required on the impact of H. pylori infection on growth impairment, diarrheal disease and cognitive impairment. Knowledge on host and bacterial factors contributing to iron deficiency in H. pylori infection in developing countries is increasing but further studies on host and bacterial polymorphisms are required in developing countries. The last Maastricht Florence Consensus Report (Maastricht IV)Citation155 recommends treating H. pylori-positive patients with IDA after the exclusion of the other common causes of the disease. Gastric carcinoma is an important cause of death in developing countries. Recently it was demonstrated that iron deficiency accelerates H. pylori-induced gastric carcinogenesis by enhancing deployment and function of the pilus component of the cag PAI type 4 secretion system, as well as upregulating proteins involved in survival and persistence of the bacterium.Citation156 It thus would be interesting also to consider eradication of H. pylori in those infected children without anemia, but with decreased serum ferritin. With high levels of antibiotic resistance in developing countries and significant H. pylori re-infection risk,Citation157 the optimum future strategy will be vaccination.
Acknowledgments
DQ and JEC were funded under the Sixth Framework Programme of the European Union, Project CONTENT (INCO-DEV-3–032136), “Evaluation and Control of Neglected Mucosal Enteric Infections in Childhood”
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther 2002; 16:Suppl 1 3 - 15; http://dx.doi.org/10.1046/j.1365-2036.2002.0160s1003.x; PMID: 11849122
- Bardhan PK. Epidemiological features of Helicobacter pylori infection in developing countries. Clin Infect Dis 1997; 25:973 - 8; http://dx.doi.org/10.1086/516067; PMID: 9402340
- Klein PD, Gilman RH, Leon-Barua R, Diaz F, Smith EO, Graham DY. The epidemiology of Helicobacter pylori in Peruvian children between 6 and 30 months of age. Am J Gastroenterol 1994; 89:2196 - 200; PMID: 7977241
- Queiroz DMM, Saito M, Rocha GA, Rocha AMC, Melo FF, Checkley W, et al. Helicobacter pylori infection in infants and toddlers in South America: concordance between 13C-urea breath test and monoclonal H. pylori stool antigen test. J Clin Microbiol 2013; Forthcoming 2013
- Windle HJ, Kelleher D, Crabtree JE. Childhood Helicobacter pylori infection and growth impairment in developing countries: a vicious cycle?. Pediatrics 2007; 119:e754 - 9; http://dx.doi.org/10.1542/peds.2006-2196; PMID: 17325213
- Harris PR, Serrano CA, Villagrán A, Walker MM, Thomson M, Duarte I, Windle HJ, Crabtree JE. Helicobacter pylori-associated hypochlorhydria in children, and development of iron deficiency. J Clin Pathol 2013; 66:343 - 7; http://dx.doi.org/10.1136/jclinpath-2012-201243; PMID: 23268321
- Queiroz DMM, Rocha AMC, Melo FF, Rocha GA, Teixeira KN, Carvalho SD, Bittencourt PF, Castro LP, Crabtree JE. Increased gastric IL-1β concentration and iron deficiency parameters in H. pylori infected children. PLoS One 2013; 8:e57420; http://dx.doi.org/10.1371/journal.pone.0057420; PMID: 23451225
- Stoltzfus RJ. Iron-deficiency anemia: reexamining the nature and magnitude of the public health problem. Summary: implications for research and programs. J Nutr 2001; 131:2S-2 697S - 700S, discussion 700S-1S; PMID: 11160600
- Iron deficiency anemia assessment, prevention, and control [Internet]. Geneva: WHO/UNICEF/UNU:c2001 [cited 2013 July 2]. Available from: http://www.who.int/nutrition/publications/en/ida_assessment_prevention_control.pdf.
- Stoltzfus RJ. Iron deficiency: global prevalence and consequences. Food Nutr Bull 2003; 24:Suppl S99 - 103; PMID: 17016951
- Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet 2007; 370:511 - 20; http://dx.doi.org/10.1016/S0140-6736(07)61235-5; PMID: 17693180
- Casey GJ, Montresor A, Cavalli-Sforza LT, Thu H, Phu LB, Tinh TT, Tien NT, Phuc TQ, Biggs BA. Elimination of iron deficiency anemia and soil transmitted helminth infection: evidence from a fifty-four month iron-folic acid and de-worming program. PLoS Negl Trop Dis 2013; 7:e2146; http://dx.doi.org/10.1371/journal.pntd.0002146; PMID: 23593517
- Harvey LJ, Armah CN, Dainty JR, Foxall RJ, John Lewis D, Langford NJ, Fairweather-Tait SJ. Impact of menstrual blood loss and diet on iron deficiency among women in the UK. Br J Nutr 2005; 94:557 - 64; http://dx.doi.org/10.1079/BJN20051493; PMID: 16197581
- Moy RJ. Prevalence, consequences and prevention of childhood nutritional iron deficiency: a child public health perspective. Clin Lab Haematol 2006; 28:291 - 8; http://dx.doi.org/10.1111/j.1365-2257.2006.00793.x; PMID: 16999717
- Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr 2001; 131:2S-2 649S - 66S, discussion 666S-8S; PMID: 11160596
- Stoltzfus RJ, Kvalsvig JD, Chwaya HM, Montresor A, Albonico M, Tielsch JM, Savioli L, Pollitt E. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: double blind, placebo controlled study. BMJ 2001; 323:1389 - 93; http://dx.doi.org/10.1136/bmj.323.7326.1389; PMID: 11744561
- Idjradinata P, Pollitt E. Reversal of developmental delays in iron-deficient anaemic infants treated with iron. Lancet 1993; 341:1 - 4; http://dx.doi.org/10.1016/0140-6736(93)92477-B; PMID: 7678046
- Blecker U, Renders F, Lanciers S, Vandenplas Y. Syncopes leading to the diagnosis of a Helicobacter pylori positive chronic active haemorrhagic gastritis. Eur J Pediatr 1991; 150:560 - 1; http://dx.doi.org/10.1007/BF02072207; PMID: 1954961
- Dufour C, Brisigotti M, Fabretti G, Luxardo P, Mori PG, Barabino A. Helicobacter pylori gastric infection and sideropenic refractory anemia. J Pediatr Gastroenterol Nutr 1993; 17:225 - 7; http://dx.doi.org/10.1097/00005176-199308000-00018; PMID: 8229554
- Carnicer J, Badía R, Argemí J. Helicobacter pylori gastritis and sideropenic refractory anemia. J Pediatr Gastroenterol Nutr 1997; 25:441; http://dx.doi.org/10.1097/00005176-199710000-00017; PMID: 9327380
- Kostaki M, Fessatou S, Karpathios T. Refractory iron-deficiency anaemia due to silent Helicobacter pylori gastritis in children. Eur J Pediatr 2003; 162:177 - 9; PMID: 12655422
- Russo-Mancuso G, Branciforte F, Licciardello M, La Spina M. Iron deficiency anemia as the only sign of infection with Helicobacter pylori: a report of 9 pediatric cases. Int J Hematol 2003; 78:429 - 31; http://dx.doi.org/10.1007/BF02983815; PMID: 14704035
- Choe YH, Kim SK, Hong YC. The relationship between Helicobacter pylori infection and iron deficiency: seroprevalence study in 937 pubescent children. Arch Dis Child 2003; 88:178; http://dx.doi.org/10.1136/adc.88.2.178-a; PMID: 12538339
- Yang YJ, Sheu BS, Lee SC, Yang HB, Wu JJ. Children of Helicobacter pylori-infected dyspeptic mothers are predisposed to H. pylori acquisition with subsequent iron deficiency and growth retardation. Helicobacter 2005; 10:249 - 55; http://dx.doi.org/10.1111/j.1523-5378.2005.00317.x; PMID: 15904483
- Baggett HC, Parkinson AJ, Muth PT, Gold BD, Gessner BD. Endemic iron deficiency associated with Helicobacter pylori infection among school-aged children in Alaska. Pediatrics 2006; 117:e396 - 404; http://dx.doi.org/10.1542/peds.2005-1129; PMID: 16452320
- Cardenas VM, Mulla ZD, Ortiz M, Graham DY. Iron deficiency and Helicobacter pylori infection in the United States. Am J Epidemiol 2006; 163:127 - 34; http://dx.doi.org/10.1093/aje/kwj018; PMID: 16306309
- Muhsen K, Barak M, Henig C, Alpert G, Ornoy A, Cohen D. Is the association between Helicobacter pylori infection and anemia age dependent?. Helicobacter 2010; 15:467 - 72; http://dx.doi.org/10.1111/j.1523-5378.2010.00793.x; PMID: 21083753
- Afifi RAR, Ali DK, Shaheen IAM. A localized case-control study of extra-gastric manifestations of Helicobacter pylori infection in children. Indian J Pediatr 2011; 78:418 - 22; http://dx.doi.org/10.1007/s12098-010-0308-6; PMID: 21165719
- Parkinson AJ, Gold BD, Bulkow L, Wainwright RB, Swaminathan B, Khanna B, Petersen KM, Fitzgerald MA. High prevalence of Helicobacter pylori in the Alaska native population and association with low serum ferritin levels in young adults. Clin Diagn Lab Immunol 2000; 7:885 - 8; PMID: 11063492
- Queiroz DMM, Harris PR, Sanderson IR, Windle HJ, Walker MM, Rocha AMC, Rocha GA, Carvalho SD, Bittencourt PF, de Castro LP, et al. Iron status and Helicobacter pylori infection in symptomatic children: an international multi-centered study. PLoS One 2013; 8:e68833; http://dx.doi.org/10.1371/journal.pone.0068833; PMID: 23861946
- Süoglu OD, Gökçe S, Saglam AT, Sökücü S, Saner G. Association of Helicobacter pylori infection with gastroduodenal disease, epidemiologic factors and iron-deficiency anemia in Turkish children undergoing endoscopy, and impact on growth. Pediatr Int 2007; 49:858 - 63; http://dx.doi.org/10.1111/j.1442-200X.2007.02444.x; PMID: 18045286
- Choi JW. Does Helicobacter pylori infection relate to iron deficiency anaemia in prepubescent children under 12 years of age?. Acta Paediatr 2003; 92:970 - 2; http://dx.doi.org/10.1111/j.1651-2227.2003.tb00633.x; PMID: 12948075
- Araf LN, Pereira CAB, Machado RS, Raguza D, Kawakami E. Helicobacter pylori and iron-deficiency anemia in adolescents in Brazil. J Pediatr Gastroenterol Nutr 2010; 51:477 - 80; http://dx.doi.org/10.1097/MPG.0b013e3181d40cd7; PMID: 20562724
- Janjetic MA, Goldman CG, Balcarce NE, Rua EC, González AB, Fuda JA, Meseri EI, Torti HE, Barrado J, Zubillaga MB, et al. Iron, zinc, and copper nutritional status in children infected with Helicobacter pylori.. J Pediatr Gastroenterol Nutr 2010; 51:85 - 9; http://dx.doi.org/10.1097/MPG.0b013e3181c2c2cd; PMID: 20410842
- Vendt N, Kool P, Teesalu K, Lillemäe K, Maaroos HI, Oona M. Iron deficiency and Helicobacter pylori infection in children. Acta Paediatr 2011; 100:1239 - 43; http://dx.doi.org/10.1111/j.1651-2227.2011.02281.x; PMID: 21434997
- Akcam M, Ozdem S, Yilmaz A, Gultekin M, Artan R. Serum ferritin, vitamin B(12), folate, and zinc levels in children infected with Helicobacter pylori.. Dig Dis Sci 2007; 52:405 - 10; http://dx.doi.org/10.1007/s10620-006-9422-8; PMID: 17211708
- de Oliveira AM, Rocha GA, Queiroz DMM, Mendes EN, de Carvalho AS, Ferrari TC, Nogueira AM. Evaluation of enzyme-linked immunosorbent assay for the diagnosis of Helicobacter pylori infection in children from different age groups with and without duodenal ulcer. J Pediatr Gastroenterol Nutr 1999; 28:157 - 61; http://dx.doi.org/10.1097/00005176-199902000-00012; PMID: 9932847
- Choe YH, Kim SK, Son BK, Lee DH, Hong YC, Pai SH. Randomized placebo-controlled trial of Helicobacter pylori eradication for iron-deficiency anemia in preadolescent children and adolescents. Helicobacter 1999; 4:135 - 9; http://dx.doi.org/10.1046/j.1523-5378.1999.98066.x; PMID: 10382128
- Choe YH, Lee JE, Kim SK. Effect of helicobacter pylori eradication on sideropenic refractory anaemia in adolescent girls with Helicobacter pylori infection. Acta Paediatr 2000; 89:154 - 7; http://dx.doi.org/10.1111/j.1651-2227.2000.tb01208.x; PMID: 10709883
- Choe YH, Kwon YS, Jung MK, Kang SK, Hwang TS, Hong YC. Helicobacter pylori-associated iron-deficiency anemia in adolescent female athletes. J Pediatr 2001; 139:100 - 4; http://dx.doi.org/10.1067/mpd.2001.114700; PMID: 11445801
- Kurekci AE, Atay AA, Sarici SU, Yesilkaya E, Senses Z, Okutan V, Ozcan O. Is there a relationship between childhood Helicobacter pylori infection and iron deficiency anemia?. J Trop Pediatr 2005; 51:166 - 9; http://dx.doi.org/10.1093/tropej/fmi015; PMID: 15855306
- Fagan RP, Dunaway CE, Bruden DL, Parkinson AJ, Gessner BD. Controlled, household-randomized, open-label trial of the effect of treatment of Helicobacter pylori infection on iron deficiency among children in rural Alaska: results at 40 months. J Infect Dis 2009; 199:652 - 60; http://dx.doi.org/10.1086/596659; PMID: 19125674
- Duque X, Moran S, Mera R, Medina M, Martinez H, Mendoza ME, Torres J, Correa P. Effect of eradication of Helicobacter pylori and iron supplementation on the iron status of children with iron deficiency. Arch Med Res 2010; 41:38 - 45; http://dx.doi.org/10.1016/j.arcmed.2009.11.006; PMID: 20430253
- Xia W, Zhang X, Wang J, Sun C, Wu L. Survey of anaemia and Helicobacter pylori infection in adolescent girls in Suihua, China and enhancement of iron intervention effects by H. pylori eradication. Br J Nutr 2012; 108:357 - 62; http://dx.doi.org/10.1017/S0007114511005666; PMID: 22004585
- Cardenas VM, Prieto-Jimenez CA, Mulla ZD, Rivera JO, Dominguez DC, Graham DY, Ortiz M. Helicobacter pylori eradication and change in markers of iron stores among non-iron-deficient children in El Paso, Texas: an etiologic intervention study. J Pediatr Gastroenterol Nutr 2011; 52:326 - 32; http://dx.doi.org/10.1097/MPG.0b013e3182054123; PMID: 21336159
- Sarker SA, Mahmud H, Davidsson L, Alam NH, Ahmed T, Alam N, Salam MA, Beglinger C, Gyr N, Fuchs GJ. Causal relationship of Helicobacter pylori with iron-deficiency anemia or failure of iron supplementation in children. Gastroenterology 2008; 135:1534 - 42; http://dx.doi.org/10.1053/j.gastro.2008.07.030; PMID: 18775429
- Yuan W, Li Yumin, Yang Kehu, Ma Bin, Guan Quanlin, Wang D, Yang L. Iron deficiency anemia in Helicobacter pylori infection: meta-analysis of randomized controlled trials. Scand J Gastroenterol 2010; 45:665 - 76; http://dx.doi.org/10.3109/00365521003663670; PMID: 20201716
- Huang X, Qu X, Yan W, Huang Y, Cai M, Hu B, Wu L, Lin H, Chen Z, Zhu C, et al. Iron deficiency anaemia can be improved after eradication of Helicobacter pylori.. Postgrad Med J 2010; 86:272 - 8; http://dx.doi.org/10.1136/pgmj.2009.089987; PMID: 20448223
- Gwamaka M, Kurtis JD, Sorensen BE, Holte S, Morrison R, Mutabingwa TK, Fried M, Duffy PE. Iron deficiency protects against severe Plasmodium falciparum malaria and death in young children. Clin Infect Dis 2012; 54:1137 - 44; http://dx.doi.org/10.1093/cid/cis010; PMID: 22354919
- Annibale B, Capurso G, Delle Fave G. The stomach and iron deficiency anaemia: a forgotten link. Dig Liver Dis 2003; 35:288 - 95; http://dx.doi.org/10.1016/S1590-8658(03)00067-7; PMID: 12801042
- Sarker SA, Davidsson L, Mahmud H, Walczyk T, Hurrell RF, Gyr N, Fuchs GJ. Helicobacter pylori infection, iron absorption, and gastric acid secretion in Bangladeshi children. Am J Clin Nutr 2004; 80:149 - 53; PMID: 15213042
- Sobala GM, Crabtree JE, Dixon MF, Schorah CJ, Taylor JD, Rathbone BJ, Heatley RV, Axon AT. Acute Helicobacter pylori infection: clinical features, local and systemic immune response, gastric mucosal histology, and gastric juice ascorbic acid concentrations. Gut 1991; 32:1415 - 8; http://dx.doi.org/10.1136/gut.32.11.1415; PMID: 1752479
- Harford WV, Barnett C, Lee E, Perez-Perez G, Blaser MJ, Peterson WL. Acute gastritis with hypochlorhydria: report of 35 cases with long term follow up. Gut 2000; 47:467 - 72; http://dx.doi.org/10.1136/gut.47.4.467; PMID: 10986205
- Wilson KT, Crabtree JE. Immunology of Helicobacter pylori: insights into the failure of the immune response and perspectives on vaccine studies. Gastroenterology 2007; 133:288 - 308; http://dx.doi.org/10.1053/j.gastro.2007.05.008; PMID: 17631150
- Beales IL, Calam J. Interleukin 1 beta and tumour necrosis factor alpha inhibit acid secretion in cultured rabbit parietal cells by multiple pathways. Gut 1998; 42:227 - 34; http://dx.doi.org/10.1136/gut.42.2.227; PMID: 9536948
- Crabtree JE, Shallcross TM, Heatley RV, Wyatt JI. Mucosal tumour necrosis factor alpha and interleukin-6 in patients with Helicobacter pylori associated gastritis. Gut 1991; 32:1473 - 7; http://dx.doi.org/10.1136/gut.32.12.1473; PMID: 1773951
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature 2000; 404:398 - 402; http://dx.doi.org/10.1038/35006081; PMID: 10746728
- Freire de Melo F, Rocha AMC, Rocha GA, Pedroso SHSP, de Assis Batista S, Fonseca de Castro LP, Carvalho SD, Bittencourt PF, de Oliveira CA, Corrêa-Oliveira R, et al. A regulatory instead of an IL-17 T response predominates in Helicobacter pylori-associated gastritis in children. Microbes Infect 2012; 14:341 - 7; http://dx.doi.org/10.1016/j.micinf.2011.11.008; PMID: 22155622
- Fox JG, Otto G, Taylor NS, Rosenblad W, Murphy JC. Helicobacter mustelae-induced gastritis and elevated gastric pH in the ferret (Mustela putorius furo). Infect Immun 1991; 59:1875 - 80; PMID: 2037349
- Takashima M, Furuta T, Hanai H, Sugimura H, Kaneko E. Effects of Helicobacter pylori infection on gastric acid secretion and serum gastrin levels in Mongolian gerbils. Gut 2001; 48:765 - 73; http://dx.doi.org/10.1136/gut.48.6.765; PMID: 11358893
- Graham DY, Opekun AR, Osato MS, El-Zimaity HMT, Lee CK, Yamaoka Y, Qureshi WA, Cadoz M, Monath TP. Challenge model for Helicobacter pylori infection in human volunteers. Gut 2004; 53:1235 - 43; http://dx.doi.org/10.1136/gut.2003.037499; PMID: 15306577
- Thomas JE, Eastham EJ, Weaver LT. New non-invasive test of gastric acid secretion for use in children. Gut 1993; 34:738 - 41; http://dx.doi.org/10.1136/gut.34.6.738; PMID: 8314504
- Dale A, Thomas JE, Darboe MK, Coward WA, Harding M, Weaver LT. Helicobacter pylori infection, gastric acid secretion, and infant growth. J Pediatr Gastroenterol Nutr 1998; 26:393 - 7; http://dx.doi.org/10.1097/00005176-199804000-00006; PMID: 9552134
- Inamura J, Ikuta K, Jimbo J, Shindo M, Sato K, Torimoto Y, Kohgo Y. Upregulation of hepcidin by interleukin-1β in human hepatoma cell lines. Hepatol Res 2005; 33:198 - 205; http://dx.doi.org/10.1016/j.hepres.2005.08.005; PMID: 16198622
- Lee P, Peng H, Gelbart T, Wang L, Beutler E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc Natl Acad Sci U S A 2005; 102:1906 - 10; http://dx.doi.org/10.1073/pnas.0409808102; PMID: 15684062
- Schwarz P, Kübler JAM, Strnad P, Müller K, Barth TFE, Gerloff A, Feick P, Peyssonnaux C, Vaulont S, Adler G, et al. Hepcidin is localised in gastric parietal cells, regulates acid secretion and is induced by Helicobacter pylori infection. Gut 2012; 61:193 - 201; http://dx.doi.org/10.1136/gut.2011.241208; PMID: 21757452
- Annibale B, Capurso G, Lahner E, Passi S, Ricci R, Maggio F, Delle Fave G. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut 2003; 52:496 - 501; http://dx.doi.org/10.1136/gut.52.4.496; PMID: 12631657
- Nahon S, Lahmek P, Massard J, Lesgourgues B, Mariaud de Serre N, Traissac L, Bodiguel V, Adotti F, Delas N. Helicobacter pylori-associated chronic gastritis and unexplained iron deficiency anemia: a reliable association?. Helicobacter 2003; 8:573 - 7; http://dx.doi.org/10.1111/j.1523-5378.2003.00184.x; PMID: 14632670
- Baysoy G, Ertem D, Ademoğlu E, Kotiloğlu E, Keskin S, Pehlivanoğlu E. Gastric histopathology, iron status and iron deficiency anemia in children with Helicobacter pylori infection. J Pediatr Gastroenterol Nutr 2004; 38:146 - 51; http://dx.doi.org/10.1097/00005176-200402000-00008; PMID: 14734875
- Capurso G, Lahner E, Marcheggiano A, Caruana P, Carnuccio A, Bordi C, Delle Fave G, Annibale B. Involvement of the corporal mucosa and related changes in gastric acid secretion characterize patients with iron deficiency anaemia associated with Helicobacter pylori infection. Aliment Pharmacol Ther 2001; 15:1753 - 61; http://dx.doi.org/10.1046/j.1365-2036.2001.01101.x; PMID: 11683689
- Doig P, Austin JW, Trust TJ. The Helicobacter pylori 19.6-kilodalton protein is an iron-containing protein resembling ferritin. J Bacteriol 1993; 175:557 - 60; PMID: 8419304
- Worst DJ, Otto BR, de Graaff J. Iron-repressible outer membrane proteins of Helicobacter pylori involved in heme uptake. Infect Immun 1995; 63:4161 - 5; PMID: 7558334
- Dhaenens L, Szczebara F, Husson MO. Identification, characterization, and immunogenicity of the lactoferrin-binding protein from Helicobacter pylori.. Infect Immun 1997; 65:514 - 8; PMID: 9009306
- Tonello F, Dundon WG, Satin B, Molinari M, Tognon G, Grandi G, Del Giudice G, Rappuoli R, Montecucco C. The Helicobacter pylori neutrophil-activating protein is an iron-binding protein with dodecameric structure. Mol Microbiol 1999; 34:238 - 46; http://dx.doi.org/10.1046/j.1365-2958.1999.01584.x; PMID: 10564468
- Velayudhan J, Hughes NJ, McColm AA, Bagshaw J, Clayton CL, Andrews SC, Kelly DJ. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. Mol Microbiol 2000; 37:274 - 86; http://dx.doi.org/10.1046/j.1365-2958.2000.01987.x; PMID: 10931324
- Ge R, Sun X. Iron trafficking system in Helicobacter pylori.. Biometals 2012; 25:247 - 58; http://dx.doi.org/10.1007/s10534-011-9512-8; PMID: 22127376
- van Vliet AHM, Stoof J, Vlasblom R, Wainwright SA, Hughes NJ, Kelly DJ, Bereswill S, Bijlsma JJ, Hoogenboezem T, Vandenbroucke-Grauls CM, et al. The role of the Ferric Uptake Regulator (Fur) in regulation of Helicobacter pylori iron uptake. Helicobacter 2002; 7:237 - 44; http://dx.doi.org/10.1046/j.1523-5378.2002.00088.x; PMID: 12165031
- González-López MA, Olivares-Trejo JJ. The gene frpB2 of Helicobacter pylori encodes an hemoglobin-binding protein involved in iron acquisition. Biometals 2009; 22:889 - 94; http://dx.doi.org/10.1007/s10534-009-9240-5; PMID: 19357969
- Lee JH, Choe YH, Choi YO. The expression of iron-repressible outer membrane proteins in Helicobacter pylori and its association with iron deficiency anemia. Helicobacter 2009; 14:36 - 9; http://dx.doi.org/10.1111/j.1523-5378.2009.00658.x; PMID: 19191894
- Senkovich O, Ceaser S, McGee DJ, Testerman TL. Unique host iron utilization mechanisms of Helicobacter pylori revealed with iron-deficient chemically defined media. Infect Immun 2010; 78:1841 - 9; http://dx.doi.org/10.1128/IAI.01258-09; PMID: 20176792
- Tan S, Noto JM, Romero-Gallo J, Peek RM Jr., Amieva MR. Helicobacter pylori perturbs iron trafficking in the epithelium to grow on the cell surface. PLoS Pathog 2011; 7:e1002050; http://dx.doi.org/10.1371/journal.ppat.1002050; PMID: 21589900
- Yokota S, Konno M, Mino E, Sato K, Takahashi M, Fujii N. Enhanced Fe ion-uptake activity in Helicobacter pylori strains isolated from patients with iron-deficiency anemia. Clin Infect Dis 2008; 46:e31 - 3; http://dx.doi.org/10.1086/526784; PMID: 18197760
- Park SA, Lee HW, Hong MH, Choi YW, Choe YH, Ahn BY, Cho YJ, Kim DS, Lee NG. Comparative proteomic analysis of Helicobacter pylori strains associated with iron deficiency anemia. Proteomics 2006; 6:1319 - 28; http://dx.doi.org/10.1002/pmic.200500293; PMID: 16404725
- Yokota S, Toita N, Yamamoto S, Fujii N, Konno M. Positive relationship between a polymorphism in Helicobacter pylori neutrophil-activating protein a gene and iron-deficiency anemia. Helicobacter 2013; 18:112 - 6; http://dx.doi.org/10.1111/hel.12011; PMID: 23067298
- Hee MH, Choe YH, Cho YJ, Ahn BY, Lee NG. Sequencing and comparative analysis of napA genes from Helicobacter pylori strains associated with iron-deficiency anemia. J Microbiol Biotechnol 2005; 15:866 - 72
- Jeon BH, Oh YJ, Lee NG, Choe YH. Polymorphism of the Helicobacter pylori feoB gene in Korea: a possible relation with iron-deficiency anemia?. Helicobacter 2004; 9:330 - 4; http://dx.doi.org/10.1111/j.1083-4389.2004.00239.x; PMID: 15270747
- Choe YH, Hwang TS, Kim HJ, Shin SH, Song SU, Choi MS. A possible relation of the Helicobacter pylori pfr gene to iron deficiency anemia?. Helicobacter 2001; 6:55 - 9; http://dx.doi.org/10.1046/j.1523-5378.2001.00007.x; PMID: 11328366
- Choe YH, Oh YJ, Lee NG, Imoto I, Adachi Y, Toyoda N, Gabazza EC. Lactoferrin sequestration and its contribution to iron-deficiency anemia in Helicobacter pylori-infected gastric mucosa. J Gastroenterol Hepatol 2003; 18:980 - 5; http://dx.doi.org/10.1046/j.1440-1746.2003.03098.x; PMID: 12859729
- Howden CW, Hunt RH. Relationship between gastric secretion and infection. Gut 1987; 28:96 - 107; http://dx.doi.org/10.1136/gut.28.1.96; PMID: 3546004
- Martinsen TC, Bergh K, Waldum HL. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol 2005; 96:94 - 102; http://dx.doi.org/10.1111/j.1742-7843.2005.pto960202.x; PMID: 15679471
- Lin J, Lee IS, Frey J, Slonczewski JL, Foster JW. Comparative analysis of extreme acid survival in Salmonella typhimurium, Shigella flexneri, and Escherichia coli. J Bacteriol 1995; 177:4097 - 104; PMID: 7608084
- Evans CA, Gilman RH, Rabbani GH, Salazar G, Ali A. Gastric acid secretion and enteric infection in Bangladesh. Trans R Soc Trop Med Hyg 1997; 91:681 - 5; http://dx.doi.org/10.1016/S0035-9203(97)90523-X; PMID: 9509179
- Khosla SN, Jain N, Khosla A. Gastric acid secretion in typhoid fever. Postgrad Med J 1993; 69:121 - 3; http://dx.doi.org/10.1136/pgmj.69.808.121; PMID: 8506192
- Weiss C, Clark HF. Rapid inactivation of rotaviruses by exposure to acid buffer or acidic gastric juice. J Gen Virol 1985; 66:2725 - 30; http://dx.doi.org/10.1099/0022-1317-66-12-2725; PMID: 2999315
- Yearsley KA, Gilby LJ, Ramadas AV, Kubiak EM, Fone DL, Allison MC. Proton pump inhibitor therapy is a risk factor for Clostridium difficile-associated diarrhoea. Aliment Pharmacol Ther 2006; 24:613 - 9; http://dx.doi.org/10.1111/j.1365-2036.2006.03015.x; PMID: 16907893
- Sullivan PB, Thomas JE, Wight DGD, Neale G, Eastham EJ, Corrah T, Lloyd-Evans N, Greenwood BM. Helicobacter pylori in Gambian children with chronic diarrhoea and malnutrition. Arch Dis Child 1990; 65:189 - 91; http://dx.doi.org/10.1136/adc.65.2.189; PMID: 2317065
- Passaro DJ, Taylor DN, Meza R, Cabrera L, Gilman RH, Parsonnet J. Acute Helicobacter pylori infection is followed by an increase in diarrheal disease among Peruvian children. Pediatrics 2001; 108:E87; http://dx.doi.org/10.1542/peds.108.5.e87; PMID: 11694671
- Shahinian ML, Passaro DJ, Swerdlow DL, Mintz ED, Rodriguez M, Parsonnet J. Helicobacter pylori and epidemic Vibrio cholerae 01 infection in Peru. Lancet 2000; 335:377 - 8; http://dx.doi.org/10.1016/S0140-6736(99)05143-0
- León-Barúa R, Recavarren-Arce S, Chinga-Alayo E, Rodríguez-Ulloa C, Taylor DN, Gotuzzo E, Kosek M, Eza D, Gilman RH. Helicobacter pylori-associated chronic atrophic gastritis involving the gastric body and severe disease by Vibrio cholerae. Trans R Soc Trop Med Hyg 2006; 100:567 - 72; http://dx.doi.org/10.1016/j.trstmh.2005.09.013; PMID: 16376396
- Clemens J, Albert MJ, Rao M, Qadri F, Huda S, Kay B, van Loon FP, Sack D, Pradhan BA, Sack RB. Impact of infection by Helicobacter pylori on the risk and severity of endemic cholera. J Infect Dis 1995; 171:1653 - 6; http://dx.doi.org/10.1093/infdis/171.6.1653; PMID: 7769312
- Shmuely H, Samra Z, Ashkenazi S, Dinari G, Chodick G, Yahav J. Association of Helicobacter pylori infection with Shigella gastroenteritis in young children. Am J Gastroenterol 2004; 99:2041 - 5; http://dx.doi.org/10.1111/j.1572-0241.2004.40120.x; PMID: 15447770
- Vollaard AM, Verspaget HW, Ali S, Visser LG, Veenendaal RA, Van Asten HA, Widjaja S, Surjadi Ch, Van Dissel JT. Helicobacter pylori infection and typhoid fever in Jakarta, Indonesia. Epidemiol Infect 2006; 134:163 - 70; http://dx.doi.org/10.1017/S0950268805004875; PMID: 16409664
- Isenbarger DW, Bodhidatta L, Hoge CW, Nirdnoy W, Pitarangsi C, Umpawasiri U, Echeverria P. Prospective study of the incidence of diarrheal disease and Helicobacter pylori infection among children in an orphanage in Thailand. Am J Trop Med Hyg 1998; 59:796 - 800; PMID: 9840601
- Perry S, Sanchez L, Yang S, Haggerty TD, Hurst P, Parsonnet J. Helicobacter pylori and risk of gastroenteritis. J Infect Dis 2004; 190:303 - 10; http://dx.doi.org/10.1086/421705; PMID: 15216465
- Rothenbacher D, Blaser MJ, Bode G, Brenner H. Inverse relationship between gastric colonization of Helicobacter pylori and diarrheal illnesses in children: results of a population-based cross-sectional study. J Infect Dis 2000; 182:1446 - 9; http://dx.doi.org/10.1086/315887; PMID: 11015236
- Perri F, Pastore M, Leandro G, Clemente R, Ghoos Y, Peeters M, Annese V, Quitadamo M, Latiano A, Rutgeerts P, et al. Helicobacter pylori infection and growth delay in older children. Arch Dis Child 1997; 77:46 - 9; http://dx.doi.org/10.1136/adc.77.1.46; PMID: 9279151
- Richter T, Richter T, List S, Müller DM, Deutscher J, Uhlig HH, Krumbiegel P, Herbarth O, Gutsmuths FJ, Kiess W. Five- to 7-year-old children with Helicobacter pylori infection are smaller than Helicobacter-negative children: a cross-sectional population-based study of 3,315 children. J Pediatr Gastroenterol Nutr 2001; 33:472 - 5; http://dx.doi.org/10.1097/00005176-200110000-00010; PMID: 11698766
- Ertem D, Pehlivanoglu E. Helicobacter pylori may influence height in children independent of socioeconomic factors. J Pediatr Gastroenterol Nutr 2002; 35:232 - 3; http://dx.doi.org/10.1097/00005176-200208000-00028; PMID: 12187306
- Takahashi M, Kimura H, Watanabe K. Helicobacter pylori infection in patients with idiopathic short stature. Pediatr Int 2002; 44:277 - 80; http://dx.doi.org/10.1046/j.1442-200X.2002.01557.x; PMID: 11982896
- Fialho AMN, Braga ABC, Queiroz DMM, Rodrigues MN, Herbster ID, Braga LLBC. The association between Helicobacter pylori infection and height in children from an urban community in north-east Brazil. Ann Trop Paediatr 2007; 27:55 - 61; http://dx.doi.org/10.1179/146532807X170510; PMID: 17469733
- Mohammad MA, Hussein L, Coward A, Jackson SJ. Prevalence of Helicobacter pylori infection among Egyptian children: impact of social background and effect on growth. Public Health Nutr 2008; 11:230 - 6; http://dx.doi.org/10.1017/S1368980007000481; PMID: 17666124
- Vilchis J, Duque X, Mera R, Morán S, Torres J, González-Cossío T, Kageyama-Escobar MdeL, Navarro F, Mendoza ME, Correa P. Association of Helicobacter pylori infection and height of Mexican children of low socioeconomic level attending boarding schools. Am J Trop Med Hyg 2009; 81:1091 - 6; http://dx.doi.org/10.4269/ajtmh.2009.09-0107; PMID: 19996442
- Ozen A, Furman A, Berber M, Karatepe HO, Mutlu N, Sarıçoban HE, Büyükgebiz B. The effect of Helicobacter pylori and economic status on growth parameters and leptin, ghrelin, and insulin-like growth factor (IGF)-I concentrations in children. Helicobacter 2011; 16:55 - 65; http://dx.doi.org/10.1111/j.1523-5378.2010.00814.x; PMID: 21241414
- Oderda G, Palli D, Saieva C, Chiorboli E, Bona G. Short stature and Helicobacter pylori infection in italian children: prospective multicentre hospital based case-control study. The Italian Study Group on Short Stature and H pylori. BMJ 1998; 317:514 - 5; http://dx.doi.org/10.1136/bmj.317.7157.514; PMID: 9712599
- Vaira D, Menegatti M, Salardi S, Alì A, Altomare Stella F, Figura N, Landi F, Holton J, Farinelli S, Cuccaro V, et al. Helicobacter pylori and diminished growth in children: is it simply a marker of deprivation?. Ital J Gastroenterol Hepatol 1998; 30:129 - 33; PMID: 9615281
- Quiñonez JM, Chew F, Torres O, Bégué RE. Nutritional status of Helicobacter pylori-infected children in Guatemala as compared with uninfected peers. Am J Trop Med Hyg 1999; 61:395 - 8; PMID: 10497978
- Ozçay F, Demir H, Özen H, Gürakan F, Saltik IN, Yüce A, Koçak N. Normal growth in young children with Helicobacter pylori infection. J Pediatr Gastroenterol Nutr 2002; 35:102; http://dx.doi.org/10.1097/00005176-200207000-00024; PMID: 12142822
- Chimonas MAR, Baggett HC, Parkinson AJ, Muth PT, Dunaway E, Gessner BD. Asymptomatic Helicobacter pylori infection and iron deficiency are not associated with decreased growth among Alaska Native children aged 7-11 years. Helicobacter 2006; 11:159 - 67; http://dx.doi.org/10.1111/j.1523-5378.2006.00395.x; PMID: 16684263
- Soylu ÖB, Ozturk Y. Helicobacter pylori infection: effect on malnutrition and growth failure in dyspeptic children. Eur J Pediatr 2008; 167:557 - 62; http://dx.doi.org/10.1007/s00431-007-0552-6; PMID: 17618457
- Cherian S, Forbes D, Sanfilippo F, Cook A, Burgner D. Helicobacter pylori, helminth infections and growth: a cross-sectional study in a high prevalence population. Acta Paediatr 2009; 98:860 - 4; http://dx.doi.org/10.1111/j.1651-2227.2009.01221.x; PMID: 19191761
- Dehghani SM, Karamifar H, Raeesi T, Haghighat M. Growth parameters in children with dyspepsia symptoms and Helicobacter pylori infection. Indian Pediatr 2013; 50:324 - 6; http://dx.doi.org/10.1007/s13312-013-0090-4; PMID: 23024103
- Choe YH, Kim SK, Hong YC. Helicobacter pylori infection with iron deficiency anaemia and subnormal growth at puberty. Arch Dis Child 2000; 82:136 - 40; http://dx.doi.org/10.1136/adc.82.2.136; PMID: 10648367
- Gulcan M, Ozen A, Karatepe HO, Gulcu D, Vitrinel A. Impact of H. pylori on growth: is the infection or mucosal disease related to growth impairment?. Dig Dis Sci 2010; 55:2878 - 86; http://dx.doi.org/10.1007/s10620-009-1091-y; PMID: 20112067
- Goodman KJ, Correa P, Mera R, Yepez MC, Cerón C, Campo C, Guerrero N, Sierra MS, Bravo LE. Effect of Helicobacter pylori infection on growth velocity of school-age Andean children. Epidemiology 2011; 22:118 - 26; http://dx.doi.org/10.1097/EDE.0b013e3181fe7e31; PMID: 21068668
- Patel P, Mendall MA, Khulusi S, Northfield TC, Strachan DP. Helicobacter pylori infection in childhood: risk factors and effect on growth. BMJ 1994; 309:1119 - 23; http://dx.doi.org/10.1136/bmj.309.6962.1119; PMID: 7987103
- Bravo LE, Mera R, Reina JC, Pradilla A, Alzate A, Fontham E, Correa P. Impact of Helicobacter pylori infection on growth of children: a prospective cohort study. J Pediatr Gastroenterol Nutr 2003; 37:614 - 9; http://dx.doi.org/10.1097/00005176-200311000-00021; PMID: 14581807
- Thomas JE, Dale A, Bunn JEG, Harding M, Coward WA, Cole TJ, Weaver LT. Early Helicobacter pylori colonisation: the association with growth faltering in The Gambia. Arch Dis Child 2004; 89:1149 - 54; http://dx.doi.org/10.1136/adc.2002.015313; PMID: 15557054
- Egorov AI, Sempértegui F, Estrella B, Egas J, Naumova EN, Griffiths JK. The effect of Helicobacter pylori infection on growth velocity in young children from poor urban communities in Ecuador. Int J Infect Dis 2010; 14:e788 - 91; http://dx.doi.org/10.1016/j.ijid.2010.03.013; PMID: 20638884
- Mera RM, Bravo LE, Goodman KJ, Yepez MC, Correa P. Long-term effects of clearing Helicobacter pylori on growth in school-age children. Pediatr Infect Dis J 2012; 31:263 - 6; http://dx.doi.org/10.1097/INF.0b013e3182443fec; PMID: 22315005
- Yang YJ, Sheu BS, Yang HB, Lu CC, Chuang CC. Eradication of Helicobacter pylori increases childhood growth and serum acylated ghrelin levels. World J Gastroenterol 2012; 18:2674 - 81; http://dx.doi.org/10.3748/wjg.v18.i21.2674; PMID: 22690077
- Sood MR, Joshi S, Akobeng AK, Mitchell J, Thomas AG. Growth in children with Helicobacter pylori infection and dyspepsia. Arch Dis Child 2005; 90:1025 - 8; http://dx.doi.org/10.1136/adc.2004.066803; PMID: 15956048
- Mera RM, Correa P, Fontham EE, Reina JC, Pradilla A, Alzate A, Bravo LE. Effects of a new Helicobacter pylori infection on height and weight in Colombian children. Ann Epidemiol 2006; 16:347 - 51; http://dx.doi.org/10.1016/j.annepidem.2005.08.002; PMID: 16246582
- Sawczenko A, Azooz O, Paraszczuk J, Idestrom M, Croft NM, Savage MO, Ballinger AB, Sanderson IR. Intestinal inflammation-induced growth retardation acts through IL-6 in rats and depends on the -174 IL-6 G/C polymorphism in children. Proc Natl Acad Sci U S A 2005; 102:13260 - 5; http://dx.doi.org/10.1073/pnas.0503589102; PMID: 16150725
- Lunn PG, Northrop-Clewes CA, Downes RM. Intestinal permeability, mucosal injury, and growth faltering in Gambian infants. Lancet 1991; 338:907 - 10; http://dx.doi.org/10.1016/0140-6736(91)91772-M; PMID: 1681266
- Gordon JI, Dewey KG, Mills DA, Medzhitov RM. The human gut microbiota and undernutrition. Sci Transl Med 2012; 4:37ps12; http://dx.doi.org/10.1126/scitranslmed.3004347; PMID: 22674549
- Guerrant RL, DeBoer M. D., Moore SR, Scharf RJ, Lima A.A.M. The impoverished gut- a triple burden of diarrhoea, stunting and chronic disease. Nature Rev Gastroenterol Hepatol 2013; 10:220 - 9; http://dx.doi.org/10.1038/nrgastro.2012.239
- Verdu EF, Bercik P, Huang XX, Lu J, Al-Mutawaly N, Sakai H, Tompkins TA, Croitoru K, Tsuchida E, Perdue M, et al. The role of luminal factors in the recovery of gastric function and behavioral changes after chronic Helicobacter pylori infection. Am J Physiol Gastrointest Liver Physiol 2008; 295:G664 - 70; http://dx.doi.org/10.1152/ajpgi.90316.2008; PMID: 18653723
- Di Leo V, D’Inca R, Bettini MB, Podswiadek M, Punzi L, Mastropaolo G, Sturniolo GC. Effect of Helicobacter pylori and eradication therapy on gastrointestinal permeability. Implications for patients with seronegative spondyloarthritis. J Rheumatol 2005; 32:295 - 300; PMID: 15693091
- Rieder G, Merchant JL, Haas R. Helicobacter pylori cag-type IV secretion system facilitates corpus colonization to induce precancerous conditions in Mongolian gerbils. Gastroenterology 2005; 128:1229 - 42; http://dx.doi.org/10.1053/j.gastro.2005.02.064; PMID: 15887107
- Shimoyama T, Everett SM, Dixon MF, Axon ATR, Crabtree JE. Chemokine mRNA expression in gastric mucosa is associated with Helicobacter pylori cagA positivity and severity of gastritis. J Clin Pathol 1998; 51:765 - 70; http://dx.doi.org/10.1136/jcp.51.10.765; PMID: 10023340
- Peek RM Jr., Crabtree JE. Helicobacter infection and gastric neoplasia. J Pathol 2006; 208:233 - 48; http://dx.doi.org/10.1002/path.1868; PMID: 16362989
- Engstrand L, Lindberg M. Helicobacter pylori and the gastric microbiota. Best Pract Res Clin Gastroenterol 2013; 27:39 - 45; http://dx.doi.org/10.1016/j.bpg.2013.03.016; PMID: 23768551
- Smith MI, Yatsunenko T, Manary MJ, Trehan I, Mkakosya R, Cheng J, Kau AL, Rich SS, Concannon P, Mychaleckyj JC, et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 2013; 339:548 - 54; http://dx.doi.org/10.1126/science.1229000; PMID: 23363771
- Trehan I, Goldbach HS, LaGrone LN, Meuli GJ, Wang RJ, Maleta KM, Manary MJ. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 2013; 368:425 - 35; http://dx.doi.org/10.1056/NEJMoa1202851; PMID: 23363496
- Tilg H, Moschen AR. Malnutrition and microbiota--a new relationship?. Nat Rev Gastroenterol Hepatol 2013; 10:261 - 2; http://dx.doi.org/10.1038/nrgastro.2013.48; PMID: 23507796
- Koletzko S, Richy F, Bontems P, Crone J, Kalach N, Monteiro ML, Gottrand F, Celinska-Cedro D, Roma-Giannikou E, Orderda G, et al. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut 2006; 55:1711 - 6; http://dx.doi.org/10.1136/gut.2006.091272; PMID: 16603633
- Goddard AF, Jessa MJ, Barrett DA, Shaw PN, Idström JP, Cederberg C, Spiller RC. Effect of omeprazole on the distribution of metronidazole, amoxicillin, and clarithromycin in human gastric juice. Gastroenterology 1996; 111:358 - 67; http://dx.doi.org/10.1053/gast.1996.v111.pm8690200; PMID: 8690200
- Niehaus MD, Moore SR, Patrick PD, Derr LL, Lorntz B, Lima AA, Guerrant RL. Early childhood diarrhea is associated with diminished cognitive function 4 to 7 years later in children in a northeast Brazilian shantytown. Am J Trop Med Hyg 2002; 66:590 - 3; PMID: 12201596
- Lorntz B, Soares AM, Moore SR, Pinkerton R, Gansneder B, Bovbjerg VE, Guyatt H, Lima AM, Guerrant RL. Early childhood diarrhea predicts impaired school performance. Pediatr Infect Dis J 2006; 25:513 - 20; http://dx.doi.org/10.1097/01.inf.0000219524.64448.90; PMID: 16732149
- Berkman DS, Lescano AG, Gilman RH, Lopez SL, Black MM. Effects of stunting, diarrhoeal disease, and parasitic infection during infancy on cognition in late childhood: a follow-up study. Lancet 2002; 359:564 - 71; http://dx.doi.org/10.1016/S0140-6736(02)07744-9; PMID: 11867110
- Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med 1991; 325:687 - 94; http://dx.doi.org/10.1056/NEJM199109053251004; PMID: 1870641
- Lozoff B, Jimenez E, Smith JB. Double burden of iron deficiency in infancy and low socioeconomic status: a longitudinal analysis of cognitive test scores to age 19 years. Arch Pediatr Adolesc Med 2006; 160:1108 - 13; http://dx.doi.org/10.1001/archpedi.160.11.1108; PMID: 17088512
- Muhsen K, Ornoy A, Akawi A, Alpert G, Cohen D. An association between Helicobacter pylori infection and cognitive function in children at early school age: a community-based study. BMC Pediatr 2011; 11:43 - 51; http://dx.doi.org/10.1186/1471-2431-11-43; PMID: 21612616
- Beydoun MA, Beydoun HA, Shroff MR, Kitner-Triolo MH, Zonderman AB. Helicobacter pylori seropositivity and cognitive performance among US adults: evidence from a large national survey. Psychosom Med 2013; 75:486 - 96; http://dx.doi.org/10.1097/PSY.0b013e31829108c3; PMID: 23697465
- Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al, European Helicobacter Study Group. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012; 61:646 - 64; http://dx.doi.org/10.1136/gutjnl-2012-302084; PMID: 22491499
- Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, Colvin DC, Romero-Gallo J, Suarez G, Loh J, Slaughter JC, et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J Clin Invest 2013; 123:479 - 92; http://dx.doi.org/10.1172/JCI64373; PMID: 23257361
- Morgan DR, Torres J, Sexton R, Herrero R, Salazar-Martínez E, Greenberg ER, Bravo LE, Dominguez RL, Ferreccio C, Lazcano-Ponce EC, et al. Risk of recurrent Helicobacter pylori infection 1 year after initial eradication therapy in 7 Latin American communities. JAMA 2013; 309:578 - 86; http://dx.doi.org/10.1001/jama.2013.311; PMID: 23403682